SUMMARY
During brainstem development, newborn neurons originating from the rhombic lip embark on exceptionally long migrations to generate nuclei important for audition, movement, and respiration. Along the way, this highly motile population passes several cranial nerves yet remains confined to the CNS. We found that Ntn1 accumulates beneath the pial surface separating the CNS from the PNS, with gaps at nerve entry sites. In mice null for Ntn1 or its receptor DCC, hindbrain neurons enter cranial nerves and migrate into the periphery. CNS neurons also escape when Ntn1 is selectively lost from the sub-pial region (SPR), and conversely, expression of Ntn1 throughout the mutant hindbrain can prevent their departure. These findings identify a permissive role for Ntn1 in maintaining the CNS-PNS boundary. We propose that Ntn1 confines rhombic lip-derived neurons by providing a preferred substrate for tangentially migrating neurons in the SPR, preventing their entry into nerve roots.
In Brief
Yung et al. show that Ntn1 prevents pontine neurons from migrating into the periphery along cranial nerves by providing a preferred substrate in the sub-pial region. These findings introduce a local, permissive role for Ntn1 in the maintenance of the CNS-PNS boundary in the developing mouse hindbrain.
INTRODUCTION
A basic organizing principle of the nervous system is the segregation of the peripheral nervous system (PNS) and CNS, which are anatomically and functionally distinct yet linked by nerves. This is particularly apparent in the vertebrate brainstem, which houses ten cranial nerves as well as a constellation of nuclei that govern functions critical to life, from motor coordination to auditory processing (Farago et al., 2006; Wang et al., 2005). Many of these nuclei are composed of neurons originating from the rhombic lip, a transient strip of proliferating neuroepithelium lining the fourth ventricle during development (Ray and Dymecki, 2009). The formation of hindbrain nuclei, therefore, depends on the successful tangential migration of newborn neurons from the rhombic lip to their final destinations. This route is unusually long and complex, especially since the surface of the hindbrain is broken by multiple cranial nerve roots that the rhombic lip derivatives must ignore. Although several guidance cues play critical roles sculpting the trajectory of tangentially migrating neurons in vivo (reviewed in Kratochwil et al., 2017), nothing is known about the molecular mechanisms that confine these neurons to the CNS, despite opportunities to deviate into the periphery.
Pontine neurons (PNs) traverse one of the longest migratory routes in the hindbrain, ultimately settling at the midline to supply excitatory mossy fiber input to the cerebellum (Kratochwil et al., 2017). PNs originate from the rhombic lip in rhombomeres (r)6–r8 from embryonic day (E)12.5 to E16.5. They extend long leading processes (Ono and Kawamura, 1990; Yee et al., 1999) as they migrate beneath the pial surface, maneuvering between the trigeminal (Vth), facial (VIIth), and vestibulocochlear (VIIIth) nerve roots and arriving at the ventral midline of r3–r4 several days later (Nichols and Bruce, 2006) (Figure 1A). This navigation depends on the activity of several guidance cues, including Slits, which are secreted by the facial motor nucleus to prevent premature ventral migration (Geisen et al., 2008), and meninges-derived chemokine SDF-1, which keeps PNs from migrating into the neuroepithelium (Zhu et al., 2009). What prevents PNs from escaping in the opposite direction, into the periphery, is unknown.
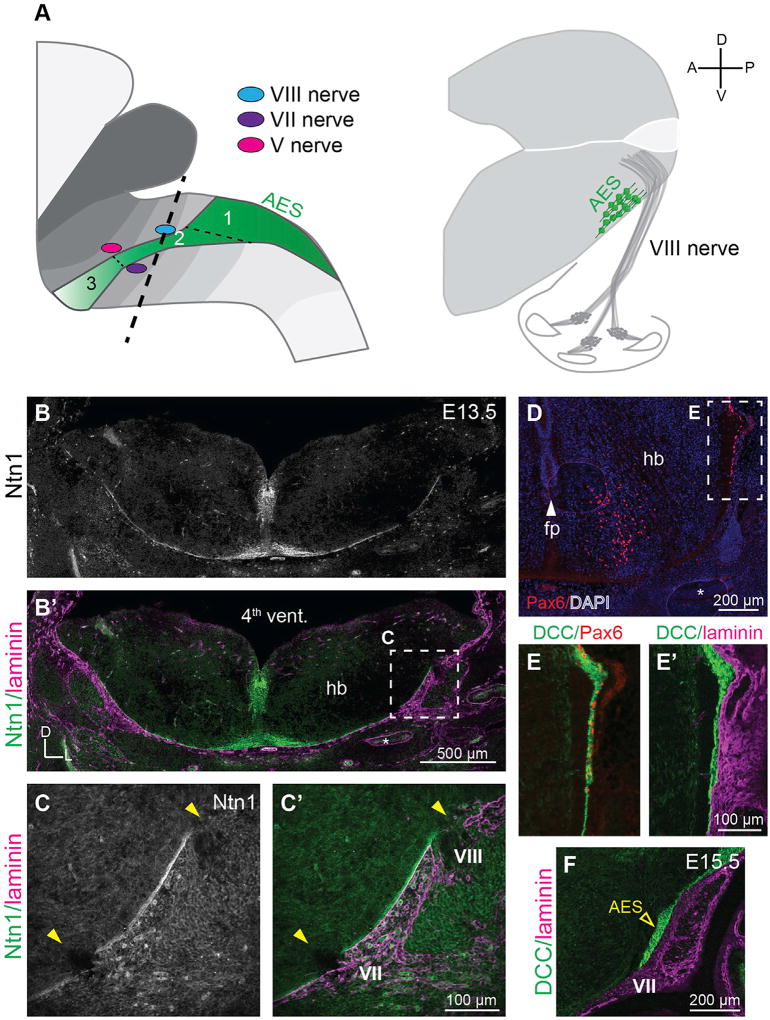
Figure 1. Ntn1 Protein Is Enriched in the SPR in the Developing Hindbrain.
