Abstract
Kidney stones are one of the oldest known and common diseases in the urinary tract system. Various human studies have suggested that diets with a higher intake of vegetables and fruits play a role in the prevention of kidney stones. In this review, we have provided an overview of these dietary plants, their main chemical constituents, and their possible mechanisms of action. Camellia sinensis (green tea), Rubus idaeus (raspberry), Rubia cordifolia (common madder), Petroselinum crispum (parsley), Punica granatum (pomegranate), Pistacia lentiscus (mastic), Solanum xanthocarpum (yellow-fruit nightshade), Urtica dioica (stinging nettle), Dolichos biflorus (horse gram), Ammi visnaga (khella), Nigella sativa (black-cumin), Hibiscus sabdariffa (roselle), and Origanum vulgare (oregano) have received considerable interest based on scientific evidence. Beside these dietary plants, phytochemicals—such as catechin, epicatechin, epigallocatechin-3-gallate, diosmin, rutin, quercetin, hyperoside, and curcumin—as antioxidant dietary phyto-phenols were found to be effective for the prevention of urolithiasis (the process of stone formation in the urinary tract). The main underlying mechanisms of these dietary plants and their isolated phytonutrients in the management of urolithiasis include diuretic, antispasmodic, and antioxidant activity, as well as an inhibitory effect on crystallization, nucleation, and aggregation of crystals. The results as presented in this review demonstrate the promising role of dietary plants and phytophenols in the prevention and management of kidney stones. Further investigations are required to confirm the safety and efficacy of these compounds.
Keywords: nephrolithiasis, dietary plants, urolithiasis, natural dietary supplement, phytochemicals, kidney stone
1. Introduction
Kidney stones, the formation of stones in the kidneys, is one of the oldest known and widespread diseases in the urinary tract system with a relapse rate of 50% in 5–10 years [1,2]. It is the third most common disorder among urinary diseases [3]. It has been reported that 10–12% of people in industrialized countries (10% of men and 3% of women) have a urinary stone during their lives. The etiology of this disorder is multifactorial and is related to genetics, diet, and low activity [4,5]. Calcium-containing stones are the most common kidney stones (75–90%), followed by magnesium ammonium phosphate (struvite) (10–15%), uric acid (3–10%), and cystine (0.5–1%) [6]. The mechanisms related to the development of kidney stones are not completely understood. Generally, it is believed that urolithiasis, the process of stone formation in the urinary tract, causes crystal aggregation, nucleation, and growth of insoluble particles [7]. The stones may cause various symptoms, including pain, obstruction, infection, and hemorrhage, through the passage of stones in the urinary tract system [8]. Treatment and management of renal stones relies on surgical techniques, such as extracorporeal shock wave lithotripsy, percutaneous lithotripsy, and transureteral lithotripsy [9,10]. These surgeries are complex and expensive and do not affect the recurrence of stones [9]. Various medicines, including thiazide as diuretic and alkali-citrate, are applied to prevent the frequency of hypercalciuria and hyperoxaluria—which cause calculi formation—but they are not promising enough due to their limited effectiveness and low tolerability [10,11,12,13]. Because of the disadvantages of surgical techniques and limited choice in pharmacotherapy, exploring new pharmacological therapies for the management of kidney stones is worthwhile. Various medicinal plants with diuretic, antispasmodic, and antioxidant activities exert inhibitory effects on crystallization, nucleation, and aggregation of crystals, making them useful for treatment of urolithiasis. The aim of the present article is to provide a critical review of the role of dietary plants as natural supplements in the prevention or management of kidney stones and elaborate underlying pharmacological mechanisms as well as their phytochemical constituents responsible for this activity.
2. Literature Search Methodology
Electronic databases, including PubMed, Science Direct, and Scopus, were searched for dietary plants and their bioactive compounds used for prevention and management of urolithiasis from 2005 to December 2017. The keywords were “kidney stone” or “urolithiasis”, or “nephrolithiasis”, or “renal calculi”, or “renal stone”, or “antilithiatic”; and “dietary plant”, or “dietary herb”, or “phytochemical”, or “fruits”, or “vegetables”. The retrieved articles were subclassified into in vitro, in vivo, and clinical studies. The studies included were evaluated with respect to the potential of the plant to be used as a dietary agent, the phytochemical composition of the plant, the kind of kidney stone that the dietary agent is effective on, as well as underlying mechanisms of action.
3. Role of Natural Diet in the Prevention of Kidney Stones
Emerging human studies have suggested that diets with a higher intake of vegetables and fruits play a role in the prevention of urolithiasis [14,15,16]. Epidemiological studies showed that diet could be one of the main risk factors for kidney diseases. Small-scale human investigations reported that diets with greater ingestion of plant-sourced protein, in comparison with animal-sourced protein, can cause amelioration of metabolic acidosis—attenuating further nephropathy progression in patients with chronic kidney disease—and reduction of glomerular filtration rate (GFR). Such dietary interventions, focusing on acid lessening with sodium-based alkalis, are among the main protective strategies in patients with reduced GFR [17]. It has been found that regular intake of natural diets rich in plants can increase urine pH and volume, as well as the amounts of stone inhibitors such as phytate, citrate, potassium, and magnesium, which are associated with supersaturation of calcium oxalate and uric acid [18]. Phytate is the main form of phosphate in natural sources, and its dietary intake is associated with the development of insoluble complexes with calcium within the gut, which can cause suppression of crystal formation in the urine and decrease the risk of urolithiasis [14,19]. Alkali load induced by a natural diet is able to raise urinary citrate which has a significant preventive effect on the development of kidney stones [20]. Additionally, dietary fiber which is abundant in fruits and vegetables can diminish the formation of stones due to its non-digestible ingredients which link to minerals and fat within the gastrointestinal tract, resulting in the suppression of urinary excretion of oxalate and calcium [14]. A human study evaluating the association between intake of dietary plants, fruits, and vegetables and the risk of incidence of urolithiasis in female subjects showed no history of kidney stones. These relationships were evaluated with stone recurrence in women with a history of kidney stones and it has been found that higher intake of fruits and vegetables was related to a decreased risk of development of urolithiasis [21].
4. Dietary Plants for the Prevention of Kidney Stones
Dietary interventions are taken into account as promising methods for kidney protection, either in concert with, or apart from, inherited or genetic factors. Nutritional plants are efficient remedies in the diet, which can influence risk of recurrence in calcium oxalate stones. In the following section, we discuss various dietary plants, food additives, fruits, and vegetables with well-established protective effects on urolithiasis. The details of the prophylactic roles of these plants on renal stones are also presented in Table 1.
Table 1.
Experimental and clinical evidence on nutritional plants used for prevention and treatment of kidney stones.
| Plant | Part of Plant | Study Type | Study Design | Results | Reference |
|---|---|---|---|---|---|
| Green tea (Camellia sinensis) | Leaves of kidney stones | In vivo | Ethylene glycol (EG)-induced nephrolithiasis in rat | ↓ Calcium crystal depositions in the kidneys ↓ The osteopontin mRNA level |
[22] |
| Leaves | In vivo | EG-induced nephrolithiasis in rat | ↓ Urinary oxalate excretion, calcium oxalate deposit formation ↑ Sodium Oxide Dismutase (SOD) activity |
[23] | |
| Rasberry (Rubus idaeus) | Aqueous extract | In vivo | Glyoxylate-induced calcium oxalate (CaOx) nephrolithiasis in mice | ↓ Generation of malondialdehyde (MDA) and protein carbonyls ↓ Urinary calcium and phosphorus levels ↓ The growth rate of calculus |
[24] |
| Methanolic extract | In vivo | Bicarbonate saline solution (containing 110 mM NaCl and 30 mM NaHCO3) induced nephrolithiasis in rats | ↓ Activity of aldosterone or epithelial sodium channels ↑ Urine volume |
[25] | |
| Common madder (Rubia cordifolia) | Hydro-alcoholic extract | In vivo | EG-induced urolithiasis | ↓ The growth of calcium oxalate crystals ↓ The formation of urinary oxalate ↑ Tubular citrate |
[26] |
| Parsley (Petroselinum sativum Hoffm.) | Ethanolic extract | In vivo | EG+ ammonium chloride (AlCl3)-induced urolithiasis in rat | ↓ Urinary calcium and protein excretion ↑ Urinary pH |
[27] |
| Aqueous Extract | In vivo | EG-induced urolithiasis in rats | ↓ Serum urea and uric acid concentrations ↑ Serum magnesium concentration |
[28] | |
| Parsley (Petroselinum sativum Hoffm.) | Aerial parts and roots aqueous extract | In vivo | EG-feeding rats | ↓ The number of calcium oxalate deposits | [29] |
| Pomegranate (Punica granatum) | Fruits chloroform and methanol extract | In vivo | EG-induced urolithiasis | ↓ Urine oxalate, calcium and phosphate, renal tissue oxalates ↓ Serum creatinine, urea and uric acid |
[24] |
| Yellow-fruit nightshade (Solanum xanthocarpum) | The methanolic extract | In vivo | EG-induced urolithiasis in rats | ↓ Renal hyperoxaluria and crystalluria, ↓ Supersaturation of calcium oxalate |
[30] |
| Stinging nettle (Urtica dioica) | Methanolic extract | In vivo | EG-induced urolithiasis in rats | ↓ Urinary creatinine level and the supersaturation of lithogenic enhancing agents | [31] |
| Khella (Ammi visnaga L.) | Aqueous extract of fruits | In vivo | EG+ aluminum chloride-induced urolithiasis in rats | ↓ Calcium oxalate crystal deposition ↑ Urinary excretion of citrate ↓ Oxalate excretion |
[32] |
| Black-cumin (Nigella Sativa L.) | Ethanolicextract of seeds | In vivo | Ethylene glycol for induction of calcium oxalate calculus formation in rats | ↓ Number of calcium oxalate deposits ↓ Urine concentration of calcium oxalate |
[33] |
| Thymoquinone (major component of seeds) | In vivo | Ethylene glycol-induced kidney calculi in rats | ↓ Number and size of calcium oxalate deposits in the renal tubules | [34] | |
| Citrus aurantium L. | Aqueous extract of unripe fruit | In vivo | EG -induced calcium oxalate crystallization | Preventing the formation of calcium oxalate nephrolithiasis and pathological alterations in rats | [35] |
| Oregano (Origanum vulgare L.) | Aerial part aqueous-methanolic extract | In vivo | EG-induced urolithiasis in rats | Preventing loss of body weight, polyurea, crystalluria, oxaluria ↑ Serum urea and creatinine levels |
[34] |
| Roselle (Hibiscus sabdariffa L.) | Plant aqueous extracts | In vivo | EG-induced hyperoxaluria | ↓ Deposition of stone-forming constituents in the kidneys and serum | [36] |
| Khella (Ammi visnaga L.) | aqueous extract | In vitro | A flask containing a cystine stone | ↑ Dissolution rate of cystine stones | [37] |
| Mastic (Pistacia lentiscus) | ethanolic fruit extract | In vitro | Calcium oxalate monohydrate-induced in Human Kidney (HK)-2 cells | ↓ Cell death induced by COM, ↓ The level of E-cadherin and H2O2 |
[38] |
| Roselle (Dolichos biflorus L.) | Hydro-alcoholic extract of seeds | In vitro | Calcium oxalate crystallization using a synthetic urine system | ↓ Nucleation and aggregation of calcium oxalate monohydrate crystals | [39] |
| Aqueous, chloroform, and benzene extracts of seed | In vitro | Experimental preparation of kidney stones; calcium oxalate and calcium phosphate | Dissolving calcium oxalate stones | [40] | |
| Oregano (Origanum vulgare L.) | Crude aqueous-methanolic extract | In vitro | Supersaturated solution of calcium oxalate, kidney epithelial cell lines (MDCK) and urinary bladder of rabbits | ↓ Calcium oxalate crystallization Exerting antioxidant, renal epithelial cell protective and antispasmodic activities |
[41] |
| Solanum xanthocarpum | Saponin rich fraction prepared from fruits | In vitro | calcium oxalate crystal nucleation. artificial urine solution | ↓ Calcium oxalate crystal formation ↑ Glycosaminoglycan level |
[42] |
| Pomegranate (Punica granatum) | Extract capsule | Clinical | 23 recurrent stone formers (RSFs) and 7 non-stone formers (NSFs) (1000 mg daily) for 90 days | ↓ Serum paraoxonasearylesterase activity ↓ Supersaturation of calcium oxalate |
[43] |
| Horse gram (Dolichos biflorus L.) | Seed | Clinical | 24 patients received Dolichosbiflorus and 23 patients were given potassium citrate | ↓ Recurrence of calcium oxalate stone | [44] |
| Roselle (Hibiscus sabdariffa L.) | A tea bag of dried plant | Clinical | 9 patients with renal stones and 9 with non-renal stone received tea (A cup of tea made from 1.5 g of dry herb two times daily | ↑ Uric acid excretion and clearance | [45] |
↑ demonstrates increasing trend; ↓ demonstrates deccreasing trend.
