Summary
Chromatin organization and gene activity are responsive to developmental and environmental cues. Although many genes are transcribed throughout development and across cell types, much of gene regulation is highly cell-type specific. To readily track chromatin features at the resolution of cell types within complex tissues, we developed and validated chromatin affinity purification from specific cell types by chromatin immunoprecipitation (CAST-ChIP), a broadly applicable biochemical procedure. RNA polymerase II (Pol II) CAST-ChIP identifies ~1,500 neuronal and glia-specific genes in differentiated cells within the adult Drosophila brain. In contrast, the histone H2A.Z is distributed similarly across cell types and throughout development, marking cell-type-invariant Pol II-bound regions. Our study identifies H2A.Z as an active chromatin signature that is refractory to changes across cell fates. Thus, CAST-ChIP powerfully identifies cell-type-specific as well as cell-type-invariant chromatin states, enabling the systematic dissection of chromatin structure and gene regulation within complex tissues such as the brain.
Introduction
Developmental programs and environmental responses depend on the cell-type-specific expression of genes, mediated by the temporal and spatial regulation of transcription and chromatin structure (Bonn et al., 2012; Heintzman et al., 2009; modENCODE Consortium et al., 2010). At the same time, a subset of ubiquitously expressed (cell-type-invariant) genes defines processes that are regulated similarly across distinct cell types. These include housekeeping genes (e.g., ribosomal genes), a subset of inducible genes (e.g., heat shock genes), and genes with broadly required functions, such as cell polarity or mRNA localization. Much of our understanding of development and physiology hinges on our ability to dissect cell-type-invariant transcriptional responses from highly cell-type-specific ones, but existing tools to obtain such information are limited.
Chromatin contributes to both cell-type-specific and cell-type-invariant gene expression (Dixon et al., 2012; Filion et al., 2010), determining cell fate, yet exhibiting plasticity. Its structure is regulated through alterations in nucleosome positioning, post-translational modifications, or the incorporation of histone variants such as H3.3 and H2A.Z (Bonn et al., 2012; Heintzman et al., 2009; modENCODE Consortium et al., 2010; Talbert and Henikoff, 2010). Further, specific chromatin marks correlate with different activation states of RNA Polymerase II (Pol II), such as binding, poising/pausing, and elongation, promoting correct levels of gene expression in distinct cell types throughout development (Dixon et al., 2012; Filion et al., 2010; Gaertner et al., 2012).
Genome-wide analyses have revealed regions that are important for establishing/maintaining cell fate and genes with cell-type-invariant transcriptional function (Ernst et al., 2011; Pujadas and Feinberg, 2012). However, most studies have been performed in cultured cells, dissected tissues, or whole organs/animals (Chintapalli et al., 2007; modENCODE Consortium et al., 2010). In fact, investigators have few tools to track cell-type-specific chromatin states within complex tissues easily. It is challenging to obtain sufficient pure material for a particular cell population from limited amounts of tissue due to diverse morphologies and histological complexity. Further, we need readily applicable, fast extraction procedures that preserve chromatin states, since physiological events generate small, transient changes in gene activity that may be lost during nuclear isolation. We set out to develop a rapid, sensitive, robust, and convenient protocol that exploits the advantages of highly validated and widely used methods, i.e., protein tagging and cell-type-specific Gal4 drivers. We combined these methods into a tool that allows chromatin structure to be profiled within highly complex organs, such as the brain, as well as across development or environmental change.
Thus far, cell-type-specific chromatin analysis has relied on isolating nuclei from tissues or cell types by enriching tagged nuclei (Deal and Henikoff, 2010; Henry et al., 2012; Steiner et al., 2012) or through fluorescence-activated cell sorting (FACS) (Bonn et al., 2012; Haenni et al., 2012; Jiang et al., 2008; Weake et al., 2011). Nuclear isolation provides flexibility by allowing the use of distinct chromatin immunoprecipitation (ChIP) antibodies following enrichment. However, these protocols have disadvantages in terms of the required time, material, and cost.
To improve on these limitations, we developed and validated a fast biochemical method we call chromatin affinity purification from specific cell types by ChIP (CAST-ChIP). It combines the cell-type-specific expression of a tagged transgene using an enhancer chosen among thousands of Gal4 driver lines (Jenett et al., 2012) with the power of affinity-tag purification. To test our method, we focused on Pol II as a transcription feature and on histone H2A.Z as a chromatin mark. Refinement of existing ChIP assays allowed us to apply CAST-ChIP to profile tagged Pol II in two distinct cell types of the adult fly head: neurons and glia. The genome-wide distribution of Pol II monitors the activation state of genes by detecting active and poised genes (Adelman and Lis, 2012). Additionally, we profiled the genome-wide distribution of Drosophila H2A.Z (historically named H2Av; Talbert et al., 2012) within distinct cell types of the adult head as well as embryos. H2A.Z associates preferentially with active promoters and distal regulatory regions (in particular at the 5′ end of active genes across species), is required for viability (reviewed in Marques et al., 2010), acts as an early developmental mark (Bošković et al., 2012; Creyghton et al., 2008; Ernst et al., 2011; Hu et al., 2013; Jin et al., 2009; Ku et al., 2012; Leach et al., 2000; Nashun et al., 2010; Swaminathan et al., 2005; Whittle et al., 2008), and might define chromatin states throughout an organism’s life.
CAST-ChIP reveals cell-type-specific genomic binding profiles for Pol II. In contrast, H2A.Z profiles are highly similar across distinct adult cell types and embryos. H2A.Z enriches at genes that are expressed (or ready for activation) across cell types, developmental stages, and origins. We define these genes as cell-type invariant. CAST-ChIP delineates cell-type-invariant and cell-type-specific chromatin domains. Together, our method and results provide a timely resource that will allow the dissection of chromatin and Pol II activity within organs composed of distinct differentiated cell types, such as the adult CNS.
Results
CAST-ChIP Reveals Cell-Type-Specific, Genome-Wide Pol II Profiles
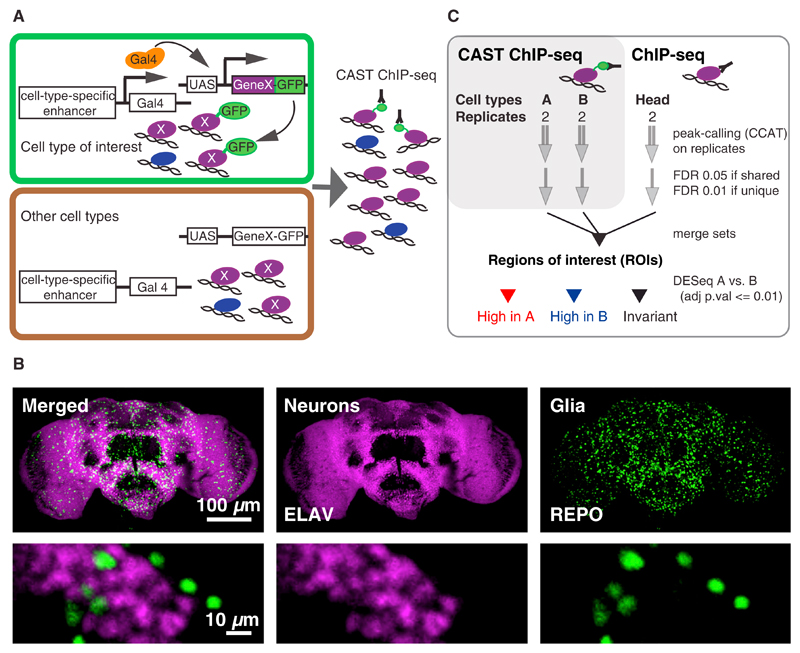
We employed CAST-ChIP (Figure 1A) to profile Pol II by expressing a GFP-tagged RPB3 Pol II subunit (Yao et al., 2006) under control of the UAS/Gal4 system in glia and neurons (Figure 1B), both of which are distinct cell types with well-characterized markers and Gal4-driver lines. We prepared chromatin from adult fly heads expressing the tagged Pol II in the cell types of interest and performed anti-GFP immunoprecipitations (Figure 1A). Controls for CAST-ChIP included input DNA from total head, input DNA from FACS-isolated neuronal and glial nuclei, and CAST-ChIP with anti-GFP on wild-type head chromatin. To compare the CAST-ChIP Pol II enrichments with endogenous Pol II, we performed ChIP sequencing (ChIP-seq) of wild-type heads using anti-RPB3 (Adelman et al., 2006; Figure 2A) or anti-RPB1 (data not shown). We identified regions of high ChIP enrichment in the head, glia, and neurons, and merged them into regions of interest (ROIs; see Experimental Procedures; Figure 1C; Table S1). As expected, Pol II predominantly locates close to annotated transcription start sites (TSSs; Figure 2B). A comparison of biological replicates of CAST-ChIP for Pol II with replicates of conventional ChIP-seq using RPB3 and RPB1 antibodies shows a high correlation (Spearman rho ≥ 0.84; Table S2). In contrast, Spearman’s rank correlation coefficient between glial and neuronal data sets is rho = 0.65 (Figure S1A), indicating differences in Pol II binding, as expected for distinct cell types.
