Abstract
Type A non-catalytic carbohydrate-binding modules (CBMs), exemplified by CtCBM3acipA, are widely believed to specifically target crystalline cellulose through entropic forces. Here we have tested the hypothesis that type A CBMs can also bind to xyloglucan, a soluble β-1,4-glucan containing α-1,6-xylose side chains. CtCBM3acipA bound to xyloglucan in cell walls and arrayed on solid surfaces. Xyloglucan and cellulose were shown to bind to the same planar surface on CBM3acipA. A range of type A CBMs from different families were shown to bind to xyloglucan in solution with ligand binding driven by enthalpic changes. The nature of CBM-polysaccharide interactions is discussed.
Keywords: Carbohydrate-binding module, CBM3a, xyloglucan, crystalline cellulose, plant cell walls
Introduction
Plant cell walls, which comprise the most abundant source of organic carbon in the biosphere, are composites of structurally complex polysaccharides. A major feature of plant cell walls are cellulose microfibrils that consist of parallel, hydrogen-bonded β-1,4-glucan chains. Plant cell walls also contain a range of matrix polysaccharides including xyloglucan, a β-1,4-glucan that is decorated with α-1,6-linked xylose residues, which is abundant in many land plant cell walls.
A wide range of microbial species and systems contribute to the degradation of the insoluble and often intractable plant structural polysaccharides and this is a major contributor to terrestrial carbon cycling (1). Glycoside hydrolases play a central role in the depolymerisation of plant cell walls. A key feature of these microbial enzymes is their modular architecture in which non-catalytic carbohydrate binding modules (CBMs) are appended via flexible linker sequences to the catalytic modules. CBMs have been extensively characterised and are currently grouped into 71 sequence-based families in the Carbohydrate Active Enzymes database (http://www.cazy.org/; (2). CBMs have also been grouped into three types reflecting both their mode of ligand recognition and the target glycan. Type A CBMs bind to the surface of crystalline polysaccharides, type B proteins interact with internal regions of single glycan chains (endo-type) and type C modules recognise the termini of glycan chains (exo-type) (3,4). The binding site of type A CBMs presents a planar surface that generally contain three hydrophobic residues that interact with the surface of crystalline cellulose of microfibrils (5–7). Ligand recognition appears to be mediated by entropic forces (8).
The specificity of type A CBMs for crystalline cellulose is predicated on their lack of significant affinity for cellulooligosaccharides and chemically modified soluble forms of cellulose (9). Indeed, this planar interaction between type A CBMs and their ligands in a conformation that adopts a two-screw axis, has been visualized through crystal structures of a CBM63 in complex with cellohexaose and other β-glucooligosaccharides (10). Based on the thermodynamics of binding it has been assumed that type A CBMs do not bind to xyloglucan, “nature’s soluble cellulose”. Defining the specificity of CBMs is important in understanding their role in enzyme targeting, and also when used as molecular probes in the in situ analyses of plant cell wall polysaccharides (11,12). Specifically, type A CBMs have been used to detect the presence of crystalline cellulose in plant materials (13,14). To test the hypothesis that type A CBMs are highly specific for crystalline cellulose we have evaluated their binding to crystalline cellulose and xyloglucan. The data showed that the CBMs bound to both crystalline cellulose and xyloglucan, demonstrating that type A modules display broader specificities than previously recognized.
Materials and Methods
Source of CBMs, expression and purification
The CBMs used in this work were as follows: CBM3acipA, derived from Clostridium thermocellum scaffoldin CipA; CfCBM10, derived from the Cellulomonas fimi AA10 lytic monooxygenase (GenBank AEE44415), TfCBM2a from Thermomonospora fusca Cel6A (GenBank EOR72170); CtCBM3aeg1 from C. thermocellum endoglucanase I (NCIB WP_003511958); BcCBM3b from Bacteroides cellulosolvens scaffoldin (GenBank AAG01230), TbCBM2a from a Thermobispora bispora AA10 lytic oxygenase (GenBank ADG87762); PfCBM2a from a Pyrococcus furiosus chitinase (NCIB WP_011012376). CtCBM3aeg1, PfCBM2a, BcCBM3b and TfCBM2a were obtained from NZYTech as green fluorescent protein (GFP) fusions. All the proteins, which contained N-terminal His6 tags and were derived from pET28-based recombinant expression vectors, were produced in Escherichia coli and purified by Immobilized Metal ion Affinity Chromatography using standard methodology (15).