4.1. Green Tea
Green tea (Camellia sinensis) has long been used as an herbal remedy with several polyphenols making them potent antioxidants. Although it is an oxalate-rich natural agent and could not be recommended for renal calculi formed by calcium oxalate [41], due to the anti-lithogenic, anti-atherosclerotic, and antioxidant effects of green tea, it has received considerable attention for use as a dietary supplement in patients suffering from nephrolithiasis and urinary stones [22,23,41,45]. The protective effect of green tea is most likely due to the presence of polyphenols and other phytochemicals. Green tea catechins, including epigallocatechin gallate (EGCG), epigallocatechin (GGC), epicatechin gallate (ECG), and epicatechin (EC), provide protective effects against oxalate-induced toxicity [23,41]. Green tea supplementation inhibited the growth of crystals in kidney of rats, diminished the excretion of oxalate [24,41], and exerted inhibitory effects on the activities of γ-glutamyltranspeptidase and N-acetyl-β-d-glucosaminidase [41,45]. It decreased the supersaturation of brushite [45], down-regulated the osteopontin (OPN) protein expression, increased superoxide dismutase (SOD), elevated Bcl-2 expression, and decreased the apoptotic index in the rat model of kidney stones [23]. These results, altogether, demonstrated that green tea rich in antioxidants possesses a protective effect against development of calcium stones in the kidneys.
4.2. Raspberry
Raspberry (Rubusidaeus, from Rosaceae family), is a commercial fruit crop grown in many European and Mediterranean countries and has been widely used for nutritional and medicinal purposes [46]. Raspberry has been found to be capable of expelling stones from the urinary tract even after acute administration. The prophylactic effect of raspberry on calcium oxalate renal stone formation has been reported [24]. Its aqueous extract exerted significant preventive effects on the deposition and precipitation of calcium oxalate in the kidney and eliminated the calcium oxalate matrix. The generation of malondialdehyde (MDA) and protein carbonyls was suppressed in raspberry-treated animals with decreased levels of urinary calcium and phosphorus. The presence of polyphenols and alteration in the level of stone formation inhibitors (such as citrate, magnesium, and glycosaminoglycans) may be involved in the mechanism by which raspberry inhibited the growth of calculus [24]. The methanolic extract of raspberry was found to be a potent diuretic via inhibiting the activity of aldosterone or epithelial sodium channels [25].
4.3. Rubia cordifolia
Rubia cordifolia (madder or Indian madder) belongs to the coffee family (Rubiaceae) and has been used as a natural food colorant. Phytochemical screening of R. cordifolia has revealed the presence of various bioactive phytochemicals, including glycosides, triterpenoids, anthraquinones, saponins, quinones, and tannins, which make it advantageous for the treatment of several disorders, such as jaundice, diabetic foot ulcer, and cardiovascular ailments [47,48]. It was found to be effective in the treatment of different kidney diseases and possessed preventive effects on urinary stones [26,49,50,51]. The hydro-alcoholic extract of R. cordifolia roots successfully inhibited the excretion of calcium in ethylene glycol-induced renal stone formation in rats, and, more importantly, prevented hyperoxaluria and hypocitraturia by decreasing the formation of urinary oxalate and regulating the re-adsorption of tubular citrate (increasing the level of urinary citrate), respectively. The preventive role of this extract against kidney stone formation was also due to its inhibitory effect on the deposition and growth of calcium oxalate crystals by restoring magnesium levels, its preventive effect on proteinuria, and its suppressive effect on acid uric excretion. The nephro-protective effect of this medicinal and nutritional plant could be largely attributed to its antioxidant properties [26].
4.4. Parsley
Parsley (Petroselinum crispum) belonging to the family Umbelliferae is commonly known as an herb, spice, and vegetable, and is widely distributed in Western Asia, the Mediterranean, and several European countries [52,53]. Various pharmacological activities, such as antioxidant, anti-inflammatory, diuretic, nephro-protective, enzyme-modulatory, and anti-hypertensive actions, have been reported for this plant [52,54]. These beneficial activities could be due to its bioactive constituents, including flavonoids, carotenoids, coumarins, tocopherol, and ascorbic acid [55]. Parsley and its extracts have been used potentially as a complementary/alternative treatment for various renal diseases [55,56,57]. P.crispum has been used as a promising anti-urolithiasis remedy. Its ethanolic extract prevented the nucleation and precipitation of calcium oxalate, urine supersaturation, and urinary protein excretion in a rat model of calcium stone formation [27]. The high content of chlorophyll and magnesium in parsley is a reason for its inhibitory effect toward the dehydration of calcium oxalate and hyperoxaluria, respectively [58]. Parsley was found to be effective in regulating urinary pH at a value at which calcium oxalate crystals could be maintained as dispersed particles, and the elimination of these crystals could be facilitated [27,28].
4.5. Pomegranate (Punica granatum)
Pomegranate has long been used in traditional medicine. Pomegranate fruit, known as “a pharmacy unto itself” [59], is a rich source of polyphenols, alkaloids, and anthocyanins (flavonoid antioxidants), which are highly capable of scavenging free radicals [60,61]. All parts of this plant can be used in traditional remedies for preventive and therapeutic purposes. Pomegranate seeds were used for regulating urine discharge and the burning sensation of urine; its seed oil, juice, flowers, and peel are used for protection against nephrotoxicity [62,63,64,65], and the extracts for renal failure [66] and renal arteries [67]. The anti-hypercalciuria and anti-urolithiasis effects of this plant attracted considerable attention toward pomegranate for use in the prevention of renal calculus formation. Its therapeutically beneficial phytochemicals are responsible for muscle relaxation in the urinary and biliary tract; consequently, stones can be easily removed from the kidney [12]. Administration of the methanolic extract of pomegranate to the rat model of urolithiasis (induced by 28 days of treatment with ethylene glycol) dose-dependently inhibited the inflammation mediated by ethylene glycol, and consequently regulated the levels of oxalates, calcium, and phosphates. The methanolic extract was also found to be more protective in comparison with the chloroform extract, which might be due to the lipophilic nature of pomegranate constituents [12]. The extracts and juice of pomegranate significantly inhibited the hyperoxaluria-induced oxidative renal tubular damages (due to its antioxidants and anti-lipid-peroxidation [68]) by reducing the levels of reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), and nuclear factor-κB (NF-κB) [61,69] and p38-mitogen-activated protein kinase (p38-MAPK) [69], and regulating urea, creatinine, and ureic acid [12]. Beside the animal studies, the nephro-protective roles of pomegranate extract on the calcium-containing lithiasis formation in humans (18–70 years old) with recurrent stone formation have been clinically studied. The daily supplementation of patients with pomegranate extract caused significant down-regulation of serum paraoxonase1 (PON1) arylesterase activity together with decreasing supersaturation of calcium oxalate, indicating that this intervention could successfully control the risk of renal stone formation [42].
4.6. Pistacia lentiscus
Pistacia lentiscus (Anacardiacceae) is a common evergreen dioecious shrub, distributed in a wide range of habitats, specifically in the eastern Mediterranean region [70]. In folk medicine, it is known as a medicinal and nutritional plant with various therapeutic potentials, such as antioxidant, anti-microbial, diuretic, anti-lipid peroxidation, and anti-urolithiasis activities [70,71]. The fruit extract has demonstrated in vitro potential in protecting human kidney (HK)-2 cells against proximal tubular injury mediated by calcium oxalate monohydrate (COM). It significantly inhibited the cell death induced by COM and suppressed the level of E-cadherin, as well as H2O2. It attenuated the attachment and internalization of calcium oxalate monohydrate crystals to epithelial tubular cells by the mechanism in which the interaction of active phytochemicals of the extract (mainly polyphenols) with cells inhibited its binding to the surface of the cells [38]. Therefore, P. lentiscus could be considered as a promising natural remedy for antilithiatic purposes.
4.7. Solanum xanthocarpum
Solanum xanthocarpum, also known as “yellow-fruit nightshade” and “Thai green eggplant”, is a famous and widely used edible traditional medicinal plant in India. The seeds and fruits are consumed as foods and vegetables [72]. This plant is used as a common remedy for the treatment of various renal diseases, including difficulty in urination, urinary infections, nephrotoxicity, and urolithiasis [30,73,74,75]. The fruit of S. xanthocarpum is a rich source of steroidal glycol-alkaloids, coumarins, triterpenes, and saponins [30]. The petroleum-ether extract of the fruits exhibited nephro-protective activity, possibly due to the anti-lipid peroxidation and antioxidant effects of the plant constituents [75]. The methanolic extract was found to be successful in preventing and inhibiting nephrolithiasis, renal hyperoxaluria, crystalluria, and supersaturation of calcium oxalate. It exerted antioxidant (by increasing SOD and glutathione (GSH) levels) and diuretic activities and also diminished the excretion of phosphorous in the calculi-induced rats [30]. The fruits of this plant contain saponins with high antilithiatic activity. The saponin-rich fraction prepared from fruits of S. xanthocarpum showed prevention of in vitro calcium oxalate crystal nucleation and aggregation in artificial urine solution, and inhibition of pathological changes due to lithogenic treatment, including polyuria, damage of renal function, oxidative stress, and crystalluria in ethylene glycol-induced urolithiasis in rats. The aforementioned fraction also increased levels of glycosaminoglycan, a stone inhibitor macromolecule found in urine, and accelerated the glomerular filtration [30].