Figure 1. The CAST-ChIP Methodology.
(A) GFP-tagged reporters (GeneX-GFP) are expressed using cell-type-specific drivers. The GFP-tagged protein is then purified from the cell type of interest using a refined ChIP procedure.
(B) Fly brain immunohistochemistry of neurons (magenta, anti-ELAV) and glia (green, anti-REPO) indicates two distinct cell types (bottom row, detail).
(C) Determination of CAST-ChIP ROIs and regions enriched in specific cell types.
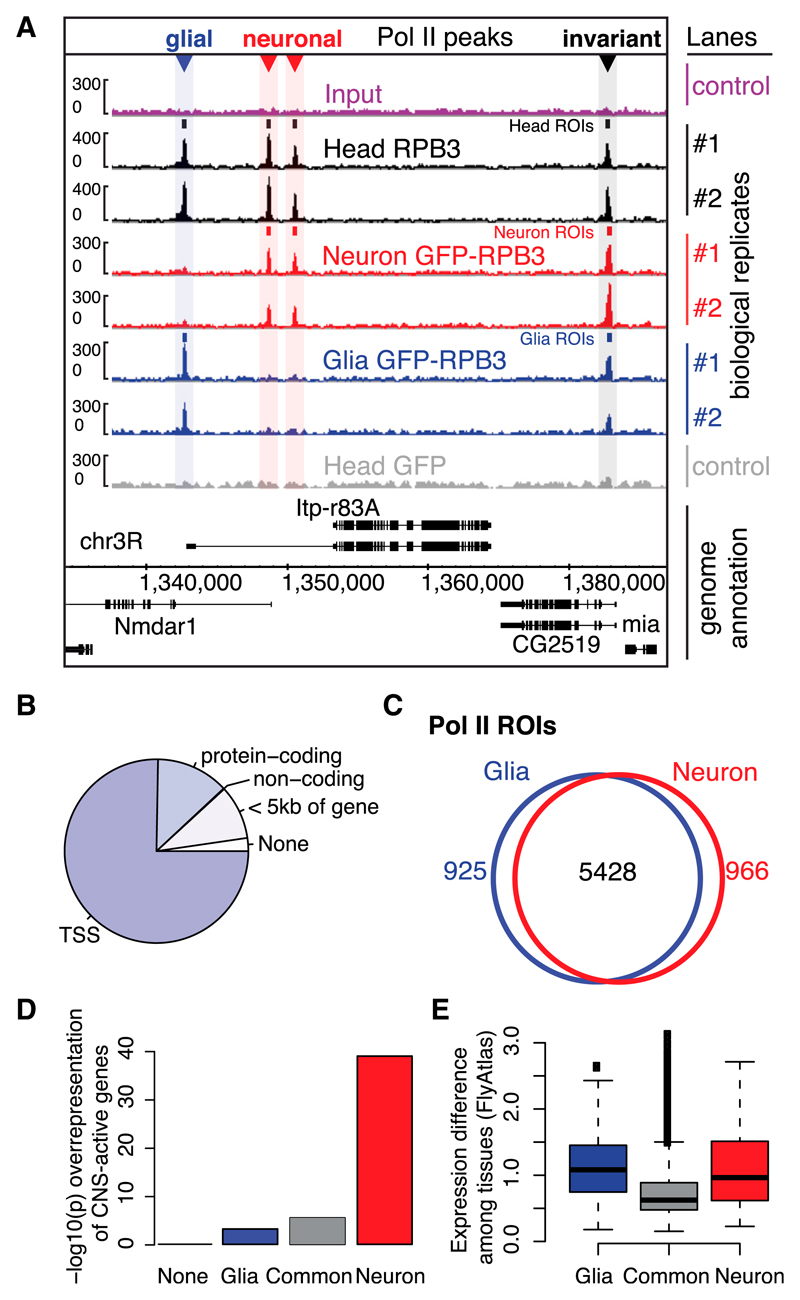
Figure 2. Distinct RNA Pol II-Enriched Regions in Neurons and Glia.
(A) IGB Browser screenshot of the Nmdar1 locus showing CAST-ChIP and ChIP biological replicates of whole-head anti-RPB3 (Head RPB3), neuronal (Neuron) and glial (Glia) GFP-RPB3, whole-head GFP control (Head GFP), and Input. Small rectangles: ROIs; colored triangles: differential ROIs.
(B) Pol II ROIs are located mainly at TSSs and in genic regions.
(C) Overlap between neuronal and glial Pol II ROIs.
(D) Head and neuronal Pol II ROIs are associated with curated CNS-active gene sets (Pfeiffer et al., 2008) (–log10 p values [Fisher’s exact test] are displayed on the y axis).
(E) Gene-expression difference (SD of probe-level values) across FlyAtlas tissues (Chintapalli et al., 2007) for genes marked by glial, common, or neuronal Pol II (*p < 10−16, Wilcoxon rank-sum test for glial-versus-common and neuronal-versus-common comparisons).
Two technical issues are crucial for CAST-ChIP: identifying a good ChIP-grade anti-GFP and expressing sufficient tagged protein in the cell type of interest (Figures S2A and S2B). CAST-ChIP compares favorably in terms of rapidity and efficiency with existing methods (Figures S2C–S2E). In fact, CAST-ChIP is as efficient as a ChIP of the endogenous protein (Figure S2B) and requires substantially less material and time than FACS (Figures S2C and S2D), thus providing a readily applicable method.
Computational and Experimental Validation of Cell-Type-Specific Pol II Profiles
To identify differentially regulated genes, we compared Pol II enrichment obtained by CAST-ChIP in glia and neurons using DESeq (Anders and Huber, 2010) on ROIs (see Experimental Procedures; Figure 1C; Table S3). Three-quarters of Pol II-bound regions (5,428) do not vary between the two cell types. In contrast, 925 regions are identified as glia specific and 966 are identified as neuron specific (Figure 2C). Because the majority of ROIs are located in genic regions, these numbers correspond to 712 genes bound by Pol II only in glia, 779 only in neurons, and 4,942 genes with invariant Pol II. Similarly to cell-type-invariant Pol II-bound regions, the majority (>60%) of neuron- or glia-specific Pol II ROIs overlap genes, exons, and TSSs (Figure S1B).
To validate the observed differences in Pol II binding between neurons and glia, we used computational and experimental approaches. We used a list of “CNS-active” genes expressed within the adult brain (Pfeiffer et al., 2008). CNS-expressed genes are overrepresented among genes that show neuron-specific, glia-specific, or invariant Pol II binding (Figure 2D). The enrichment is most significant for neuron-specific genes (Figure 2D), indicating that Pol II-bound regions identified using neuronally tagged Pol II most closely match the expected CNS activity. Next, we analyzed the cell-type specificity of genes marked by glial, neuronal, and invariant Pol II in CAST-ChIP using FlyAtlas, a microarray-based gene-expression data set of dissected fly tissues (Chintapalli et al., 2007). As expected, mRNA expression levels for glial and neuronal Pol II-bound genes deviate more (p < 10−16, Wilcoxon rank-sum test) among FlyAtlas tissues compared with Pol II-invariant genes (Figure 2E). Further, genes carrying neuron-specific Pol II enrichment show expression in the larval CNS, thoracicoabdominal ganglion, and adult brain (Figure S1D). Third, Gene Ontology (GO) analysis reveals enrichment of terms related to neuronal function for genes marked by neuron-specific Pol II ROIs (Figure S1C; Table S4). In sum, three distinct analyses show that neuron- and glia-specific Pol II-bound regions mark genes with cell-type-specific CNS expression.