(A) Schematic depicting the three phases of PN migration (green) across multiple rhombomere segments (shaded in gray) and a view of the AES in an E15.5 transverse section. Thick dashed line indicates plane of section. D, dorsal; A, anterior; P, posterior; V, ventral.
(B–F) Immunostains of transverse embryonic head sections. At E13.5, low-power (B and B’) and high-power (C and C’) images show strong Ntn1 staining at the FP, on crossing commissural axons at the midline, and in the sub-pial region (SPR) adjacent to the laminin-positive pial basement membrane (magenta). Curiously, Ntn1 appears to be absent from nerve roots (yellow arrowheads). Low (D) and high (E–E’) magnification images show ventrally migrating pontine neurons in the SPR, even as they avoid cranial nerve roots later in their migration (F; E15.5). Pontine neurons express Pax6 (red, D and E) and DCC (green, E–F).
AES, anterior extramural stream; fp, floor plate; hb, hindbrain; 4th vent., fourth ventricle; VII, facial nerve; VIII, vestibulocochlear nerve.
One of the first guidance cues implicated in PN migration is the classic chemoattractant Netrin-1 (Ntn1). PNs are highly sensitive to Ntn1 and can migrate toward a source of Ntn1 over unusually long distances in vitro (Yee et al., 1999); in vivo, the pontine nuclei are missing in mice severely hypomorphic for Ntn1 (Serafini et al., 1996; Yee et al., 1999). These data were originally interpreted to indicate that a floor-plate-derived gradient of Ntn1 guides PNs to the midline during the final leg of their migration (Zelina et al., 2014), much as Ntn1 was proposed to guide commissural axon growth in the developing spinal cord (Kennedy et al., 2006; Serafini et al., 1996). However, Ntn1 is also expressed in the ventricular zone (Kennedy et al., 1994; Serafini et al., 1996), and this source is required for proper commissure formation (Charron et al., 2003). The protein itself is deposited in the sub-pial region (SPR) adjacent to the basement membrane (BM) surrounding the neural tube (Kennedy et al., 2006; MacLennan et al., 1997; Varadarajan et al., 2017). Recent genetic studies have underscored the importance of SPR-localized Ntn1 for commissural axon guidance (Dominici et al., 2017; Varadarajan et al., 2017; Yamauchi et al., 2017), consistent with documented functions for Ntn1 in the BM of other tissues (Liu et al., 2004; Srinivasan et al., 2003; Yebra et al., 2003). This suggests that additional roles for locally produced Ntn1 in the developing nervous system likely remain to be found, particularly in cases such as PN migration, where neurons migrate along the pial surface rather than through the neuroepithelium. Indeed, the complete range of Ntn1’s effects on PN migration is still unclear, due both to the hypomorphic nature of the original allele and to the lack of information about the ultimate fate of PNs.
Here, we show that Ntn1 functions as a permissive cue to confine PNs to the CNS. We propose that Ntn1 in the sub-pial region provides a preferred corridor for migrating rhombic lip-derived neurons, allowing them to distinguish the appropriate migratory substrate and avoid opportunities to migrate instead into the periphery. These findings introduce another local function for Ntn1 and establish an additional molecular explanation for how CNS-PNS segregation is achieved in the brainstem, a hub for CNS-PNS interactions that are vital for life.
RESULTS
Ntn1 Protein Is Enriched in the SPR in the Developing Hindbrain
Migrating PNs follow a stereotyped pathway to the ventral midline that can be divided into three phases: (1) a short ventral migration marking the departure from the rhombic lip; (2) a relatively straight rostral migration between and past the vestibulocochlear (VIIIth), facial (VIIth), and trigeminal (Vth) nerves, respectively; and (3) a final ventral turn before resting at the midline (Figure 1A) (Geisen et al., 2008; Nichols and Bruce, 2006). The entire migration takes place in the space beneath the pia, which we call the sub-pial region (SPR). While the molecules that define many aspects of this complex migratory route have been identified, the cues that instruct PNs to stay in the SPR and avoid cranial nerve roots remain unknown. During the peak of migration at E15.5, PNs traverse hundreds of microns as they move past the cranial nerves. Throughout this journey, PNs express the Ntn1 receptor Deleted in Colorectal Carcinoma (DCC) and are exposed to Ntn1 produced in the floor plate (FP) and ventricular zone (Yee et al., 1999). When Ntn1 levels are reduced, rare spinal cord interneuron axons can be found in dorsal root ganglia (Laumonnerie et al., 2014), hinting at a role in setting the CNS-PNS boundary. We therefore hypothesized that Ntn1 not only guides PNs to the midline but also keeps them contained within the CNS.
Since Ntn1 is a potent secreted cue, we examined the localization of Ntn1 relative to its sources in the hindbrain at the onset of PN migration at E13.5 (de Diego et al., 2002; Yee et al., 1999). Immunostaining revealed that Ntn1 protein is widely distributed (Figures 1B and 1B’; n = 2 animals), accumulating in the FP, on commissural axons, and in the SPR in the vicinity of the laminin-positive pial BM, as described previously (Dominici et al., 2017; Kennedy et al., 2006; MacLennan et al., 1997; Varadarajan et al., 2017). Notably, Ntn1 is absent from nerve roots, where cranial nerves project into or out of the CNS via gaps in the pial BM at stereotyped locations (Figures 1C and 1C’; n = 2 animals). Thus, Ntn1 protein is present where PNs migrate, but not at sites they avoid.