Plant materials and immunocytochemistry
Immunochemistry was carried out on resin sections of tamarind seed cotyledon parenchyma and 25 days post anthesis (dpa) cotton fibres (JFW15 cultivar from G. arboreum species). Samples were dehydrated, resin-embedded and sectioned as described previously (16). Enzymatic treatments prior to xyloglucan immunochemistry on cotton sections included: 0.1 M sodium carbonate for 2 h at room temperature and recombinant pectate lyase from Cellvibrio japonicus (E-PLYCJ Megazyme) at 10 μg/ml in a 2 mM CaCl2, 50 mM CAPS (3-(Cyclohexylamino)-1-aminopropane sulfonic acid) buffer, pH 10.0, for 2 h at room temperature. Treatment with sodium carbonate de-esterified all pectins facilitating the action of the pectate lyase to remove pectin from fibre primary cell walls so xyloglucans can be unmasked. In some cases thin sections were incubated with 10 μg/ml of recombinant xyloglucanase (GH5) from Paenibacillus sp. (E-XEGP Megazyme) in 0.1 M sodium acetate pH 5.5 or buffer alone for 2 h at 37°C prior to labelling with molecular probes. Prior to immunohistochemistry, sections were incubated with 5% (w/v) milk protein in phosphate-buffered saline (MP/PBS) for 30 min at room temperature to prevent non-specific binding. Xyloglucan-directed rat monoclonal antibody LM15 (Marcus et al. 2008) was used at a 1:5 dilution of hybridoma cell culture supernatant and CtCBM3a at 20 μg/ml in MP/PBS for 1.5 h. Anti-rat IgG Alexa Fluor488 (Life Technologies) was used as secondary antibody for LM15 in a 1:100 dilution in MP/PBS and samples were incubated for 1 h. A three step labelling was carried out when using his-tagged CtCBM3a. Anti-his mouse antibody (H1029 Sigma-Aldrich) in a 1:1000 dilution in MP/PBS for 1 h was used as secondary antibody. Anti-mouse IgG Alexa Fluor488 (Life Technologies) in a 1:100 dilution in MP/PBS for 1 h was used as tertiary antibody. Calcofluor White (Sigma–Aldrich) was used at 0.02 mg/ml in PBS for 5 min for visualization of cell walls. Anti-fade reagent Citifluor glycerol/PBS (Agar Scientific) was added before placing a coverslip. Immunofluorescence imaging was performed using an Olympus BX61 microscope (http://www.olympus-global.com/) equipped with epifluorescence irradiation. Micrographs were obtained with a Hamamatsu ORCA285 camera (Hamamastu, http://www.hamamatsu.com) and PerkinElmer Volocity software. All related and comparative micrographs were captured using equivalent settings, and relevant micrographs were processed in equivalent ways for the generation of datasets.
Quantitative in vitro analyses of xyloglucan recognition
In vitro analysis of CBM and antibody binding was carried out using ELISAs as described (12,17). For antibody/CBM capture ELISAs, 10-fold serial dilutions of 100 μg/ml tamarind xyloglucan (Megazyme) in PBS were coated (100 μl) on to microtitre plates (Nunc, Denmark). LM15 was used at 1:20 dilution of hybridoma cell culture supernatant in MP/PBS. CtCBM3a was used at 10 μg/ml in MP/PBS. Anti-rat-IgG-peroxidase (A9037 Sigma-Aldrich) and anti-his-peroxidase (A7058 Sigma-Aldrich) were used as secondary antibodies for LM15 and CtCBM3a respectively. For competitive-inhibition assessment of recognition of polysaccharides/oligosaccharides microtitre plates were coated with 0.5 μg/ml xyloglucan to act as immobilised antigen. After blocking with MP/PBS and washing, six ten-fold serial dilutions from 50 μg/ml of xyloglucan from tamarind seeds (P-XYGLN Megazyme), xyloglucan heptasaccharide (O-X3G4 Megazyme), guar galactomannan medium viscosity (P-GGMMV Megazyme) and cellohexaose (O-CHE Megazyme) haptens were prepared and 50 μl added to microtritre plate wells. Immediately after, 50 μl of 1:50 dilution of LM15 antibody or CBMs at 20 μg/ml were added to equivalent sets of microtitre plate wells containing the soluble xyloglucan or oligosaccharide haptens and incubated for 1.5 h. Probe binding was determined as described above.