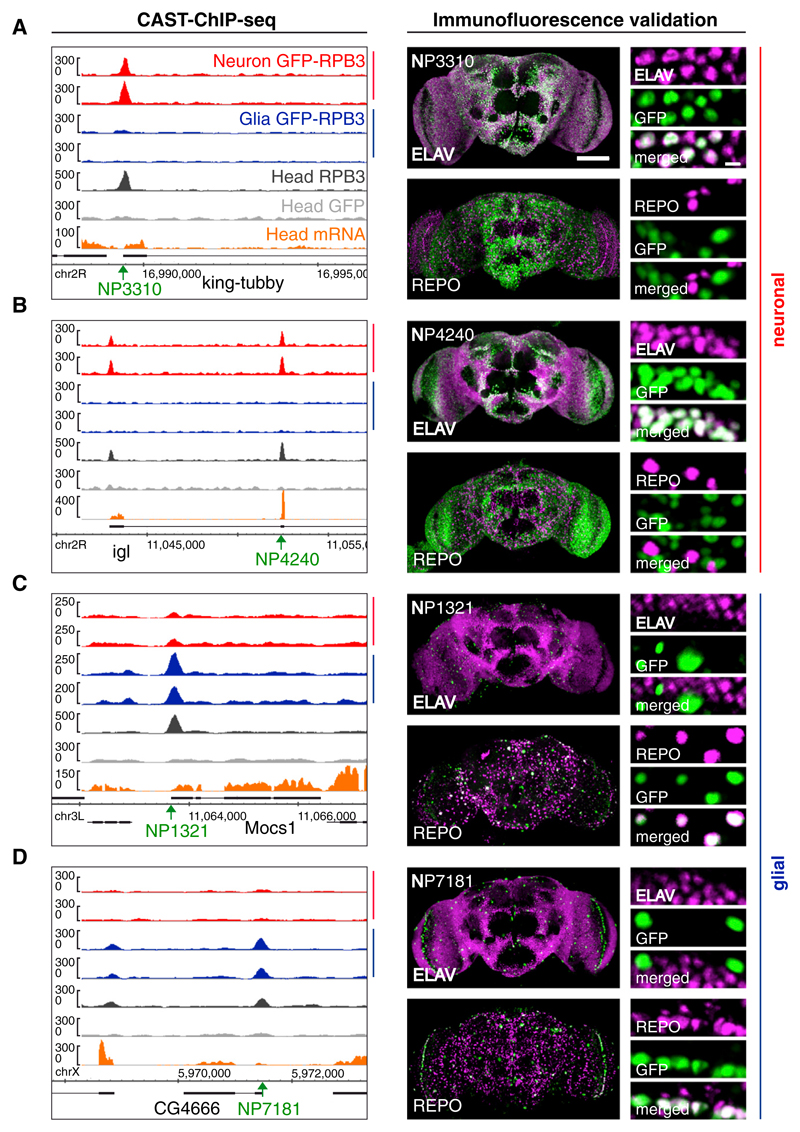
Next, we evaluated the cell-type-specific activity of Pol II-marked genes experimentally. To avoid limitations, including the lack of antibodies for cell-type-specific gene products and the nuclear localization of reference markers, we used “enhancer trap” insertions to express histone-GFP (Figures 3 and S3). We evaluated overlap with nuclear GFP by costaining with pan-neuronal ELAV or pan-glial REPO antibodies. All 12 viable and healthy lines of the 15 selected for testing confirm the differential enrichment (Figures 3 and S3; Experimental Procedures). Thus, the insertion-line-driven histone-GFP verifies CAST-ChIP. Pol II binding qualitatively reflects the spatial expression pattern of the gene to which it localizes, and CAST-ChIP biochemically enriches chromatin-associated Pol II from the cell types marked by GFP-tagged Pol II.
Figure 3. Validation of Neuronal and Glial Pol II Enrichment.
(A–D) To validate neuron- and glia-specific Pol II ROIs, we assayed the neuronal and glial specificity of enhancer-trap insertion lines driving nuclear GFP at (A) king-tubby, (B) Igl, (C) Mocs1, and (D) CG4666. Left panels: Pol II profiles for neurons and glia, whole-head Pol II ChIP, GFP control, and whole-head mRNA tracks, including gene annotation and location of the Gal4 driver (green arrow, insertion point). Right panels: costaining of nuclear GFP (green) expressed using Gal4 drivers located in proximity to the cell-type-specific Pol II ROIs, alongside REPO or ELAV (magenta) to reveal expression overlaps (white).
See also Figure S3.
H2A.Z Is Similarly Distributed Across Distinct Cell Types of the Adult Fly Brain
To better understand the contribution of chromatin structure to cell-type-specific gene regulation, we used CAST-ChIP to study H2A.Z distribution, an active mark that has not previously been probed in the adult fly CNS. GFP-tagged H2A.Z expressed by its genomic promoter (Clarkson and Saint, 1999) faithfully reports the location of endogenous H2A.Z (95% overlap between ROIs; Figures 4A, 4B, and S4A), indicating that neither the tag nor transgene (over-) expression globally alter H2A.Z distribution.
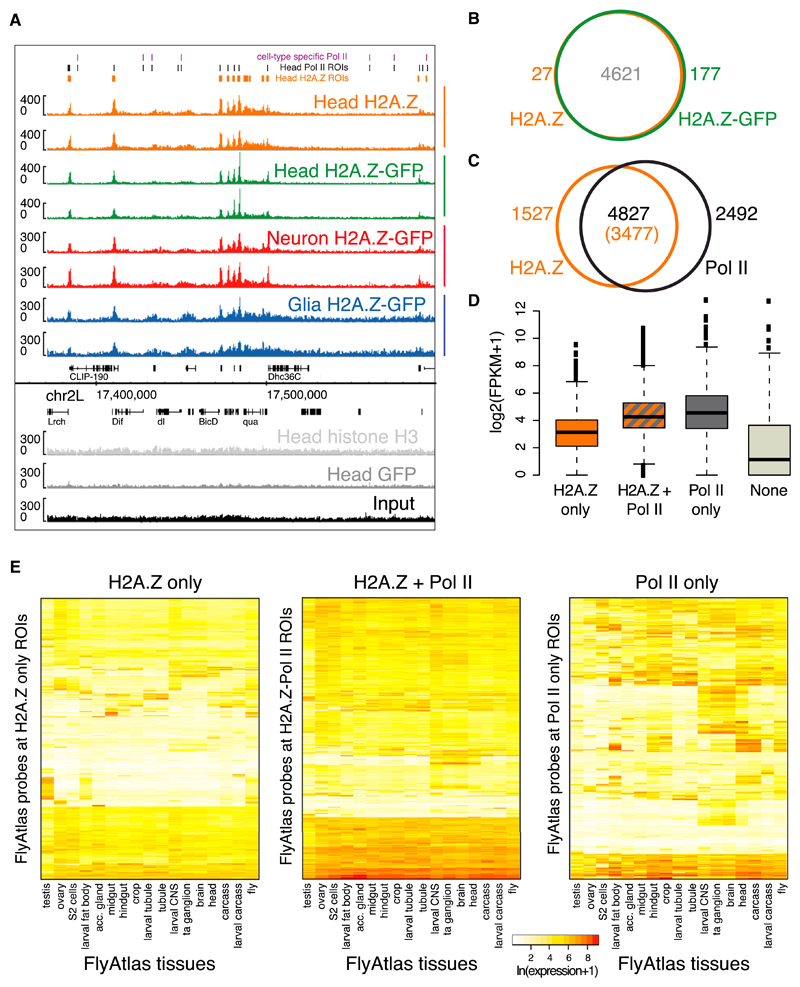
Figure 4. Enrichment of Histone H2A.Z in Neurons and Glia.
(A) Screenshot on chr2L showing biological replicates of whole-head endogenous- and GFP-tagged H2A.Z, neuronal, and glial H2A.Z, as well as whole-head H3, GFP, and Input.
(B) Overlap between endogenous and GFP-tagged H2A.Z ROIs in the whole head.
(C) Overlap between endogenous H2A.Z and Pol II ROIs in whole heads relative to the total number of Pol II (or H2A.Z) ROIs.
(D) Gene-expression levels (log2(FPKM+1)) in whole heads for H2A.Z-only, H2A.Z+Pol II, Pol II-only, and None (neither H2A.Z nor Pol II) gene classes. The p values for all comparisons are p < 2.2 × 10−16, except for H2A.Z+Pol II versus Pol II-only, where p = 0.003 (Wilcoxon rank-sum test).
(E) Heatmaps showing gene-expression levels (color range) in FlyAtlas tissues (Chintapalli et al., 2007). Genes that do not overlap H2A.Z ROIs (third panel) show larger differences in expression across tissues. Genes marked by Pol II show higher expression (second and third panels) compared with H2A.Z-only genes (first panel).
See also Figure S4.