To determine whether migrating PNs might encounter and respond to Ntn1 in the SPR, we stained for PN markers Pax6 and DCC at E13.5, when PNs have begun exiting the rhombic lip, and at E15.5, when they are passing by cranial nerves. At E13.5, Pax6+/DCC+ cells cluster beneath the pial BM (Figures 1D–1E’; n = 4 animals) and maintain this position as they navigate near the facial (VII) and vestibulocochlear (VIII) nerves at E15.5 (Figure 1F). Since Ntn1 is enriched in the SPR, migrating DCC+ PNs likely encounter Ntn1 from early on. Given the conspicuous absence of Ntn1 at nerve roots—which migrating PNs avoid—this pattern of distribution suggests that Ntn1 may contribute to the confinement of tangentially migrating neurons in the hindbrain by providing an attractive substrate.
Loss of Ntn1 Causes PNs to Exit the Hindbrain and Enter the Periphery
Earlier analyses of hypomorphic Ntn1 animals (Ntn1trap/trap) suggested that Ntn1 mediates the final ventral migration to the midline, as PNs complete most of the first and second phases of their migration (Zelina et al., 2014). However, phenotypic analyses of Ntn1 null animals (Ntn1−/−) showed that residual Ntn1 in hypomorphs masks the full extent of its role in guidance (Bin et al., 2015; Yung et al., 2015). We posited that complete loss of Ntn1 might reveal additional functions earlier in migration, particularly since PNs are exposed to Ntn1 far before their final ventral turn.
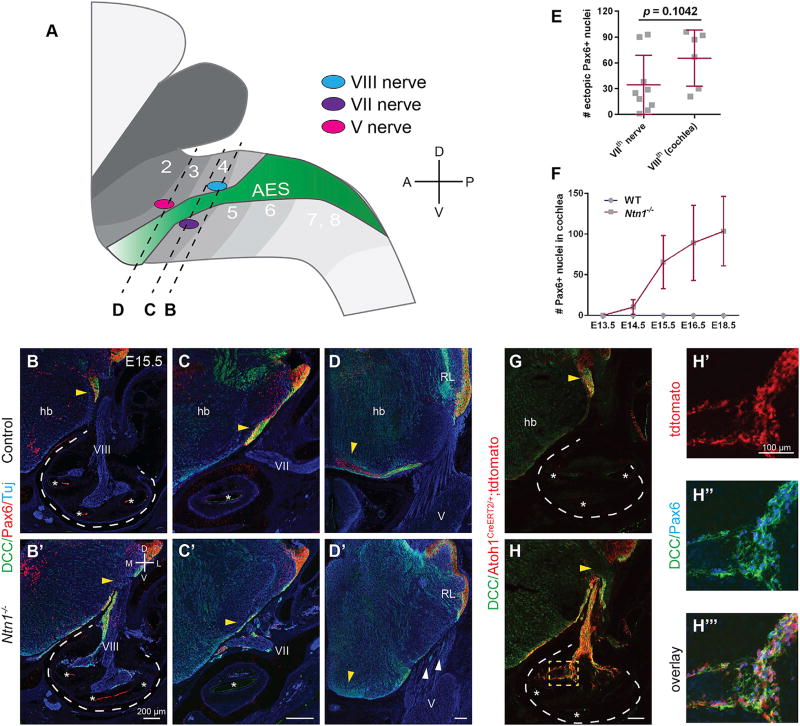
To visualize PN distribution, we collected transverse sections of embryonic Ntn1−/− heads spanning the anterior extramural stream (AES) through which PNs travel. In E15.5 control animals (n = 4), the AES is identifiable as a dense stripe of Pax6+ nuclei and DCC+ processes traveling beneath the pial surface (Figures 2B–2D), giving rise to the pontine nuclei at the midline, which first appear at late E14.5 (Nichols and Bruce, 2006). In contrast, in Ntn1−/− animals (n = 6), the AES was missing, and there were ectopic streams of Pax6+ and DCC+ neurons immediately ventral to the stereotyped location of the AES, as if the PNs had been diverted into the periphery (Figures 2B’ and 2C’). Ectopic Pax6+ nuclei and a few DCC+ processes were rarely found in the trigeminal (Vth) ganglion (Figure 2D’). However, the facial and vestibulocochlear nerves contained similar numbers of ectopic nuclei (Figure 2E; n = 6 nerves each). We focused on the vestibulocochlear (VIIIth) nerve as a site of exit, as it is the first nerve root that migrating PNs pass and because the cochlea is an enclosed, easily recognizable landmark. These findings suggest that, in addition to mediating the final ventral turn to the midline, Ntn1 acts earlier to prevent PNs from migrating along cranial nerves and into the periphery.