4.8. Urtica dioica
Urtica dioica or “Stinging Nettle”, which belongs to the nettle genus of Urticaceae family, is used as tea in Austrian medicine [31,76]. It has shown a long history of beneficial therapeutic effects toward urinary ailments, specifically with the urinary tract and kidney stones. Its major bioactive phytochemicals include flavonoids, anthocyanins, and saponins [31]. These phytoconstituents provide the possibility of inhibition of calcium and oxalate deposition and crystals growth. Supplementation of the methanolic extract U. dioica to rats with kidney stones (induced by ethylene glycol and ammonium chloride) was found to be associated with decreased urinary creatinine level and reduction of supersaturation of lithogenic enhancing agents. This extract potentially dissolved the lithiasis and overcame the hyperoxaluria and crystalluria induced by ethylene glycol [31].
4.9. Dolichos biflorus
Dolichos biflorus (horse gram) is a nutritional and medicinal plant native to India, where its seeds are used to prepare soup [77]. The seeds are acclaimed in ayurvedic literature to have litholytic, free radical-scavenging, and anti-nephrotoxic effects [40,43,77]. The beneficial effect of this plant can be attributed to the existence of various phytoconstituents in the seeds, including phenolic compounds (such as quercetin), alkaloids, phytosterols (such as β-sitosterol), saponins, glucosides (such as β-galactosidases and α-mannosides) [39,40]. Various extracts from seeds, including aqueous, chloroform, and benzene, dissolved calcium oxalate stones in experimental models of kidney stones. Aqueous extract showed the highest dissolution of stones compared to other extracts [40]. In a synthetic urine system for calcium oxalate crystallization, the hydro-alcoholic extract of seeds showed inhibitory activity on nucleation and aggregation of calcium oxalate monohydrate crystals [39]. Administration of D. biflorus to patients with calcium oxalate renal calculi decreased the recurrence of calcium oxalate stones and had a better result than the use of potassium citrate in these patients [43].
4.10. Ammi visnaga
Teas prepared from the fruits of Ammi visnaga have been traditionally used by patients with renal stones in Egypt [32]. The aqueous extract of this fruit accelerated the dissolution of cystine stones [37]. The fruit and its two major constituents, namely khellin and visnagin, showed beneficial effects in the management of kidney stone disease caused by hyperoxaluria in male rats through reduction of the incidence of calcium oxalate crystal deposition, increasing urinary excretion of citrate along with a decrease of oxalate excretion [32].
4.11. Nigella sativa
Nigella sativa has been used in Iranian traditional medicine for treatment of urinary stones [33,34,78,79]. Ethanolic extract of seeds reduced the number of calcium oxalate deposits in ethylene glycol-induced lithiatic rats and decreased the urine concentration of calcium oxalate [33]. Thymoquinone, the major component of the seeds, showed preventive and therapeutic effects on ethylene glycol-induced kidney calculi in rats. This phytochemical compound decreased the size and number of calcium oxalate deposits in the renal tubules in rats [34].
4.12. Hibiscus sabdariffa
Based on Thai traditional medicine, Hibiscus sabdariffa is used for the prophylaxis and treatment of urinary stones [44]. It has been found that the main active constituents of this plant include polyphenols, hibiscus anthocyanins, as well as L-ascorbic acid, quercetin, and protocatechuic acid. The aqueous plant extract had demonstrated antiurolithiatic activity due to the decreased deposition of stone-forming constituents in the kidneys and serum of ethylene glycol-induced urolithiatic rats [36]. Moreover, the plant extract had an antilithic effect on rats on a glycolate diet through the decrease in oxalate retention time in the kidneys and more excretion into urine [80]. A clinical trial—which had tested a cup of tea made from 1.5 g of dry H. sabdariffa two times daily on 18 patients for 15 days—revealed uricosuric effect and significant increase in uric acid excretion and clearance [44].
4.13. Origanum vulgare
This plant has been widely used as spice and in traditional medicine as a lithotriptic, diuretic, and antispasmodic [11]. The crude aqueous-methanolic extract of the aerial part of O. vulgare exhibited in vitro inhibitory activity in the nucleation and aggregation of calcium oxalate crystals, and also decreased the number of crystals produced in calcium oxalate metastable solutions. Evaluation of rats with ethylene glycol and ammonium chloride-induced urolithiasis showed that the extract of the aerial part of O. vulgare had antiurolithic activity, possibly through prevention of calcium oxalate crystallization, renal epithelial cell protection, antioxidant, and antispasmodic properties. The preventive effect could be attributed to its active phytochemicals including flavonoids, terpenes, coumains, saponins, alkaloids, sterol, and tannins [11].
5. Medicinal Plants and Phytoconstituents as Dietary Supplements for the Prevention of Kidney Stones
In current years, there is great interest in herbal and traditional medicine for prevention and management of variety of diseases [81]. Medicinal plants have been used for thousands of years for the prevention of the development and recurrence of kidney stones in different countries [82,83]. Various medicinal plants and phytochemical constituents have been evaluated for their preventive and therapeutic potential in kidney stones [84]. Medicinal plants with well-established preclinical and/or clinical evidence of their protective or therapeutic effect in urolithiasis include Bergenia ciliata (Haw.) Sternb [85], Bergenia ligulata Engl. [86,87], Commiphora wightii (Arn.) Bhandari [88], Costus arabicus L. [89], Herniaria hirsuta L. [90], Terminalia chebula Retz. [91], Tribulus terrestris L. [92], Acalypha indica L. [93], Aerva lanata (L.) Juss. [94], Ageratum conyzoides (L.) L. [11], Alcea rosea L. [95], Asparagus racemosus Willd. [96], Bombax ceiba L. [97], Carthamus tinctorius L. [98], Cynodon dactylon (L.) Pers. [99], Helichrysum graveolens (M. Bieb.) Sweet and Helichrysum stoechas ssp. barellieri (Ten.) Nyman [100], Hordeum vulgare L. [101], Hygrophila spinosa T.Anderson [102], Hypericum perforatum L. [103], Launaea procumbens L. [104], Lygodium japonicum (Thunb.) Sw. [105], Orthosiphon grandiflorus Bold. [106], Paronychia argentea Lam. [107], Pedalium murex L. [9], Pergularia daemia (Forssk.) Chiov. [108], Quercus salicina Blume [109], Salvadora persica L. [110], Selaginella lepidophylla (Hook. et Grev) Spring [111], Agropyron repens (L.) P.Beauv. [112], and Phyllanthus niruri L. [113].
These nephro-protective herbs, were found to be effective inhibitors of the formation and growth of calcium hydrogen phosphate dihydrate (Brushite) crystals, calcium hydrogen phosphate dehydrate (CHPD) crystals, calcium oxalate monohydrate crystals, and cysteine and uric acid stones [85,86,87,101,104,114,115].
The details on the study design and pharmacological evidences of these medicinal plants are presented in Table 2 and Table 3.
Table 2.
Cellular studies on medicinal plants used for the prevention and treatment of kidney stones.
| Plant | Part or Chemical Constituents | Study Type | Study Design | Results | Reference |
|---|---|---|---|---|---|
| Bergenia ciliata (Haw.) Sternb | Hydro-alcoholic extract of rhizomes | In vitro | Calcium oxalate induced in a synthetic urine system | ↑ Nucleation and aggregation of COM crystals ↓ The number and size of COM crystals |
[85] |
| Bergenia ligulata Engl. | Aqueous-methanolic extract of rhizome | In vitro | Calcium oxalate induced crystal in a synthetic urine system | Inhibition of crystal aggregation and formation ↑ Radical scavenging ability and lipid peroxidation |
[86] |
| Commiphora wightii (Arn.) Bhandari | Extract | In vitro | Struvite crystals induced using gel growth technique | ↓ Growth and the size of the struvite crystals | [88] |
| Costus arabicus L. | Aqueous dried plant extract | In vitro | Calcium oxalate monohydrate (COM) crystals induced in MDCK cells | ↓ Crystal growth and calculogenesis | [89] |
| Herniaria hirsuta L. | Ether and methanol extracts of aerial parts | In vitro | Calcium oxalate-induced stone in urine | ↓ The size and supersaturation rate of crystals | [90] |
| Terminalia chebula Retz. | Aqueous fruits extract | In vitro | Calcium oxalate induced cell injury in NRK-52E and MDCK renal epithelial cells | ↓ Lactate dehydrogenase release ↑ Cell viability |
[91] |
| Tribulus terrestris L. | Protein biomolecules | In vitro | Oxalate induced injury on NRK-52E cells | ↓ Lactate dehydrogenase release ↑ Cell viability |
[92] |
↑ demonstrates increasing trend; ↓ demonstrates deccreasing trend.
Table 3.
Pre-clinical and clinical evidence on medicinal plants used for prevention and treatment of kidney stones.
