Abstract
Astragalus membranaceus, dried root extract, also known as Astragali radix, is used in traditional Chinese medicine as a tonic remedy. Moreover, it has been reported that Astragalus membranaceus could attenuate intestinal inflammation; however, the underlying mechanism for its anti-inflammatory activity in intestinal epithelial cells (IECs) remains unclear. In this study, we evaluated Astragalus membranaceus extract (5–100 µg/mL) in a model of inflammation and oxidative stress for IECs. We showed that Astragalus membranaceus extract reduced the inflammatory response induced by lipopolysaccharide from E. coli (LPS) plus interferon-γ (IFN), decreasing tumor necrosis factor-α (TNF-α) release, cycloxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression, nitrotyrosine formation, nuclear factor-κB (NF-κB) activation, and reactive oxygen species (ROS) release in the non-tumorigenic intestinal epithelial cell line (IEC-6). The antioxidant potential of Astragalus membranaceus extract was also evaluated in a model of hydrogen peroxide (H2O2)-induced oxidative stress in IEC-6, indicating that this extract reduced ROS release and increased nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activation and the expression of antioxidant cytoprotective factors in these cells. The results contributed to clarify the mechanisms involved in Astragalus membranaceus extract-reduced inflammation and highlighted the potential use of this extract as an anti-inflammatory and antioxidant remedy for intestinal diseases.
Keywords: Astragalus membranaceus, intestinal epithelial cells, inflammation, oxidative stress
1. Introduction
Inflammatory bowel disease (IBD) is one of the most prevalent gastrointestinal disorders, and it includes ulcerative colitis (UC) [1] and Crohn’s disease (CD) [2]. IBD pathogenesis results from a multifactorial process involving genetic, environmental, and immunogenic factors [3]. Over the last few decades, our understanding of IBD aetiology has increased, yet its exact mechanisms remain unclear. Nevertheless, it is well known that IBD is characterized by inflammation and abnormalities in epithelial barrier function [4]. Current knowledge shows that intestinal epithelial cells (IECs), which under physiological conditions are indispensable to maintain a selective barrier between the host and harmful substances present in the lumen, have emerged as key players in the generation and persistence of intestinal inflammation during IBD [5]. Different noxious agents, such as chemical, physical, infectious, and inflammatory injuries can damage the intestinal epithelial integrity. This damage can lead to increased penetration and absorption of toxic substances, the activation of immunogenic responses, and a final disequilibrium in the host’s homeostasis. In response to these external factors, IECs and immune cells are activated and can trigger an inflammatory response [6]. The release of pro-inflammatory factors, such as cytokines and chemokines, as well as oxidative stress with the release of reactive oxygen species (ROS), are the main events taking place in intestinal inflammation during the active phase. In particular, by influencing transcription factors and redox-sensitive signaling pathways, ROS and their oxidized products can further sustain inflammation within the intestinal layer.
Plants are considered a potential source of antioxidant and anti-inflammatory molecules. The genus Astragalus belongs to the legume family (Fabaceae). This genus, the largest of flowering plants in the world, includes 2500–3000 species mainly found in Central and Southwestern Asia [7,8]. Different Astragalus species are used in traditional medicine, mostly Chinese, and the dried roots of A. membranaceus (Fisch.) Bge and A. membranaceus (Fisch.) var. mongholicus (Bge) Hsiao are included in the drug Huangqi (Radix Astragali), which is present in the pharmacopoeia of the People’s Republic of China [9]. Astragalus membranaceus is used as a tonic and has many effects, such as enhancing defensive energy and inducing diuresis to treat edema [10]. It is widely used in East Asia to prevent some severe chemotherapy side effects [11] and liver fibrosis [12]. Moreover, recent pharmacological studies and clinical evidence centered on Astragalus membranaceus have reported a wide spectrum of biological activities for this plant [13,14], including at an intestinal level [15,16,17]. Astragali radix, the dried root of Astragalus membranaceus, is a popular health-promoting herb, and its use as a crude drug is one of the oldest and most frequently used remedies in oriental medicine [18]. Pharmacological studies have demonstrated that the water extract of Astragali radix possesses many biological functions [19,20,21,22,23]. Also, Astragalus polysaccharides, which are major constituents of Astragali radix, possess many biological effects and pharmacological properties, including at intestinal levels [24,25]. Despite this, there is little mechanistic knowledge regarding the molecular action(s) of Astragalus membranaceus root extract on IECs [26] and especially during inflammatory conditions. Considering the pivotal role of IECs in maintaining and regulating intestinal homeostasis, in this study, we evaluated the effects of Astragalus membranaceus extract (5–100 µg/mL) on inflammation and oxidative stress in the intestinal epithelial cell line (IEC-6) in order to elucidate the effect of Astragalus membranaceus extract during intestinal inflammation at the cellular level.
2. Results
2.1. Astragalus membranaceus Extract Did Not Have Any Antiproliferative Activity on IEC-6 Cells
To evaluate the antiproliferative potential of Astragalus membranaceus extract on IEC-6, cells were treated with the extract (5–100 μg/mL) for 24 h. The results indicated that the extract did not have any significant antiproliferative activity on IEC-6 cells (mean ± SEM of % antiproliferative activity vs. control: 1.12 ± 1.04, 3.38 ± 1.20, 4.06 ± 1.15, 6.16 ± 2.03, respectively for Astragalus membranaceus extract 5, 10, 50, 100 μg/mL).
2.2. Astragalus membranaceus Extract Reduced Tumor Necrosis Factor-α (TNF-α) Levels in Lipopolysaccharide from E. coli (LPS) + Interferon-γ (IFN)-Stimulated IEC-6
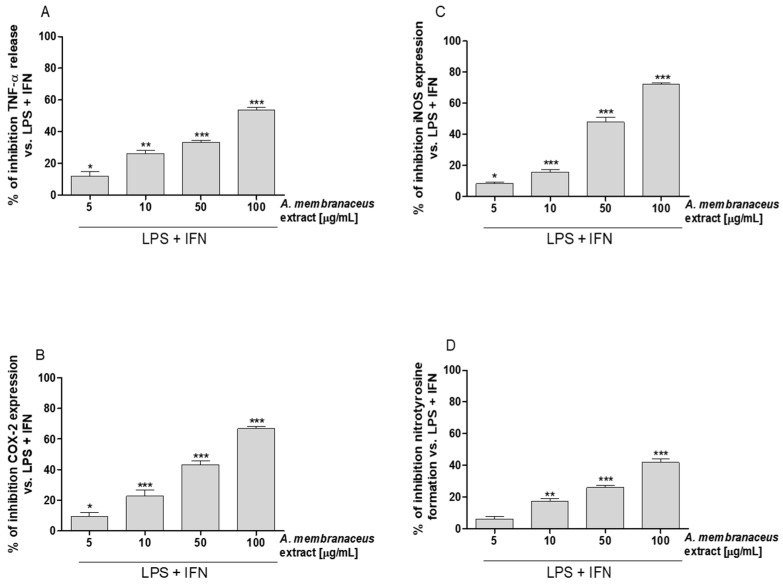
The effect of Astragalus membranaceus extract on TNF-α levels in IEC-6 cellular medium was evaluated using an enzyme-linked immunosorbent assay (ELISA). Our results showed that Astragalus membranaceus extract (5–100 μg/mL) significantly inhibited TNF-α release, induced by LPS + IFN, in IEC-6 cells medium (p < 0.05 vs. LPS + IFN; Figure 1A).