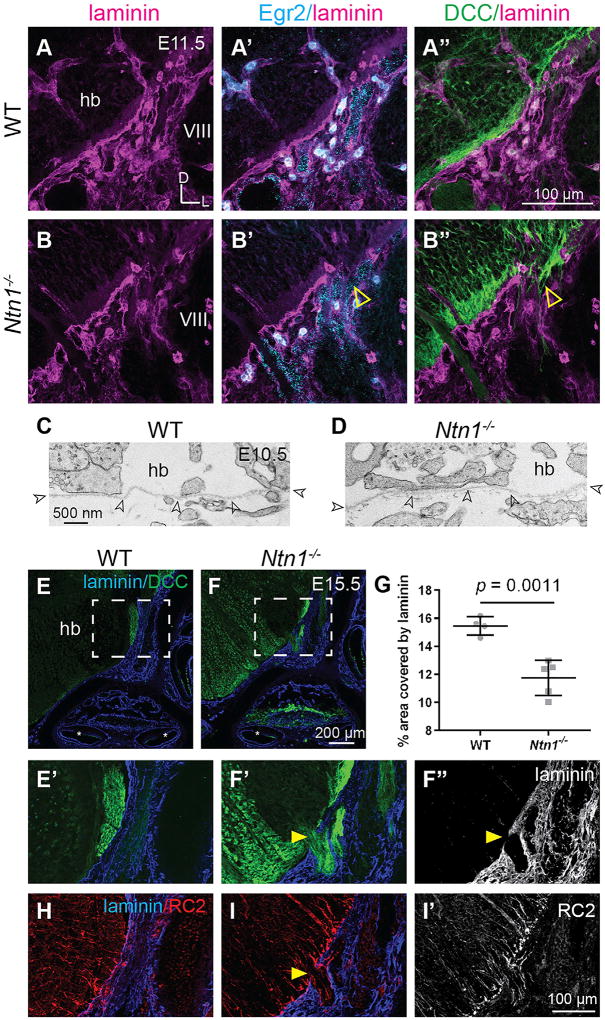
Figure 2. PNs Exit the CNS along Cranial Nerves in the Absence of Ntn1.
(A) Schematic of the PN migratory route (anterior extramural stream, green) across multiple rhombomeres (numbered, shaded in gray) and relative to cranial nerve roots.
(B–D’) E15.5 transverse head sections immunostained for DCC and Pax6 to label migrating PNs (yellow arrowheads), which normally travel rostrally beneath the pial surface toward the midline, shown at three rostro-caudal levels (B, C, and D), as indicated by the dashed lines in (A). A mix of WT and Ntn1+/− tissues are shown as controls. In Ntn1−/− animals, the AES is missing. PNs, instead, diverge into the VIIIth (B’) and VIIth (C’) nerves. Rare ectopic processes are present in the Vth nerve (D’, white arrowheads).
(E and F) Quantification of the number of Pax6+ neurons in the VIIth and VIIIth nerves (E) (mean ± SD, Student’s t test) and in the base and middle turns of control and Ntn1−/− cochlear sections over time (F) (mean ± SD).
(G–H’’’) Low- (G and H) and high-power (H’–H’’’) images of fate-mapped PNs in the cochlea which were labeled with tdTomato (G, H, H’), DCC (G, H, H’’), and Pax6 (H’’) in Atoh1CreERT2/+; Ai14; Ntn1+/− (G) and Ntn1−/− (H–H’’’) embryos exposed to tamoxifen at E13.5. A merged image is shown in (H’’’).
Dotted lines indicate the cochlea; roman numerals indicate cranial nerves. hb, hindbrain. Asterisks indicate cochlear duct. All scale bars indicate 200 µm unless otherwise noted.
See also Figures S1 and S2.
To confirm the origin and identity of these neurons, we genetically labeled PNs by providing tamoxifen to E13.5 Atoh1CreERT2;Ai14 mice crossed onto the Ntn1 null background. tdTomato+ cells also expressed both Pax6 and DCC (Figures 2G–2H’’’; n = 3 animals), demonstrating that the ectopic neurons in the cochlea derive from the rhombic lip. Likewise, in Ntn1 mutants, Pax6+ neurons first reach the cochlea at E13.5 and increase in number steadily up until E15.5 (Figure 2F; n = 6 cochleae per time point), matching the timing of PN production and migration. These neurons accumulate mostly in the base and middle turns of the cochlea, which lie closest to the hindbrain. Taken together, these data demonstrate that the Pax6+ neurons invading Ntn1−/− cochleae are bona fide PNs.
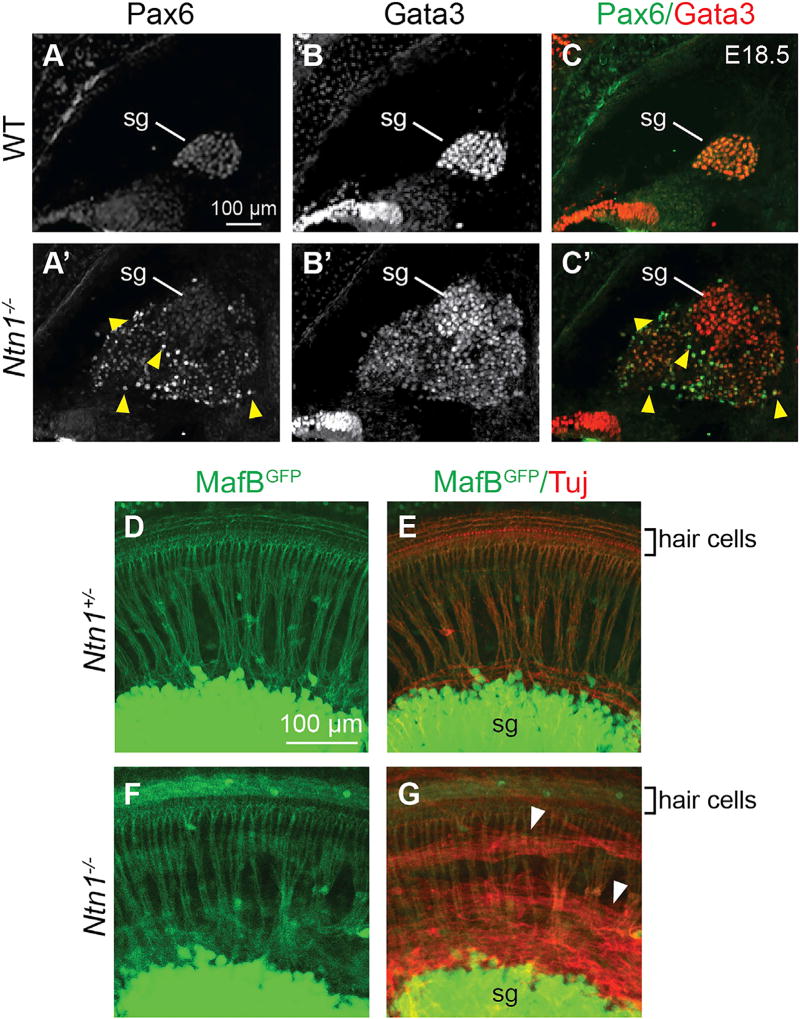
Despite their ectopic location, the misrouted PNs survived and differentiated within the cochlea. At E18.5, 103.5 ± 42.6 (mean ± SD) Pax6+ PNs were present in the cochlea, but instead of integrating into the spiral ganglion, the PNs, which express low levels of Gata3, formed a rind around the Gata3-high spiral ganglion neurons (SGNs; Figures 3A–3C’; n = 3 Ntn1−/−). The gross organization of the spiral ganglion was remarkably normal, as visualized by crossing a MafBGFP allele (Moriguchi et al., 2006) into the Ntn1−/− background. As in control embryos, GFP+ SGNs extended orderly bundles of radial fibers toward the hair cells in mutants (Figures 3D and 3F; n = 3 Ntn1−/−), beneath an overlying swath of processes from GFP−/Tuj+ PNs (Figures 3E and 3G). Since Ntn1 mutants die at birth, we could not examine the fate of ectopic PNs in adults. Nonetheless, these data show that PNs thrive in the cochlea but stay segregated from the SGNs.
