Abstract
Synthetic biodegradable elastomers are an emerging class of materials that play a critical role in supporting innovations in bioabsorbable medical implants. This work describes the synthesis and characterization of poly (glycerol-co-sebacate)-cinnamate (PGS-CinA), a biodegradable elastomer based on hyperbranched polyesters derivatized with pendant cinnamate groups. PGS-CinA can be prepared via photodimerization in the absence of photoinitiator using monomers that are found in common foods. The resulting network exhibits a Young’s modulus of 50.5 to 152.1 kPa and a projected in vitro degradation half-life time between 90 and 140 days. PGS-CinA elastomers are intrinsically cell adherent and support rapid proliferation of fibroblasts. Spreading and proliferation of fibroblasts are loosely governed by the substrate stiffness within the range of Young’s moduli in PGS-CinA networks that were prepared. The thermo-mechanical properties, biodegradability, and intrinsic support of cell attachment and proliferation suggest that PGS-CinA networks abroadly applicable for use in next generation bioabsorable materials including temporary medical devices and scaffolds for soft tissue engineering.
Keywords: polymer, biodegradable, cinnamate, elastomers, photocrosslink
1. Introduction
Biodegradable elastomers are an emerging class of biomaterials that show promise for use in temporary medical implants such as scaffolds and surgical materials [1, 2]. This family of synthetic polymers includes polyester-based materials such as poly(glycerol-co-sebacate) (PGS) [3], poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) (APS) [4], and poly(1,8-octanediol-co-citric acid) (POC) [5]. Other types of biodegradable elastomers include poly(urethane urea) [6], poly(trimethylene carbonate) [7] and their associated co-polymers and derivatives [8]. These polymers are rapidly gaining acceptance as valuable bulk materials in a variety of biomedical applications including surgical meshes [9], controlled release matrices [10, 11], synthetic vascular grafts [12, 13], and scaffolds for peripheral nerve regeneration [14, 15]. The utility of this class of materials in biomedical applications is derived from the combination of mechanical compliance, total macroscopic biodegradation, and subsequent metabolism of non-toxic monomers. Furthermore, a wide range of the aforementioned thermo-mechanical properties and bioabsorption kinetics can be accessed by careful selection of monomer stoichiometry and the conditions during network formation [16–18].
Polyester-based biodegradable elastomers are typically synthesized using polycondensation schemes that use in vacuo environments and high temperatures [3–5]. These processing conditions largely prohibit the inclusion of biological materials such as bioactive proteins or viable pre-seeded cell populations [5]. Room temperature free radical crosslinking of polymers in mild environments is a suitable strategy to obviate restrictive processing conditions. There are several prominent examples of photocrosslinkable elastomers including poly(trimethylene carbonate) [19], poly(octamethylene maleate citrate) [20], and poly(β-amino ester)s [21]. PGS can be modified with pendant acrylate groups to produce poly(glycerol-co-sebacate)-acrylate (PGSA) [22], which can be used as a precursor for photocrosslinkable biodegradable elastomeric networks. This strategy has also been applied to natural and synthetic hydrogels including poly(ethylene glycol) [23] and hyaluronic acid networks [24]. In situ photocrosslinking of diene functionalities presents two potential limitations. First, introducing an exogenous toxic photoinitiator can negatively impact the viability of seeded cell populations [25]. Second, free radical polymerization of pendant dienes produces non-degradable high molecular weight aliphatic backbones, which can retard hydrolytic-mediated degradation [22]. Therefore, alternative photocrosslinking schemes that can address these potential limitations are of interest. Cinnamates are a class of naturally occurring aromatic compounds that are found in some fruits [26]. Cinnamate derivatives undergo reversible photodimerization upon irradiation of ultraviolet light with wavelengths longer than 260 nm [27]. The aforementioned dimerization forms a cyclobutane ring by [2+2] photocycloaddition [28, 29]. The resulting α-truxillic acid derivatives, which are the products of photodimerization of cinnamates, are one kind of naturally occurring compounds [30]. Photocleavage of the cyclobutane ring is possible by irradiation at wavelength less than 260 nm [28]. Derivatizing hyperbranched polyester pre-polymers with pendant cinnamate can therefore produce photocrosslinkable elastomeric networks that are hydrolytically labile, intrinsically cell adherent, and composed of simple monomers.
2. Experimental Section
2.1 Synthesis of Poly(glycerol-co-sebacate)-cinnamate
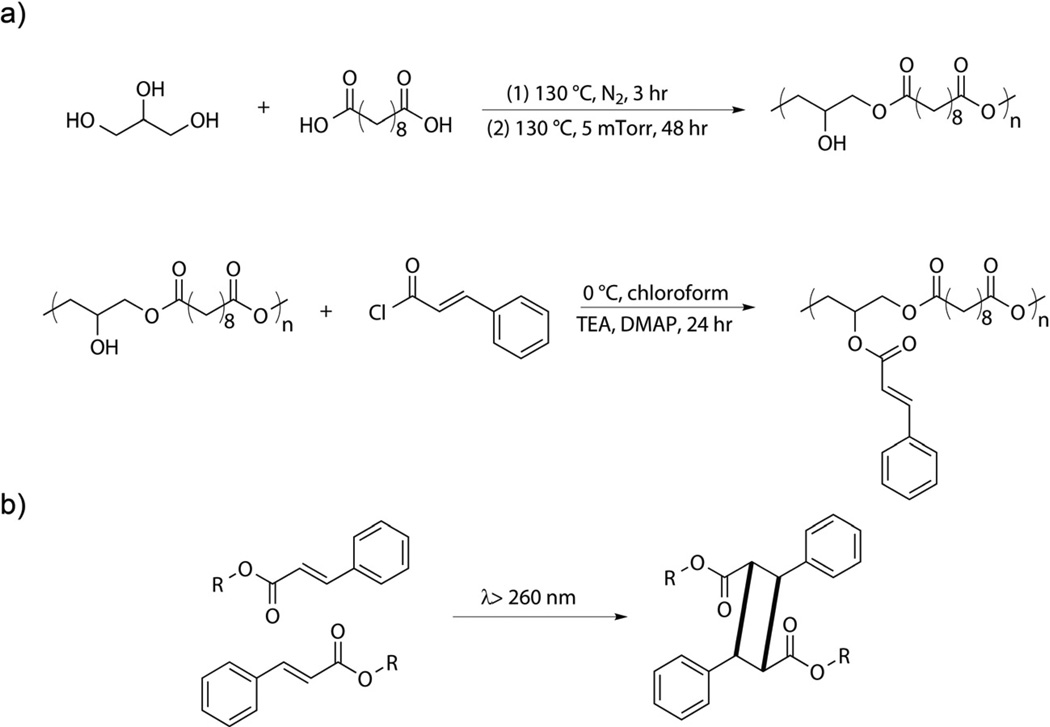
PGS pre-polymer was synthesized according to previously reported method [3]. Briefly, equimolar amounts of sebacic acid and glycerol were combined by melting at 130 °C under a blanket of nitrogen. Polycondensation was accelerated through a gradual reduction in pressure from atmospheric pressure to 50 mTorr over 1 hr. After 48 hr, a transparent viscous product was recovered. PGS-CinA pre-polymer was prepared as follows: 5 g of PGS pre-polymer was dehydrated at 100 °C under vacuum for two hours and dissolved in 30 ml anhydrous chloroform in a three-neck round bottom flask. The solution was purged under nitrogen for three hours before adding 20 mg of 4-dimethylaminopyridine. The reaction mixture was cooled to 0 °C and charged with cinnamoyl chloride (1.62 g, 50% mol/mol on a basis of pendant hydroxyl groups) and an equimolar amount of triethylamine (1.36 ml). The reaction proceeded for 24 hr as the temperature gradually increased from 0 °C to room temperature. Chloroform was removed by rotary evaporation and 30 ml ethyl acetate was added to precipitate the salt, which was removed by filtration. Ethyl acetate was removed by evaporation to produce a viscous, transparent, light yellow product. PGS-CinA pre-polymer was purified by re-crystallization in ethanol.