Affinity gel electrophoresis
The binding of CBMs to soluble polysaccharides was evaluated by affinity gel electrophoresis (AGE) following the method described previously (18). Polysaccharide ligands were used at a concentration of 0.1% (w/v). Electrophoresis was carried out at room temperature in native 10% (w/v) polyacrylamide gels. The gels were also loaded with BSA and GFP, which act as a non-interacting negative controls. After electrophoresis, proteins were visualized through staining with Coomassie Blue.
Isothermal Titration Calorimetry (ITC)
ITC experiments were carried out essentially as described previously (18), except that proteins were in 50 mM Na-HEPES buffer, pH 7.5, containing 200 mM NaCl at 25°C. The reaction cell contained protein at 50 μM, and the syringe contained the polysaccharide at 10 mg/ml, unless stated otherwise. For experiments with regenerated cellulose, the ligand was retained in the cell at 12 mg/ml, and the protein (200 μM) was injected. Titrations were carried out at same conditions. Integrated heat effects, after correction for heats of dilution, were analyzed by nonlinear regression using a single site-binding model (Microcal ORIGIN, Version 5.0; Microcal Software). The fitted data yielded the association constant (Ka) and the enthalpy of binding (ΔH). Other thermodynamic parameters were calculated by using the standard thermodynamic equation: −RTlnKa = ΔG = ΔH − TΔS.
Mutational analysis of CBM3a recognition of crystalline cellulose and xyloglucan
Five residues of the crystalline cellulose binding site of CtCBM3acipA were replaced with alanine (D56A, H57A, Y67A, R112A, W118A) in order to block recognition of crystalline cellulose. The mutant protein, which was designated CtCBM3acipA-M5, was created with a PCR-based QuikChange™ site-directed mutagenesis kit (Stratagene) as described previously (19).
Circular Dichroism (CD) spectroscopy CD spectra were recorded with a JASCO 810 spectropolarimeter. The spectra were obtained in 10 mM sodium phosphate buffer, pH 8.0, at 20°C using a 0.2 mm path length quartz cuvette (Hellma 106-QS). Each spectrum was accumulated from 9 scans between 180 and 250 nm, at a scan rate of 20 nm/min. Protein concentrations for CtCBM3acipA and CtCBM3acipA-M5 were 2.4133.10-5 M and 2.2378.10-5 M respectively. Samples were corrected by subtraction from a buffer blank which was accumulated from 3 scans.
Results
CtCBM3acipA binds to xyloglucan in intact plant cell walls
In situ labelling of plant materials with CtCBM3acipA, a type A CBM from the Clostridium thermocellum scaffoldin CipA, showed that the protein module bound not only to crystalline cellulose but also to an additional cell wall component, which was shown to be xyloglucan. This is demonstrated in Fig 1 by the fluorescence labelling of thin sections of xyloglucan-rich tamarind seed cotyledon parenchyma and crystalline cellulose-rich cotton fibres. The binding of CtCBM3acipA to tamarind seed parenchyma cell walls was abrogated by prior treatment with a specific xyloglucanase, whereas the binding to secondary cell walls of cotton fibres was not affected by this enzyme. In contrast the xyloglucan monoclonal antibody LM15 labelled both sections (specifically to the primary cell walls in the case of cotton fibres) and its binding to tamarind seeds and cotton fibres was abolished by the enzyme treatment. These data demonstrate dual recognition of cell wall glucans by CtCBM3acipA.
Fig. 1.
Indirect immunofluorescence detection of crystalline cellulose and xyloglucan in sections of xyloglucan-rich tamarind seed cotyledon parenchyma and cellulose-rich cotton fibres, respectively. Calcofluor White staining indicates location of all cell walls. LM15 binding to cell walls of tamarind cotyledon parenchyma cells and primary cell walls of cotton fibres (arrows) is abolished after a xyloglucanase pre-treatment. CtCBM3acipA WT binding to cell walls of tamarind cotyledon parenchyma cells is abolished by xyloglucanase pre-treatment however its binding to the secondary cell wall of cotton fibres is not. CtCBM3acipA-M5 did not bind to cell walls of tamarind cotyledon parenchyma cells nor to cotton fibre secondary cell walls.