As previously observed in mammalian cells and flies (Hardy et al., 2009; Mavrich et al., 2008; Weber et al., 2010), H2A.Z is mostly associated with Pol II-bound regions in the adult fly head and is predominantly enriched at the +1 nucleosome relative to the TSS (Figures 4C, S4B, and S4D). In addition, we detect a class of genes with high Pol II enrichment but no H2A.Z. Expression profiling using whole-head RNA-seq reveals that genes marked by H2A.Z and Pol II show similarly high expression compared with genes bound by Pol II only (Figure 4D). As expected, promoter-proximal Pol II enrichments reflect the expression level (Figure S4D). In contrast, although H2A.Z does mark expressed genes, there is no linear correlation between H2A.Z binding and the expression level (Figure S4D).
Intriguingly, not all Pol II-bound regions in the fly head overlap with H2A.Z regions, suggesting the presence of a class of active genes with no or little H2A.Z enrichment (Figure 4C). To gain insight into the nature of the genes marked either by H2A.Z and Pol II together or by Pol II or H2A.Z alone, we used FlyAtlas expression data (Chintapalli et al., 2007). Genes marked by H2A.Z alone or by H2A.Z and Pol II together are expressed homogeneously across cell fates (Figures 4E and S4E). In contrast, Pol II-bound genes that lack H2A.Z enrichment show divergent expression across tissues and developmental stages (Figures 4E and S4E).
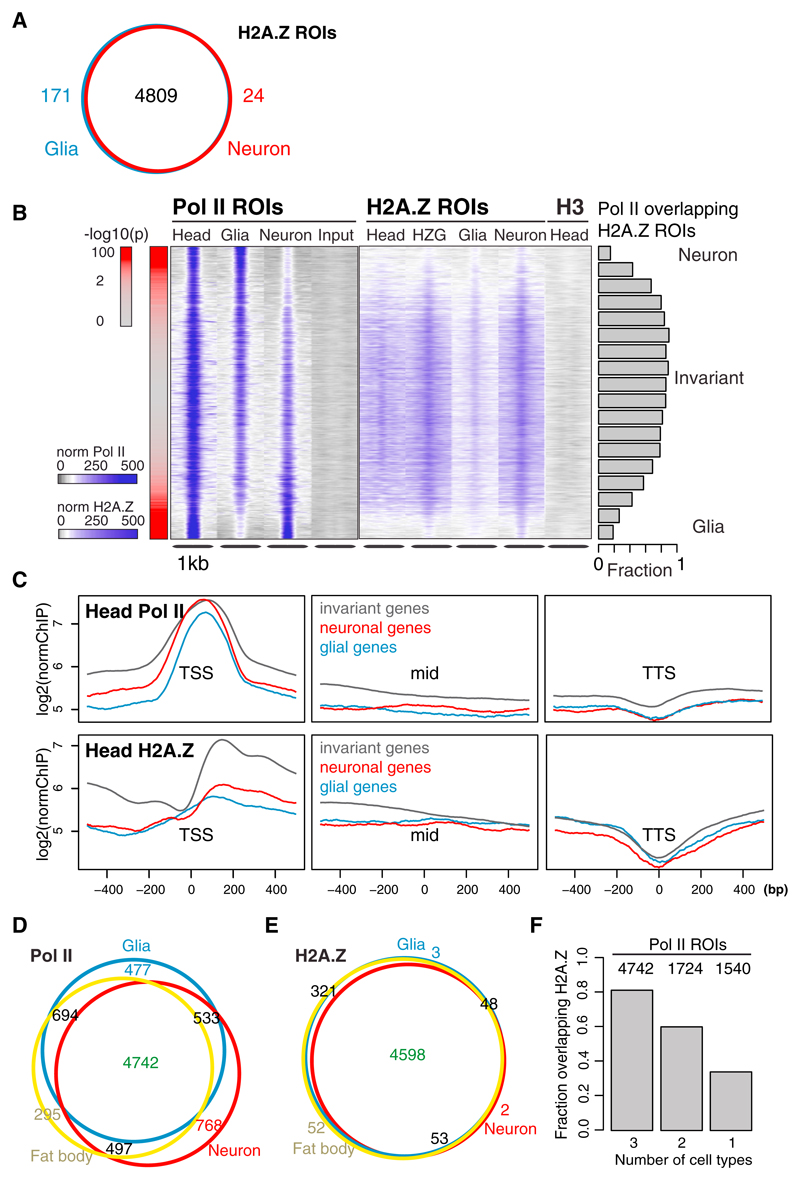
The observation that H2A.Z is nearly always present at genes with similar expression across cell types and universal cellular functions led us to hypothesize that H2A.Z may characterize chromatin environments permissive for cell-type-invariant gene expression. If this is the case, H2A.Z would be expected to show similar distribution across distinct cell types. To test this, we expressed a GFP-tagged H2A.Z construct (Clarkson and Saint, 1999) in neurons or glia and identified regions of differential enrichment as we did for Pol II (Figure 1C). Indeed, we find that H2A.Z enrichments are highly similar across the two cell types (Figures 4A, 5A, and S5A; Table S2). This observation largely holds true when we compare enrichments across exonic regions with no prior filtering, relying on peak calling to reduce noise levels (Figure S5B). Further, the few observed cell-type-specific H2A.Z ROIs show small differences in counts and often do not associate with genes.
Figure 5. H2A.Z Marks Cell-Type-Invariant, but Not Active Cell-Type-Specific, Genes.
(A) Overlap between neuronal and glial H2A.Z ROIs.
(B) Head, glial, and neuronal Pol II and H2A.Z enrichments, as well as GFP-tagged head H2A.Z (HZG), H3, and Input at whole-head Pol II ROIs. Regions are centered on the point with maximal Pol II intensity and sorted from highest to lowest signal in glia (–log10 p values are shown in red on the left). The fractions of H2A.Z ROIs overlapping bins of 25 Pol II ROIs are shown as barplots. The data were only normalized to the total number of reads, and the same scale was used for all Pol II, H2A.Z, and H3 data.
(C) Average ChIP enrichment of head Pol II and head H2A.Z centered on the TSS, gene bodies (1 kb around the center of the transcript, labeled “mid”) and transcription termination site (TTS) of genes associated with invariant, neuronal, and glial Pol II CAST-ChIP ROIs.
(D) Overlap between neuronal, glial, and fat body Pol II ROIs.
(E) Overlap between neuronal, glial, and fat body H2A.Z ROIs.
(F) Fraction of Pol II-bound regions found in one (1), two (2), or all three (3) cell types (neuron, glia, and fat body) that overlap at least one whole-head H2A.Z ROI. The total number of Pol II ROIs in each class is indicated on the graph. Pol II-bound regions present in all three cell types (4,725) largely overlap with H2A.Z.
See also Figure S5.
A comparison of averaged H2A.Z profiles over genes with glial, neuronal, or invariant Pol II enrichment shows that H2A.Z is highly enriched at cell-type-invariant Pol II ROIs, as well as in TSS-proximal genic regions (Figure 5C). Further, cell-type-invariant Pol II sites nearly always (90%) overlap an H2A.Z ROI (Figures 5B and S5C). We note that H3 is equally distributed across these ROIs on both cell-type-invariant and cell-type-specific Pol II peaks (Figure 5B). Strikingly, genomic regions that show strong cell-type-specific Pol II binding are depleted of H2A.Z (Figure 5B) despite the high expression of the marked genes (Figure S5D).
To check whether the tissue invariance of H2A.Z distribution holds true across cell types of distinct developmental origin, we profiled Pol II and H2A.Z in adipocytes, which are derived from the mesoderm (“fat body” in flies; Figures 5D and 5E). We find that of the 4,742 regions bound by Pol II in all cell types, >80% associate with H2A.Z (Figure 5F). In contrast, only ~35% of the 1,540 regions that show Pol II enrichment in only one of the cell types overlap with H2A.Z ROIs (Figure 5F). Thus, H2A.Z is similarly distributed across neurons, glia, and fat body, and preferentially localizes to the TSSs of cell-type-invariant genes (Figure 5C).