Figure 1.
Inhibitory and concentration related effect of Astragalus membranaceus extract (5–100 μg/mL) in LPS + IFN-stimulated IEC-6 on (A) tumor necrosis factor-α (TNFα) levels, evaluated using an ELISA, (B) cyclooxygenase-2 (COX-2) expression, (C) inducible nitric oxide synthase (iNOS) expression, and on (D) nitrotyrosine formation, evaluated by the cytofluorimetric technique. Data are expressed as mean ± SEM. ***, **, * indicate p < 0.001, p < 0.01 and p < 0.05 vs. LPS + IFN.
2.3. Astragalus membranaceus Extract Reduced Cycloxygenase-2 (COX-2) and Inducible Nitric Oxide Synthase (iNOS) Expression and Nitrotyrosine Formation in LPS + IFN-Stimulated IEC-6
Expression of COX-2 and iNOS were evaluated by a cytofluorimetric technique. Our results showed that Astragalus membranaceus extract (5–100 μg/mL) inhibited COX-2 and iNOS expression in IEC-6 cells at all tested concentrations (p < 0.05 vs. LPS + IFN; Figure 1B,C). Under the same experimental conditions, the extract (5–100 μg/mL) also inhibited nitrotyrosine formation in IEC-6 cells (p < 0.01 vs. LPS + IFN; Figure 1D).
2.4. Astragalus membranaceus Extract Reduced p65 Nuclear Factor-κB (NF-κB) Translocation in LPS + IFN-Stimulated IEC-6
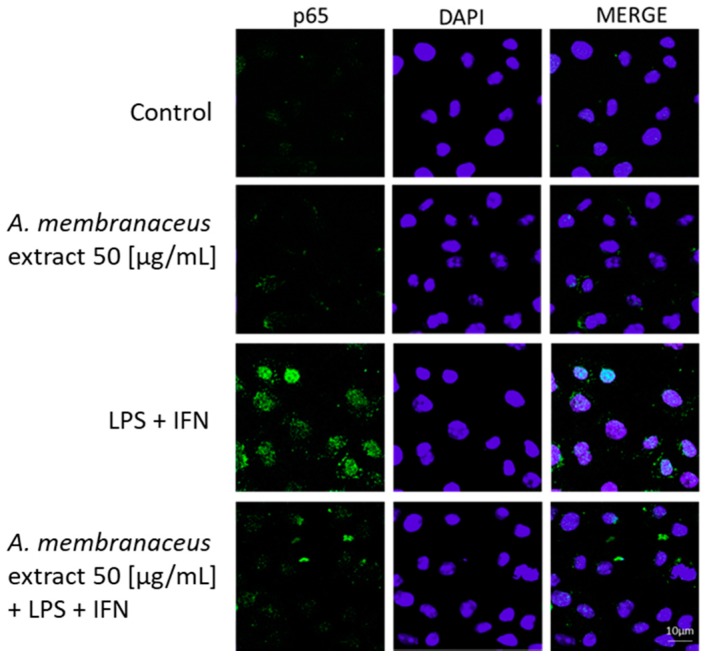
To evaluate NF-κB activation, p65 NF-κB was labeled with a green fluorescent marker. Astragalus membranaceus extract alone did not induce p65 nuclear translocation in IEC-6 cells (Figure 2). However, at a concentration of 50 μg/mL, the extract inhibited p65 NF-κB nuclear translocation when compared to LPS + IFN treatment alone (Figure 2).
Figure 2.
Effects of Astragalus membranaceus extract (50 μg/mL) alone and with LPS + IFN on nuclear factor-κB (NF-κB) p65 nuclear translocation, evaluated by immunofluorescence analysis. The blue fluorescence identified the nuclei, while the green fluorescence indicated the p65 NF-κB subunit.
2.5. Astragalus membranaceus Extract Reduced ROS Release by IEC-6 Cells
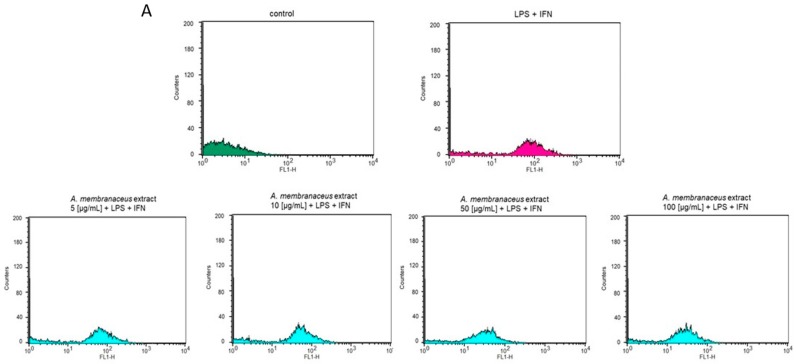
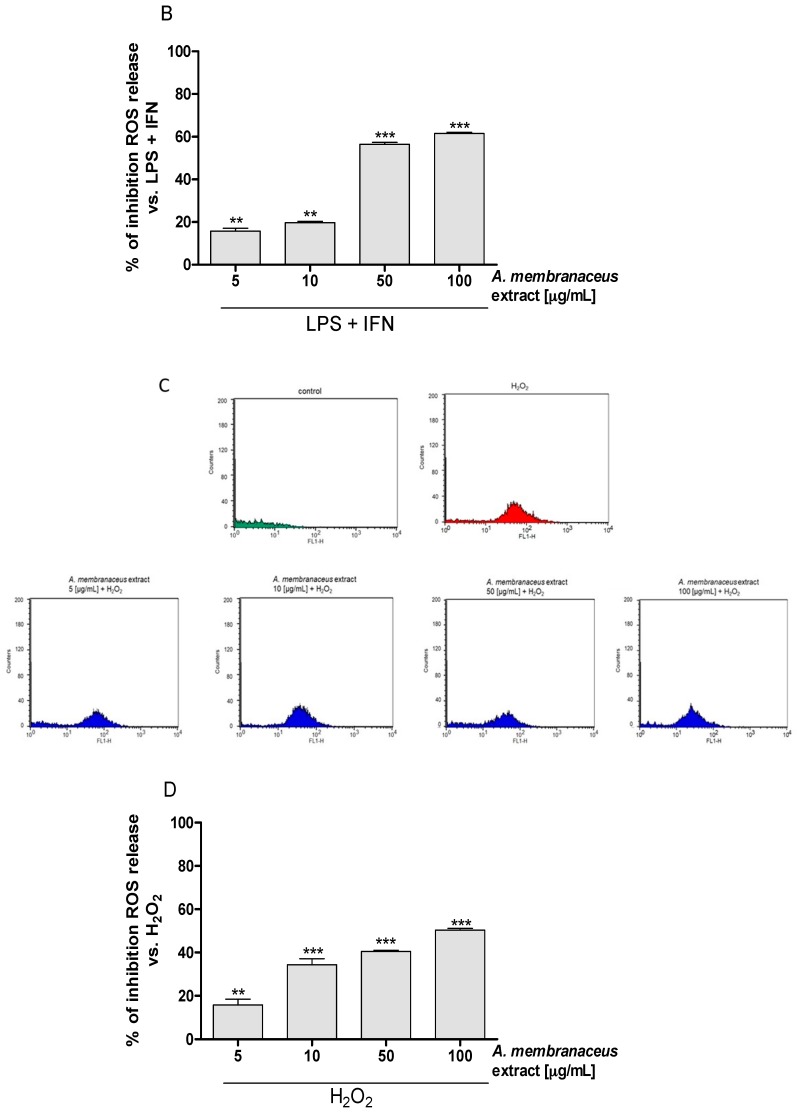
The antioxidant potential of Astragalus membranaceus extract was evaluated by measuring the intracellular ROS production in LPS + IFN-stimulated IEC-6 cells. It was found that the root extract (5–100 μg/mL) significantly inhibited ROS production in IEC-6 cells (p < 0.01 vs. LPS + IFN; Figure 3A,B). To further evaluate its antioxidant potential, Astragalus membranaceus extract (5–100 μg/mL) was also evaluated in IEC-6 cells treated with the pro-oxidant stimulus H2O2 (1 mM). Again, under these different experimental conditions, as assessed during inflammatory conditions, Astragalus membranaceus exhibited significant antioxidant activity by inhibiting ROS release (p < 0.01 vs. H2O2; Figure 3C,D).