The crystalline cellulose-binding site of the carbohydrate binding module CBM3a can also accommodate xyloglucan
In a previous study reporting the crystal structure of CtCBM3acipA it was proposed that the planar surface consisting of Asp56, His57, Tyr67, Arg112 and Trp118 comprised the cellulose binding surface (20). To explore the recognition of xyloglucan by CtCBM3acipA a mutant of the protein was constructed in which the five amino acids that comprise the hydrophobic cellulose binding surface were substituted with alanine (D56A/H57A/Y67A/R112A/W118A). CD spectra of wild type CtCBM3acipA and the mutant showed that both proteins were folded, Fig. S1. This mutant, CtCBM3acipA-M5, did not bind to either cell walls of tamarind cotyledon parenchyma or to cotton fibres (Fig. 1), indicating that the same binding site recognises both polysaccharides. Variants of the protein where only one of the five residues were mutated retained cellulose binding.
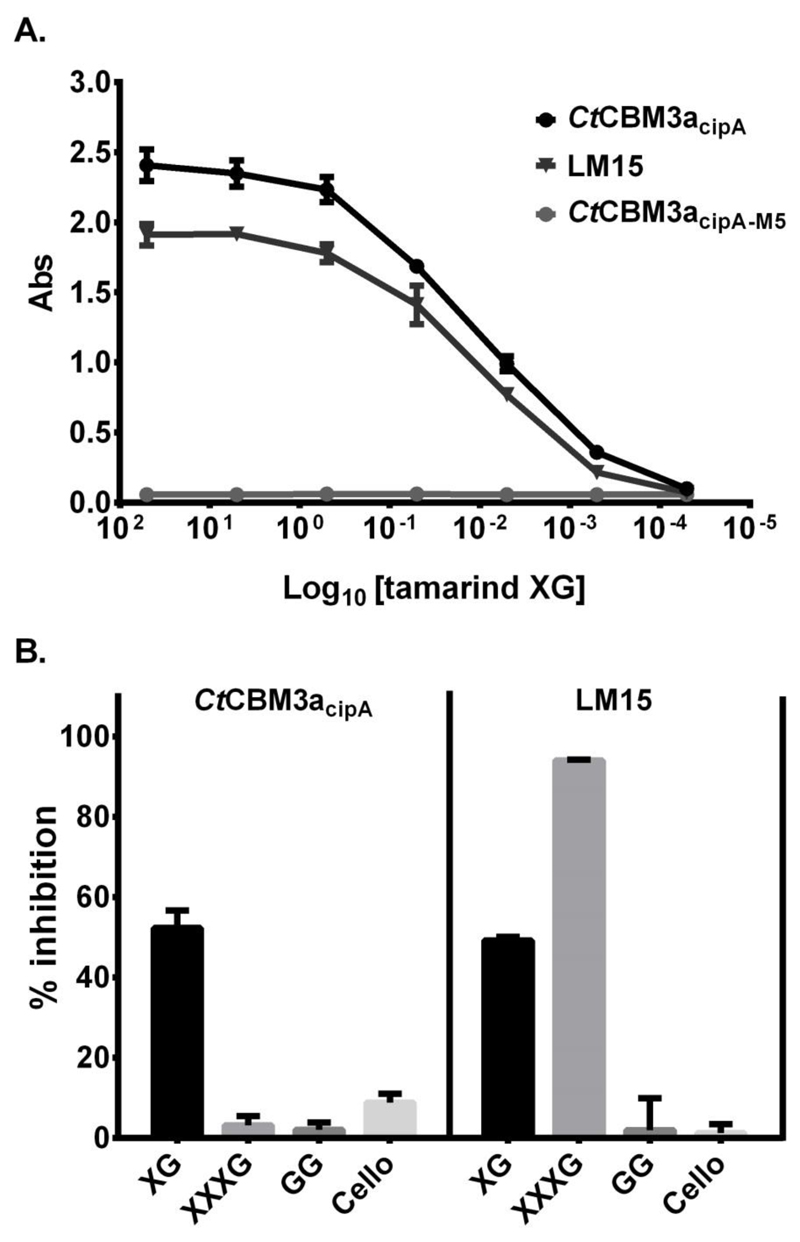
To explore further the binding of wild type CtCBM3acipA and CtCBM3acipA-M5 to xyloglucan, in vitro studies using microtitre plate-based assays were used. Wild type CtCBM3a (20 μg/ml) was as effective at detecting tamarind xyloglucan immobilised on microtitre plates as a 10-fold dilution of LM15 hybridoma cell culture supernatant (Fig. 2A). There was no in vitro recognition of xyloglucan by CtCBM3acipA-M5 (Fig 2A) confirming the in situ observations. In competitive inhibition ELISA assays, the binding of both CtCBM3acipA and LM15 to tamarind xyloglucan was reduced by ~50% by the presence of 25 μg/ml xyloglucan polysaccharide (Fig 2B). In contrast, while LM15 binding was largely abolished by the presence of 25 μg/ml of xyloglucan-derived XXXG heptasaccharide, CtCBM3acipA binding was unaffected (Fig 2B). Galactomannan and cellohexaose, which do not display significant binding to CtCBM3a or LM15, did not inhibit the binding of the two proteins to the immobilized xyloglucan (Fig 2B). These observations indicate, that CtCBM3a can bind to xyloglucan in solution, in addition to binding to xyloglucan immobilised on solid supports or as a component of plant cell walls. The data also indicate that CtCBM3acipA recognition is restricted to xyloglucan polysaccharides and not xyloglucan-derived oligosaccharides. This suggests that the protein binds to a conformation of xyloglucan that is present in the polymer but not in the oligosaccharide.
Fig. 2.