| Plant | Part or Chemical Constituents | Study Type | Study Design | Results | Reference |
|---|---|---|---|---|---|
| Acalypha indica L. | Ethanolic extract | In vivo | Ethylene glycol (EG)-induced urolithiasis in Wistar albino rats | ↑ Ca2+ ATPase, Mg2+ ATPase, Na+K+ ATPase ↑ Aspartate Transaminase (AST), Alanine Transaminase (ALT), Acid phosphatase (ACP) and Alkaline Phosphatase (ALP) |
[93] |
| Aerva lanata (L.) Juss. | Aqueous suspension of aerial parts | In vivo | EG-induced urolithiasis in rats | ↓ Glycolic acid oxidase (GAO), and lactate dehydrogenase (LDH) | [94] |
| Ageratum conyzoides (L.) L. | Hydroalcolohlic extract of whole plant | In vivo | EG-induced urolithiasis in rats | ↓ Stone forming constituents, Blood urea nitrogen (BUN), uric acid and creatinine | [11] |
| Alcea rosea L. | Hydroalcoholic extract of roots | In vivo | EG-induced lithiasis in rats | ↓ The number of calcium oxalate deposits ↓ Urinary oxalate level |
[95] |
| Asparagus racemosus Willd. | Ethanolic extract of tuberous roots | In vivo | EG-induced urolithiasis in rats | ↓ The level of calcium, oxalate, phosphate, and serum creatinine; ↑ Urinary concentration of magnesium | [96] |
| Bergenia ciliata (Haw.) Sternb. | The hydro-methanolic extract of rhizomes | In vivo | EG-induced urolithiasis in rats | ↓ Nucleation and aggregation of crystals ↓ The number and size of COM crystals |
[85] |
| Bergenia ligulata Engl. | Aqueous-methanolic extract of rhizome | In vivo | EG-induced urolithiasis in rats | ↓ Calcium oxalate crystal deposition, and lithogenic signs ↑ Urinary magnesium |
[96] |
| Ethanolic extract of rhizome; bergenin | In vivo | EG+ aluminium chloride-induced urolithiasis in rats | ↓ MDA level, ↑ H2O2 scavenging ability ↑ SOD, Catalase (CAT) and GP levels |
[87] | |
| Bombax ceiba L. | Fruit aqueous and ethanol extract | In vivo | EG-induced urolithiasis in rats | ↓ Urinary oxalate ↓ Stone forming constituents |
[97] |
| Carthamus tinctorius L. | Commercial herbal powder- gastric gavage | In vivo | EG-induced stones in rats | ↓ Deposition of calcium oxalate crystal | [98] |
| Cynodon dactylon (L.) Pers. | N-butanol and ethyl acetate extract of root | In vivo | EG-induced calculus in rats | Preventing calcium oxalate deposition ↓ The size of crystals |
[99] |
| Helichrysum graveolens (M.Bieb.) Sweet and Helichrysum stoechas ssp. barellieri (Ten.) Nyman | Capitulum aqueous extract | In vivo | Sodium oxalate- induced urolithiasis in rats | ↓ Formation and growth of crystals ↓ Urine oxalate and uric acid levels, ↑ Citrate level |
[100] |
| Hordeum vulgare L. | Seeds ethanolic extract | In vivo | EG-induced urolithiasis in rats | ↓ Stone forming constituents ↓ Lipid peroxidation ↑ SOD and CAT |
[101] |
| Hygrophila spinosa T.Anderson | Methanolic extract of aerial parts | In vivo | EG-induced nephrolithiasis in rats | ↓ Urinary oxalate ↓ Calcium and oxalate in kidney; ↑ Urinary magnesium |
[102] |
| Hypericum perforatum L. | Hydroalcoholic extract of leaves | In vivo | EG+ ammonium chloride- induced stone in rats | ↓ The size and number of calcium oxalate deposits | [103] |
| Launaea procumbens L. | Methanolic extract of leaves | In vivo | EG-induced urolithiasis in rats | ↓ Urinary calcium, oxalate and phosphate excretion ↓ Creatinine and uric acid |
[104] |
| Lygodium japonicum (Thunb.) Sw. | Ethanolic extract of spore | In vivo | EG-induced kidney calculi in rats | ↓ Urinary calcium, oxalate and uric acid ↓ Kidney peroxides, and the number of oxalate deposits ↑ Urinary citrate levels |
[105] |
| Orthosiphon grandiflorus Bold. | Aqueous extract of leaves | In vivo | EG-induced stones in rats | ↓ Crystal deposits ↑ SOD and CAT |
[106] |
| Paronychia argentea Lam. | Butanolic extract of aerial parts | In vivo | Sodium oxalate-induced lithiasis in rats | ↓ Renal necrosis ↓ Serum creatinine and blood urea levels |
[107] |
| Pergularia daemia (Forssk.) Chiov. | Whole-plant hydroalcoholic extract | In vivo | EG- induced kidney stone in rats | ↓ Serum urea nitrogen, creatinine and uric acid levels | [108] |
| Quercus salicina Blume | Leaves aqueous extract | In vivo | EG and the vitamin D3 analog(α-calcidol)-induced urolithiasis in rats | ↓ MDA and serum creatinine level ↓ Oxidative stress ↓ Calcium level in kidney |
[109] |
| Salvadora persica L. | Aqueous and alcoholic extract of the leaves | In vivo | EG- induced urolithiasis in rats | ↓ Urinary oxalate levels and deposition | [110] |
| Selaginella lepidophylla (Hook. et Grev) Spring | Chloroform extract of the plant | In vivo | EG and ammonium chloride- induced urolithiasis in rats | ↑ Urinary flow rate, glomerular filtration rate (GFR) ↓ ROS and lipid-peroxidation ↓ Renal cortical organic anion transporter (OAT3) expression |
[111] |
| Agropyron repens (L.) P.Beauv. | Extract | Clinical | Unblinded treatment to the patients (treatment group received potassium citrate + Agropyrum repens and control group recieved potassium citrate alone (100 mg/day for 5 month)) | ↓ Number and size of urinary stones ↓ Uric acid urinary secretion |
[112] |
| Phyllanthus niruri L. | Extract | Clinical | 150 patients received 1 to 3 extracorporeal shock wave lithotripsy sessions. After treatment 78 patients received extract and 72 were served as a control group (2 g/day for 3 month) | ↑ Stone-free rate (stone-free defined as the absence of any stone or residual fragments less than 3 mm) | [113] |
↑ demonstrates increasing trend; ↓ demonstrates deccreasing trend.
6. Effect of Pharmacologically Active Phytochemicals on the Inhibition of Urolithiasis
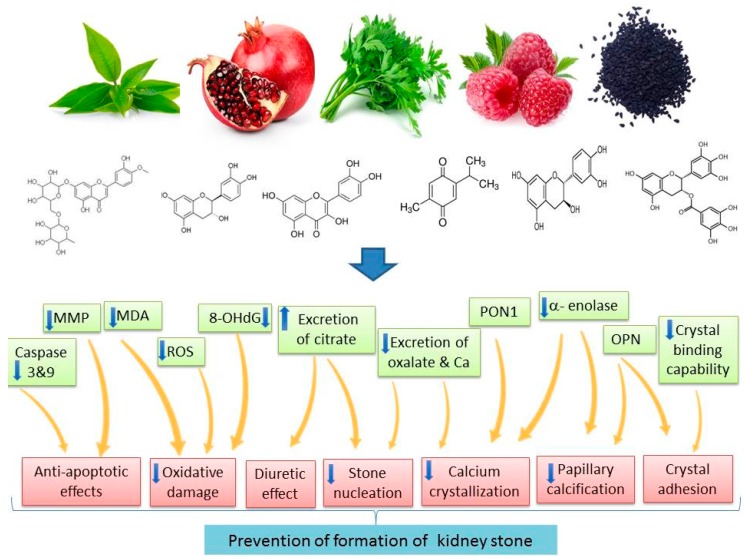
Several recent studies have highlighted the effectiveness of dietary interventions as a promising method for kidney protection, either in concert with, or apart from, inherited or genetic factors. Nutritional plants and their phytochemicals could be included either in the main diet or as dietary supplements to treat urolithiasis, reduce the risk of recurrence of kidney stones, and affect the formation and growth of crystals. Several medicinal herbs and natural compounds have been successfully applied for these purposes, while the molecular basis underlying the prophylactic effect of these phyto-therapeutics is poorly understood. The anti-urolithiasis effects of nutraceuticals have been thought to be most likely due to their antioxidative effects. Calcium oxalate is the major constituent of urolithiasis, and antioxidant therapy could be one of the effective methods for preventing the nucleation as well as binding and growth of calcium oxalate crystals. Dietary phyto-phenols, with more than 8,000 structural variants, are the most abundant antioxidants in human diets which can occur in a variety of vegetables and fruits [116]. These nutraceuticals exhibited high inhibitory effects against the oxidative stress-associated dysfunctions in kidney (Table 4). Catechin and epicatechin are two of these antioxidants mostly included in plant sources, such as tea (green and black) and grape seeds [117]. The antioxidant activity of catechin could be attributed to either its radical scavenging and metal-chelating properties, or its modulatory effect on transcription factors and enzymes [118]. This provides the reno-protective capability toward different renal injuries, oxidative stress associated with renal failure, and renal calcium crystallization [119,120]. Catechin increased the SOD activity in COM-treated NRK-52E renal proximal tubular cell line and restored the mitochondrial membrane potential and cleavage of caspase-3 [121]. The renal papillary calcification and enhancement of COM papillary calculi have been successfully inhibited by catechin [122], which could be due to its promoting effect on SOD activity [119]. The OPN, MDA, and 8-hydroxy-2′-deoxyguanosine (8-OHdG) were also well-regulated by catechin in the ethylene glycol-induced rat model of nephrolithiasis [121]. EGCG, as an important catechin, also exerted the protective effect by attenuating the binding capability of Madin–Darby canine kidney (MDCK) cells to COM crystals. This was found to be attributed to the reduced α-enolase protein expression (responsible for binding) on the renal tubular cell surface following EGCG administration [123].
Table 4.
Cellular and animal evidence on phytochemicals used for prevention and treatment of kidney stones.
| Photochemical | In Vitro/In Vivo | Model | Result | Reference |
|---|---|---|---|---|
| Catechin | In vitro | Calcium oxalate monohydrate(COM)-induced NRK-52E cells | ↑ SOD activity ↓ Mitochondrial membrane potential (MMP), Caspase-3 activity, and renal calcium crystallization |
[121] |
| In vivo | Ethylene glycol (EG) induced nephrolithiasis in rat | ↑ OPN, ↓ MDA, 8-OHdG ↓ Renal calcium crystallization |
[121] | |
| In vivo | EG-induced nephrolithiasis in rat | ↓ Calcium oxalate monohydrate and Papillary calculus formation ↓ Renal papillary calcification |
[122] | |
| Epigallocatechin-3-gallate | In vitro | COM-induced Madin–Darby canine kidney (MDCK) cells | ↓ α-enolase protein expression ↓ crystal-binding capability |
[123] |
| In vitro | Oxalate-induced NRK-52E cells | ↓ Free-radical production | [41] | |
| In vivo | Oxalate-induced renal stone in rats | ↓ Excretion of urinary oxalate ↓ Activities of urinary gammaglutamyl transpeptidase and N-acetylglucosaminidase |
[41] | |
| Diosmin | In vivo | EG-induced nephrolithiasis in rat | ↓ Capillary hyper-permeability ↓ Degeneration of glomeruli and tubules, Restoring the diameter of the capillaries and vessels in the cortex |
[129] |
| Rutin | In vivo | EG-induced nephrolithiasis in rat | Prevention of stone formation Inhibition of calcium oxalate urolithiasis |
[133] |
| Quercetin | In vivo | EG induced calcium oxalate (CaOx) formation | Hypo-Uricemic, and anti-inflammatory activities Inhibitory effect on the deposition of CaOx crystal |
[134] |
| In vitro | Sodium oxalate | ↓ Cell viability ↓ Lipid peroxidation |
[135] | |
| In vivo | Hyperoxaluria-induced rats | ↓ Urinary crystal deposit formation | [135] | |
| In vivo | EG-induced nephrolithiasis in rat | ↓ Oxidative damage ↑ Serum paraoxonase 1 (PON1) |
[119] |
↑ demonstrates increasing trend; ↓ demonstrates deccreasing trend.