Figure 3.
(A,B) Effect of a graded concentration of Astragalus membranaceus extract (5–100 μg/mL) in the presence of LPS + IFN on reactive oxygen species (ROS) levels, detected by 2′,7′ dichlorofluorescein-diacetate (H2DCF-DA). (C,D) Effect of Astragalus membranaceus extract (50 μg/mL) in the presence of H2O2 on ROS formation, evaluated with the probe H2DCF-DA. Data are expressed as mean ± SEM. ***, ** denote p < 0.001 and p < 0.01 vs. LPS + IFN or vs. H2O2.
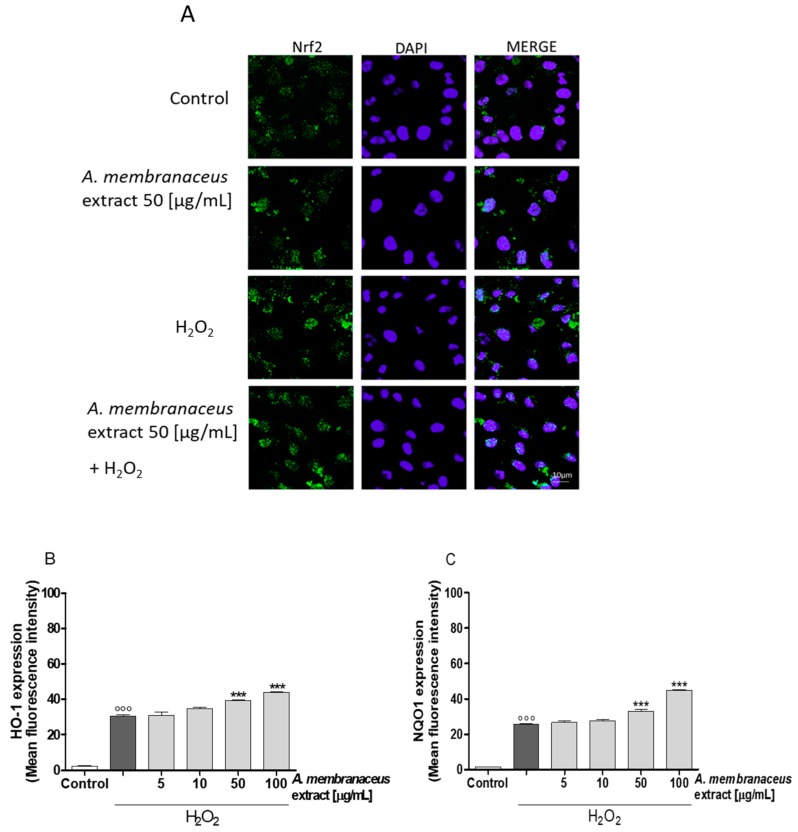
2.6. Astragalus membranaceus Extract Induced Nuclear Factor (Erythroid-Derived 2)-Like 2 (Nrf2) Activation and Heme Oxygenase 1 (HO-1), NAD(P)H Quinone Dehydrogenase 1 (NQO1) Expression in IEC-6 Cells
Oxidative stress is due to a disequilibrium between pro-oxidant and antioxidant factors. To assess the antioxidant potential of Astragalus membranaceus, the antioxidant Nrf2 response was also evaluated. As shown in Figure 4A, Nrf2 nuclear translocation in IEC-6 cells was slightly increased one hour after the addition of Astragalus membranaceus extract (50 μg/mL) when compared to the untreated control cells. Moreover, Nrf2 activation was increased even more with the addition of the extract under pro-oxidant conditions when compared to cells treated by H2O2 alone (Figure 4A). Expression of Nrf2-related cytoprotective factors, such as HO-1 and NQO1, was significantly increased in the presence of H2O2 (p < 0.001 vs. control; Figure 4B,C), and it was further increased by Astragalus membranaceus extract (p < 0.001 vs. H2O2; Figure 4B,C), thus supporting the antioxidant potential of the latter.
Figure 4.
(A) Effect of Astragalus membranaceus extract (50 μg/mL) alone and in the presence of H2O2 on the translocation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) into the cellular nuclei, evaluated by immunofluorescence analysis. The blue fluorescence identified the nuclei while the green fluorescence indicated Nrf2. (B) Effect of Astragalus membranaceus extract (5–100 μg/mL) in the presence of H2O2 on heme oxygenase 1 (HO-1) expression, evaluated by the cytofluorimetric technique (C) and on NAD(P)H quinone dehydrogenase 1 (NQO1) in IEC-6 cells, evaluated by the cytofluorimetric technique. The dark grey bars (B, C) represent IEC-6 cells treated with H2O2 alone. Data are expressed as mean ± SEM.°°° denotes p < 0.001 vs. control. *** denotes p < 0.001 vs. H2O2.