In vitro recognition of xyloglucan. A. ELISA analysis of CtCBM3acipA, CtCBM3acipA-M5 and LM15 binding to tamarind xyloglucan. B. Competitive-inhibition ELISAs of LM15 and CtCBM3acipA binding to immobilised xyloglucan with xyloglucan (XG), xyloglucan heptasaccharide (XXXG), guar galactomannan (GG) and cellohexaose (Cello) in the soluble phase at 25 μg/ml. Y axis represents the percentage of inhibition achieved by the polysaccharides/haptens. Error bars: SD (n=3)
Type A CBM recognition of soluble β-glucans
To explore further the general significance of the data presented above, the capacity of type A CBMs from different families to bind to xyloglucan was evaluated by affinity gel electrophoresis. All the CBMs used in this study bound to crystalline cellulose, typical of type A CBMs. Affinity gel electrophoresis (AGE) data, Fig. 3, showed that migration of the CBM2s and CBM3s were significantly retarded in gels containing xyloglucan, indicating binding to the polysaccharide, while CfCBM10 did not interact with the polysaccharide. None of the eight CBMs displayed binding to barley β1,4-β1,3-glucan. The binding of these CBMs to β-glucans was also assessed by ITC. Examples of the binding isotherms and the full data set are shown in Fig. 4 and Table 1. The data mirrored the AGE profiles with the CBMs displaying similar binding profiles in both methods. None of the proteins bound to cellohexaose. Binding to xyloglucan was driven by the change in enthalpy, with the loss in entropy causing a reduction in affinity. In contrast, the binding of CtCBM3a to regenerated cellulose was an endothermic process that was driven by entropic forces.
Fig. 3.
Affinity gel electrophoresis of type A CBMs. The proteins were electrophoresed in gels containing 0.1% xyloglucan, 0.1% barley β1,3-β1,4-mixed linkage glucan (β-glucan) or no glucan (control). ~10 μg of protein was loaded in each well. CBM65, which binds to a range of β-glucans was used as a positive control and GFP and BSA were deployed as non-binding negative controls.
Fig. 4.
Representative isothermal titration calorimetry (ITC) data of CtCBM3acipA-M5 titrated with carbohydrates. The soluble ligand (10 mg/mL in the syringe) was titrated into 54 μM CtCBM3acipA-M5 in the cell. In the case of cellulose the CBM was titrated into cellulose in the cell. The top half of each titration shows the raw injection heats; the bottom half, the integrated peak areas fitted using a single-site model (MicroCal Origin v7.0). ITC was carried out in 50 mM Na-HEPES pH 7.5 at 25°C.