Diosmin, as a flavonoid glycoside, is another polyphenol with inherent antiurolithiatic activity which can be found in vegetables and citrus fruits [124]. It has been commonly used as a natural medicament for various renal diseases and protects kidneys from diabetic nephropathy, nephro-toxicity, and oxidative stress [125,126,127,128]. The renoprotective effects of diosmin were found attributable to its suppressing activity on lipid peroxidation, potentiating activity on antioxidant enzymes, and its modulatory effect on the expressions of Bax and p53 proteins [127]. Beside these properties, it exhibited the potential to block the process of calcium oxalate stone formation in the rat model of nephrolithiasis. It exerted its antiurolithiatic effect by diminishing the capillary hyper-permeability, attenuating the degeneration of glomeruli and tubules, and restoring the diameter of the capillaries and vessels in the cortex [129]. Diosmin could also prevent the crystallization of stone-forming promoters by keeping the urinary pH at acidic values. This increased urine volume, elevated urinary level of potassium and magnesium, and suppressed level of urinary protein [130]. Rutin, quercetin, and hyperoside, like diosmin, are known as flavone glycosides with high antioxidant and anti-lithiatic activities. Rutin can be found in variety of plants, and black tea and apple peels are common dietary sources of this nutraceutical [131]. Rutin therapy, alone or in combination with curcumin, was found to be a successful remedy for prevention of stone formation [132,133]. Co-administration of these two phyto-phenolic compounds in calculi-induced rats caused restoration of the urinary calcium and oxalate levels and attenuated lipid-peroxidation. The aggregation and growth of COM crystals and glomerular filtration rate were found to be affected by these phyto-phenols [133]. Besides the antioxidative potential, the anti-inflammatory effect of these phytochemicals could be, in part, included in their prophylactic mechanism. Quercetin and hyperoside (mostly found in vegetables and fruits) have also exhibited promising antioxidant, diuretic, hypo-uricemic, and anti-inflammatory activities [134]. These two bioflavonoids are taken into account as efficient phyo-therapeutics for management of renal lithiasis based on their inhibitory effect on the deposition of calcium oxalate crystals, antioxidant activity against renal tubular cell injury (via increasing SOD and catalase activities), and anti-apoptotic effects [134,135]. The ability of quercetin to promote serum PON1 also provided an efficient antioxidant effect on hyperoxaluria-induced rats [119].
7. Major Pharmacological Mechanisms of Plants and Natural Products in the Prevention of Kidney Stones
Medicinal plants could affect different aspects of urolithiasis pathophysiology [136]. The medicinal plants can be effective in prophylaxis, treatment, and prevention of kidney stone recurrence. Their mechanisms of actions include increasing excretion of urinary citrate, decreasing excretion of urinary calcium and oxalate, inhibition of the nucleation and growth of the calcium oxalate crystals, dissolving stones, raising the level of glycosaminoglycan, and being diuretic. In addition to the hypermagneseuric inhibitory effect on crystallization and aggregation of crystals, cytoprotective, nephroprotection, antioxidant, and antispasmodic pharmacological effects of dietary natural components are among other mechanisms involved in protection against urolithiasis.
Recurrence of kidney stones is an important problem. Studies have revealed that treatments like phyto-medication or dietary modification can reduce the recurrence rate. Therefore, recurrence of renal stones is partially preventable [9]. Drugs, including thiazide as diuretic and alkali-citrate, are being used to prevent the recurrence of hypercalciuria and hyperoxaluria, but evidence for their efficacy is low [12]. Medicinal plants can prevent recurrence of renal calculi by lithotriptic activity, regulation of oxalate metabolism, modulating the crystalloid colloid imbalance, and decreasing supersaturation which inhibits crystallization. For example, B.ligulata rhizome extract has been reported to suppress calcium oxalate crystal precipitation through interference with crystal growth and aggregation [86]. The leaf extract of Launaea procumbens is effective in preventing the recurrence of renal calculi by its activity on early stages of stone development [104]. Moriyama et al. [43] demonstrated that Quercus salicina extract inhibited renal calcium accumulation and urinary MDA excretion in a rat model of calcium oxalate urolithiasis, possibly by reducing oxidative stress. Dietary plants and their isolated natural polyphenols were also effective natural remedies based on their prophylactic effects on urolithiasis. The preventive role of these nutritional plants and their polyphenols is attributed to their well-established pharmacological mechanisms in the kidney, including attenuation of hyperoxaluria, proteinuria, and hypocitraturia; their inhibitory effect on the deposition and the growth of calcium oxalate; down-regulation of serum PON1; up-regulation of antioxidant enzymes; and suppression of the attachment and internalization of calcium oxalate monohydrate crystals to epithelial tubular cells (Figure 1).
Figure 1.
Main mechanisms of action of dietary polyphenols in the prevention of kidney stones (Up arrow demonstrates increasing trend; down arrow demonstrates decreasing trend).
8. Concluding Remarks
Dietary plants, including food additives, fruits and vegetables, have a pivotal role in human health and the prevention of diseases, including kidney stones. However, the pharmacological evidence of dietary plants and their phytonutrients in the prevention of kidney stones have not been well-established yet. In this review, we have presented evidence on the efficacy and pharmacological mechanisms of various dietary plants and their phytochemicals in the prevention or management of kidney stones. Current literature showed that a large number of in vitro and animal studies were conducted to evaluate the preventive effects of dietary plants and their phytochemicals as dietary supplements in the development of urolithiasis. However, limited human studies on the efficacy of medicinal as well as dietary plants in the management of kidney stone were performed. A. repens (L.), D. biflorus L., H. sabdariffa L., P.granatum L., and Phyllanthus niruri L. are among the plants whose efficacy have been confirmed by clinical trials. Nutraceuticals (mainly dietary polyphenols), including catechin, epicatechin, EGCG, diosmin, rutin, quercetin, hyperoside, and curcumin, could be proposed as promising dietary supplements for prevention of urolithiasis.
To conclude, results obtained from the available literature revealed that dietary plants and their phytonutrients could be useful in the prevention and intervention of urolithiasis. Since natural dietary recommendations for patients with the risk of kidney stones are poorly provided, and patients often request instructions for a beneficial dietary regimen, it is essential for physicians to have evidence-based knowledge regarding the efficacy, pharmacological mechanisms, and side effects of the administration of a protective dietary regimen. More investigations using clinical trials are needed to confirm the efficacy and safety of these dietary agents in patients with kidney stones. Moreover, to achieve more conclusive results, further preclinical and human studies are compulsory to reveal molecular and cellular mechanisms, as well as the bioactive phytonutrients of these dietary plants in urolithiasis.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Mina Cheraghi Nirumand and Mohammad Hosein Farzaei designed the structure of the paper and drafted the manuscript. Marziyeh Hajialyani performed the literature search and contributed in writing the manuscript. Anupam Bishayee, Seyed Mohammad Nabavi, Stéphane Zingue, and Roja Rahimi reviewed and revised the manuscript. All authors had full access to the final version of the manuscript and gave their approval before publishing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.López M., Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr. Nephrol. 2010;25:49–59. doi: 10.1007/s00467-008-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiselius H.G. Epidemiology and medical management of stone disease. BJU Int. 2003;91:758–767. doi: 10.1046/j.1464-410X.2003.04208.x. [DOI] [PubMed] [Google Scholar]
- 3.Moe O.W. Kidney stones: Pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 4.Gindi S., Methra T., Chandu B.R., Boyina R., Dasari V. Antiurolithiatic and invitro anti-oxidant activity of leaves of Ageratum conyzoides in rat. World J. Pharm. Pharm. Sci. 2013;2:636–649. [Google Scholar]
- 5.Heron S., Yarnell E. Recurrent kidney stones: A naturopathic approach. Altern. Complement. Ther. 1998;4:60–67. doi: 10.1089/act.1998.4.60. [DOI] [Google Scholar]
- 6.Aggarwal A., Tandon S., Singla S., Tandon C. Diminution of oxalate induced renal tubular epithelial cell injury and inhibition of calcium oxalate crystallization in vitro by aqueous extract of Tribulus terrestris. Int. Braz. J. Urol. 2010;36:480–489. doi: 10.1590/S1677-55382010000400011. [DOI] [PubMed] [Google Scholar]
- 7.Baumann J.M. Stone preventionhy so little progress? Urol. Res. 1998;26:77–81. doi: 10.1007/s002400050027. [DOI] [PubMed] [Google Scholar]
- 8.Baynes R., Riviere J. Risks associated with melamine and related triazine contamination of food. Emerg. Health Threats J. 2010;3:e5. doi: 10.3402/ehtj.v3i0.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandavia D.R., Patel M.K., Patel J.C., Anovadiya A.P., Baxi S.N., Tripathi C.R. Anti-urolithiatic effect of ethanolic extract of Pedalium murex linn. fruits on ethylene glycol-induced renal calculi. Urol. J. 2013;10:946–952. [PubMed] [Google Scholar]