3. Discussion
Astragalus membranaceus, also known as Astragali radix, is a popular health-promoting herb that has been used by people in China to strengthen immunity for more than 2000 years. The effect of Astragalus membranaceus has been investigated in models of intestinal injury. In particular, Astragalus membranaceus has been reported to have a protective effect during intestinal mucosa reperfusion injury [16]. It has also been reported that Astragalus membranaceus has protective effects on small intestine villi and that it increases the level of antioxidant factors, such as superoxide dismutase activity (SOD) [17].
While the potential activity of Astragalus membranaceus extract as an anti-inflammatory and antioxidant agent has been widely demonstrated in in vivo studies, its effects on IECs, which have a pivotal role in maintaining intestinal homeostasis, are not well understood. For this reason, the aim of this study was to clarify the contribution of Astragalus membranaceus extract in the inflammatory process in the intestinal cells and to highlight its potential use as a new anti-inflammatory and antioxidant natural remedy for IBD treatment.
This study provided evidence that Astragalus membranaceus extract decreased inflammatory response in IECs by reducing the following: (1) TNF-α production; (2) COX-2 and iNOS expression; (3) nitrotyrosine formation; and (4) NF-κB activation. Interestingly, the extract also exerted an appreciable antioxidant effect by reducing ROS release and by activating antioxidant elements, such as Nrf2 activation and HO-1 and NQO1 expression.
During the inflammatory process, several pro-inflammatory cytokines were produced by IEC-6 cells, among them TNF-α. TNF-α has a pivotal role in intestinal inflammation, such as in IBD, and clinical results using anti-TNF-α drugs support its role in IBD pathogenesis. TNF-α promotes the activation and recruitment of immune cells and induces apoptosis of IECs and abnormal expression levels of tight junction molecules in these cells factors that ultimately lead to disruption of the intestinal mucosal barrier and increased permeability [27,28,29]. In our experiments, Astragalus membranaceus extract significantly reduced TNF-α levels in IEC-6 cells during inflammation. These data are in accordance with previous findings indicating the protective effect of the root extract of Astragalus membranaceus in a model of hapten-induced colitis mediated by TNF-α modulation [30] and with the fact that its administration could regulate TNF-α in UC patients [31]. Cytokines and TNF-α lead to the activation and release of other inflammatory mediators, such as iNOS and COX-2, amplifying and perpetuating the inflammatory condition in IBD. COX-2 and iNOS are expressed mainly at the inflammation sites, affecting colon integrity and contributing to the progress of intestinal damage [32], potentially leading to carcinogenesis associated with IBD. In fact, it was also shown that both COX-2 and iNOS stimulated tumor angiogenesis in colorectal cancer, a process mainly induced by vascular endothelial growth factor [33]. Our results showed that COX-2 expression was significantly reduced by Astragalus membranaceus extract in LPS + IFN-stimulated IEC-6 at all tested concentrations. The inhibition of COX-2 expression by Astragalus membranaceus extract indicated that the extract positively modulated the arachidonic acid cascade during inflammation, thus significantly contributing to its anti-inflammatory effects in IECs. Our findings agree with other studies reporting that Astragalus membranaceus reduced COX-2 expression and inflammatory response in general [19,34]. iNOS is the inducible isoform of the nitric oxide synthase, responsible for the synthesis of pro-inflammatory nitric oxide (NO), mainly induced in inflammatory cells, such as macrophages and mononuclear cells neutrophils by pro-inflammatory stimulants [35]. It has been reported that NO levels and iNOS activity increased in the colonic mucosa of UC and CD patients [36]. NO has a pivotal role in many organs and tissues and can exert beneficial or harmful effects depending on its levels and on the redox environment of the site. Its role as a modulator of key signaling pathways and as a regulator of the physiological functions of the gut, such as gastrointestinal motility, absorption, and secretion, is exerted at low NO concentration. The expression of iNOS results in high levels of NO and for a longer time. Therefore, under these conditions, NO regulates many pathophysiologic processes. It is also known that NO produced by the inducible isoform iNOS generates free radicals such as peroxynitrite and hydroxyl radical. Peroxynitrite affects protein function through its ability to nitrate tyrosine residues, thus inducing nitrotyrosine formation. Since tyrosine nitration represents an alternative pathway to phosphorylation of key residues, this process can affect the enzymatic activity of proteins and thus significantly affect intracellular processes [37]. Our results provided evidence for the ability of Astragalus membranaceus extract to inhibit iNOS protein expression at all tested concentrations, and this inhibitory effect could lead to significant reduction in NO overproduction and thus to nitrotyrosine formation in LPS + IFN-treated IEC-6 cells. These data are in accordance with previous studies reporting that aqueous extracts of Astragalus membranaceus could modulate pro-inflammatory cytokine gene expression and nitric oxide production in macrophages [38]. A nuclear pro-inflammatory activated factor common to all these mediators is NF-κB. NF-κB is a key regulator of inflammation and can be activated by a broad panel of stimuli, including bacterial components such as LPS, proinflammatory cytokines such as TNF-α and interleukin-1, viruses, and DNA-damaging agents [39]. Increased expression and activation of NF-κB were observed in IBD patients, especially in mucosal macrophages and epithelial cells, accompanied by enhanced production of proinflammatory cytokines such as TNF-α, IL-1, and IL-6 [40]. The NF-κB pathway functions as a molecular link between inflammation and tumorigenesis due to its ability to stimulate the expression of proinflammatory cytokines, antiapoptotic factors, angiogenesis factors, and proteases, which promote tumor initiation and ensure the survival and proliferation as well as invasion of malignant cells. Here, we reported that Astragalus membranaceus extract can inhibit NF-κB activation, in accordance with previous studies that demonstrated the protective effect of Astragalus spp. polysaccharide in inhibiting NF-κB signaling in a colitis animal model [41]. The roles of oxidative stress and the oxidant/antioxidant balance in IBD development have recently received increasing attention. Oxidative stress has been demonstrated to influence different gastrointestinal diseases, such as IBD, gastroduodenal ulcers, and malignancies [42]. In our experimental model, Astragalus membranaceus extract exerted a significant antioxidant effect by inhibiting ROS release induced both by LPS + IFN and by H2O2. These results agree with previous data reporting the antioxidant potential of Astragalus membranaceus at the intestinal level [43,44]. Oxidative stress is due to an imbalance between pro- and antioxidant factors; thus, cellular antioxidant response plays a pivotal role in controlling oxidative stress. Nrf2 is a redox-sensitive transcription factor that plays a key role in the antioxidant response. When cells were exposed to oxidative stress, Nrf2 was released from Kelchlike ECH-associated protein 1 (Keap1), which sequesters Nrf2 in the cytoplasm and then binds to Maf or Jun to form a heterodimer in the nucleus. The heterodimer combines with the antioxidant response element (ARE) to promote the expression of genes encoding many phase II detoxification and antioxidant enzymes, including HO-1 and NOQ1, thereby improving the ability of the cell to remove ROS [45]. A Nrf2 deficiency has been shown to exacerbate colonic injury in a mouse model of experimental colitis [46,47], whereas the pharmacological activation of Nrf2 produced protective effects on the colon [48,49]. HO-1 and NQO1 enzymes also exert a protective effect during intestinal inflammation [50]. In the presence of H2O2, Astragalus membranaceus extract enhanced Nrf2 activation and HO-1 and NQO1 expression, thus increasing an antioxidant response in IEC-6 cells. This evidence fits with other data reporting that the Astragalus constituents activate Nrf2 cellular response [51]. Several bioactive compounds have been highlighted in the dried root of Astragalus, such as polysaccharides, triterpene saponins, isoflavonoids, and various trace elements [52,53]. The phytochemical characterization of the present extracts reveals the presence of these active compounds [52,53]. Studies suggested that Astragalus polysaccharides have effects on the activation of B cells and macrophages, promotion of humoral and immune responses, protection of blood vessels, and prevention of inflammation and cancer [54,55]. Moreover, Astragalus polysaccharides also have effects at the intestinal level; in particular, they attenuate murine colitis through inflammasome inhibition [25]. The hepatoprotective properties have been widely studied, and it has been found that the Astragalus polysaccharides’ antioxidant activity can prevent liver and intestinal damage [13,56,57]. Similarly, the anti-inflammatory effect of triterpene derivatives, also derived from Astagalus, has been reported [58,59] and the antioxidant potential of Astragalus constituents has been reported and reviewed [60,61,62]. However, despite the activity of Astragalus constituents, it is important to underscore that the efficacy of 50% HA (Axtragyl®) is strongly related to the whole phytocomplex [53].