2.2 Pre-polymer Characterization
The molecular weight distribution of PGS pre-polymer was measured by Gel Permeation Chromatography (Polymer Standards Services, Amherst, MA) using a poly(methyl methacrylate) (PMMA) standard in N,N-dimethylformamide (DMF) as the eluent. 1H nuclear magnetic resonance (Bruker Avance 300 MHz, Billerica, MA) was performed in CDCl3 to determine the degree of substitution (DS) of cinnamate groups on PGS pre-polymer. 1H NMR (300 MHz, CDCl3, δ): 7.7 (1H, d, –CO–CH=), 7.56 (2H, t, Ar–H), 7.4 (3H, t, Ar–H), 6.5 (1H, d, Ar–CH=), 5.2 (1H, m, –CH–O–), 4.2 (4H, d, –CH2–O–), 2.3 (4H, m, –CO–CH2–), 1.6 (4H, m, –CH2–CH2–), 1.32 (8H, m, –CH2–CH2–). Cinnamate incorporation was confirmed by FT-IR (Mattson ATI Affinity 60AR, Madison, WI). Samples used in FT-IR were prepared by drop-casting dilute solution of PGS-CinA (DS35%)/chloroform on zinc selenide substrates (Alfa Aesar, Ward Hill, MA). The intrinsic viscosity of PGS-CinA pre-polymer was measured using an Ubbelohde viscometer at 25 °C. Briefly, solutions of PGS-CinA in DMSO (3.1 to 7.5 g/dL) were filtered and efflux time was measured at least three times.
2.3 Characterization of Photocrosslinked PGS-CinA Networks
PGS-CinA films (DS = 26%, 29%, 35%, and 45%) were prepared using melt-pressed method between two quartz slides with a spacer of 350 µm. Films were exposed to UV light on both sides each for 2 hr using a 600 W UVB lamp (Integrated Dispensing Solution, Agoura Hills, CA). The mass density of DS26% and DS45% films was measured using a 25 ml pycnometer. The sol content (Solcont) of crosslinked PGS-CinA was determined by weighing the mass difference of dry films before (mgel+sol)and after sol extraction (mgel) and calculated using the following equation:
| (1) |
Sol content was measured as a function of UV exposure time to elucidate the behavior of the sol-gel transition in PGS-CinA networks during photocrosslinking. The glass transition temperature (Tg) of crosslinked PGS-CinA sol-free films was measured by Differential Scanning Calorimetry (DSC, TA instrument, Q20, New Castle, DE) at a heating rate of 10 °C min−1. The kinetics of photopolymerzation was measured by UV-vis Spectroscopy (Cary 300, Foster city, CA). Briefly, 30 mg PGS-CinA DS35% pre-polymer was dissolved in 1 ml chloroform and spin coated on quartz slides with the speed of 1500 RPM for 30 seconds followed by solvent evaporation under vacuum for two hours. UV-vis spectra were recorded after exposure to UVB lamp for various times. Photocrosslinked PGS-CinA elastomers were cut into rectangular geometries with a length-width ratio 2:1 for macroscopic mechanical characterization. Tensile tests (n = 4) were conducted (Instron 5943 equipped with Bluehill 3 software, Norwood, MA) using a 10 N load cell. A strain rate of 1 mm min−1 was applied to the sample until failure. The Young’s modulus was calculated using the stress-strain slope until 5% of strain.
2.4 Replica Molding of PGS-CinA
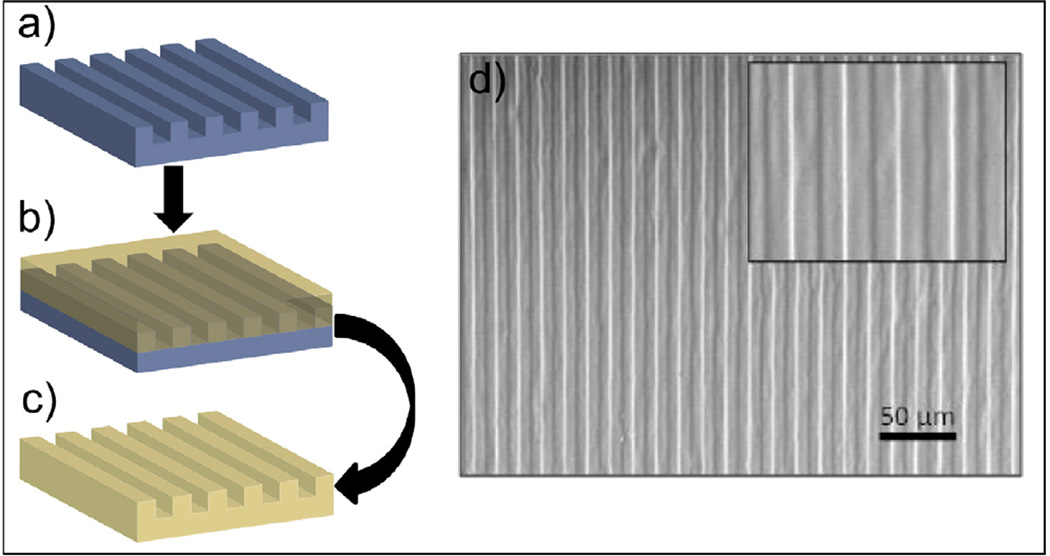
Replica-molding of PGS-CinA was demonstrated through photopolymerization of PGS-CinA on microfabricated substrates using a method analogous to soft lithography. Briefly, negative molds composed of silicon were fabricated as previously described [31]. PGS-CinA pre-polymer (DS45%) was applied to the microfabricated silicon substrate and pressed to form a film 100 µm in thickness. Films were exposed to UVB light for two hours and carefully delaminated by mild thermal expansion. The fidelity of pattern transfer in PGS-CinA films was verified via scanning electron microscope (SEM, Philips XL30).
2.5 In vitro Degradation and Cell Adhesion
PGS-CinA elastomers (DS26%, DS29%, DS35%, DS45%, n = 4) were cut into disks with the diameter of 9.5 mm and thickness of 350 µm. Sol-free samples were incubated at 37 °C in 20 ml 3 M sodium acetate solution with pH equal to 5.2 with medium exchange after every 48 hr. After degradation samples were first washed with deionized water and soaked in ethanol for 12 hr then equilibrated in DI water for 12 hr. Hydrated mass was measured after gently removing excess water. Samples were allowed to dry for 6 hr before the dry mass was recorded.
Elastomeric disks (n = 6) 5 mm in diameter prepared from DS26% and DS45% pre-polymer formulations were incubated in ethanol for 12 hr to remove sol followed by rinsing in DI water for 12 hr for equilibration. PGS-CinA substrates were transferred to 96-well plates and disinfected using a 100 W Black Ray UVA lamp for one hour. NIH/3T3 fibroblast cells (ATCC, Manassas, VA) were seeded on elastomers at the density of 65 000 cells/cm2 using tissue culture polystyrene (TCPS) as a control. Cell metabolism was measured using a tetrazolium salt WST-1 assay (Roche Applied Science, Indianapolis, IN) which can be cleaved to a soluble formazan by viable metabolically active cells. Briefly, after 1, 2, 3, 5, and 7 days of cell incubation, 15 µl WST-1 reagent was added to each well with 150 µl growth medium (Dulbecco's Modified Eagle's Medium with 10% concentration of bovine calf serum) and incubated for 4 hr at 37 °C in a 5% CO2 atmosphere. Absorbance measurements were made using 100 µl solution at 450 nm (VICTOR Multilabel Plate Reader, PerkinElmer, Waltham, MA). Cell viability was measured using the Live/Dead Viability Assay (Invitrogen, Grand Island, NY). Briefly, fibroblast monolayers were stained by adding 1 ml calcein AM and 1 µl EthD-1 directly to cells and incubated at 37 °C 5% CO2 for 20 minutes. Cells were imaged using fluorescent microscopy (TIE, Nikon, Melville, NY). Cell spreading was assessed by measuring projected surface area across at least three different images (n > 100 cells) using NIH ImageJ software.