- 10.Miller N.L., Lingeman J.E. Management of kidney stones. Br. Med. J. 2007;334:468. doi: 10.1136/bmj.39113.480185.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan A., Bashir S., Khan S.R., Gilani A.H. Antiurolithic activity of Origanum vulgare is mediated through multiple pathways. BMC Complement. Altern. Med. 2011;11:96. doi: 10.1186/1472-6882-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathod N., Biswas D., Chitme H., Ratna S., Muchandi I., Chandra R. Anti-urolithiatic effects of Punica granatum in male rats. J. Ethnopharmacol. 2012;140:234–238. doi: 10.1016/j.jep.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Coe F.L., Parks J.H., Asplin J.R. The pathogenesis and treatment of kidney stones. N. Engl. J. Med. 1992;327:1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 14.Curhan G.C., Willett W.C., Knight E.L., Stampfer M.J. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch. Intern. Med. 2004;164:885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 15.Taylor E.N., Stampfer M.J., Curhan G.C. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J. Am. Soc. Nephrol. 2004;15:3225–3232. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 16.Meschi T., Nouvenne A., Ticinesi A., Prati B., Guerra A., Allegri F., Pigna F., Soldati L., Vezzoli G., Gambaro G. Dietary habits in women with recurrent idiopathic calcium nephrolithiasis. J. Transl. Med. 2012;10:63. doi: 10.1186/1479-5876-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goraya N., Wesson D.E. Dietary interventions to improve outcomes in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2015;24:505–510. doi: 10.1097/MNH.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 18.Meschi T., Maggiore U., Fiaccadori E., Schianchi T., Bosi S., Adorni G., Ridolo E., Guerra A., Allegri F., Novarini A. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402–2410. doi: 10.1111/j.1523-1755.2004.66029.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V., Sinha A.K., Makkar H.P., Becker K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010;120:945–959. doi: 10.1016/j.foodchem.2009.11.052. [DOI] [Google Scholar]
- 20.Seltzer M.A., Low R.K., McDonald M., Shami G.S., Stoller M.L. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J. Urol. 1996;156:907–909. doi: 10.1016/S0022-5347(01)65659-3. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen M.D., Hsi R.S., Chi T., Shara N., Wactawski-Wende J., Kahn A.J., Wang H., Hou L., Stoller M.L. Dietary intake of fiber, fruit and vegetables decreases the risk of incident kidney stones in women Women’s Health Initiative report. J. Urol. 2014;192:1694–1699. doi: 10.1016/j.juro.2014.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.W., Choi J.S., Yoon G.S., Yang E.K., Kim D.Y. Effect of green tea on calcium oxalate stone formation and excretion in ethylene glycol-treated rats. Korean J. Urol. 2005;46:299–305. [Google Scholar]
- 23.Itoh Y., Yasui T., Okada A., Tozawa K., Hayashi Y., Kohri K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J. Urol. 2005;173:271–275. doi: 10.1097/01.ju.0000141311.51003.87. [DOI] [PubMed] [Google Scholar]
- 24.Ghalayini I.F., Al-Ghazo M.A., Harfeil M.N. Prophylaxis and therapeutic effects of raspberry (Rubus idaeus) on renal stone formation in Balb/c mice. Int. Braz. J. Urol. 2011;37:259–267. doi: 10.1590/S1677-55382011000200013. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Zhang Z., Yang Y., Zu X., Guan D., Guan Y. Diuretic activity of Rubus idaeus L (Rosaceae) in rats. Trop. J. Pharm. Res. 2011;10 doi: 10.4314/tjpr.v10i3.15. [DOI] [Google Scholar]
- 26.Divakar K., Pawar A., Chandrasekhar S., Dighe S., Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem. Toxicol. 2010;48:1013–1018. doi: 10.1016/j.fct.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Al-Yousofy F., Gumaih H., Ibrahim H., Alasbahy A. Parsley! Mechanism as antiurolithiasis remedy. Am. J. Clin. Exp. Urol. 2017;5:55. [PMC free article] [PubMed] [Google Scholar]
- 28.Jafar S., Mehri L., Hadi B. The antiurolithiasic activity of aqueous extract of Petroselinum sativum on ethylene glycolinduced kidney calculi in rats; Proceedings of the International Conference on Chemical, Environmental and Biological Sciences; Pattaya, Thailand. 7–8 October 2011; pp. 1577–1583. [Google Scholar]
- 29.Saeidi J., Bozorgi H., Zendehdel A., Mehrzad J. Therapeutic effects of aqueous extracts of Petroselinum sativum on ethylene glycol-induced kidney calculi in rats. Urol. J. 2012;9:361–366. [PubMed] [Google Scholar]
- 30.Patel P., Patel M., Saralai M., Gandhi T. Antiurolithiatic effects of Solanum xanthocarpum fruit extract on ethylene-glycol-induced nephrolithiasis in rats. J. Young Pharm. 2012;4:164–170. doi: 10.4103/0975-1483.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., Li N., Li K., Li P. Protective effect of Urtica dioica methanol extract against experimentally induced urinary calculi in rats. Mol. Med. Rep. 2014;10:3157–3162. doi: 10.3892/mmr.2014.2610. [DOI] [PubMed] [Google Scholar]
- 32.Vanachayangkul P., Chow N., Khan S., Butterweck V. Prevention of renal crystal deposition by an extract of Ammi visnaga L. and its constituents khellin and visnagin in hyperoxaluric rats. Urol. Res. 2011;39:189–195. doi: 10.1007/s00240-010-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoei A., Hadjzadeh Z., Parizady M. Ethanolic extract of nigella sativa L seeds on ethylene glycol-induced kidney calculi in rats. Urol. J. 2009;4:86–90. [PubMed] [Google Scholar]
- 34.Hajzadeh M., Mohammadian N., Rahmani Z., Rassouli F.B. Effect of thymoquinone on ethylene glycol-induced kidney calculi in rats. Urol. J. 2008;5:149–155. [PubMed] [Google Scholar]
- 35.Li X., Liang Q., Sun Y., Diao L., Qin Z., Wang W., Lu J., Fu S., Ma B., Yue Z. Potential mechanisms responsible for the antinephrolithic effects of an aqueous extract of Fructus aurantii. Evid.-Based Complement. Altern. Med. 2015 doi: 10.1155/2015/491409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laikangbam R., Devi M.D. Inhibition of calcium oxalate crystal deposition on kidneys of urolithiatic rats by Hibiscus sabdariffa L. extract. Urol. Res. 2012;40:211–218. doi: 10.1007/s00240-011-0433-3. [DOI] [PubMed] [Google Scholar]
- 37.Meiouet F., El Kabbaj S., Daudon M. In vitro study of the litholytic effects of herbal extracts on cystine urinary calculi. Prog. Urol. 2011;21:40–47. doi: 10.1016/j.purol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Cheraft-Bahloul N., Husson C., Ourtioualous M., Sinaeve S., Atmani D., Stévigny C., Nortier J.L., Antoine M.-H. Protective Effects of Pistacia lentiscus L. fruit extract against calcium oxalate monohydrate induced proximal tubular injury. J. Ethnopharmacol. 2017;209:248–254. doi: 10.1016/j.jep.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Saha S., Verma R.J. Evaluation of hydro-alcoholic extract of Dolichos biflorus seeds on inhibition of calcium oxalate crystallization. J. Herb. Med. 2015;5:41–47. doi: 10.1016/j.hermed.2014.11.001. [DOI] [Google Scholar]
- 40.Atodariya U., Barad R., Upadhyay S., Upadhyay U. Anti-urolithiatic activity of Dolichos biflorus seeds. J. Pharmacogn. Phytochem. 2013;2:45051. [Google Scholar]
- 41.Jeong B.C., Kim B.S., Kim J.I., Kim H.H. Effects of green tea on urinary stone formationn in vivo and in vitro study. J. Endourol. 2006;20:356–361. doi: 10.1089/end.2006.20.356. [DOI] [PubMed] [Google Scholar]
- 42.Tracy C.R., Henning J.R., Newton M.R., Aviram M., Zimmerman M.B. Oxidative stress and nephrolithiasis: A comparative pilot study evaluating the effect of pomegranate extract on stone risk factors and elevated oxidative stress levels of recurrent stone formers and controls. Urolithiasis. 2014;42:401–408. doi: 10.1007/s00240-014-0686-8. [DOI] [PubMed] [Google Scholar]
- 43.Singh R.G., Behura S.K., Kumar R. Litholytic property of Kulattha (Dolichous biflorus) vs. potassium citrate in renal calculus disease comparative study. JAPI. 2010;58:286–289. [PubMed] [Google Scholar]
- 44.Prasongwatana V., Woottisin S., Sriboonlue P., Kukongviriyapan V. Uricosuric effect of Roselle (Hibiscus sabdariffa) in normal and renal-stone former subjects. J. Ethnopharmacol. 2008;117:491–495. doi: 10.1016/j.jep.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Rodgers A., Mokoena M., Durbach I., Lazarus J., de Jager S., Ackermann H., Breytenbach I., Okada A., Usami M., Hirose Y. Do teas rich in antioxidants reduce the physicochemical and peroxidative risk factors for calcium oxalate nephrolithiasis in humans? Pilot studies with Rooibos herbal tea and Japanese green tea. Urolithiasis. 2016;44:299–310. doi: 10.1007/s00240-015-0855-4. [DOI] [PubMed] [Google Scholar]
- 46.Anttonen M.J., Karjalainen R.O. Environmental and genetic variation of phenolic compounds in red raspberry. J. Food Compos. Anal. 2005;18:759–769. doi: 10.1016/j.jfca.2004.11.003. [DOI] [Google Scholar]
- 47.Bhatt P., Kushwah A. Rubia cordifolia overview: A new approach to treat cardiac disorders. Int. J. Drug Dev. Res. 2013;5:47–54. [Google Scholar]
- 48.Deshkar N., Tilloo S., Pande V. A comprehensive review of Rubia cordifolia Linn. Pharmacogn. Rev. 2008;2:124–134. [Google Scholar]
- 49.Dev S. Selection of Prime Ayurvedic Plant Drugs. Anamaya Publishers; New Delhi, India: 2006. [Google Scholar]
- 50.Son J.K., Jung S.J., Jung J.H., Fang Z., Lee C.S., Seo C.S., Moon D.C., Min B.S., Kim M.R., Woo M.H. Anticancer Constituents from the Roots of Rubia cordifolia L. Chem. Pharm. Bull. 2008;56:213–216. doi: 10.1248/cpb.56.213. [DOI] [PubMed] [Google Scholar]
- 51.Lodi S., Sharma V., Kansal L. The protective effect of Rubia cordifolia against lead nitrate-induced immune response impairment and kidney oxidative damage. Indian J. Pharm. 2011;43:441–444. doi: 10.4103/0253-7613.83118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farzaei M.H., Abbasabadi Z., Ardekani M.R.S., Rahimi R., Farzaei F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 2013;33:815–826. doi: 10.1016/S0254-6272(14)60018-2. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H., Chen F., Wang X., Yao H.-Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res. Int. 2006;39:833–839. doi: 10.1016/j.foodres.2006.03.007. [DOI] [Google Scholar]
- 54.Moazedi A., Mirzaie D., Seyyednejad S., Zadkarami M., Amirzargar A. Spasmolytic effect of Petroselinum crispum (Parsley) on rat’s ileum at different calcium chloride concentrations. Pak. J. Biol. Sci. 2007;10:4036–4042. doi: 10.3923/pjbs.2007.4036.4042. [DOI] [PubMed] [Google Scholar]
- 55.Rezazad M., Farokhi F. Protective effect of Petroselinum crispum extract in abortion using prostadin-induced renal dysfunction in female rats. Avicenna J. Phytomed. 2014;4:312–319. [PMC free article] [PubMed] [Google Scholar]
- 56.Kreydiyyeh S.I., Usta J. Diuretic effect and mechanism of action of parsley. J. Ethnopharmacol. 2002;79:353–357. doi: 10.1016/S0378-8741(01)00408-1. [DOI] [PubMed] [Google Scholar]