In conclusion, our results indicated that the Astragalus membranaceus extract exerted significant anti-inflammatory and antioxidant effects in IECs, thus highlighting its potential application in intestinal inflammatory conditions.
4. Materials and Methods
4.1. Reagents
Unless otherwise specified, all reagents and compounds were purchased from Sigma Chemicals Company (Sigma, Milan, Italy).
4.2. Plant Material
Astragalus membranaceus dried root hydroalcoholic extract (Axtragyl®) was provided by Giellepi Health Science Division (Lissone, Italy). The extract (50% HA) has already been characterized by Di Cesare Mannelli et al. [52,53] and contains 70% polysaccharides and other bioactive molecules (i.e., saponins and isoflavonoids).
4.3. Cell Culture
IEC-6 cells (CRL-1592) were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). These cells, derived from normal rat intestinal crypt cells [63], were grown in Dulbecco’s modified Eagle’s medium (DMEM, 4 g/L glucose) with 10% (v/v) foetal bovine serum (FBS), 2 mM l-glutamine, 1.5 g/L NaHCO3, and 0.1 unit/mL bovine insulin. Cells between the 17th and 21st passages were used for these experiments, as previously reported [64].
4.4. Cell Treatment
IEC-6 cells were plated and, after adhesion, were treated with Astragalus membranaceus extract (5–100 μg/mL) for 1 h and then co-exposed to Astragalus membranaceus extract and lipopolysaccharides from E. coli (LPS; 10 μg/mL) plus interferon-γ (IFN; 10 U/mL) for different times, as outlined below. In order to estimate specifically the antioxidant potential of Astragalus membranaceus extract, in other experiments, the IEC-6 cells were incubated with Astragalus membranaceus extract (5–100 μg/mL) alone for 1 h and then co-exposed to the extract and hydrogen peroxide (H2O2; 1 mM).
4.5. Antiproliferative Activity
IEC-6 cells (5.0 × 103cells/well) were plated on 96-well plates and allowed to adhere for 72 h at 37 °C in a 5% CO2 atmosphere. Thereafter, the medium was substituted with either a new one alone or one containing serial dilutions of Astragalus membranaceus extract (5–100 μg/mL) and incubated for 24 h. Cell antiproliferative activity was evaluated using the colorimetric assay of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), as formerly reported [64]. MTT (5 mg/mL) was then added to IEC-6 cells. After 3 h, cells were lysed with 100 µL of a solution containing 50% (v/v) N,N-dimethylformamide, and 20% (w/v) sodium dodecyl sulphate(SDS) (pH = 4.5). A microplate spectrophotometer reader (Titertek Multiskan MCC/340-DASIT, Cornaredo, Milan, Italy) was used to measure the optical density (OD) of released formazan in each well, as we previously reported [65]. The antiproliferative activity was measured as % dead cells = 100 × [(OD treated/OD control) × 100].
4.6. TNF-α Determination
TNF-α release in IEC-6 cells was measured with an Enzyme-Linked Immuno Sorbent Assay (ELISA). IEC-6 cells (8.0 × 104 cells/well/24-well plates) were treated as previously reported for 24 h. Supernatants from IEC-6 cells were then collected and a commercial kit (e-Biocscience, San Diego, CA, USA) was used to perform the ELISA, according to the manufacturer’s instructions (e-Biosciences, San Diego, CA, USA). The results were expressed as pg/mL, as formerly reported [66].
4.7. Measurement of COX-2, iNOS, HO-1, NQO1 Expression and Nitrotyrosine Formation by Cytofluorimetry
IEC-6 cells were plated into 96-well plates (1 × 104 cells/well) and treated as previously indicated for 24 h in order to evaluate COX-2 and iNOS expression and nitrotyrosine formation in IEC-6 cells. In another set of experiments, the IEC-6 cells were incubated with the extract and H2O2 as previously indicated, in order to measure HO-1 and NQO1 expression. For this analysis, IEC-6 cells were then collected and washed with phosphate buffered saline (PBS). Fixing solution was added to cells for 20 min and then IEC-6 cells were incubated in fix perm solution for a further 30 min. Anti-COX-2 (BD Transduction Laboratories, Milan, Italy), anti-iNOS (BD Transduction Laboratories, Milan, Italy), anti-nitrotyrosine (Merck Millipore, Milan, Italy), anti-HO-1 (Santa Cruz Biotechnologies, Dallas, TX, USA), or anti-NQO1 (Santa Cruz Biotechnologies, Dallas, TX, USA) antibodies were then added for 1 h. The secondary antibody, in fixing solution, was added to IEC-6 cells and cell fluorescence was then evaluated by a fluorescence-activated cell sorter (FACSscan; Becton Dickinson, Milan, Italy) and analyzed by Cell Quest software (version 4; Becton Dickinson, Milan, Italy), as formerly reported [67].