3. Results and Discussion
3.1 Pre-polymer Characterization
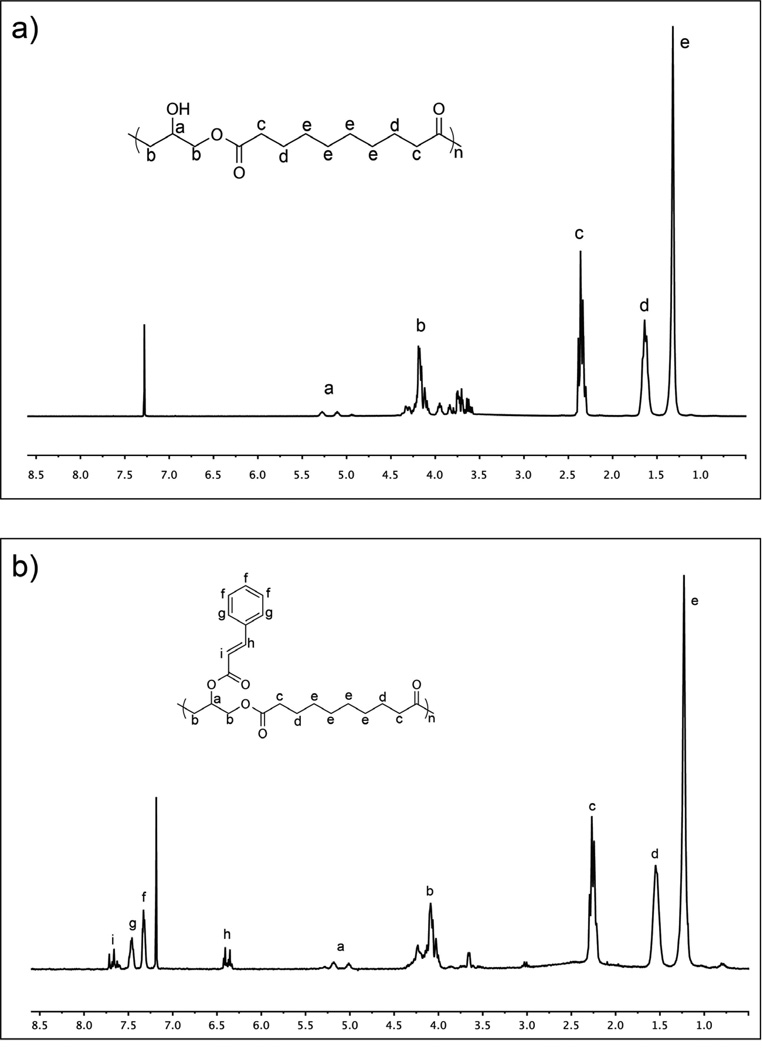
Step growth polymerization between sebacic acid and glycerol yields a hyperbranched PGS pre-polymer with number average molecular weight (M̅n) of 3900 g/mol and a polydispensity index of 1.93, as measured by GPC. This measurement is expected to serve as a lower bound due to the hyperbranched nature of PGS relative to linear PMMA standards. Polycondensation reactions of PGS pre-polymer are terminated at times significantly prior to the anticipated gel point. This strategy produces a low molecular weight pre-polymer and also preserves a high population of free hydroxyl groups available for subsequent cinnamate modifications. Furthermore, hyperbranched PGS pre-polymer will likely reduce the extent of intramolecular overlap in cinnamate reaction cages compared to pendant functional groups prepared in linear pre-polymers. This preferred topology will promote intermolecular dimerization and subsequent formation of a crosslinked elastomeric network as opposed to consumption of pendant groups through intramolecular dimerization, which will not contribute significantly to stable network formation. The functionalization of PGS pre-polymer with cinnamoyl chloride to yield PGS-CinA pre-polymers (Scheme 1a) is evident by comparing the 1H NMR spectra of the reactants and products (Figure 1). Additional peaks at δ 7.7, 7.56, 7.4 and 6.5 ppm identified in the PGS-CinA spectrum (Figure 1b) indicate the presence of pendant cinnamate groups. The degree of substitution is measured by comparing the integrals of the peaks at δ 1.32 ppm (position “e”, methylene groups on sebacic acid chain) and δ 7.7 ppm (position “i”, vinylene groups on cinnamate group) of 1H NMR spectra. The calculated DS of PGS-CinA pre-polymers ranges from 26% to 45%. Gelation was observed occasionally during the synthesis of high DS PGS-CinA pre-polymer, which may be attributed to hydrophobic interactions in pendant cinnamate groups. Higher DS can be achieved by performing multiple modification procedures in series. The intrinsic viscosity of PGS-CinA pre-polymer was measured to be 0.058 dL/g.
Scheme 1.
(a) Synthesis of poly(glycerol-co-sebacate)-cinnamate. PGS pre-polymer is synthesized by polycondensation of glycerol and sebacic acid. Functionalization of PGS pre-polymer with cinnamoyl chloride yields PGS-CinA pre-polymer. (b) Photodimerization of pendant cinnamate functional groups proceeds upon irradiation with medium wave UV.
Figure 1.
1H NMR spectrum of (a) PGS; (b) PGS-CinA pre-polymer. The presence of pendant cinnamate group is clear from peaks at 7.7, 7.56, 7.4 and 6.5 ppm (see text). The degree of substitution is calculated by comparing the integrals of the methylene groups peak at 1.32 ppm and the vinylene groups peak at 7.7 ppm.
3.2 Photocrosslinking of PGS-CinA Elastomers
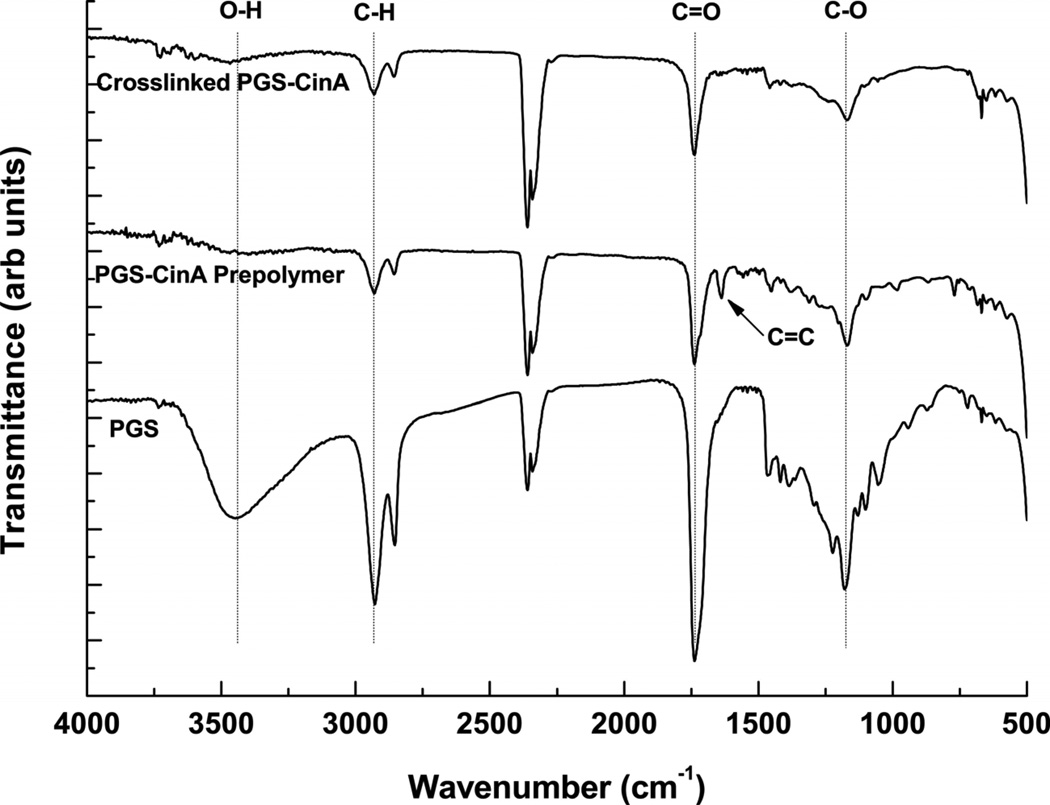
The incorporation of pendant cinnamate molecules produces a macromolecular precursor that can be processed into polyester-based elastomer via initiator-free photocrosslinking. Incorporating pendant cinnamates into hyperbranched polyesters can leverage a [2+2] photocrosslinking mechanism that can proceed without the addition of secondary aliphatic networks. These groups are essential for creating crosslinked polyester networks and conferring elastomeric properties upon these polymers. Furthermore, cinnamic acid and the photocrosslinked dimer (α-truxillic acid) are naturally occurring bioactive compounds [32]. Photodimerization of cinnamate moieties proceeds through excitation of π electrons to antibonding molecular oribitals that ultimately form a cyclobutane ring between two adjacent molecules [33] (Scheme 1b). This mechanism in PGS-CinA networks is supported by FT-IR (Figure 2). The FT-IR spectrum of PGS pre-polymer reveals a broad peak at 3423 cm−1 that corresponds to the H-bonded hydroxyl group [34]. This prominent feature is attenuated via cinnamate modification as shown in the PGS-CinA spectrum. Additionally, the absorbance peak at 1637 cm−1, which corresponds to the vinylene C=C stretching vibration [35], is present in the PGS-CinA pre-polymer spectrum and is abolished upon preparation of photocrosslinked PGS-CinA networks. These data indicate that C=C bonds on the cinnamate groups form crosslinks via the formation of cyclobutane rings.
Figure 2.
FT-IR spectra of photocrosslinked PGS-CinA, PGS-CinA pre-polymer (DS = 35%) and PGS pre-polymer. The absorbance peak at 3423 cm−1 that is associated with hydrogen-bonded hydroxyl groups is attenuated via cinnamate modification of PGS pre-polymer. The vinylene C=C stretching peak at 1637 cm−1 is abolished after the photodimerization of PGS-CinA pre-polymer into the final crosslinked network.