Figure 3. Ectopic Neurons Do Not Integrate into the Spiral Ganglion.
(A–C’) E18.5 transverse sections of the base of the cochlea immunostained for Pax6 (A and A’) and Gata3 (B and B’). Only mutant cochleae contain Pax6+ neurons (yellow arrowheads), which form a rind around SGNs that express higher levels of Gata3 (C’).
(D–G) Whole-mount immunostains of E18.5 cochleae from control and Ntn1−/− embryos also harboring a MafBGFP allele, which is expressed in SGNs. GFP+ SGN processes (green, D–G) form bundles extending radially to hair cells in both Ntn1+/− controls (D and E) and mutant mice (F and G). In addition, Ntn1−/− cochleae contain Tuj-positive PNs (red, E and G) that extend MafBGFP -negative GFP-processes longitudinally over the SGNs and their radial fibers (G; white arrowheads). Merged images shown in (E) and (G). sg, spiral ganglion.
Multiple Populations of Neurons Escape the CNS in Ntn1 Mutants
In addition to PNs, many other rhombic lip derivatives migrate through the SPR, including neurons of the cochlear nucleus, inferior olive, and external cuneate nucleus (Machold and Fishell, 2005; Nichols and Bruce, 2006; Wang et al., 2005). As these neurons also respond to Ntn1 (Alcántara et al., 2000; Bloch-Gallego et al., 1999; Causeret et al., 2002; Howell et al., 2007; Riyadh et al., 2014; Marcos et al., 2009), we wondered if Ntn1’s role in confinement extends to other rhombic lip derivatives. Since Ntn1 is expressed in the neural tube as early as E9.5 (Kennedy et al., 1994; Serafini et al., 1994; Yee et al., 1999), we examined the trajectory of some of the earliest born rhombic lip-derived neurons in the CNS by injecting tamoxifen at E9.5 into Atoh1CreERT2;Ai14 mice crossed to the Ntn1 null background. As expected, many commissural neurons were labeled, shown by the presence of tdTomato+ processes crossing the midline at E11.5 (Figures S1A’–S1A’’’; n = 2 controls). In Ntn1−/− animals, tdTomato+ neurons resided in more dorsal locations, and the commissure failed to form. Additionally, many labeled cell bodies and processes were found outside the hindbrain near and along cranial nerve roots (Figures S1B’–S1B’’’; n = 3 mutants). As in older animals, the tdTomato+ cells escaped through the Vth, VIIth, and VIIIth nerves, raising the possibility that later born neurons such as PNs depend on these existing ectopic tracts to exit the CNS. However, we found that, within the VIIIth nerve, PNs were not physically associated with any other ectopic DCC+ axons and were consistently present independent of other ectopic projections, such as those from the earlier born neurons in the ventral cochlear nucleus (Figure S2). These observations argue that the departure of PNs is not secondary to earlier phenotypes. Thus, multiple populations of neurons exit the CNS in the absence of Ntn1, indicating that Ntn1 plays a general role in establishing the CNS-PNS boundary.