Table 1. ITC analysis of the binding of type A CBMs to soluble β-glucans.
| Protein | Ligand | Ka (103 M-1) | ΔG (kcal mol-1) |
ΔH (kcal mol-1) |
TΔG (kcal mol-1) |
|---|---|---|---|---|---|
| CtCBM3acipA | Xyloglucan | 9.5 ± 0.2 | -5.4 | 12.4 ± 0.3 | -7.0 |
| CtCBM3acipA-M5 | Xyloglucan | NBa | - | - | - |
| CtCBM3acipA | XXXG | NBa | |||
| TbCBM2a | Xyloglucan | 11.6 ± 0.4 | -5.5 | 11.1 ± 0.5 | -5.6 |
| TfCBM2a | Xyloglucan | 13.5 ± 0.3 | -5.6 | -9.5 ± 0.4 | -3.9 |
| BcCBM3a | Xyloglucan | 5.7 ± 0.4 | -5.1 | -8.7 ± 0.6 | -3.6 |
| PfCBM2a | Xyloglucan | 8.2 ± 0.3 | -5.3 | -10.5 ± 0.3 | -5.2 |
| CtCBM3aeg1 | Xyloglucan | 10.3 ± 0.1 | -5.5 | -13.6 ± 0.2 | -8.1 |
| CfCBM10 | Xyloglucan | NB | - | - | - |
| All the CBMs | β-glucanb | NB | - | - | - |
| All the CBMs | Cellohexaose | NB | - | - | - |
NB: No ligand binding detected
Barley β1,3-β1,4-glucan
Discussion
Classically type A CBMs are defined as proteins that bind exclusively to the planar surface of crystalline glycans, and these macromolecular interactions are driven by entropic forces (8). This report shows that the binding properties of at least some type A CBMs are more complicated than previously thought. Here we show that a cohort of type A CBMs belonging to families 2 and 3 can bind to both crystalline cellulose and xyloglucan. The entropic forces mediating the binding of type A CBMs to crystalline polysaccharides result from the release of caged water molecules from protein and ligand with a fixed conformation. In type B and C CBMs the affinity for their soluble ligands is driven by changes in enthalpy (19–21); the conformational restriction of the bound ligand incurs an entropic penalty that has a negative impact on affinity. Based on the tertiary structure of type A CBMs these proteins appear to make few if any polar interactions with their ligands, binding is through hydrophobic (van der Waal) interactions with planar aromatic residues. Given the important role entropy plays in ligand recognition by type A CBMs, it was thought that the loss of thermodynamic freedom when a soluble ligand binds to these proteins offsets any increase in the randomness of the system through the release of water molecules, leading to no net affinity for these glycans. The demonstration that CBMs from two different type A CBM families bind to soluble xyloglucan contradicts this assumption (10).
It should be noted that the affinity of the CBMs for xyloglucan is ~50-100 fold lower than for crystalline cellulose. This reduction in affinity and the decreased role of entropy in ligand binding is likely to reflect conformational restriction of the polysaccharide bound to the CBM. This is supported by the ITC data showing that ligand binding is associated with a negative change in entropy, which results in a reduction in affinity according to the thermodynamic equation −RTlnKA = ΔG = ΔH-TΔS (H, enthalpy; S, entropy; G, Gibbs free energy of binding; T, temperature in Kelvins; KA, affinity constant). Furthermore, the polysaccharide may also lose binding energy by adopting a non-favoured two-fold screw axis conformation, which contrasts to the three-fold helical structure displayed by β-glucuooligosaccharides, such as XXXG and cellohexaose, when bound to type B CBMs and enzymes (19–21). Forcing the ligand into a non-favoured conformation will also result in reduced entropy, consistent with the negative TΔS value obtained by ITC. It is possible that the type A CBMs bind to xyloglucan polysaccharides but not β-glucuooligosaccharides because the conformational flexibility of the oligosaccharides in solution leads to an entropic penalty that is too great for binding to occur. It is also possible that the xylose side chains in xyloglucan, by making interactions with the surface of the type A CBMs, contribute to ligand binding. Indeed, side chain recognition may explain why the β1,4-β1,3-glucan did not bind to the type A CBMs.
It is worth noting that the CBM10 (CfCBM10) studied here displayed no binding to xyloglucan. CBM10s are small proteins of 40 residues. Although they contain the typical three planar aromatic residues that interact with crystalline cellulose, the region is not extensive (5) and thus the xylose side chains in xyloglucan may not interact with the protein and assist in ligand binding (see discussion above). Whether the properties of CfCBM10 are typical of other members of the family is currently unclear as we were unable to produce other CBM10 proteins in E. coli. Similarly it is unclear whether CBM63, which binds to crystalline cellulose (10), also recognizes xyloglucan.