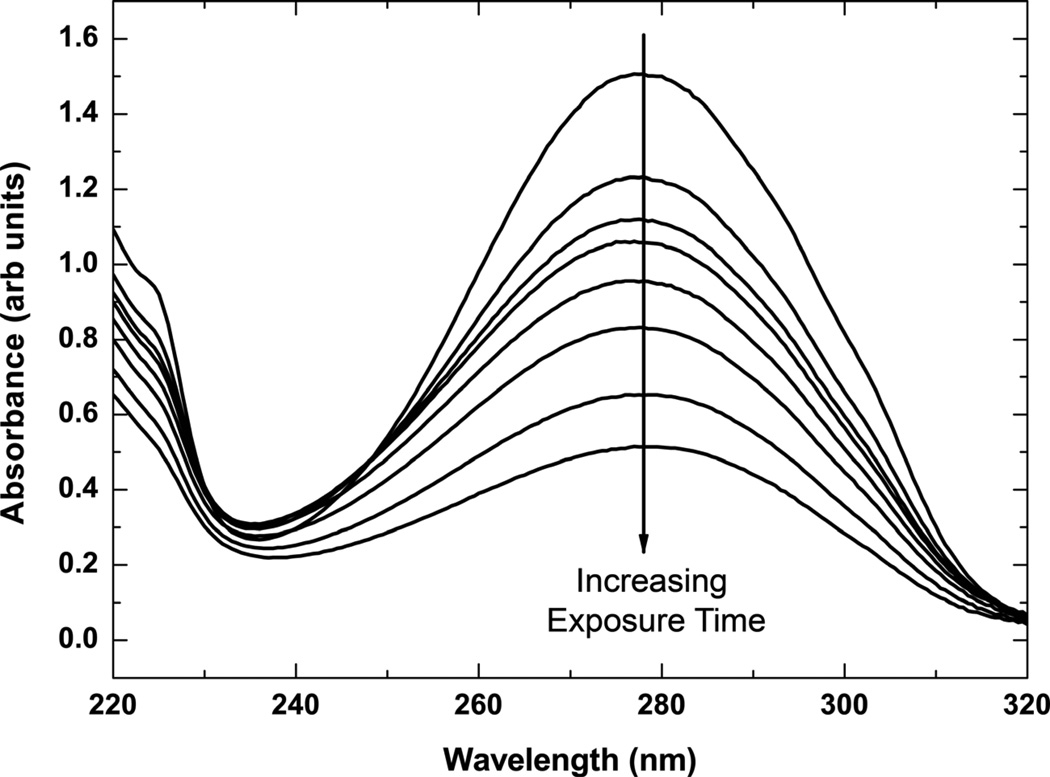
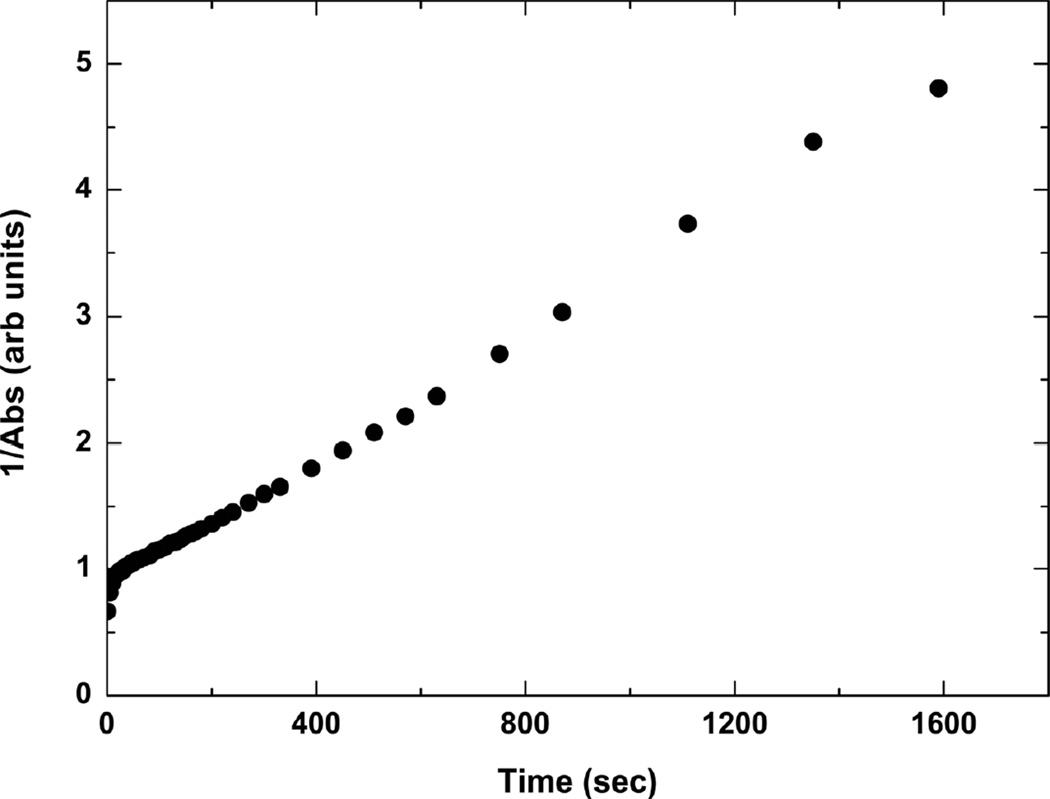
The UV-vis spectra associated with kinetics of cinnamate photocrosslinking in PGS-CinA thin films reveal a strong absorbance peak at 273 nm (Figure 3). The kinetics of photodimerization can be monitored by measuring the peak absorption at this wavelength. The intensity at λ = 273 nm decreases by 90% upon crosslinking via [2+2] photodimerization after 30 min of UV irradiation. The time scale for absorbance consists of two distinct regimes (Figure 4). The initial phase consists of a rapid decrease in absorbance that lasts for several seconds and represents cis-trans isomerizaton [36]. The secondary phase is associated with the solid state photodimerization reaction in PGS-CinA [36], which obeys second order reaction kinetics [37]. The experimental kinetics are accurately described by the following second rate equation in which the absorbance A has a rate constant of k = 2.5 msec−1 and an initial value of A0 = 0.876.
| (2) |
Figure 3.
UV absorbance spectra of PGS-CinA thin film. Cinnamate groups in thin films of PGS-CinA (DS35%) reveal a strong absorbance peak at 273 nm. The intensity of this peak decreases upon crosslinking via [2+2] photodimerization under UV irradiation. The intensity decreases 90% from the initial state after 30 minutes of irradiation.
Figure 4.
Kinetics of photodimerization in PGS-CinA thin films. Photodimerization of PGS-CinA (DS35%) proceeds in two distinct phases. The initial phase represents cis-trans isomerization and the secondary phase is associated with the solid state photodimerization which obeys second order reaction kinetics.