4.8. Immunofluorescence Analysis for NF-κB and Nrf2 by Confocal Microscopy
IEC-6 cells were plated on coverslips in a 12-well plate (2 × 105 cells/well) and allowed to adhere for 24 h. After the adhesion time, the cellular medium was substituted with a new one, either alone or in the presence of Astragalus membranaceus extract (50 μg/mL), and treated for 1 h. It was then co-exposed to the extract and LPS (10 μg/mL) plus IFN (10 U/mL) for 1 h in order to evaluate NF-κB activation in IEC-6 cells. In another set of experiments, the IEC-6 cells were incubated with Astragalus membranaceus extract (50 μg/mL) alone for 1 h and then exposed simultaneously to Astragalus membranaceus extract and H2O2 (1 mM) for 1 h more in order to measure Nrf2 activation. Cells were then fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% saponin in PBS. The blocking was performed with bovine serum albumin (BSA) and PBS. Cells were then incubated with rabbit anti-phospho p65 NF-κB antibody (Santa Cruz Biotechnologies, Dallas, TX, USA) or rabbit anti-Nrf2 antibody (Santa Cruz Biotechnologies, Dallas, TX, USA) for 1 h at 37 °C. Then, PBS was used to wash the slides. Next, we added the fluorescein-conjugated (FITC) secondary antibody for 1 h. 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) was used for the nuclei, as formerly reported [68]. At the end, we mounted the coverslips in mounting medium and images were taken using the laser confocal microscope (Leica TCS SP5, Leica, Wetzalar, Germany), as formerly reported [68].
4.9. Intracellular ROS Release Measurement
ROS intracellular production was evaluated by the probe 2′,7′-dichlorofluorescein-diacetate (H2DCF-DA) [69]. The IEC-6 cells were seeded in 24-well plates (8 × 104 cells/well) and allowed to adhere for one day. After adhesion, we incubated cells with Astragalus membranaceus extract (5–100 μg/mL) alone for 1 h and then co-exposed to the extract and LPS (10 μg/mL) plus IFN (10 U/mL) for 24 h. In another set of experiments, the IEC-6 cells were treated with Astragalus membranaceus extract (5–100 μg/mL) alone for 1 h and then exposed simultaneously to the extract and H2O2 (1 mM) for 1 h more. After cellular treatment, IEC-6 cells were collected, washed with PBS, and then incubated in PBS containing H2DCF-DA (10 μM). Cell fluorescence was evaluated after 15 min at 37 °C, using a fluorescence-activated cell sorter (FACSscan; Becton Dickinson, Franklin Lakes, NJ, USA), and was analyzed by Cell Quest software version 4 (Becton Dickinson, Milan, Italy), as formerly reported [69].
4.10. Data Analysis
We reported data as mean ± standard error mean (SEM) of at least three independent experiments. Each experiment was conducted in triplicate. For the statistical analysis, we used the analysis of variance test. Bonferroni’s test was used to make multiple comparisons. We considered significant a p-value less than 0.05.
5. Conclusions
Our results indicated that Astragalus membranaceus root extract significantly reduced the inflammatory response and the pro-oxidant status in IEC-6 cells. Our study provides evidence that might further the development of Astragalus membranaceus root extract as a therapeutic agent for IBD treatments.
Acknowledgments
The authors acknowledge the financial support of Giellipi S.p.A. “Ricerche sull’attività di integratori alimentari e dispositivi medici”.
Author Contributions
Study conception and design: Stefania Marzocco and Simona Adesso. Acquisition, analysis, and interpretation of data: Stefania Marzocco and Simona Adesso. Drafting of manuscript: Stefania Marzocco and Simona Adesso. Critical revision: Giuseppina Autore, Rosario Russo, and Andrea Quaroni. English editing: Andrea Quaroni. All authors have seen and approved the manuscript.
Conflicts of Interest
Rosario Russo is an employee of Giellepi S.p.A. He had no influence on the interpretation of study results nor the decision to submit the manuscript for publication. The other authors had no conflicts of interest.
References
- 1.Huang B.L., Chandra S., Shih D.Q. Skin manifestations of inflammatory bowel disease. Front. Physiol. 2012;3:13. doi: 10.3389/fphys.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matricon J., Barnich N., Ardid D. Immunopathogenesis of inflammatory bowel disease. Self/Nonself. 2010;1:299–309. doi: 10.4161/self.1.4.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanauer S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:S3–S9. doi: 10.1097/01.MIB.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 4.Neurath M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 5.Luo K., Cao S.S. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease. Gastroenterol. Res. Pract. 2015;2015:328791. doi: 10.1155/2015/328791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov R., Janeway C., Jr. Innate immunity. New Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary L.B., Rana T.S., Anand K.K. Current status of the systematics of Astragalus L. (Fabaceae) with special reference to the Himalayan species in India. Taiwania. 2008;53:338–355. [Google Scholar]
- 8.Mabberley D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses. 3rd ed. Cambridge University Press; Cambridge, UK: 2008. pp. 77–476. [Google Scholar]
- 9.State Pharmacopoeia Commission of the PRC . People’s Medical Publishing House; Beijing, China: 2005. Pharmacopoeia of the People’s Republic of China. [Google Scholar]
- 10.Kim C., Ha H., Kim J.S., Kim Y.T., Kwon S.C., Park S.W. Induction of growth hormone by the roots of Astragalus membranaceus in pituitary cell culture. Arch. Pharm. Res. 2003;26:34–39. doi: 10.1007/BF03179928. [DOI] [PubMed] [Google Scholar]
- 11.Zee-Cheng R.K. Shi-quan-da-bu-tang (ten significant tonic decoction), SQT. A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxifi-cation of anticancer drugs. Methods Find Exp. Clin. Pharmacol. 1992;14:725–736. [PubMed] [Google Scholar]
- 12.Li C.X., Li L., Lou J., Yang W.X., Lei T.W., Li Y.H., Liu J., Cheng M.L., Huang L.H. The protective effects of traditional Chinese medicine prescription, Han-Dan-Gan-Le, on CCl4-induced liver fibrosis in rats. Am. J. Chin. Med. 1998;26:325–332. doi: 10.1142/S0192415X98000361. [DOI] [PubMed] [Google Scholar]
- 13.McKenna D., Hughes K., Jones K. Astragalus. Altern. Ther. Health Med. 2002;8:34–40. [PubMed] [Google Scholar]
- 14.Cho W.C.S., Leung K.N. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J. Ethnopharmacol. 2007;113:132–141. doi: 10.1016/j.jep.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Hei Z.Q., Zhang J.J., Lin S.Q., Liu K.X., Chen B.X. Effects of Astragalus membranaceus injection on nitric oxide and endothelin concentration of intestinal mucosa after hemorrhage shock-reperfusion in rats. Zhongguo Zhongyao Zazhi. 2004;29:444–447. [PubMed] [Google Scholar]