From a biological perspective, this report indicates that CtCBM3acipA and the other type A CBMs not only direct enzymes onto highly crystalline cellulose that is recalcitrant to attack by typical endoglucanases, but also targets xyloglucan which, in contrast, is a highly accessible glycan that is hydrolyzed by β-glucanases with a range of different specificities (22). Thus, the targeting role of type A CBMs may be broader than previously believed, explaining why these modules are appended to a range of different enzymes. This study, in addition to raising issues regarding the enzyme targeting role of type A CBMs, also raises issues concerning the use of these CBMs as molecular probes for crystalline cellulose. It is evident that when using type A CBMs to study plant cell wall architecture it is important to compare data before and after treatment with a highly specific xyloglucanase. Finally it should also be emphasised that the binding of type A CBMs to cellulose may be compromised by the masking of non-cellulosic glycans (23). Thus several factors must be considered when inferring the architecture of cell walls based on the binding patterns of type A CBMs.
Supplementary Material
Highlights.
CtCBM3a, a family 3a CBM, can bind to xyloglucan in addition to crystalline cellulose
Five point mutations at the CtCBM3a crystalline cellulose binding site abolished recognition of both crystalline cellulose and xyloglucan
XG polysaccharides, but not XG-derived oligosaccharides, in solution can be bound by CtCBM3a
Acknowledgements
We greatly appreciate the help we received from Dr Helen Waller with the CD spectroscopy experiment. We acknowledge funding from the European Union Seventh Framework Programme (FP7 2007-2013) under Grant Agreement n°263916 and the Danish Strategic Research Council and The Danish Council for Independent Research, Technology and Production Sciences project GlycAcT under contract 10-093465. This paper reflects the author’s views only. The European Community is not liable for any use that may be made of the information contained herein.
Abbreviations
- CBM
carbohydrate-binding module
- ITC
Isothermal titration calorimetry
- XG
xyloglucan
References
- 1.Gilbert HJ. The biochemistry and structural biology of plant cell wall deconstruction. Plant Physiol. 2010;153:444–455. doi: 10.1104/pp.110.156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic acids research. 2014;42:D490–495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert HJ, Knox JP, Boraston AB. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr Opin Struct Biol. 2013;23:669–677. doi: 10.1016/j.sbi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J. 2004;382:769–781. doi: 10.1042/BJ20040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghothama S, Simpson PJ, Szabo L, Nagy T, Gilbert HJ, Williamson MP. Solution structure of the CBM10 cellulose binding module from Pseudomonas xylanase A. Biochemistry. 2000;39:978–984. doi: 10.1021/bi992163+. [DOI] [PubMed] [Google Scholar]
- 6.Tormo J, Lamed R, Chirino AJ, Morag E, Bayer EA, Shoham Y, Steitz TA. Crystal structure of a bacterial family-III cellulose-binding domain: a general mechanism for attachment to cellulose. EMBO J. 1996;15:5739–5751. [PMC free article] [PubMed] [Google Scholar]
- 7.Xu GY, Ong E, Gilkes NR, Kilburn DG, Muhandiram DR, Harris-Brandts M, Carver JP, Kay LE, Harvey TS. Solution structure of a cellulose-binding domain from Cellulomonas fimi by nuclear magnetic resonance spectroscopy. Biochemistry. 1995;34:6993–7009. [PubMed] [Google Scholar]