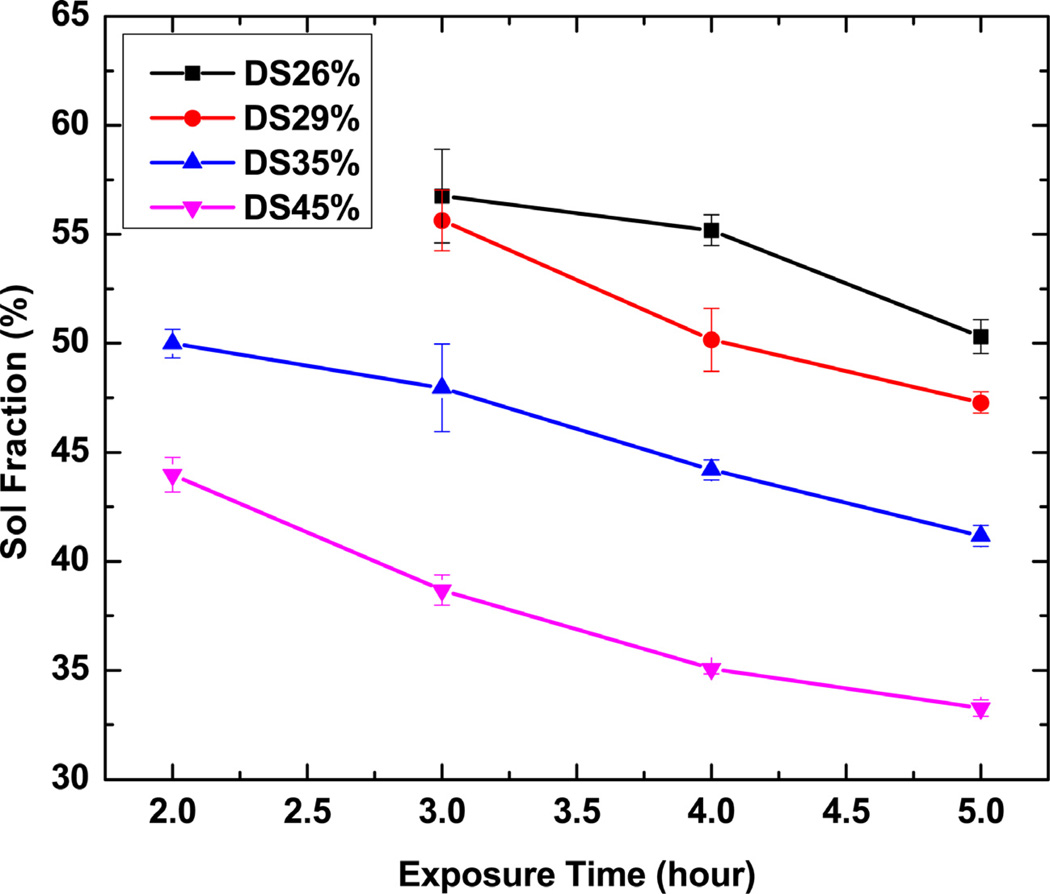
The photocrosslinking kinetics was also studied by monitoring the sol-gel content of PGS-CinA films over time. Figure 5 shows that there is a continuous decrease of sol fractions over exposure 10 time for crosslinked PGS-CinA films. Networks formed from pre-polymers with a higher DS exhibit less sol content than polymers of low DS for the same amount of exposure time. The sol fractions of polymers after 4 hours of crosslinking decrease from 55% to 35% by increasing the DS from 26% to 45%, indicating the increasing of crosslinks between polymer chains. The amount of incorporated cinnamate functional groups dominates polymers’ intrinsic molecular structures, resulting in significant differences in thermal, mechanical, and degradation properties. The formation of PGS-CinA networks via mild photo-polymerizaton conditions enables the rapid fabrication of micron-scale features on unmodified silicon substrates by a modified replica-molding process (Figure 6). The grating features have a line width and line spacing of 7 µm each. The fidelity of the features is confirmed through SEM microscopy (Figure 6d). Efficient replica molding is aided by the robust mechanical properties of photocrosslinked PGS-CinA networks [38]. Replica-molded PGS-CinA films may serve as the basis for biodegradable microdevices or topographically-rich cell-culture substrates.
Figure 5.
Sol fractions as a function of exposure time for PGS-CinA networks. The sol contents decrease gradually over time under UV irradiation. The degrees of substitution by cinnamate functional groups significantly affect polymers’ sol contents and furthermore crosslink density at given length of exposure time.
Figure 6.
Replica-molding of PGS-CinA films. (a) Patterned silicon wafers are used as molds during photocrosslinking of PGS-CinA (DS45%) pre-polymers. (c) Photocrosslinked PGS-CinA networks demonstrate a high degree of feature fidelity (d) as assessed by SEM.
3.3 Thermal and Mechanical Properties of Photocrosslinked PGS-CinA Films
A relatively broad range of thermo-mechanical properties can be accessed through subtle alterations in the DS of PGS-CinA pre-polymer (Table 1). The Tg ranges from −24.1 to −15.5 °C suggesting that all PGS-CinA networks characterized in this study are rubbery at room temperature. DSC results show that networks formed PGS-CinA pre-polymer with DS = 45% exhibit the highest Tg. Under identical crosslinking conditions, polymers with a higher DS produce networks with a higher crosslink density and therefore a higher Tg. In general, the observed Tg of networks prepared from PGS-CinA are higher than Tg produced from comparable pre-polymers composed of aliphatic polyesters such as PGS or PGSA [22, 39]. This is likely due to the presence of bulky aromatic groups that elevate the Tg via reduced bond rotation [40].
Table 1.
Thermal and mechanical properties of photocrosslinked PGS-CinA
| Abbreviation | Degree of Substitutiona (DS) |
Young’s Modulus, E [kPa] |
Crosslink Density, n [mol m−3] |
Glass Transition Temperature, Tg [°C] |
|---|---|---|---|---|
| DS26% | 0.26 | 50.48 ± 3.72 | 6.79 ± 0.5 | −24.1 |
| DS29% | 0.29 | 76.62 ± 4.6 | 10.31 ± 0.62 | −21.4 |
| DS35% | 0.35 | 118.5 ± 18.76 | 15.94 ± 2.53 | −20.1 |
| DS45% | 0.45 | 152.1 ± 8.2 | 20.46 ± 1.1 | −15.5 |
Degree of substitution calculated on a per sebacic acid monomer basis.
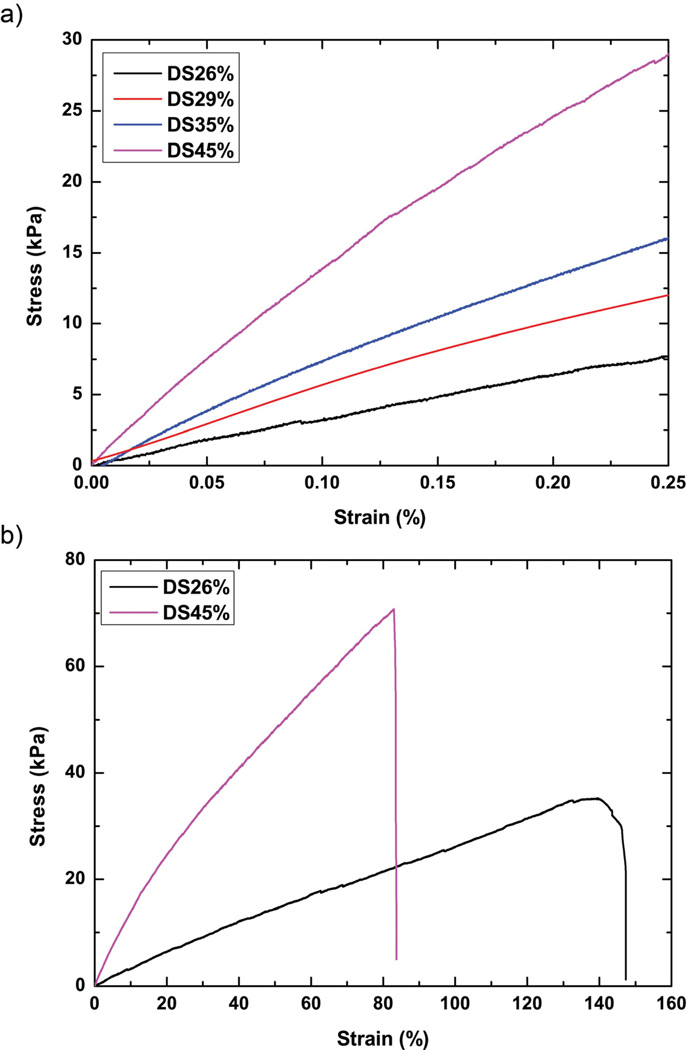
Photocrosslinked PGS-CinA networks are tough, elastomeric and capable of high strains at break (Figure 7). The tensile modulus of dehydrated PGS-CinA films ranges from 50.5 ± 3.7 kPa to 152.1 ± 8.2 kPa while the ultimate tensile strain ranges from 80% to 140%. The mechanical properties are strongly dependent upon the DS as it is elevated from 26% to 45%. The volumetric crosslink density is calculated using the equation where E is the tensile modulus, R is the gas constant and T is the temperature (Table 1) [41].
| (3) |
Figure 7.
Stress-strain curves for PGS-CinA elastomers. (a) The tensile modulus of PGS-CinA films ranges from 50.5 ± 3.7 kPa to 152.1 ± 8.2 kPa. (b) The ultimate tensile strain ranges from 80% to 140%. Both elastic modulus and ultimate tensile strain are affected by the degree of substitution in PGS-CinA pre-polymers.
The range of tensile modulus achieved using PGS-CinA is notable because of the potential impact on directing intrinsic cellular function including adhesion, spreading, and differentiation. For example, recent advancements in cell mechanics have shown that the substrate stiffness can assume a potent cue for directing stem cell differentiation [42]. The range of elasticity that is accessible with PGS-CinA can map to cell phenotypes ranging from muscle (~10 kPa) [43] to cardiac cells (~100 kPa) [44]. The range of mechanical properties and intrinsic ability to promote cell adhesion suggest that PGS-CinA could serve as a suitable material platform for studying cell mechanics in a wide range of phenotypes and lineages. The kinetics of [2+2] photodimerization in PGS-CinA is relatively slow compared to free radical propagation thereby allowing for more precise tuning of mechanical properties compared to free radical polymerization of pre-polymers with pendant dienes. Furthermore, photocrosslinked PGS-CinA networks are capable of being sterilized by autoclaving. This process does not significantly impact the mechanical integrity or macroscopic properties of the crosslinked network. A typical autoclave process increases the Young’s modulus of PGS-CinA networks by 3.25 ± 0.08%. These data suggest that the elevated temperatures may promote additional crosslinking through polycondensation reactions.