H2A.Z Localization Is Maintained from Early Development to Adulthood
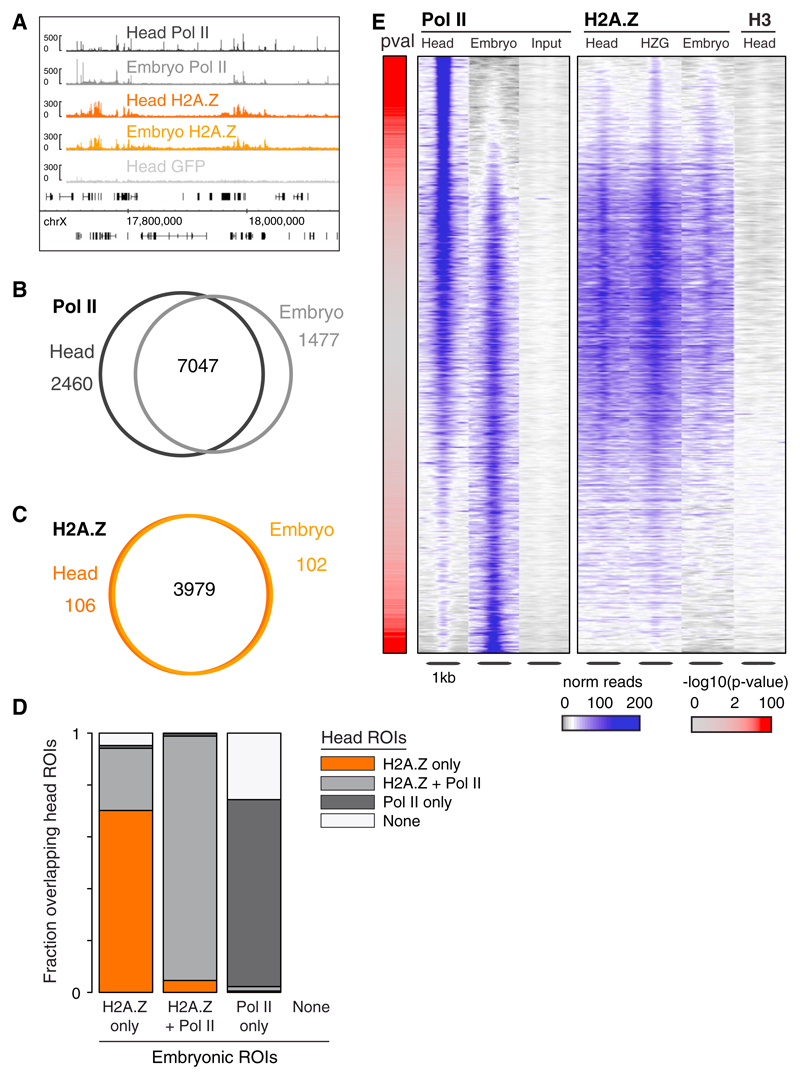
Having observed that H2A.Z profiles are invariant across cell types within the adult brain, we hypothesized that H2A.Z marks may be established early during embryogenesis and maintained throughout development. To test this, we profiled H2A.Z and Pol II in 0–6 hr embryos using antibodies against the endogenous protein (Figure 6A). H2A.Z enrichments are very similar in adult head and embryo (Spearman rho = 0.91; Figure S6A; Table S2), marking largely (97%) the same genes (Figures 6A, 6C, and S6A). In contrast, Pol II binding differs extensively (38% of differentially bound genes; Figure 6B). The whole-head versus embryo comparison confirms that H2A.Z is mainly present in regions bound by Pol II across developmental stages (Figure 6E), and is absent in genes showing adult-head or embryo-specific Pol II binding. We also analyzed modENCODE ChIP-chip data for H2A.Z in embryos and confirmed the early deposition and cell-type invariance of H2A.Z distribution (modENCODE Consortium et al., 2010; Figure S6C).
Figure 6. H2A.Z Locations Are Conserved from the Embryo to the Adult Brain.
(A) IGB Browser screenshot on chromosome X showing adult head and embryo Pol II and H2A.Z, as well as head GFP.
(B) Overlap between embryonic and whole-head Pol II-bound genes.
(C) Overlap between embryonic and whole-head H2A.Z-bound genes.
(D) Fraction of embryonic ROIs (H2A.Z-only, H2A.Z+Pol II, and Pol II-only) overlapping head ROIs (H2A.Z-only, H2A.Z+Pol II, Pol II-only, and None [neither H2A.Z nor Pol II]). Total number of embryonic ROIs: 1,126 in the H2A.Z-only group, 2,828 in the H2A.Z+Pol II group, and 4,489 in the Pol II-only group.
(E) Adult head and embryonic Pol II, and H2A.Z enrichment at Pol II-enriched regions. Controls: Input, H3, H2A.Z.-GFP (HZG). Regions are centered and sorted as in Figure 5B.
See also Figure S6.
Furthermore, we investigated the fate of embryonic H2A.Z ROIs by splitting genomic regions into three classes (H2A.Z-only, Pol II+H2A.Z, or Pol II-only) and compared their overlap with head H2A.Z ROIs (Figure 6D). The majority of H2A.Z-bound regions (with or without Pol II) remain in the same class. H2A.Z-only regions have an increased propensity to acquire Pol II in the adult. In contrast, embryonic PoI II-only bound regions rarely gain H2A.Z, but rather lose Pol II binding in the adult head. Consistent with this observation, genes whose TSS overlaps only with Pol II and not with H2A.Z ROIs in the embryo show greater variability in expression across developmental stages (Chintapalli et al., 2007) than their H2A.Z-positive counterparts (Figure S6B). H2A.Z may thus mark a chromatin state that is permissive for cell-type-invariant gene expression across development.
H2A.Z Marks Cell-Type-Invariant Chromatin Domains
H2A.Z-bound genes share characteristics that support the role of H2A.Z in marking cell-type-invariant genes. H2A.Z-enriched genes tend to have broad TSSs, whereas Pol II-only TSSs resemble those of cell-type-specific promoters (Figure S7A; Hoskins et al., 2011; Rach et al., 2011). In addition, H2A.Z ROIs are enriched in constitutively active chromatin (Figures 7A and 7B; Filion et al., 2010). In contrast, the majority of Pol II binding in the absence of H2A.Z localizes to chromatin associated with silent but developmentally regulated genes, dynamically active regions, and Polycomb group chromatin (Figure 7B). H2A.Z+Pol II ROIs associate with so-called “Tau gene clusters,” defined as chromosomal regions encoding coregulated genes (Figures 7A and S7B; Weber and Hurst, 2011), and the H2A.Z-enriched regions themselves form clusters (p value < 10−16; Figure 7A). Finally, promoters of genes that are significantly up- or downregulated across distinct cell types show overall lower enrichment of H2A.Z than do promoters of coordinately expressed genes (Figure S7B; Kapushesky et al., 2012).
Figure 7. H2A.Z Marks Constitutive Transcriptional Competence.
(A) IGB Browser view of low-tissue-specificity genes (Tau clusters) (Weber and Hurst, 2011), gene-based H2A.Z clusters, 0–6 hr embryo and adult head H2A.Z tracks (embryo and head H2A.Z), and five-state chromatin domains (Filion et al., 2010), respectively. H2A.Z overlaps constitutively active (yellow) chromatin regions and Tau clusters.
(B) Fractions of H2A.Z-only (HZ-only), H2A.Z and Pol II (HZ+Pol II), and Pol II-only (Pol II-only) ROIs associated with color-coded chromatin domains (Filion et al., 2010). Top-enriched domains are shown with solid colors and transitions are shown with striped colors. Classes are ordered proportionally and the less abundant classes are indicated as “other classes” (gray).
(C) Fraction of ROIs (H2A.Z-only [HZ-only], H2A.Z and Pol II [HZ+Pol II], and Pol II-only [Pol II-only]) overlapping insulator-binding proteins. The overlap between H2A.Z+Pol II and CP190, and BEAF-32 and CTCF is highly significant (one-sided Fisher’s exact test; p < 2.2 × 10−16).
See also Figure S7.
Since we observed that H2A.Z ROIs often overlapped two neighboring chromatin domains, such as constitutive and silent or constitutive and dynamically active chromatin (Figures 7B, S7C, and S7D), we investigated the relationship between H2A.Z and insulator-binding proteins, both of which have previously been shown to localize at promoters with a broad TSS (Nègre et al., 2010; Rach et al., 2011). We find that at nearly all sites, H2A.Z+Pol II-shared ROIs are bound by the insulator proteins BEAF-32 and CP190 (Figure 7C), whereas H2A.Z-only and Pol II-only ROIs overlap with these insulators much less frequently (Figure 7C). In addition, the majority (75%) of BEAF-32- and CP190-bound regions overlap with H2A.Z+Pol II ROIs, whereas ~50% of CTCF- and <10% of Su(Hw)-bound regions overlap with H2A.Z+Pol II. The striking association of H2A.Z with BEAF-32 and CP190 in the presence of Pol II suggests a putative role for these insulator-binding proteins in the activation of cell-type-invariant genes.
Discussion
CAST-ChIP Rapidly and Efficiently Profiles Cell-Type-Specific Chromatin
We describe an affinity-based, biochemical procedure for the cell-type-specific mapping of chromatin-binding proteins. As a proof of principle, we employed this technique to obtain genome-wide profiles for two landmark chromatin features—the binding of Pol II and the histone variant H2A.Z—in distinct cell types of the adult fly brain. Our approach complements existing tools in this rapidly evolving area and offers key advantages in terms of cost, speed, and chromatin yield. As we start to dissect chromatin structure within tissues and in different environmental conditions, the cost, quantity of material, and amount of time needed to prepare chromatin will become restrictive.