DCC Is Also Required for PN Confinement
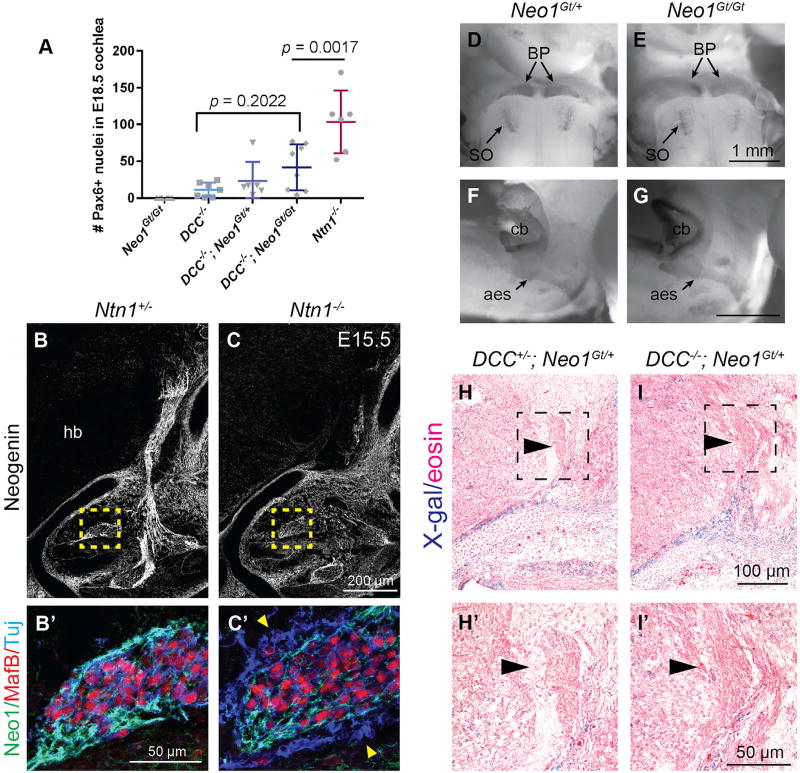
Since PNs express DCC and fail to reach the midline in DCC−/− animals (Fazeli et al., 1997; Yee et al., 1999), we hypothesized that Ntn1-DCC signaling underlies their confinement. Consistent with this idea, DCC−/− animals contain ectopic neurons in the cochlea that were assumed to be displaced SGNs (Kim et al., 2016). We found not only that ectopic neurons in DCC−/− cochlea express Pax6, indicative of PN identity instead, but also that Pax6+ neurons are also present elsewhere in the periphery, such as in the VIIth and VIIIth nerves, demonstrating that DCC enables Ntn1-mediated confinement. However, there were not as many neurons in DCC−/− cochleae as in Ntn1 mutants (Figure 4A; n ≥ 6 cochlea per genotype), hinting that another receptor also contributes.
Figure 4. DCC−/−; Neo1Gt/Gt Double Mutants Phenocopy Ntn1−/− Mutants, but Neogenin Acts Non-Cell-Autonomously.
(A) The number of Pax6+ PNs found in the base and middle turns of the cochlea in E18.5 DCC and Neo1 gene trap (Neo1Gt) single and double mutants (mean ± SD, p < 0.0001, F = 14.33; DF = 28; one-way ANOVA, Tukey’s multiple comparisons test).
(B–C’) Immunostained E15.5 transverse sections show that Neogenin is expressed strongly in MafB+ SGNs and in the surrounding mesenchyme in control (B) and Ntn1−/− (C) tissue. High-power images of the boxed areas show that MafB+ SGNs normally express Neogenin (B’), but ectopic MafB- PNs in the ear do not (C’, yellow arrowheads).
(D–G) Ventral (D and E) and sagittal (F and G) views of heterozygous (D and F) and homozygous (E and G) Neo1 E15.5 brains immunostained for Pax6. Rostral is up in (D) and (E) and to the right in (F) and (G).
(H–I’) X-gal reactions (blue) in eosin-stained tissue from E15.5 control and DCC−/− embryos carrying the Neo1Gt allele, which drives expression of β-galactosidase in Neogenin+ cells. No signal is detected in the AES (black arrowheads) in DCC+/−; Neo1Gt/+ (H, H’) or DCC−/−; Neo1Gt/+ (I and I’) animals, shown at low (H and I) and high (H’ and I’) magnification.
aes, anterior extramural stream; BP, basilar pons; cb, cerebellum; hb, hindbrain; SO, superior olive.
See also Figures S3 and S7.
Ntn1 signals through many receptors, including Unc5 family members, Down syndrome cell adhesion molecule (DSCAM), integrins, and the DCC ortholog Neogenin (reviewed in Lai Wing Sun et al., 2011). We predicted that Neogenin might influence Ntn1-mediated confinement, since DCC and Neogenin collaborate to mediate midline crossing in commissural neurons (Xu et al., 2014). As in previous reports (Brugeaud et al., 2014; Fitzgerald et al., 2007; van den Heuvel et al., 2013), we observed low levels of Neogenin throughout the E15.5 hindbrain (Figures 4B and 4B’; n = 2 Ntn1+/−), with stronger expression in the surrounding mesenchyme and SGNs. We did not detect Neogenin in migrating PNs and found no obvious qualitative differences in the size or location of the pontine nuclei or the trajectory of the AES in either Neo1 hypomorphs (Neo1Gt/Gt; Bae et al., 2009; Leighton et al., 2001) (Figures 4D–4G; n = 3 Neo1Gt/Gt) or null animals (Kam et al., 2016) (Figures S3A and S3B; n = 3 Neo1−/−). Ectopic PNs in the cochleae also did not express Neogenin (Figures 4C and 4C’; n = 3 Ntn1−/−), suggesting that Neogenin is not required for PN migration or confinement. Nonetheless, increasing numbers of Pax6+ neurons accumulated in the cochlea, as more copies of Neo1 were lost in the DCC null background (Figure 4A). Thus, Neogenin affects Ntn1-mediated PN confinement when DCC is absent, but not when DCC is present.