3.4 In Vitro Degradation
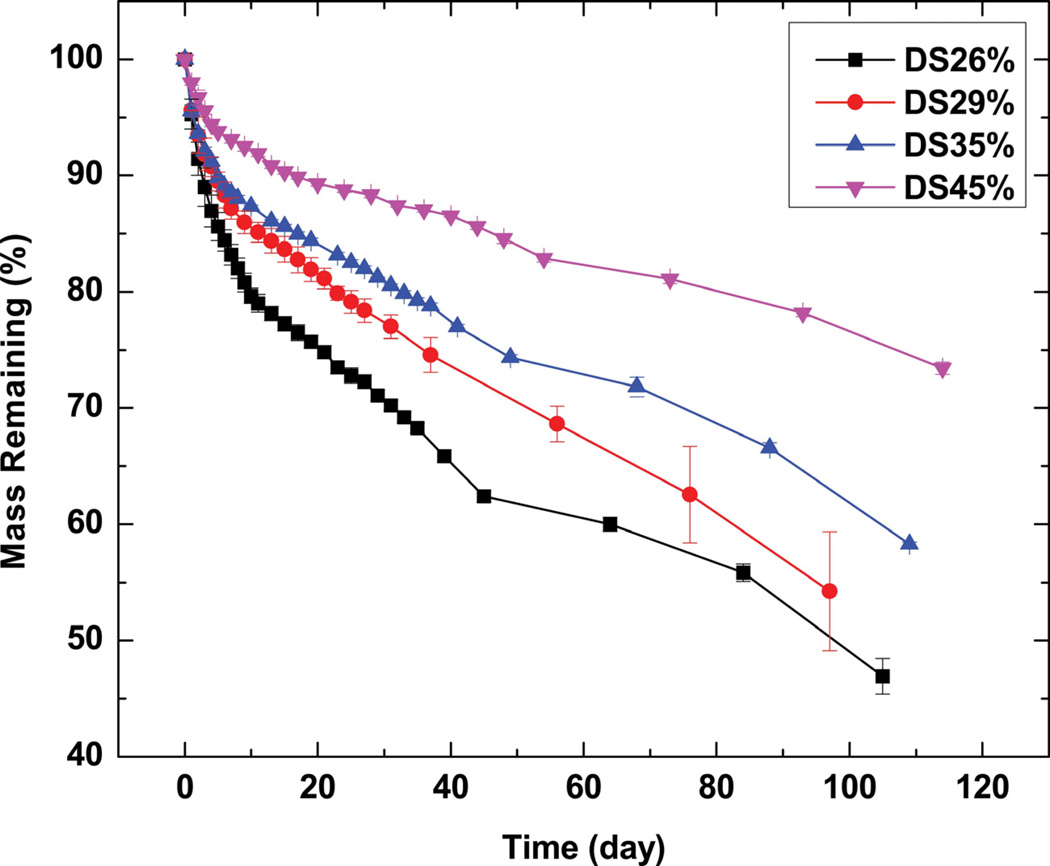
The measured sol content of crosslinked PGS-CinA varies from 31% to 49% depending on the DS. The relatively high sol content compared to previous studies [17, 22] can be attributed to the crosslinking mechanism. Crosslinking by photodimerization does not permit the formation of high molecular weight backbones. Furthermore, increasing the DS in hyperbranched networks may result in unfavorable intramolecular photodimerization as opposed to the preferred intermolecular coupling [45]. Therefore, increasing the DS of PGS-CinA pre-polymers produces networks with only modestly higher crosslink densities and gel content compared to networks formed from PGS-CinA pre-polymers with low DS. The crosslink density of the network level also affects the in vitro degradation rate (Figure 8). Networks formed from low DS PGS-CinA (DS26%) exhibit the most rapid degradation rate, losing 50% of mass after 90 days. Conversely, sol-free networks prepared from PGS-CinA (DS45%) lose only 20% of mass by that time. Degradation experiments were conducted for more than 90 days for all samples. This time course induced mass losses of up to 50% for polymer compositions with the lowest DS. It is difficult to assess degradation rate in this class of materials for mass losses higher than 80% because of the loss of mechanical integrity. The stabilized degradation rate can be used to project full mass loss at time points between 180 and 280 days depending on the DS. The degradation rate is anticipated to be much faster in vivo compared to in vitro because of the presence of reactive oxygen species and enzymatic species [46, 47]. The degradation of PGS-CinA is expected to proceed via bulk erosion owing to the hygroscopic nature and high density of hydrophilic ester bonds. The relatively high ratio of sol also suggests that there may be a secondary macroscopic network that permits rapid water diffusion and thus biases the erosion number to values much less than unity (Figure 8b) [48]. The degradation experiments were conducted in 3 M sodium acetate solution, which has a pH of 5.2. The local pH of materials encapsulated in fibrous capsules is known to be acidic and can have a pH as low as 5 due to the hypoxic environment [17, 49]. One of the potential applications of PGS-CinA networks is as an implantable material for use as scaffold or drug delivery matrix. Therefore, the in vitro degradation buffer best simulates the local pH of the eventual microenvironment that PGS-CinA is expected to encounter in vivo.
Figure 8.
In vitro degradation kinetics of PGS-CinA elastomers in sodium acetate solution (3M, pH5.2). The rate of in vitro degradation in PGS-CinA elastomers is inversely proportional to the degree of substitution in PGS-CinA pre-polymers used during network formation. PGS-CinA is expected to undergo bulk erosion due to the high degree of hydration and the relatively slow hydrolysis of polyester bonds.
3.5 In vitro Cell Adhesion and Proliferation
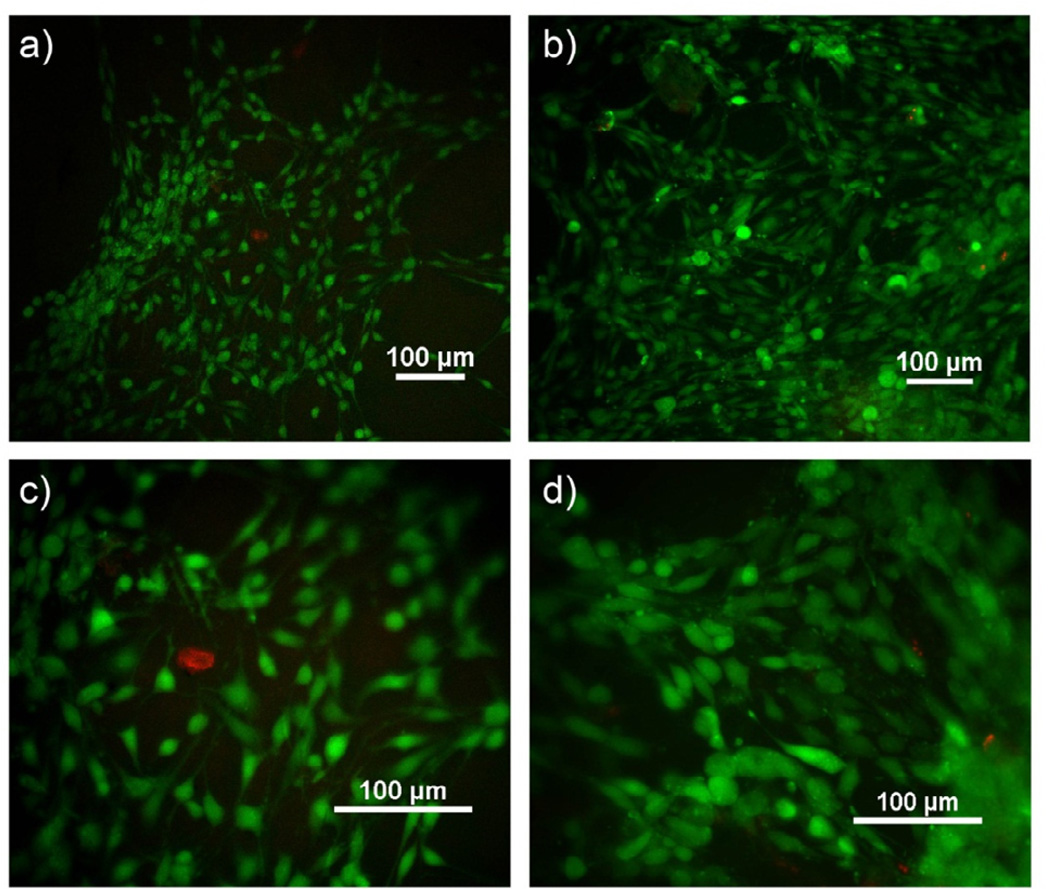
Cell culture substrates prepared from pristine sol-free PGS-CinA networks support the spontaneous attachment and expansion of NIH 3T3 fibroblasts in typical cell culture conditions (Figure 9). No additional bioactive components are required to promote cell adhesion. Fibroblasts maintain excellent viability on PGS-CinA substrates prepared from pre-polymers with DS equal to 26% and 45% after 4 days of culture, as determined by live/dead assay (Figure 9). The fraction of viable cells cultured on PGS-CinA substrates is greater than 99% as assessed by live/dead staining. Fibroblasts cultured on stiffer substrates (DS45%) exhibit a higher degree of spreading compared to fibroblasts cultured on softer substrates (DS26%). The projected area of fibroblasts cultured on PGS-CinA substrates composed of DS26% and DS45% is 182.6 ± 49.6 µm2 and 404.7 ± 75.8 µm2, respectively (***p < 0.001). The projected area of fibroblasts cultured on TCPS control samples is 1405 ± 333 µm2. The observed dependence of spreading with substrate stiffness in PGS-CinA and TCPS substrates is consistent with previous reports [50].