Existing cell-type-specific approaches rely on isolating tissue by dissection (Nagoshi et al., 2010), extracting nuclei through a transgenic tag on the nuclear membrane (Deal and Henikoff, 2010; Henry et al., 2012), or FACS of labeled nuclei (Jiang et al., 2008). These methods have revealed differential H3K4me3 enrichment in neuronal and nonneuronal cells (Cheung et al., 2010), SAGA occupancy in embryonic fly muscle and neurons (Weake et al., 2011), embryonal transcription factor binding and histone modifications (Bonn et al., 2012), as well as gene expression and histone modification profiles in Kenyon cells and octopaminergic neurons of the adult fly brain (Henry et al., 2012). However, FACS assays at the scale suitable for existing ChIP-seq procedures are time consuming and require a lot of material due to loss during isolation, making it difficult to profile several cell types, test different environmental conditions, and perform necessary replicates. In contrast, CAST-ChIP requires little mechanical sample manipulation, is rapid, improves sample preservation, and achieves high-yield enrichment from small amounts of tissue in a single biochemical step (Figure S2). This facilitates the analysis of cell-type-specific chromatin by decreasing the number of animals required and eliminating the need for extended access to FACS.
Like other methods that rely on tagged transgenes, CAST-ChIP is limited by its dependence on the expression of tagged proteins. Organisms need to be genetically manipulatable, and highly specific enhancers are required to obtain cell-type resolution. Further, the tagged protein should not interfere with the function of the endogenous protein, but should be expressed at a high enough level to obtain good enrichment. Both of these conditions are met in our system, which takes advantage of well-characterized Gal4 lines and functional tagged proteins (Clarkson and Saint, 1999; Yao et al., 2006). Clearly, there will be situations in which other assays might be more advantageous, for example, when overexpression of a low-abundance transcription factor leads to artifacts or the biological question requires analysis of a posttranslational modification.
Using CAST-ChIP, we identified ~1,500 genes with glia- and neuron-specific Pol II enrichment. We confirmed the cell-type specificity of these genes using FlyAtlas (Chintapalli et al., 2007), GO annotations, a “CNS-active” gene list (Pfeiffer et al., 2008), and enhancer trap lines. For example, we found that neuron-specific Pol II enrichment identified genes with neuronal functions, such as Nmdar1 (Figure 2A), igloo (neuromodulin), and king-tubby. Similarly, glia-specific Pol II enrichment identified glial genes such as tramtrack (Figure S3) and Eaat1. Our study provides a valuable resource for the community, delineating regions of both active and poised transcription in fruit fly neurons, glia, and fat body.
H2A.Z Marks Cell-Type-Invariant Transcriptional Domains across Distinct Cell Types
We extended the application of CAST-ChIP to the enquiry of chromatin structure by mapping the distribution of H2A.Z in embryos and adult cell types. Studies in yeast, animal cell types, and embryonic tissue have attributed seemingly contradictory functions to H2A.Z in gene expression and chromatin structure, placing it at both active and repressed promoters as well as chromatin boundaries (reviewed in Marques et al., 2010; Talbert and Henikoff, 2010). We show here that H2A.Z is distributed similarly in distinct brain cell types, where it colocalizes with Pol II generally in the context of active TSSs, confirming the correlation of both H2A.Z and Pol II with transcription (Henikoff et al., 2009; Jin et al., 2009). However, we also find that thousands of expressed genes are bound by Pol II in the absence of H2A.Z (Figures 4D and 4E). This could be explained by nucleosome/histone eviction, differences in nucleosome positioning (e.g., “fuzzy” nucleosomes; Zhang and Pugh, 2011), and/or an H2A.Z-independent regulatory mechanism in this subset of genes. Whatever the mechanism, the steady state we probe contains a class of active cell-type-specific promoters that are devoid of H2A.Z. Interestingly, these regions correspond to the most cell-type-specific Pol II regions. Conversely, H2A.Z is highly enriched (and its levels do not differ) at cell-type-invariant regions bound by Pol II in distinct cell types, including those of different developmental origin (Figures 5B–5E). We propose that H2A.Z marks transcriptionally permissive chromatin states across cell fates.
H2A.Z enrichment may be indicative of how genes are regulated, rather than whether they are transcriptionally active at a particular moment. According to this model, H2A.Z marks cell-type-invariant genes, including constitutively expressed genes, genes that are reused in several cell types, and a set of environmentally inducible genes. Indeed, we find that H2A.Z is enriched at constitutively expressed genes (e.g., ribosomal genes), inducible genes (e.g., heat shock and hormonally regulated genes), and developmental genes (e.g., trithorax, Hairy, and Mef2). Some of these H2A.Z-marked genes are required for cellular processes that occur during development and are reused in subsets of cell types in the adult, such as mRNA localization (e.g., staufen and pumilio). Indeed, in plants and animals, H2A.Z is involved in the regulation of inducible genes, ranging from heat shock to hormone-responsive genes, as well as the MHC class I locus (Coleman-Derr and Zilberman, 2012; Gévry et al., 2009; Kotekar et al., 2008; Kotova et al., 2011; Leach et al., 2000). Our data suggest that H2A.Z may not be sufficient for transcription, but maintains a chromatin state that facilitates the transcription of both highly expressed genes and genes poised for activation across cell types.
Consistent with this model, we find H2A.Z clusters in chromosomal regions that are enriched for coregulated (Weber and Hurst, 2011) and constitutively active genes (Filion et al., 2010). Further, H2A.Z is enriched at promoters of genes that are not differentially expressed across cell types (Chintapalli et al., 2007). Other investigators and we have shown that H2A.Z is present at insulators in both mammals and flies (Barski et al., 2007; Fu et al., 2008; Jin et al., 2009; Rach et al., 2011). An individual study of the p16 gene locus, for example, revealed that CTCF binding, H2A.Z incorporation, and the establishment of a permissive chromatin environment can result in transcriptional activation (Witcher and Emerson, 2009; reviewed in Yang and Corces, 2011). Here, our genome-wide data on H2A.Z show that in the presence of Pol II the histone variant is particularly enriched at insulators containing BEAF-32 and CP190 (90%; Figure 7C), and that 75% of all BEAF-32 and CP190 bind to these regions. In contrast, Pol II-bound ROIs lacking H2A.Z do not show this association, suggesting that the mechanism(s) of gene activation for H2A.Z-bound genes may specifically involve insulator-binding proteins. Our data raise the possibility that H2A.Z, BEAF-32, CP190, and other chromatin factors, such as the NSL complex (Feller et al., 2012; Lam et al., 2012) and/or MRG15 (Filion et al., 2010), may coregulate cell-type-invariant genes. The presence of H2A.Z at genes that are both constitutive and inducible across cell types suggests that H2A.Z is not sufficient for transcription, but rather maintains a chromatin state that in combination with the insulator-binding proteins BEAF-32 and CP190 allows for cell-type-invariant transcription. This mechanism for regulating cell-type-invariant gene expression may be evolutionarily conserved since H2A.Z is also preferentially found at broad TSSs in mammals (Rach et al., 2011). Whether H2A.Z marks cell-type-invariant genes in mammals remains to be tested. CAST-ChIP is a timely tool for addressing such questions.
The high overlap of H2A.Z enrichment between cell types of different developmental origins (neurons/glia versus fat body, or 0–6 hr embryo versus adult head) suggests that the H2A.Z mark is established in the embryo and maintained throughout development. Other studies indicate that H2A.Z is established on chromatin very early in embryogenesis in several different species (Bošković et al., 2012; Chioda et al., 2010; Nashun et al., 2010; Whittle et al., 2008). In addition, the establishment of chromatin boundaries between heterochromatin and euchromatin appears to depend on the interaction of H2A.Z with Polycomb group proteins in Drosophila (Chioda et al., 2010; Swaminathan et al., 2005) and mammalian embryonic stem cells (Creyghton et al., 2008; Hu et al., 2013; Ku et al., 2012; reviewed in Pandey and Dou, 2013). Together, these studies and our results suggest that H2A.Z may facilitate chromatin organization during differentiation across complex organisms. Our data argue that H2A.Z domains are faithfully maintained after being established in the embryo to the adult stage, allowing for cell-type-invariant gene expression throughout development and in the adult. Other chromatin marks have not been shown to be as refractory to changes across cell fates. How such a mark is established and maintained is not clear. A simple mechanism relies on the sequence of the promoters that are enriched in H2A.Z-marked genes (Rach et al., 2011), but it is also possible that H2A.Z localization may rely on epigenetic mechanisms.