- 57.Vamenta-Morris H., Dreisbach A., Shoemaker-Moyle M., Abdel-Rahman E.M. Internet claims on dietary and herbal supplements in advanced nephropathyruth or myth. Am. J. Nephrol. 2014;40:393–398. doi: 10.1159/000368724. [DOI] [PubMed] [Google Scholar]
- 58.Kuźma P., Drużyńska B., Obiedziński M. Optimization of extraction conditions of some polyphenolic compounds from parsley leaves (Petroselinum crispum) Acta Sci. Pol. Technol. Aliment. 2014;13:145–154. doi: 10.17306/J.AFS.2014.2.4. [DOI] [PubMed] [Google Scholar]
- 59.Jurenka J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 60.Bhandari P.R. Pomegranate (Punica granatum L). Ancient seeds for modern cure? Review of potential therapeutic applications. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012;2:171–184. doi: 10.4103/2231-0738.99469. [DOI] [Google Scholar]
- 61.Tugcu V., Kemahli E., Ozbek E., Arinci Y.V., Uhri M., Erturkuner P., Metin G., Seckin I., Karaca C., Ipekoglu N. Protective effect of a potent antioxidant, pomegranate juice, in the kidney of rats with nephrolithiasis induced by ethylene glycol. J. Endourol. 2008;22:2723–2732. doi: 10.1089/end.2008.0357. [DOI] [PubMed] [Google Scholar]
- 62.Elwej A., Ghorbel I., Marrekchi R., Boudawara O., Jamoussi K., Boudawara T., Zeghal N., Sefi M. Improvement of kidney redox states contributes to the beneficial effects of dietary pomegranate peel against barium chloride-induced nephrotoxicity in adult rats. Arch. Physiol. Biochem. 2016;122:130–140. doi: 10.3109/13813455.2016.1150298. [DOI] [PubMed] [Google Scholar]
- 63.Boroushaki M.T., Asadpour E., Sadeghnia H.R., Dolati K. Effect of pomegranate seed oil against gentamicin-induced nephrotoxicity in rat. J. Food Sci. Technol. 2014;51:3510–3514. doi: 10.1007/s13197-012-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alimoradian A., Changizi-Ashtiyani S., Ghiasabadi Farahani A., Kheder L., Rajabi R., Sharifi A. Protective Effects of Pomegranate Juice on Nephrotoxicity Induced by Captopril and Gentamicin in Rats. Iran. J. Kidney Dis. 2017;11:422–429. [PubMed] [Google Scholar]
- 65.Sadeghi F., Nematbakhsh M., Noori-Diziche A., Eshraghi-Jazi F., Talebi A., Nasri H., Mansouri A., Dehghani A., Saberi S., Shirdavani S., et al. Protective effect of pomegranate flower extract against gentamicin-induced renal toxicity in male rats. J. Ren. Inj. Prev. 2015;4:45–50. doi: 10.12861/jrip.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Primarizky H., Yuniarti W.M., Lukiswanto B.S. Benefits of pomegranate (Punica granatum Linn) fruit extracts to weight changes, total protein, and uric acid in white rats (Rattus norvegicus) as an animal model of acute renal failure. Vet. World. 2016;9:1269–1274. doi: 10.14202/vetworld.2016.1269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharifiyan F., Movahedian-Attar A., Nili N., Asgary S. Study of pomegranate (Punica granatum L.) peel extract containing anthocyanins on fatty streak formation in the renal arteries in hypercholesterolemic rabbits. Adv. Biomed. Res. 2016;5:8. doi: 10.4103/2277-9175.175241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagri P., Ali M., Aeri V., Bhowmik M., Sultana S. Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009;47:50–54. doi: 10.1016/j.fct.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 69.Ilbey Y.O., Ozbek E., Simsek A., Cekmen M., Somay A., Tasci A.I. Effects of pomegranate juice on hyperoxaluria-induced oxidative stress in the rat kidneys. Ren. Fail. 2009;31:522–531. doi: 10.1080/08860220902963871. [DOI] [PubMed] [Google Scholar]
- 70.Ljubuncic P., Song H., Cogan U., Azaizeh H., Bomzon A. The effects of aqueous extracts prepared from the leaves of Pistacia lentiscus in experimental liver disease. J. Ethnopharmacol. 2005;100:198–204. doi: 10.1016/j.jep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Benhammou N., Bekkara F.A., Panovska T.K. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. Afr. J. Pharm. Pharmacol. 2008;2:22–28. [Google Scholar]
- 72.Kumar S., Pandey A.K. Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as foodn in vitro antioxidant, anticancer and anti HIV perspective. BMC Complement. Altern. Med. 2014;14:112. doi: 10.1186/1472-6882-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saha S., Goswami G., Pandrangi A. Isolation and prevention of calcium oxalate-induced apoptotic death and oxidative stress in MDCK cells by diosgenin. Chem. Biol. Interact. 2014;224:51–57. doi: 10.1016/j.cbi.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 74.Ranka D., Aswar M., Aswar U., Bodhankar S. Diuretic potential of aqueous extract of roots of Solanum xanthocarpum Schrad & Wendl, a preliminary study. Indian J. Exp. Biol. 2013;51:833–839. [PubMed] [Google Scholar]
- 75.Hussain T., Gupta R.K., Sweety K., Eswaran B., Vijayakumar M., Rao C.V. Nephroprotective activity of Solanum xanthocarpum fruit extract against gentamicin–induced nephrotoxicity and renal dysfunction in experimental rodents. Asian Pac. J. Trop. Med. 2012;5:686–691. doi: 10.1016/S1995-7645(12)60107-2. [DOI] [PubMed] [Google Scholar]
- 76.Durak I., Biri H., Devrim E., Sözen S., Avcı A. Aqueous extract of Urtica dioica makes significant inhibition on adenosine deaminase activity in prostate tissue from patients with prostate cancer. Cancer Biol. Ther. 2004;3:855–857. doi: 10.4161/cbt.3.9.1038. [DOI] [PubMed] [Google Scholar]
- 77.Mathew L.E., Sindhu G., Helen A. Dolichos biflorus exhibits anti-inflammatory and antioxidant properties in an acute inflammatory model. J. Food Drug Anal. 2014;22:455–462. doi: 10.1016/j.jfda.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayatdavoudi P., Rad A.K., Rajaei Z., Hadjzadeh M.A.-R. Renal injury, nephrolithiasis and Nigella sativa: A mini review. Avicenna J. Phytomed. 2016;6:1–8. [PMC free article] [PubMed] [Google Scholar]
- 79.Amin G.R. Popular Medicinal Plants of Iran. Volume 1 Iranian Research Institute of Medicinal Plants Tehran; Tehran, Iran: 1991. [Google Scholar]
- 80.Woottisin S., Hossain R.Z., Yachantha C., Sriboonlue P., Ogawa Y., Saito S. Effects of Orthosiphon grandiflorus, Hibiscus sabdariffa and Phyllanthus amarus extracts on risk factors for urinary calcium oxalate stones in rats. J. Urol. 2011;185:323–328. doi: 10.1016/j.juro.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Farzaei M.H., Abdollahi M., Rahimi R. Role of dietary polyphenols in the management of peptic ulcer. World J. Gastroenterol. 2015;21:6499. doi: 10.3748/wjg.v21.i21.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gürocak S., Küpeli B. Consumption of historical and current phytotherapeutic agents for urolithiasis: A critical review. J. Urol. 2006;176:450–455. doi: 10.1016/j.juro.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 83.Alok S., Jain S.K., Verma A., Kumar M., Sabharwal M. Pathophysiology of kidney, gallbladder and urinary stones treatment with herbal and allopathic medicine: A review. Asian Pac. J. Trop. Dis. 2013;3:496–504. doi: 10.1016/S2222-1808(13)60107-3. [DOI] [Google Scholar]
- 84.Lien E.J.-C., Lien L.L.-M., Wang R., Wang J. Phytochemical analysis of medicinal plants with kidney protective activities. Chin. J. Integr. Med. 2012;18:790–800. doi: 10.1007/s11655-011-0713-3. [DOI] [PubMed] [Google Scholar]
- 85.Saha S., Verma R.J. Inhibition of calcium oxalate crystallisation in vitro by an extract of Bergenia ciliata. Arab J. Urol. 2013;11:187–192. doi: 10.1016/j.aju.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bashir S., Gilani A.H. Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J. Ethnopharmacol. 2009;122:106–116. doi: 10.1016/j.jep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 87.Aggarwal D., Kaushal R., Kaur T., Bijarnia R.K., Puri S., Singla S.K. The most potent antilithiatic agent ameliorating renal dysfunction and oxidative stress from Bergenia ligulata rhizome. J. Ethnopharmacol. 2014;158:85–93. doi: 10.1016/j.jep.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Chauhan C., Joshi M., Vaidya A. Growth inhibition of struvite crystals in the presence of herbal extract Commiphora wightii. J. Mater. Sci. Mater. Med. 2009;20:85. doi: 10.1007/s10856-008-3489-z. [DOI] [PubMed] [Google Scholar]
- 89.De Cógáin M.R., Linnes M.P., Lee H.J., Krambeck A.E., de Mendonça Uchôa J.C., Kim S.-H., Lieske J.C. Aqueous extract of Costus arabicus inhibits calcium oxalate crystal growth and adhesion to renal epithelial cells. Urolithiasis. 2015;43:119–124. doi: 10.1007/s00240-015-0749-5. [DOI] [PubMed] [Google Scholar]
- 90.Atmani F., Slimani Y., Mbark A., Bnouham M., Ramdani A. In vitro and in vivo antilithiasic effect of saponin rich fraction isolated from Herniaria hirsute. J. Bras. Nefrol. 2006;28:199–203. [Google Scholar]
- 91.Tayal S., Duggal S., Bandyopadhyay P., Aggarwal A., Tandon S., Tandon C. Cytoprotective role of the aqueous extract of Terminalia chebula on renal epithelial cells. Int. Braz. J. Urol. 2012;38:204–214. doi: 10.1590/S1677-55382012000200008. [DOI] [PubMed] [Google Scholar]
- 92.Aggarwal A., Tandon S., Kumar Singla S., Tandon C. A novel antilithiatic protein from Tribulus terrestris having cytoprotective potency. Protein Pept. Lett. 2012;19:812–819. doi: 10.2174/092986612801619552. [DOI] [PubMed] [Google Scholar]
- 93.Sathya M., Kokilavani R., Teepa K.A., Balakrishnan A. Biopotency of Acalypha indica Linn on Membrane Bound ATPases and Marker Enzymes urolithic Rats. Anc. Sci. Life. 2011;31:3–9. [PMC free article] [PubMed] [Google Scholar]
- 94.Soundararajan P., Mahesh R., Ramesh T., Begum V.H. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J. Exp. Biol. 2006;44:981–986. [PubMed] [Google Scholar]
- 95.Ahmadi M., Rad A.K., Rajaei Z., Hadjzadeh M.-A.-R., Mohammadian N., Tabasi N.S. Alcea rosea root extract as a preventive and curative agent in ethylene glycol-induced urolithiasis in rats. Indian J. Pharmacol. 2012;44:304–307. doi: 10.4103/0253-7613.96298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Christina A., Ashok K., Packialakshmi M., Tobin G., Preethi J., Murugesh N. Antilithiatic effect of Asparagus racemosus Willd on ethylene glycol-induced lithiasis in male albino Wistar rats. Methods Find. Exp. Clin. Pharmacol. 2005;27:633–638. doi: 10.1358/mf.2005.27.9.939338. [DOI] [PubMed] [Google Scholar]
- 97.Gadge N., Jalalpure S. Curative treatment with extracts of Bombax ceiba fruit reduces risk of calcium oxalate urolithiasis in rats. Pharm. Biol. 2012;50:310–317. doi: 10.3109/13880209.2011.604332. [DOI] [PubMed] [Google Scholar]