Figure 9.
Live/Dead assay florescent images of fibroblast cells on substrates of PGS-CinA elastomers after 4 days. (a, c) DS26%, (b, d) DS45%. Fibroblasts cultured on PGS-CinA substrates with a higher elastic modulus exhibit a higher degree of spreading as measured by projected surface area (see text).
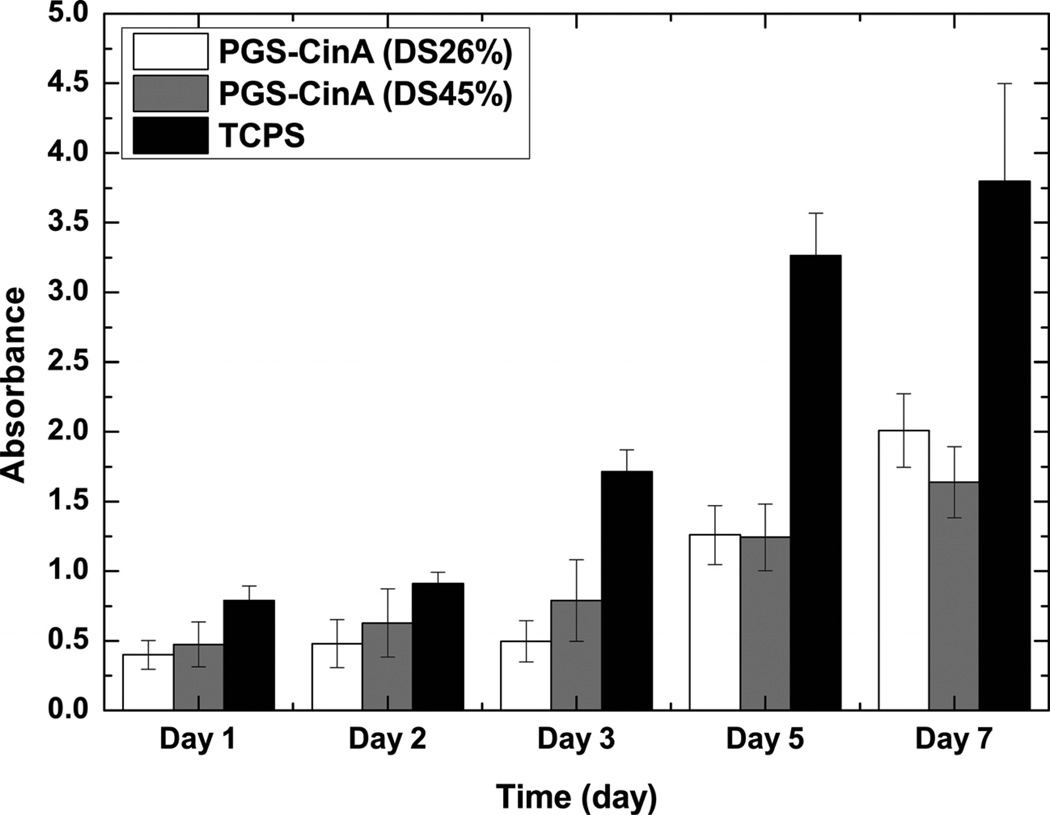
PGS-CinA substrates support fibroblast proliferation as inferred through increased metabolic activity measured by WST-1 assay (Figure 10). Measuring DNA content could lead to potentially erroneous or misleading measurements due to excess DNA arising from non-adherent cells. This is problematic due to the nanoporous and hygroscopic nature of crosslinked PGS-CinA networks with high sol content. Hence, metabolic assays can most accurately assess cell adherence and overall viability. These data are consistent with fluorescence microscopy images (Figure 9). The subtle differences in metabolic activity of fibroblasts cultures on networks prepared from different degrees of substitution in PGS-CinA are dwarfed by the significant increases in WST-1 activity in cells cultures on TCPS substrates. The observed increase in proliferation in fibroblasts cultured on TCPS substrates is likely attributed to the significantly higher stiffness (EPolystyrene ~ 3.2 GPa) [51].
Figure 10.
WST-1 absorbance of fibroblast cells after 1, 2, 3, 5, and 7 days of culture on substrates composed of PGS-CinA elastomers. Both PGS-CinA elastomers (DS26% and DS45%) and TCPS controls support the attachment and rapid fibroblast proliferation as measured by increased metabolic activity.
4. Conclusion
Hyperbranched polyesters modified with pendant cinnamate groups can be processed into biodegradable elastomeric networks by UV photocrosslinking under mild conditions in the absence of photoinitiators. A broad range of thermo-mechanical properties in PGS-CinA networks can be accessed by altering the density of cinnamate moieties. Elastomeric mechanical properties and mild photopolymerization schemes suggest that PGS-CinA polyesters are bulk materials that are suitable for microfabrication techniques such as replica molding. PGS-CinA networks are amenable to biodegradation via hydrolysis and exhibit rapid adhesion and proliferation of fibroblasts without the need for additional surface modification strategies. The relatively long exposure to UV during photocrosslinking may limit the direct incorporation of bioactive species such as viable cells and functional proteins into the network. However, the presence of pendant cinnamate groups may permit spatially localized covalent conjugation of appropriately functionalized small molecule therapeutics or peptides. Taken together PGS-CinA networks are a class of elastomeric biomaterials that is appropriate for a broad range of biomedical applications including scaffolds for regeneration of soft tissues, matrices for drug delivery, and bulk materials for bioabsorbable medical implants.