For further information, see the Extended Results and Discussion.
Conclusions
In this work, we developed, validated, and used a method we call CAST-ChIP to study the distribution of Pol II and H2A.Z in specific cell types within highly complex tissues, such as the Drosophila CNS. By taking advantage of established, widely available techniques, CAST-ChIP permits the profiling of chromatin-associated factors in any cell type provided that suitable genetic drivers are available. It requires vastly less material and shorter processing times compared with nuclear purification techniques, enabling chromatin profiling in small amounts of ex vivo material. This facilitates the analysis of replicates, which is mandatory for studying physiological processes, making it an accurate, fast, and cost-effective method. Because our tool builds on broadly used technology, it is readily transferable to the mouse and other model organisms. CAST-ChIP, together with other cell-type-specific profiling methods, will allow for the systematic dissection of chromatin and gene regulation upon metabolic, behavioral, and other environmental changes at the resolution of individual cell types.
Experimental Procedures
CAST-ChIP
Flies carrying the GFP-tagged reporter UAS-GFP-RPB3 (Yao et al., 2006) or UAS-H2A.Z-GFP were crossed with the driver lines elav-Gal4 (Bloomington stock No. 458), repo-Gal4 (Sepp et al., 2001), or takeout-Gal4 (Dauwalder et al., 2002), or maintained as a homozygous stock in the case of elav-Gal4; UAS-GFP-RPB3. Fly heads were separated and chromatin was prepared as described in the Extended Experimental Procedures. Cell-type-specific ChIP was performed using anti-GFP antibody (goat, lab stock) and Protein G Dynabeads (Invitrogen). Input and ChIP with anti-GFP antibody on wild-type heads were used as controls. Library preparation and sequencing were carried out by the EMBL Genomics Core Facility according to standard Illumina protocols.
Data Analysis
Reads were aligned to the Drosophila melanogaster genome (BDGP5.13) using Bowtie version 0.12.7. Aligned reads were transformed into coverage files using igvtools and visualized in the IGV 2.0 Browser (Robinson et al., 2011) and IGB Browser (Nicol et al., 2009). Regions of high ChIP enrichment were detected with CCAT 3.0 (Xu et al., 2010) on individual replicates using Input or ChIP against GFP as control. Regions from different cell types were merged together to obtain a set of ROIs. Counts in individual ROIs for either Pol II or H2A.Z were compared using DESeq (Anders and Huber, 2010) to determine significant differences in ChIP enrichment between pairs of cell types.
Further details of the methods are described in the Extended Experimental Procedures.
Supplementary Material
Supplemental Information includes Extended Discussion, Extended Experimental Procedures, seven figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.09.001.
Acknowledgments
We thank Karen Adelman, Takeshi Awasaki, Brigitte Dauwalder, Robert Glaser, John Lis, Robert Saint, Hiromu Tanimoto, and Tim Tully for reagents; EMBL GeneCore, Simon Anders, Vladimir Benes, and Wolfgang Huber for sequencing and discussion; EMBL ALMF and Ulrike Gaul for microscopy; Dagmar Pich and Wolfgang Hammerschmidt for FACS assistance; and Bianca Nijmeijer and Sandra Esser for technical help. We also thank Thomas Conrad and Kasia Oktaba for ChIP protocols; Margarete Reisenauer and Zdenka Stanic for fly work; the Department of Physiological Chemistry and Vertebrate Genomics Team for discussions; and David Arnosti, Anton Eberharter, Sandra Hake, Corey Laverty, Mikhail Spivakov, and Maria-Elena Torres-Padilla for comments on the manuscript. This work was supported by EMBL, an HFSP Young Investigator Grant (A.G.L.), EU FP6 programs NoE Epigenome and Marie Curie RTN Chromatin Plasticity (A.G.L.), EU FP7 programs NoE EpigeneSys and Marie Curie ITN Nucleosome4D (A.G.L.), and the Bavarian Research Network for Molecular Biosystems BioSysNet (A.G.L.).
Footnotes
Accession Numbers
The ArrayExpress accession number for the data reported in this paper is E-MTAB-1117.
References
- Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet. 2012;13:720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman K, Wei W, Ardehali MB, Werner J, Zhu B, Reinberg D, Lis JT. Drosophila Paf1 modulates chromatin structure at actively transcribed genes. Mol Cell Biol. 2006;26:250–260. doi: 10.1128/MCB.26.1.250-260.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bonn S, Zinzen RP, Girardot C, Gustafson EH, Perez-Gonzalez A, Delhomme N, Ghavi-Helm Y, Wilczyński B, Riddell A, Furlong EEM. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat Genet. 2012;44:148–156. doi: 10.1038/ng.1064. [DOI] [PubMed] [Google Scholar]
- Bošković A, Bender A, Gall L, Ziegler-Birling C, Beaujean N, Torres-Padilla M-E. Analysis of active chromatin modifications in early mammalian embryos reveals uncoupling of H2A.Z acetylation and H3K36 tri-methylation from embryonic genome activation. Epigenetics. 2012;7:747–757. doi: 10.4161/epi.20584. [DOI] [PubMed] [Google Scholar]
- Cheung I, Shulha HP, Jiang Y, Matevossian A, Wang J, Weng Z, Akbarian S. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc Natl Acad Sci USA. 2010;107:8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Chioda M, Vengadasalam S, Kremmer E, Eberharter A, Becker PB. Developmental role for ACF1-containing nucleosome remodellers in chromatin organisation. Development. 2010;137:3513–3522. doi: 10.1242/dev.048405. [DOI] [PubMed] [Google Scholar]
- Clarkson M, Saint R. A His2AvDGFP fusion gene complements a lethal His2AvD mutant allele and provides an in vivo marker for Drosophila chromosome behavior. DNA Cell Biol. 1999;18:457–462. doi: 10.1089/104454999315178. [DOI] [PubMed] [Google Scholar]
- Coleman-Derr D, Zilberman D. Deposition of histone variant H2A.Z within gene bodies regulates responsive genes. PLoS Genet. 2012;8:e1002988. doi: 10.1371/journal.pgen.1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton MP, Markoulaki S, Levine SS, Hanna J, Lodato MA, Sha K, Young RA, Jaenisch R, Boyer LA. H2AZ is enriched at polycomb complex target genes in ES cells and is necessary for lineage commitment. Cell. 2008;135:649–661. doi: 10.1016/j.cell.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder B, Tsujimoto S, Moss J, Mattox W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 2002;16:2879–2892. doi: 10.1101/gad.1010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Henikoff S. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Dev Cell. 2010;18:1030–1040. doi: 10.1016/j.devcel.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller C, Prestel M, Hartmann H, Straub T, Söding J, Becker PB. The MOF-containing NSL complex associates globally with house-keeping genes, but activates only a defined subset. Nucleic Acids Res. 2012;40:1509–1522. doi: 10.1093/nar/gkr869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion GJ, van Bemmel JG, Braunschweig U, Talhout W, Kind J, Ward LD, Brugman W, de Castro IJ, Kerkhoven RM, Bussemaker HJ, van Steensel B. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell. 2010;143:212–224. doi: 10.1016/j.cell.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sinha M, Peterson CL, Weng Z. The insulator binding protein CTCF positions 20 nucleosomes around its binding sites across the human genome. PLoS Genet. 2008;4:e1000138. doi: 10.1371/journal.pgen.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner B, Johnston J, Chen K, Wallaschek N, Paulson A, Garruss AS, Gaudenz K, De Kumar B, Krumlauf R, Zeitlinger J. Poised RNA polymerase II changes over developmental time and prepares genes for future expression. Cell Rep. 2012;2:1670–1683. doi: 10.1016/j.celrep.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gévry N, Hardy S, Jacques P-É, Laflamme L, Svotelis A, Robert F, Gaudreau L. Histone H2A.Z is essential for estrogen receptor signaling. Genes Dev. 2009;23:1522–1533. doi: 10.1101/gad.1787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni S, Ji Z, Hoque M, Rust N, Sharpe H, Eberhard R, Browne C, Hengartner MO, Mellor J, Tian B, Furger A. Analysis of C. elegans intestinal gene expression and polyadenylation by fluorescence-activated nuclei sorting and 3′-end-seq. Nucleic Acids Res. 2012;40:6304–6318. doi: 10.1093/nar/gks282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Jacques P-É, Gévry N, Forest A, Fortin M-È, Laflamme L, Gaudreau L, Robert F. The euchromatic and heterochromatic landscapes are shaped by antagonizing effects of transcription on H2A.Z deposition. PLoS Genet. 