- 98.Lin W.-C., Lai M.-T., Chen H.-Y., Ho C.-Y., Man K.-M., Shen J.-L., Lee Y.-J., Tsai F.-J., Chen Y.-H., Chen W.-C. Protective effect of Flos carthami extract against ethylene glycol-induced urolithiasis in rats. Urol. Res. 2012;40:655–661. doi: 10.1007/s00240-012-0472-4. [DOI] [PubMed] [Google Scholar]
- 99.Rad A.K., Rajaei Z., Mohammadian N., Valiollahi S., Sonei M. The Beneficial effect of Cynodon Dactylon fractions on ethylene glycol-induced kidney calculi in rats. Urol. J. 2011;8:179–184. [PubMed] [Google Scholar]
- 100.Orhan N., Onaran M., Şen İ., Gönül İ.I., Aslan M. Preventive treatment of calcium oxalate crystal deposition with immortal flowers. J. Ethnopharmacol. 2015;163:60–67. doi: 10.1016/j.jep.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 101.Shah J.G., Patel B.G., Patel S.B., Patel R.K. Antiurolithiatic and antioxidant activity of Hordeum vulgare seeds on ethylene glycol-induced urolithiasis in rats. Indian J. Pharmacol. 2012;44:672–677. doi: 10.4103/0253-7613.103237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ingale K.G., Thakurdesai P.A., Vyawahare N.S. Effect of Hygrophila spinosa in ethylene glycol induced nephrolithiasis in rats. Indian J. Pharmacol. 2012;44:639–642. doi: 10.4103/0253-7613.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khalili M., Jalali M.R., Mirzaei-Azandaryani M. Effect of hydroalcoholic extract of Hypericum perforatum L. leaves on ethylene glycol-induced kidney calculi in rats. Urol. J. 2012;9:472–479. [PubMed] [Google Scholar]
- 104.Makasana A., Ranpariya V., Desai D., Mendpara J., Parekh V. Evaluation for the anti-urolithiatic activity of Launaea procumbens against ethylene glycol-induced renal calculi in rats. Toxicol. Rep. 2014;1:46–52. doi: 10.1016/j.toxrep.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cho H.J., Bae W.J., Kim S.J., Hong S.H., Lee J.Y., Hwang T.-K., Choi Y.J., Hwang S.Y., Kim S.W. The inhibitory effect of an ethanol extract of the spores of Lygodium japonicum on ethylene glycol-induced kidney calculi in rats. Urolithiasis. 2014;42:309–315. doi: 10.1007/s00240-014-0674-z. [DOI] [PubMed] [Google Scholar]
- 106.Akanae W., Tsujihata M., Yoshioka I., Nonomura N., Okuyama A. Orthosiphon grandiflorum has a protective effect in a calcium oxalate stone forming rat model. Urol. Res. 2010;38:89–96. doi: 10.1007/s00240-010-0265-6. [DOI] [PubMed] [Google Scholar]
- 107.Bouanani S., Henchiri C., Migianu-Griffoni E., Aouf N., Lecouvey M. Pharmacological and toxicological effects of Paronychia argentea in experimental calcium oxalate nephrolithiasis in rats. J. Ethnopharmacol. 2010;129:38–45. doi: 10.1016/j.jep.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 108.Vyas B., Vyas R., Joshi S., Santani D. Antiurolithiatic activity of whole-plant hydroalcoholic extract of Pergularia daemia in rats. J. Young Pharm. 2011;3:36–40. doi: 10.4103/0975-1483.76417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moriyama M.T., Suga K., Miyazawa K., Tanaka T., Higashioka M., Noda K., Oka M., Tanaka M., Suzuki K. Inhibitions of urinary oxidative stress and renal calcium level by an extract of Quercus salicina Blume/Quercus stenophylla Makino in a rat calcium oxalate urolithiasis model. Int. J. Urol. 2009;16:397–401. doi: 10.1111/j.1442-2042.2009.02268.x. [DOI] [PubMed] [Google Scholar]
- 110.Geetha K., Manavalan R., Venkappayya D. Control of urinary risk factors of stone formation by Salvadora persica in experimental hyperoxaluria. Methods Find. Exp. Clin. Pharmacol. 2010;32:623–629. doi: 10.1358/mf.2010.32.9.1549114. [DOI] [PubMed] [Google Scholar]
- 111.Mirian E.-C.M., Juanita N.-M., Christophe B.O., Estela M.-C.M. Molecular mechanisms involved in the protective effect of the chloroform extract of Selaginella lepidophylla (Hook. et Grev.) Spring in a lithiasic rat model. Urolithiasis. 2013;41:205–215. doi: 10.1007/s00240-013-0556-9. [DOI] [PubMed] [Google Scholar]
- 112.Brardi S., Imperiali P., Cevenini G., Verdacchi T., Ponchietti R. Effects of the association of potassium citrate and agropyrum repens in renal stone treatment: Results of a prospective randomized comparison with potassium citrate. Arch. Ital. Urol. Androl. 2012;84:61–67. [PubMed] [Google Scholar]
- 113.Micali S., Sighinolfi M., Celia A., de Stefani S., Grande M., Cicero A., Bianchi G. Can Phyllanthus niruri affect the efficacy of extracorporeal shock wave lithotripsy for renal stones? A randomized, prospective, long-term study. J. Urol. 2006;176:1020–1022. doi: 10.1016/j.juro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 114.Joshi V.S., Parekh B.B., Joshi M.J., Vaidya A.D. Inhibition of the growth of urinary calcium hydrogen phosphate dihydrate crystals with aqueous extracts of Tribulus terrestris and Bergenia ligulata. Urol. Res. 2005;33:80–86. doi: 10.1007/s00240-004-0450-6. [DOI] [PubMed] [Google Scholar]
- 115.Byahatti V.V., Pai K.V., D’Souza M.G. Effect of phenolic compounds from Bergenia ciliata (Haw.) Sternb. leaves on experimental kidney stones. Anc. Sci. Life. 2010;30:14–17. [PMC free article] [PubMed] [Google Scholar]
- 116.Han X., Shen T., Lou H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007;8:950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- 117.Mendoza-Wilson A.M., Glossman-Mitnik D. Theoretical study of the molecular properties and chemical reactivity of (+)-catechin and (−)-epicatechin related to their antioxidant ability. J. Mol. Struct. THEOCHEM. 2006;761:97–106. doi: 10.1016/j.theochem.2006.01.001. [DOI] [Google Scholar]
- 118.Higdon J.V., Frei B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 119.Amengual-Cladera E., Nadal-Casellas A., Gómez-Pérez Y., Gomila I., Prieto R.M., Proenza A.M., Lladó I. Phytotherapy in a rat model of hyperoxaluriahe antioxidant effects of quercetin involve serum paraoxonase 1 activation. Exp. Biol. Med. 2011;236:1133–1138. doi: 10.1258/ebm.2011.011090. [DOI] [PubMed] [Google Scholar]
- 120.Khan S.A., Priyamvada S., Farooq N., Khan S., Khan M.W., Yusufi A.N. Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacol. Res. 2009;59:254–262. doi: 10.1016/j.phrs.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Zhai W., Zheng J., Yao X., Peng B., Liu M., Huang J., Wang G., Xu Y. Catechin prevents the calcium oxalate monohydrate induced renal calcium crystallization in NRK-52E cells and the ethylene glycol induced renal stone formation in rat. BMC Complement. Altern. Med. 2013;13:228. doi: 10.1186/1472-6882-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grases F., Prieto R.M., Fernandez-Cabot R.A., Costa-Bauzá A., Tur F., Torres J.J. Effects of polyphenols from grape seeds on renal lithiasis. Oxid. Med. Cell. Longev. 2015;2015:813737. doi: 10.1155/2015/813737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kanlaya R., Singhto N., Thongboonkerd V. EGCG decreases binding of calcium oxalate monohydrate crystals onto renal tubular cells via decreased surface expression of alpha-enolase. J. Biol. Inorg. Chem. 2016;21:339–346. doi: 10.1007/s00775-016-1344-0. [DOI] [PubMed] [Google Scholar]
- 124.Crespo M., Galvez J., Cruz T., Ocete M., Zarzuelo A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999;65:651–653. doi: 10.1055/s-2006-960838. [DOI] [PubMed] [Google Scholar]
- 125.Abdel-Daim M.M., Khalifa H.A., Abushouk A.I., Dkhil M.A., Al-Quraishy S.A. Diosmin Attenuates Methotrexate-Induced Hepatic, Renal, and Cardiac Injury: A Biochemical and Histopathological Study in Mice. Oxid. Med. Cell. Longev. 2017;2017:3281670. doi: 10.1155/2017/3281670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahmed S., Mundhe N., Borgohain M., Chowdhury L., Kwatra M., Bolshette N., Ahmed A., Lahkar M. Diosmin Modulates the NF-κB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation. 2016;39:1783–1797. doi: 10.1007/s10753-016-0413-4. [DOI] [PubMed] [Google Scholar]
- 127.Rehman M.U., Tahir M., Quaiyoom Khan A., Khan R., Lateef A., Hamiza O.O., Ali F., Sultana S. Diosmin protects against trichloroethylene-induced renal injury in Wistar ratslausible role of p53, Bax and caspases. Br. J. Nutr. 2013;110:699–710. doi: 10.1017/S0007114512005752. [DOI] [PubMed] [Google Scholar]
- 128.Schlottfeldt Fdos S., Fernandes S.M., Martins D.M., Cordeiro P., Fonseca C.D., Watanabe M., Vattimo Mde F. Prevention of amphotericin B nephrotoxicity through use of phytotherapeutic medication. Rev. Esc. Enferm. USP. 2015;49:74–79. doi: 10.1590/S0080-623420150000700011. [DOI] [PubMed] [Google Scholar]
- 129.Noorafshan A., Karbalay-Doust S., Karimi F. Diosmin reduces calcium oxalate deposition and tissue degeneration in nephrolithiasis in rats: A stereological study. Korean J. Urol. 2013;54:252–257. doi: 10.4111/kju.2013.54.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prabhu V.V., Sathyamurthy D., Ramasamy A., Das S., Anuradha M., Pachiappan S. Evaluation of protective effects of diosmin (a citrus flavonoid) in chemical-induced urolithiasis in experimental rats. Pharm. Biol. 2016;54:1513–1521. doi: 10.3109/13880209.2015.1107105. [DOI] [PubMed] [Google Scholar]
- 131.Pastukhov A.V., Levchenko L.A., Sadkov A.P. Spectroscopic study on binding of rutin to human serum albumin. J. Mol. Struct. 2007;842:60–66. doi: 10.1016/j.molstruc.2006.12.008. [DOI] [Google Scholar]
- 132.Colombo R., de L Batista A.N., Teles H.L., Silva G.H., Bomfim G.C., Burgos R.C., Cavalheiro A.J., da Silva Bolzani V., Silva D.H., Pelicia C.R., et al. Validated HPLC method for the standardization of Phyllanthus niruri (herb and commercial extracts) using corilagin as a phytochemical marker. Biomed. Chromatogr. BMC. 2009;23:573–580. doi: 10.1002/bmc.1155. [DOI] [PubMed] [Google Scholar]
- 133.Ghodasara J., Pawar A., Deshmukh C., Kuchekar B. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacogn. Res. 2010;2:388–392. doi: 10.4103/0974-8490.75462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu W., Xu Y.F., Feng Y., Peng B., Che J.P., Liu M., Zheng J.H. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis. 2014;42:519–526. doi: 10.1007/s00240-014-0695-7. [DOI] [PubMed] [Google Scholar]
- 135.Park H.K., Jeong B.C., Sung M.-K., Park M.-Y., Choi E.Y., Kim B.S., Kim H.H., Kim J.I. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J. Urol. 2008;179:1620–1626. doi: 10.1016/j.juro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 136.Mutalib L.Y. Comparison between phytotherapy and conventional drug therapy used in urolithiasis management in Hawler city, Kurdistan Region\Iraq. J. Pharmacogn. Phytochem. 2015;4:83–86. [Google Scholar]