Supplementary Material
Acknowledgements
Funding provided by the following organizations: the Berkman Foundation; the American Chemical Society Petroleum Research Fund (ACS PRF #51980-DNI7); the Proctor & Gamble Education Grant Program; and the Carnegie Mellon University School of Engineering. NMR instrumentation at CMU was partially supported by National Science Foundation (CHE-0130903 and CHE-1039870).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bettinger CJ. Biodegradable Elastomers for Tissue Engineering and Cell–Biomaterial Interactions. Macromol Biosci. 2011;11:467–482. doi: 10.1002/mabi.201000397. [DOI] [PubMed] [Google Scholar]
- 2.Kang Y, Yang J, Khan S, Anissian L, Ameer GA. A new biodegradable polyester elastomer for cartilage tissue engineering. Journal of Biomedical Materials Research Part A. 2006;77A:331–339. doi: 10.1002/jbm.a.30607. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotech. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 4.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer RS. Amino alcohol-based degradable poly(ester amide) elastomers. Biomaterials. 2008;29:2315–2325. doi: 10.1016/j.biomaterials.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Webb AR, Ameer GA. Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Advanced Materials. 2004;16:511–516. [Google Scholar]
- 6.Guan J, Wagner WR. Synthesis, Characterization and Cytocompatibility of Polyurethaneurea Elastomers with Designed Elastase Sensitivity. Biomacromolecules. 2005;6:2833–2842. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timbart L, Tse MY, Pang SC, Babasola O, Amsden BG. Low Viscosity Poly(trimethylene carbonate) for Localized Drug Delivery: Rheological Properties and in vivo Degradation. Macromol Biosci. 2009;9:786–794. doi: 10.1002/mabi.200800318. [DOI] [PubMed] [Google Scholar]
- 8.go A PP, Poot AA, Grijpma DW, Feijen J. Copolymers of trimethylene carbonate and - caprolactone for porous nerve guides: Synthesis and properties. J Biomater Sci Polym Ed. 2001;12:35–53. doi: 10.1163/156856201744434. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume R, Fujimoto KL, Hong Y, Amoroso NJ, Tobita K, Miki T, et al. Morphological and mechanical characteristics of the reconstructed rat abdominal wall following use of a wet electrospun biodegradable polyurethane elastomer scaffold. Biomaterials. 2010;31:3253–3265. doi: 10.1016/j.biomaterials.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias IS, Lee H, Engelmayr GC, Jr, Macaya D, Bettinger CJ, Cima MJ. Zero-order controlled release of ciprofloxacin-HCl from a reservoir-based, bioresorbable and elastomeric device. J Controlled Release. 2010;146:356–362. doi: 10.1016/j.jconrel.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapanian R, Amsden BG. Combined and sequential delivery of bioactive VEGF165 and HGF from poly(trimethylene carbonate) based photo-cross-linked elastomers. J Controlled Release. 2010;143:53–63. doi: 10.1016/j.jconrel.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Kibbe MR, Martinez J, Popowich DA, Kapadia MR, Ahanchi SS, Aalami OO, et al. Citric acid-based elastomers provide a biocompatible interface for vascular grafts. Journal of Biomedical Materials Research Part A. 2010;93A:314–324. doi: 10.1002/jbm.a.32537. [DOI] [PubMed] [Google Scholar]
- 13.Dargaville BL, Vaquette Cd, Peng H, Rasoul F, Chau YQ, Cooper-White JJ, et al. Cross-Linked Poly(trimethylene carbonate-co-l-lactide) as a Biodegradable, Elastomeric Scaffold for Vascular Engineering Applications. Biomacromolecules. 2011;12:3856–3869. doi: 10.1021/bm201291e. [DOI] [PubMed] [Google Scholar]
- 14.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, et al. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Wach RA, Adamus A, Olejnik AK, Dzierzawska J, Rosiak JM. Nerve guidance channels based on PLLA–PTMC biomaterial. J Appl Polym Sci. 2012 [Google Scholar]
- 16.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SnE, et al. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer R. In vitro and in vivo degradation of poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) elastomers. Journal of Biomedical Materials Research Part A. 2009;91A:1077–1088. doi: 10.1002/jbm.a.32306. [DOI] [PubMed] [Google Scholar]
- 18.Bruggeman JP, de Bruin B-J, Bettinger CJ, Langer R. Biodegradable poly(polyol sebacate) polymers. Biomaterials. 2008;29:4726–4735. doi: 10.1016/j.biomaterials.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapanian R, Tse MY, Pang SC, Amsden BG. The role of oxidation and enzymatic hydrolysis on the in vivo degradation of trimethylene carbonate based photocrosslinkable elastomers. Biomaterials. 2009;30:295–306. doi: 10.1016/j.biomaterials.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Gyawali D, Tran RT, Guleserian KJ, Tang L, Yang J. Citric-Acid-Derived Photo-Cross-Linked Biodegradable Elastomers. J Biomater Sci Polym Ed. 2010;21:1761–1782. doi: 10.1163/092050609X12567178204169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson DG, Tweedie CA, Hossain N, Navarro SM, Brey DM, VaniıVliet KJ, et al. A Combinatorial Library of Photocrosslinkable and Degradable Materials. Advanced Materials. 2006;18:2614–2618. [Google Scholar]
- 22.Nijst CLE, Bruggeman JP, Karp JM, Ferreira L, Zumbuehl A, Bettinger CJ, et al. Synthesis and Characterization of Photocurable Elastomers from Poly(glycerol-co-sebacate) Biomacromolecules. 2007;8:3067–3073. doi: 10.1021/bm070423u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quick DJ, Anseth KS. DNA delivery from photocrosslinked PEG hydrogels: encapsulation efficiency, release profiles, and DNA quality. J Controlled Release. 2004;96:341–351. doi: 10.1016/j.jconrel.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks. Biomacromolecules. 2004;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Lunkenbein S, Bellido ML, Aharoni A, Salentijn EMJ, Kaldenhoff R, Coiner H, et al. Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose: Cinnamate glucosyltransferase from strawberry. Plant Physiol. 2006;140:1047–1058. doi: 10.1104/pp.105.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinazzi CP, Fernandez A. Synthesis of New Photocrosslinkable Polymers Derived from Cinnamic Acid. Ultraviolet Light Induced Reactions in Polymers. 1976;25:37–51. [Google Scholar]
- 28.Jiang HY, Kelch S, Lendlein A. Polymers Move in Response to Light. Advanced Materials. 2006;18:1471–1475. [Google Scholar]
- 29.Lendlein A, Jiang H, Junger O, Langer R. Light-induced shape-memory polymers. Nature. 2005;434:879–882. doi: 10.1038/nature03496. [DOI] [PubMed] [Google Scholar]
- 30.Chi Y-M, Nakamura M, Yoshizawa T, Zhao X-Y, Yan W-M, Hashimoto F, et al. Anti-inflammatory Activities of alpha-Truxillic Acid Derivatives and Their Monomer Components. Biol Pharm Bull. 2005;28:1776–1778. doi: 10.1248/bpb.28.1776. [DOI] [PubMed] [Google Scholar]
- 31.Curran JE. Production of surface patterns by chemical and plasma etching. Journal of Physics E: Scientific Instruments. 1981;14:393. [Google Scholar]
- 32.Urones JG, Marcos IS, Martin DD, Rodilla JML, de Mendoca DIMD, Williams DJ. Alpha-Truxillic acid from Halimium verticillatum. Phytochemistry. 1994;36:529–530. [Google Scholar]
- 33.Khan M, Brunklaus G, Enkelmann V, Spiess H-W. Transient States in [2 + 2] Photodimerization of Cinnamic Acid: Correlation of Solid-State NMR and X-ray Analysis. J Am Chem Soc. 2008;130:1741–1748. doi: 10.1021/ja0773711. [DOI] [PubMed] [Google Scholar]
- 34.Pretsch E, Bühlmann P, Badertscher M. Structure Determination of Organic Compounds - Tables of Spectral Data. 4th Edition. Springer - Verlag; [Google Scholar]
- 35.Gupta P, Trenor SR, Long TE, Wilkes GL. In Situ Photo-Cross-Linking of Cinnamate Functionalized Poly(methyl methacrylate-co-2-hydroxyethyl acrylate) Fibers during Electrospinning. Macromolecules. 2004;37:9211–9218. [Google Scholar]
- 36.Visconte LLY, Andrade CT, Azuma C. Kinetic behavior of UV-sensitive polymers from modified natural rubber. Polymers for Advanced Technologies. 1993;4:490–495. [Google Scholar]
- 37.Visconte LLY, Andrade CT, Azuma C. Kinetic treatment for the photodimerization reaction of modified natural polyisoprenes. Polymer Bulletin. 1991;26:637–641. [Google Scholar]
- 38.Song L, Zhu M, Chen Y, Haraguchi K. Surface-Patterning of Nanocomposite Hydrogel Film by Direct Replica Molding and Subsequent Change in Pattern Size. Polym J. 2008;40:800–805. [Google Scholar]
- 39.Cai W, Liu L. Shape-memory effect of poly (glycerol-sebacate) elastomer. Materials Letters. 2008;62:2171–2173. [Google Scholar]
- 40.Hiemenz PC, Lodge TP. Polymer Chemistry. 2nd Edition. CRC Press; 2007. [Google Scholar]
- 41.Sperling LH. Introduction to Physical Polymer Science. John Wiley & Sons; 2001. [Google Scholar]
- 42.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Smith BA, Tolloczko B, Martin JG, Grütter P. Probing the Viscoelastic Behavior of Cultured Airway Smooth Muscle Cells with Atomic Force Microscopy: Stiffening Induced by Contractile Agonist. Biophys J. 2005;88:2994–3007. doi: 10.1529/biophysj.104.046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathur AB, Collinsworth AM, Reichert WM, Kraus WE, Truskey GA. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J Biomech. 2001;34:1545–1553. doi: 10.1016/s0021-9290(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 45.Shindo Y, Sugimura T, Horie K, Mita I. Photocrosslinking of polymers containing cinnamoyl groups: The change in polymer coil dimension during the reaction in solution. European Polymer Journal. 1986;22:859–863. [Google Scholar]
- 46.Ali SAM, Doherty PJ, Williams DF. Molecular biointeractions of biomedical polymers with extracellular exudate and inflammatory cells and their effects on the biocompatibility, in vivo. Biomaterials. 1994;15:779–785. doi: 10.1016/0142-9612(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 47.Smith R, Oliver C, Williams DF. The enzymatic degradation of polymers in vitro. J Biomed Mater Res. 1987;21:991–1003. doi: 10.1002/jbm.820210805. [DOI] [PubMed] [Google Scholar]
- 48.Burkersroda Fv, Schedl L, Göpferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 49.Malda J, Woodfield TBF, van der Vloodt F, Kooy FK, Martens DE, Tramper J, et al. The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials. 2004;25:5773–5780. doi: 10.1016/j.biomaterials.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 50.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 51.Brandrup J, Immergut EH, Grulke EA, Abe A, Bloch DR. Polymer Handbook. 4th Edition. John Wiley & Sons; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.