Neogenin Functions Non-Cell-Autonomously in PN Migration
A redundant function for Neogenin within PNs would provide the simplest explanation for why DCC−/−; Neo1Gt/Gt double mutants, but not DCC−/− or Neo1Gt/Gt single mutants, more closely mimic the phenotype in Ntn1−/− animals. To test whether PNs upregulate Neogenin in the absence of DCC, we crossed the Neo1Gt/+ allele into the DCC−/− background. This allowed us to assay β-galactosidase activity as a proxy for Neo1 expression, which is more sensitive than immunostaining. As expected, β-galactosidase reaction product was present in SGNs and the surrounding mesenchyme at E15.5, but not in the AES in either control or mutants (Figures 4H–4I’; n = 3 control, 4 DCC−/−). Thus, Neogenin is unlikely to compensate for DCC in migrating PNs.
To be sure that early or low levels of Neogenin in rhombic lip-derived neurons do not explain the stronger phenotype in DCC−/−; Neo1Gt/Gt double mutants, we used Wnt1Cre and a floxed Neo1 allele (Kam et al., 2016) to remove Neogenin from Wnt1+ rhombic lip precursors in a DCC null background. Deletion of Neo1 from early PNs did not enhance the DCC phenotype (Figures S3C and S3D; n = 3 conditional mutants), making it highly unlikely that Neogenin and DCC function redundantly in PNs. Additionally, though more PNs migrated all the way into the cochlea in DCC−/−; Neo1Gt/Gt double mutants than in DCC−/− single mutants, similar numbers entered the nerve roots in E15.5 animals of both genotypes. Ntn1, therefore, appears to act largely through DCC to prevent PNs from crossing the CNS-PNS boundary but may influence their subsequent behavior through Neogenin expressed in other tissues.
Ntn1−/− Mutants Retain Boundary Cap Cells at Nerve Roots
Our results show that, in addition to its canonical role as a chemoattractant, Ntn1 contributes to CNS-PNS segregation, raising the question of how Ntn1 mediates this function. Many cellular structures contribute to the compartmentalization of the CNS. Neural crest-derived boundary cap cells (BCCs), for example, reside at all spinal nerve roots, and loss of BCCs or the cues they secrete results in the ectopic migration of motor neurons into the ventral root (Bron et al., 2007; Garrett et al., 2016; Mauti et al., 2007; Vermeren et al., 2003). The role of BCCs in the hindbrain is less well understood, though they reside at the trigeminal and facial nerve roots in mice (Garrett et al., 2016) and chicks (Niederländer and Lumsden, 1996). Loss of Ntn1 could alter the position of BCCs at cranial nerve roots, in turn permitting the departure of CNS neurons along nerves.
To test this possibility, we used RNAscope to detect Egr2, one of the only markers for BCCs (Vermeren et al., 2003), and counterstained for laminin to assess the distribution of BCCs at the Vth, VIIth, and VIIIth nerves. At E11.5, when DCC+ processes have already entered the periphery (Figure S1), Egr2+ BCCs were observed at nerve entry and exit sites in both controls and mutants (Figures 5A–5B’’; n = 3 animals per genotype). This result is not unexpected, given that, based on the number of ectopic CNS neurons in the periphery, the Ntn1−/− hindbrain phenotype is much more severe than what was previously described in animals lacking BCCs (Vermeren et al., 2003). These data show that Ntn1 maintains the CNS-PNS divide through mechanisms distinct from those of BCCs.
Figure 5. Neurons Exit the CNS Independent of Defects in BCCs, Radial Glial Endfeet, and the Basement Membrane.
(A–B’’) High-power images of E11.5 transverse head sections near the VIIIth nerve root show that Egr2+ BCCs (blue) are present at gaps in laminin (magenta) in both WT (A–A’’) and Ntn1−/− (B–B’’) animals. In Ntn1−/− mutants, ectopic DCC+ processes exit the CNS despite the presence of BCCs (hollow yellow arrowheads).
(C and D) TEM images of the basement membrane (BM, hollow black arrowheads) surrounding the WT (C) and Ntn1−/− (D) hindbrain.
(E–G) Immunostains of transverse sections from WT (E) and Ntn1−/− (F) E15.5 embryos. Low- (E and F) and high-power (E’–F’’) images of laminin (blue) and DCC (green) show an ectopic break in the BM (yellow arrowheads, F’ and F’’) in Ntn1 mutants, quantified in (G) (mean ± SD, Student’s t test).
(H–I’) Stains on the same WT (H) and mutant (I and I’) sections for RC2, a radial glia marker, show that the radial glia endfeet (red) remain attached to the laminin-positive BM (blue) in the mutant, even extending together with PN processes through breaks in the laminin (yellow arrowhead), shown also in a single-channel image for RC2 in (I’).
hb, hindbrain; VIII, vestibulocochlear nerve; WT, wild-type.
Ectopic Neurons Exit the CNS Independent of Defects in BM Organization
In addition to BCCs, an effective CNS-PNS border depends on BM integrity, which is maintained, in part, by radial glial endfeet lining the pial surface. Deletion of BM components or detachment of radial glial endfeet from the pial surface causes BM rupture, defects in neuronal migration, extrusion of cortical neurons into the subarachnoid space, and ectopic migration of spinal cord motor neurons into the ventral root (Beggs et al., 2003; Halfter et al., 2002; Lee and Song, 2013; Moore et al., 2002; Nakagawa et al., 2015; Satz et al., 2010). Since Netrins affect BM integrity in some tissues (Abraira et al., 2008; Liu et al., 2004; Srinivasan et al., 2003; Yebra et al., 2003; Ziel et al., 2009), we wondered whether loss of Ntn1 might cause defects in the pial BM or in the organization of the radial glial endfeet, thereby enabling PN exodus.