2009;5:e1000687. doi: 10.1371/journal.pgen.1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG, Sakai A, Loeb GB, Ahmad K. Genome-wide profiling of salt fractions maps physical properties of chromatin. Genome Res. 2009;19:460–469. doi: 10.1101/gr.087619.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry GL, Davis FP, Picard S, Eddy SR. Cell type-specific genomics of Drosophila neurons. Nucleic Acids Res. 2012;40:9691–9704. doi: 10.1093/nar/gks671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins RA, Landolin JM, Brown JB, Sandler JE, Takahashi H, Lassmann T, Yu C, Booth BW, Zhang D, Wan KH, et al. Genome-wide analysis of promoter architecture in Drosophila melanogaster. Genome Res. 2011;21:182–192. doi: 10.1101/gr.112466.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Cui K, Northrup D, Liu C, Wang C, Tang Q, Ge K, Levens D, Crane-Robinson C, Zhao K. H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2013;12:180–192. doi: 10.1016/j.stem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo T-TB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Matevossian A, Huang H-S, Straubhaar J, Akbarian S. Isolation of neuronal chromatin from brain tissue. BMC Neurosci. 2008;9:42. doi: 10.1186/1471-2202-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapushesky M, Adamusiak T, Burdett T, Culhane A, Farne A, Filippov A, Holloway E, Klebanov A, Kryvych N, Kurbatova N, et al. Gene Expression Atlas update—a value-added database of microarray and sequencing-based functional genomics experiments. Nucleic Acids Res. 2012;40(Database issue):D1077–D1081. doi: 10.1093/nar/gkr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotekar AS, Weissman JD, Gegonne A, Cohen H, Singer DS. Histone modifications, but not nucleosomal positioning, correlate with major histocompatibility complex class I promoter activity in different tissues in vivo. Mol Cell Biol. 2008;28:7323–7336. doi: 10.1128/MCB.00889-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotova E, Lodhi N, Jarnik M, Pinnola AD, Ji Y, Tulin AV. Drosophila histone H2A variant (H2Av) controls poly(ADP-ribose) polymerase 1 (PARP1) activation in chromatin. Proc Natl Acad Sci USA. 2011;108:6205–6210. doi: 10.1073/pnas.1019644108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M, Jaffe JD, Koche RP, Rheinbay E, Endoh M, Koseki H, Carr SA, Bernstein BE. H2A.Z landscapes and dual modifications in pluripotent and multipotent stem cells underlie complex genome regulatory functions. Genome Biol. 2012;13:R85. doi: 10.1186/gb-2012-13-10-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KC, Mühlpfordt F, Vaquerizas JM, Raja SJ, Holz H, Luscombe NM, Manke T, Akhtar A. The NSL complex regulates house-keeping genes in Drosophila. PLoS Genet. 2012;8:e1002736. doi: 10.1371/journal.pgen.1002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach TJ, Mazzeo M, Chotkowski HL, Madigan JP, Wotring MG, Glaser RL. Histone H2A.Z is widely but nonrandomly distributed in chromosomes of Drosophila melanogaster. J Biol Chem. 2000;275:23267–23272. doi: 10.1074/jbc.M910206199. [DOI] [PubMed] [Google Scholar]
- Marques M, Laflamme L, Gervais AL, Gaudreau L. Reconciling the positive and negative roles of histone H2A.Z in gene transcription. Epigenetics. 2010;5:267–272. doi: 10.4161/epi.5.4.11520. [DOI] [PubMed] [Google Scholar]
- Mavrich TN, Jiang C, Ioshikhes IP, Li X, Venters BJ, Zanton SJ, Tom-sho LP, Qi J, Glaser RL, Schuster SC, et al. Nucleosome organization in the Drosophila genome. Nature. 2008;453:358–362. doi: 10.1038/nature06929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- modENCODE Consortium. Roy S, Ernst J, Kharchenko PV, Kheradpour P, Negre N, Eaton ML, Landolin JM, Bristow CA, Ma L, et al. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science. 2010;330:1787–1797. doi: 10.1126/science.1198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E, Sugino K, Kula E, Okazaki E, Tachibana T, Nelson S, Rosbash M. Dissecting differential gene expression within the circadian neuronal circuit of Drosophila. Nat Neurosci. 2010;13:60–68. doi: 10.1038/nn.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashun B, Yukawa M, Liu H, Akiyama T, Aoki F. Changes in the nuclear deposition of histone H2A variants during pre-implantation development in mice. Development. 2010;137:3785–3794. doi: 10.1242/dev.051805. [DOI] [PubMed] [Google Scholar]
- Nègre N, Brown CD, Shah PK, Kheradpour P, Morrison CA, Henikoff JG, Feng X, Ahmad K, Russell S, White RAH, et al. A Comprehensive Map of Insulator Elements for the Drosophila Genome. PLoS Genet. 2010;6:e1000814. doi: 10.1371/journal.pgen.1000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol JW, Helt GA, Blanchard SG, Jr, Raja A, Loraine AE. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25:2730–2731. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Dou Y. H2A.Z sets the stage in ESCs. Cell Stem Cell. 2013;12:143–144. doi: 10.1016/j.stem.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo T-TB, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuro-anatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujadas E, Feinberg AP. Regulated noise in the epigenetic landscape of development and disease. Cell. 2012;148:1123–1131. doi: 10.1016/j.cell.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rach EA, Winter DR, Benjamin AM, Corcoran DL, Ni T, Zhu J, Ohler U. Transcription initiation patterns indicate divergent strategies for gene regulation at the chromatin level. PLoS Genet. 2011;7:e1001274. doi: 10.1371/journal.pgen.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, Auld VJ. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev Biol. 2001;238:47–63. doi: 10.1006/dbio.2001.0411. [DOI] [PubMed] [Google Scholar]
- Steiner FA, Talbert PB, Kasinathan S, Deal RB, Henikoff S. Cell-type-specific nuclei purification from whole animals for genome-wide expression and chromatin profiling. Genome Res. 2012;22:766–777. doi: 10.1101/gr.131748.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan J, Baxter EM, Corces VG. The role of histone H2Av variant replacement and histone H4 acetylation in the establishment of Drosophila heterochromatin. Genes Dev. 2005;19:65–76. doi: 10.1101/gad.1259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Henikoff S. Histone variants—ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol. 2010;11:264–275. doi: 10.1038/nrm2861. [DOI] [PubMed] [Google Scholar]
- Talbert PB, Ahmad K, Almouzni G, Ausió J, Berger F, Bhalla PL, Bonner WM, Cande WZ, Chadwick BP, Chan SW-L, et al. A unified phylogeny-based nomenclature for histone variants. Epigenetics Chromatin. 2012;5:7. doi: 10.1186/1756-8935-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weake VM, Dyer JO, Seidel C, Box A, Swanson SK, Peak A, Florens L, Washburn MP, Abmayr SM, Workman JL. Post-transcription initiation function of the ubiquitous SAGA complex in tissue-specific gene activation. Genes Dev. 2011;25:1499–1509. doi: 10.1101/gad.2046211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CC, Hurst LD. Support for multiple classes of local expression clusters in Drosophila melanogaster, but no evidence for gene order conservation. Genome Biol. 2011;12:R23. doi: 10.1186/gb-2011-12-3-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CM, Henikoff JG, Henikoff S. H2A.Z nucleosomes enriched over active genes are homotypic. Nat Struct Mol Biol. 2010;17:1500–1507. doi: 10.1038/nsmb.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle CM, McClinic KN, Ercan S, Zhang X, Green RD, Kelly WG, Lieb JD. The genomic distribution and function of histone variant HTZ-1 during C. elegans embryogenesis. PLoS Genet. 2008;4:e1000187. doi: 10.1371/journal.pgen.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher M, Emerson BM. Epigenetic silencing of the p16(INK4a) tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol Cell. 2009;34:271–284. doi: 10.1016/j.molcel.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Handoko L, Wei X, Ye C, Sheng J, Wei CL, Lin F, Sung WK. A signal-noise model for significance analysis of ChIP-seq with negative control. Bioinformatics. 2010;26:1199–1204. doi: 10.1093/bioinformatics/btq128. [DOI] [PubMed] [Google Scholar]
- Yang J, Corces VG. Chromatin insulators: a role in nuclear organization and gene expression. Adv Cancer Res. 2011;110:43–76. doi: 10.1016/B978-0-12-386469-7.00003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Munson KM, Webb WW, Lis JT. Dynamics of heat shock factor association with native gene loci in living cells. Nature. 2006;442:1050–1053. doi: 10.1038/nature05025. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Pugh BF. High-resolution genome-wide mapping of the primary structure of chromatin. Cell. 2011;144:175–186. doi: 10.1016/j.cell.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.