- 16.Hei Z.Q., Huang H.Q., Zhang J.J., Chen B.X., Li X.Y. Protective effect of Astragalus membranaceus on intestinal mucosa reperfusion injury after hemorrhagic shock in rats. World J. Gastroenterol. 2005;11:4986–4991. doi: 10.3748/wjg.v11.i32.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan X.L., Hei Z.Q., Huang H.Q., Chen L.X., Li S.R., Cai J. Effect of Astragalus membranaceus injection on the activity of the intestinal mucosal mast cells after hemorrhagic shock-reperfusion in rats. Chin. Med. J. 2006;119:1892–1898. [PubMed] [Google Scholar]
- 18.Ma X.Q., Shi Q., Duan J.A., Dong T.T.X., Tsim K.W.K. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chemistry. 2002;50:4861–4866. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 19.Ryu M., Kim E.H., Chun M., Kang S., Shim B., Yu Y.B., Jeong G., Lee J.S. Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J. Ethnopharmacol. 2008;115:184–193. doi: 10.1016/j.jep.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Chu C., Qi L.W., Li B., Gao W., Li P. Radix Astragali (Astraglaus): Latest advancements and trends in chemistry, analysis, pharmacology and pharmacokinetics. Curr. Org. Chem. 2010;14:1792–1807. doi: 10.2174/138527210792927663. [DOI] [Google Scholar]
- 21.You H.Z., Lu Y., Gui D.K., Peng A., Chen J., Gu Y. Aqueous extract of Astragali Radix ameliorates proteinuria in adriamycin nephropathy rats through inhibition of oxidative stress and endothelial nitric oxide synthase. J. Ethnopharmacol. 2011;134:176–182. doi: 10.1016/j.jep.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B.Q., Hu S.J., Qiu L.H., Zhu J.H., Xie X.J., Sun J., Zhu Z.H., Xia Q., Bian K. Effects of Astragalus membranaceus and its main components on the acute phase endothelial dysfunction induced by homocysteine. Vasc. Pharmacol. 2007;46:278–285. doi: 10.1016/j.vph.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Yang M., Qian X.H., Zhao D.H., Fu S.Z. Effects of Astragalus polysaccharide on the erythroid lineage and microarray analysis in K562 cells. J. Ethnopharmacol. 2010;127:242–250. doi: 10.1016/j.jep.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Gao X.H., Xu X.X., Pan R., Li Y., Luo Y.B., Xia Y.F., Murata K., Matsuda H., Dai Y. Saponin fraction from Astragalus membranaceus roots protects mice against polymicrobial sepsis induced by cecal ligation and puncture by inhibiting inflammation and upregulating protein C pathway. J. Nat. Med. 2009;63:421–429. doi: 10.1007/s11418-009-0348-2. [DOI] [PubMed] [Google Scholar]
- 25.Tian Z., Liu Y., Yang B., Zhang J., He H., Ge H., Wu Y., Shen Z. Astragalus Polysaccharide Attenuates Murine Colitis through Inhibiton of the NLRP3 Inflammasome. Planta Med. 2017;83:70–77. doi: 10.1055/s-0042-108589. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C.L., Ren H.J., Liu M.M., Li X.G., Sun D.L., Li N., Ming L. Modulation of intestinal epithelial cell proliferation, migration, and differentiation in vitro by Astragalus polysaccharides. PLoS ONE. 2014;9:e106674. doi: 10.1371/journal.pone.0106674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peake S.T., Bernardo D., Mann E.R., Al-Hassi H.O., Knight S.C., Hart A.L. Mechanisms of action of anti-tumor necrosis factor-α agents in Crohn’s disease. Inflamm. Bowel Dis. 2013;19:1546–1555. doi: 10.1097/MIB.0b013e318281333b. [DOI] [PubMed] [Google Scholar]
- 28.Günther C., Neumann H., Neurath M.F., Becker C. Apoptosis, necrosis and necroptosis: Cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 29.Fries W., Muja C., Crisafulli C., Cuzzocrea S., Mazzon E. Dynamics of enterocyte tight junctions: Effect of experimental colitis and two different anti-TNF strategies. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G938–G947. doi: 10.1152/ajpgi.00469.2007. [DOI] [PubMed] [Google Scholar]
- 30.Ko J.K., Chik C.W. The protective action of radix Astragalus membranaceus against hapten-induced colitis through modulation of cytokines. Cytokine. 2009;47:85–90. doi: 10.1016/j.cyto.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y.Q., Sun W.Z. Study on regulation effects of TNF-α, IL-8 and sIL-2r content to patients of ulcerative colitis by mediation method. J. Harbin Univ. Commun. Nat. Sci. 2002;18:248–250. [Google Scholar]
- 32.Sánchez-Fidalgo S., Cárdeno A., Villegas I., Talero E., de la Lastra C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010;633:78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Cianchi F., Cuzzocrea S., Vinci M.C., Messerini L., Comin C.E., Navarra G., Perigli G., Centorrino T., Marzocco S., Lenzi E., et al. Heterogeneous expression of cyclooxygenase-2 and inducible nitric oxide synthase within colorectal tumors: Correlation with tumor angiogenesis. Dig. Liver Dis. 2010;42:20–27. doi: 10.1016/j.dld.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Law P.C., Auyeung K.K., Chan L.Y., Ko J.K. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complement. Altern. Med. 2012;12:160. doi: 10.1186/1472-6882-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valentine J.F., Tannahill C.L., Stevenot S.A., Sallustio J.E., Nick H.S., Eaker E.Y. Colitis and interleukin 1â up-regulated inducible nitric oxide synthase and superoxide dismutase in rat myenteric neurons. Gastroenterology. 1996;111:56–64. doi: 10.1053/gast.1996.v111.pm8698225. [DOI] [PubMed] [Google Scholar]
- 36.Rachmilewitz D., Stamler J.S., Bachwich D., Karmeli F., Ackerman Z., Podolsky D.K. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut. 1995;36:718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schopfer F.J., Baker P.R., Freeman B.A. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y.S., Han O.K., Park C.W., Yang C.H., Jeon T.W., Yo W.K., Kim S.H., Kim H.J. Pro-inflammatory cytokine gene expression and nitric oxide regulation of aqueous extracted Astragali Radix in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2005;100:289–294. doi: 10.1016/j.jep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Karin M., Greten F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 40.Atreya I., Atreya R., Neurath M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 41.Lv J., Zhang Y., Tian Z., Liu F., Shi Y., Liu Y., Xia P. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κВ activation. Int. J. Biol. Macromol. 2017;98:723–729. doi: 10.1016/j.ijbiomac.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen R., Shao H., Lin S., Zhang J.J., Xu K.Q. Treatment with Astragalus membranaceus produces antioxidative effects and attenuates intestinal mucosa injury induced by intestinal ischemia-reperfusion in rats. Am. J. Chin. Med. 2011;39:879–887. doi: 10.1142/S0192415X11009275. [DOI] [PubMed] [Google Scholar]