- 8.Creagh AL, Ong E, Jervis E, Kilburn DG, Haynes CA. Binding of the cellulose-binding domain of exoglucanase Cex from Cellulomonas fimi to insoluble microcrystalline cellulose is entropically driven. Proc Natl Acad Sci U S A. 1996;93:12229–12234. doi: 10.1073/pnas.93.22.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolam DN, Ciruela A, McQueen-Mason S, Simpson P, Williamson MP, Rixon JE, Boraston A, Hazlewood GP, Gilbert HJ. Pseudomonas cellulose-binding domains mediate their effects by increasing enzyme substrate proximity. Biochem J. 1998;331:775–781. doi: 10.1042/bj3310775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgelis N, Yennawar NH, Cosgrove DJ. Structural basis for entropy-driven cellulose binding by a type-A cellulose-binding module (CBM) and bacterial expansin. Proc Natl Acad Sci U S A. 2012;109:14830–14835. doi: 10.1073/pnas.1213200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCartney L, Blake AW, Flint J, Bolam DN, Boraston AB, Gilbert HJ, Knox JP. Differential recognition of plant cell walls by microbial xylan-specific carbohydrate-binding modules. Proc Natl Acad Sci U S A. 2006;103:4765–4770. doi: 10.1073/pnas.0508887103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCartney L, Gilbert HJ, Bolam DN, Boraston AB, Knox JP. Glycoside hydrolase carbohydrate-binding modules as molecular probes for the analysis of plant cell wall polymers. Anal Biochem. 2004;326:49–54. doi: 10.1016/j.ab.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Blake AW, McCartney L, Flint JE, Bolam DN, Boraston AB, Gilbert HJ, Knox JP. Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J Biol Chem. 2006;281:29321–29329. doi: 10.1074/jbc.M605903200. [DOI] [PubMed] [Google Scholar]
- 14.Kljun A, Benians TAS, Goubet F, Meulewaeter F, Knox JP, Blackburn RS. Comparative Analysis of Crystallinity Changes in Cellulose I Polymers Using ATR-FTIR, X-ray Diffraction, and Carbohydrate-Binding Module Probes. Biomacromolecules. 2011;12:4121–4126. doi: 10.1021/bm201176m. [DOI] [PubMed] [Google Scholar]
- 15.Charnock SJ, Bolam DN, Nurizzo D, Szabo L, McKie VA, Gilbert HJ, Davies GJ. Promiscuity in ligand-binding: The three-dimensional structure of a Piromyces carbohydrate-binding module, CBM29-2, in complex with cello- and mannohexaose. Proc Natl Acad Sci U S A. 2002;99:14077–14082. doi: 10.1073/pnas.212516199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KJ, Knox JP. Resin embedding, sectioning, and immunocytochemical analyses of plant cell walls in hard tissues. Methods Mol Biol. 2014;1080:41–52. doi: 10.1007/978-1-62703-643-6_3. [DOI] [PubMed] [Google Scholar]
- 17.Marcus SE, Verhertbruggen Y, Herve C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, Willats WG, Knox JP. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008;8:60. doi: 10.1186/1471-2229-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henshaw JL, Bolam DN, Pires VM, Czjzek M, Henrissat B, Ferreira LM, Fontes CM, Gilbert HJ. The family 6 carbohydrate binding module CmCBM6-2 contains two ligand-binding sites with distinct specificities. J Biol Chem. 2004;279:21552–21559. doi: 10.1074/jbc.M401620200. [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Bolam DN, Nagy T, Szabo L, Cooper A, Simpson PJ, Lakey JH, Williamson MP, Gilbert HJ. Role of hydrogen bonding in the interaction between a xylan binding module and xylan. Biochemistry. 2001;40:5700–5707. doi: 10.1021/bi010034z. [DOI] [PubMed] [Google Scholar]
- 20.Boraston AB. The interaction of carbohydrate-binding modules with insoluble non-crystalline cellulose is enthalpically driven. Biochem J. 2005;385:479–484. doi: 10.1042/BJ20041473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najmudin S, Guerreiro CI, Carvalho AL, Prates JA, Correia MA, Alves VD, Ferreira LM, Romao MJ, Gilbert HJ, Bolam DN, Fontes CM. Xyloglucan is recognized by carbohydrate-binding modules that interact with beta-glucan chains. J Biol Chem. 2006;281:8815–8828. doi: 10.1074/jbc.M510559200. [DOI] [PubMed] [Google Scholar]
- 22.Gloster TM, Ibatullin FM, Macauley K, Eklof JM, Roberts S, Turkenburg JP, Bjornvad ME, Jorgensen PL, Danielsen S, Johansen KS, Borchert TV, et al. Characterization and three-dimensional structures of two distinct bacterial xyloglucanases from families GH5 and GH12. J Biol Chem. 2007;282:19177–19189. doi: 10.1074/jbc.M700224200. [DOI] [PubMed] [Google Scholar]
- 23.Ruel K, Nishiyama Y, Joseleau JP. Crystalline and amorphous cellulose in the secondary walls of Arabidopsis. Plant Science. 2012;193–194:48–61. doi: 10.1016/j.plantsci.2012.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.