- 44.Shahzad M., Shabbir A., Wojcikowski K., Wohlmuth H., Gobe G.C. The Antioxidant Effects of Radix Astragali (Astragalus membranaceus and Related Species) in Protecting Tissues from Injury and Disease. Curr. Drug Targets. 2016;17:1331–1340. doi: 10.2174/1389450116666150907104742. [DOI] [PubMed] [Google Scholar]
- 45.Uruno A., Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide Biol. Chem. 2011;25:153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Osburn W.O., Karim B., Dolan P.M., Liu G., Yamamoto M., Huso D.L., Kensler T.W. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int. J. Cancer. 2007;121:1883–1891. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 47.Khor T.O., Huang M.T., Prawan A., Liu Y., Hao X., Yu S., Cheung W.K.L., Chan J.Y., Reddy B.S., Yang C.S., et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev. Res. 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., Wang H., Qian C., Tang J., Zhou W., Liu X., You Q., Hu R. 3-(2-Oxo-2-phenylethylidene)-2,3,6,7-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one (compound 1), a novel potent Nrf2/ARE inducer, protects against DSS-induced colitis via inhibiting NLRP3 inflammasome. Biochem. Pharmacol. 2016;101:71–86. doi: 10.1016/j.bcp.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Yue L., Lei S., Luo H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 2016;40:24. doi: 10.1016/j.intimp.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L., Zhang Z., Liu B., Jin Y., Tian Y., Xin Y., Duan Z. The Protective Effect of Heme Oxygenase-1 against Intestinal Barrier Dysfunction in Cholestatic Liver Injury Is Associated with NF-κB Inhibition. Mol. Med. 2017;9:23. doi: 10.2119/molmed.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H., Wang P., Huang F., Jin J., Wu H., Zhang B., Wang Z., Shi H., Wu X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol. Appl. Pharmacol. 2017;340:58–66. doi: 10.1016/j.taap.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Di Cesare Mannelli L., Zanardelli M., Bartolucci G., Karioti A., Bilia A.R., Vannacci A., Mugelli A., Ghelardini C. In Vitro Evidence for the Use of Astragali Radix Extracts as Adjuvant against Oxaliplatin-Induced Neurotoxicity. Planta Med. 2015;81:1045–1055. doi: 10.1055/s-0035-1546117. [DOI] [PubMed] [Google Scholar]
- 53.Di Cesare Mannelli L., Pacini A., Micheli L., Femia A.P., Maresca M., Zanardelli M., Vannacci A., Gallo E., Bilia A.R., Caderni G., et al. Astragali radix: Could it be an adjuvant for oxaliplatin-induced neuropathy? Sci. Rep. 2017;7:42021. doi: 10.1038/srep42021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Shao B.M., Xu W., Dai H., Tu P., Li Z., Gao X.M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 55.Wang D., Hu Y., Sun J., Kong X., Zhang B., Liu J. Comparative study on adjuvanticity of compound Chinese herbal medicinal ingredients. Vaccine. 2005;23:3704–3708. doi: 10.1016/j.vaccine.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Han G.H., Xiang D.Y., He Y.J., Xu C.X., Zhang P.Z. Influence of radix Astragali on the noise-caused change in hepatic glycogencontent of rats. J. Chin. Mater. Med. 1989;14:750–752. [PubMed] [Google Scholar]
- 57.Tang X., Xiong H.E. Inhibitive effect of injection Radix Astragali in epidemic hemorrhagic fever virus infection. Hunan Yike Daxue Xuebao. 1990;15:250–252. [Google Scholar]
- 58.Braca A., Dal Piaz F., Marzocco S., Autore G., Vassallo A., De Tommasi N. Triterpene derivatives as inhibitors of protein involved in the inflammatory process: Molecules interfering with phospholipase A2, cycloxygenase, and lipoxygenase. Curr. Drug Targets. 2011;12:302–321. doi: 10.2174/138945011794815284. [DOI] [PubMed] [Google Scholar]
- 59.Li L., Hou X., Xu R., Liu C., Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017;31:17–36. doi: 10.1111/fcp.12232. [DOI] [PubMed] [Google Scholar]
- 60.Fu J., Wang Z., Huang L., Zheng S., Wang D., Chen S., Zhang H., Yang S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi) Phytother. Res. 2014;28:1275–1283. doi: 10.1002/ptr.5188. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L.J., Liu H.K., Hsiao P.C., Kuo L.M.Y., Lee I.J., Wu T.S., Chiou W.F., Kuo Y.H. New isoflavonoid glycosides and related constituents from Astragali Radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011;59:1131–1137. doi: 10.1021/jf103610j. [DOI] [PubMed] [Google Scholar]
- 62.Huang X., Liu Y., Song F., Liu Z., Liu S. Studies on principal components and antioxidant activity of different Radix Astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta. 2009;78:1090–1101. doi: 10.1016/j.talanta.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 63.Quaroni A. Pre- and postnatal development of differentiated functions in rat intestinal epithelial cells. Dev. Biol. 1985;111:280–292. doi: 10.1016/0012-1606(85)90483-X. [DOI] [PubMed] [Google Scholar]
- 64.Bianco G., Fontanella B., Severino L., Quaroni A., Autore G., Marzocco S. Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: A concentration related study. PLoS ONE. 2012;7:e52051. doi: 10.1371/journal.pone.0052051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pepe G., Sommella E., Ventre G., Scala M.C., Adesso S., Ostacolo C., Marzocco S., Novellino E., Campiglia P. Antioxidant peptides released from gastrointestinal digestion of “Stracchino” soft cheese: Characterization, in vitro intestinal protection and bioavailability. J. Funct. Foods. 2016;26:494–505. doi: 10.1016/j.jff.2016.08.021. [DOI] [Google Scholar]
- 66.Adesso S., Autore G., Quaroni A., Popolo A., Severino L., Marzocco S. The Food Contaminants Nivalenol and Deoxynivalenol Induce Inflammation in Intestinal Epithelial Cells by Regulating Reactive Oxygen Species Release. Nutrients. 2017;9:1343. doi: 10.3390/nu9121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adesso S., Magnus T., Cuzzocrea S., Campolo M., Rissiek B., Paciello O., Autore G., Pinto A., Marzocco S. Indoxyl Sulfate Affects Glial Function Increasing Oxidative Stress and Neuroinflammation in Chronic Kidney Disease: Interaction between Astrocytes and Microglia. Front. Pharmacol. 2017;12:370. doi: 10.3389/fphar.2017.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marzocco S., Calabrone L., Adesso S., Larocca M., Franceschelli S., Autore G., Martelli G., Rossano R. Anti-inflammatory activity of horseradish (Armoracia rusticana) root extracts in LPS-stimulated macrophages. Food Funct. 2015;6:3778–3788. doi: 10.1039/C5FO00475F. [DOI] [PubMed] [Google Scholar]
- 69.Marzocco S., Adesso S., Alilou M., Stuppner H., Schwaiger S. Anti-Inflammatory and Anti-Oxidant Potential of the Root Extract and Constituents of Doronicum austriacum. Molecules. 2017;22:1003. doi: 10.3390/molecules22061003. [DOI] [PMC free article] [PubMed] [Google Scholar]