To assess BM integrity prior to the earliest signs of the phenotype, we performed transmission electron microscopy (TEM) of E10.5 control and Ntn1−/− animals. At this age, the BM looks like a thin, diffuse rope surrounding the hindbrain, and we were able to follow the BM from the ventral edge of the VIIIth nerve root to the midline. The appearance of the BM was highly variable, altering in thickness, smoothness, and curvature, with no discernable pattern in WT and null animals (Figures 6C and 6D; n = 3 WT, 4 Ntn1−/−). In rare cases, we observed what appeared to be ectopic processes reaching into the periphery, yet the surrounding BM still did not look diminished in a way that would, a priori, enable neurons to exit.
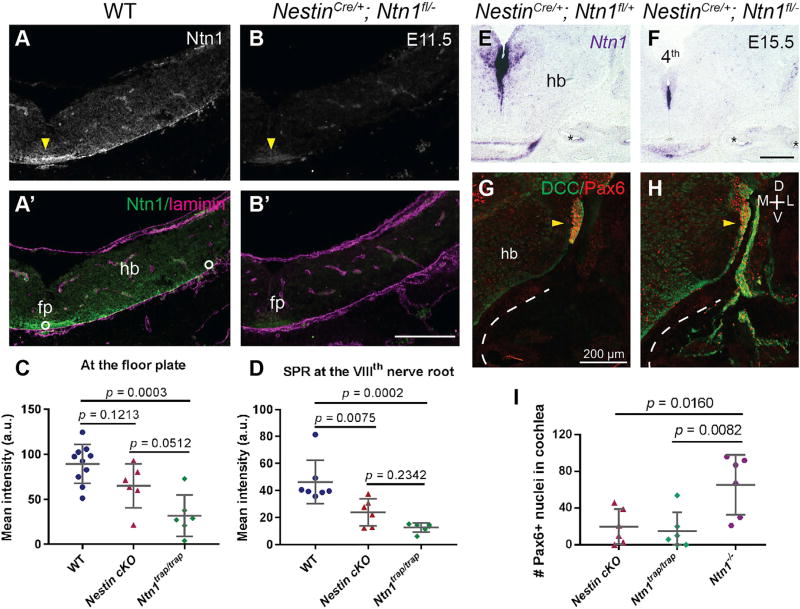
Figure 6. Ntn1 in the SPR, but Not the FP, Is Required for PN Confinement.
(A–D) Immunostaining for Ntn1 shows depletion from the SPR of E11.5 Nestin cKO animals (B and B’) compared to controls (A and A’), with maintained expression in the FP (arrowheads). Ntn1 intensity was measured at the FP or in the SPR (white circles in A’), quantified in (C) and (D), respectively (mean ± SD). For (C), F = 12.06; DF = 19; p = 0.0004; for (D), F = 14.73; DF= 16; p = 0.0002; one-way ANOVA with Tukey’s multiple comparisons test.
(E and F) In situ hybridization for Ntn1 further illustrates that relative to NestinCre/+; Ntn1fl/+ animals (E), Ntn1 is selectively reduced in the ventricular zone of E15.5 Nestin cKO embryos (F).
(G–I) DCC (green) and Pax6 (red) immunostains on E15.5 transverse head sections. Nestin cKO animals (H) retain the AES (yellow arrowheads), but it is smaller and deformed compared to controls (G), and there are many Pax6+ nuclei in the cochlea, quantified in (I). Depleting Ntn1 from the SPR is sufficient to partly recapitulate the null phenotype and fully phenocopy the hypomorph (I; mean ± SD) (F = 7.542; DF = 15; p = 0.0054; one-way ANOVA with Tukey’s multiple comparisons test). Refer to Figure S4 for raw data for the gene trap allele. Dotted lines indicate the outline of the cochlea.
fp, floor plate; hb, hindbrain; 4th, fourth ventricle. Asterisks indicate cochlear duct. All scale bars indicate 200 µm.
See also Figure S4.
Several days later, the BM in Ntn1−/− mutants still looked intact overall, as assessed by laminin staining. However, small ectopic breaks were consistently observed near the AES and the vestibulocochlear nerve (Figures 5E–5F’’; n = 3 Ntn1−/−), where ectopic DCC+ processes protrude, resulting in a significant decrease in the area covered by laminin adjacent to the AES (Figure 5G; n = 4 control, 5 Ntn1−/− ears). These breaks appeared independent of impaired radial glia architecture, whose RC2/Nestin+ endfeet remained attached to the pial surface in E15.5 mutants, as in controls (Figures 5H and 5I; n = 2 control, 3 Ntn1−/−). Moreover, at the sites of BM breaks, the radial glial endfeet projected further without showing obvious changes in their morphology or organization (Figure 5I’). Since BM integrity is normal at E10.5, with no apparent changes in radial glia organization at E15.5, it is unlikely that Ntn1 is required for BM integrity per se, consistent with the fact that Ntn1 has no effects on laminin assembly in vitro (Schneiders et al., 2007). Altogether, the lack of defects in key cell types that contribute to CNS integrity indicate that Ntn1 in the SPR acts directly on migrating neurons to keep them in the CNS.
SPR-Localized Ntn1 Produced by Hindbrain Progenitors Is Required for Confinement
Our results contrast with those from previous studies that reported no phenotypes in the spiral ganglion of Ntn1 hypomorphs (Howell et al., 2007; Kim et al., 2016). These differences could be attributed to the hypomorphic nature of the Ntn1trap/trap mice, which show a weaker phenotype: many PNs get close to their final destination (Figures S4D and S4D’), and there are significantly fewer Pax6+ cells in E15.5 Ntn1trap/trap cochleae (Figures S4B–S4C’; n = 6 cochleae). The number of ectopic neurons remained unchanged at E18.5 (Figure S4E; n = 6 cochleae), further indicating that, unlike null animals, the phenotype does not worsen as later born PNs migrate out. Despite this difference, the pontine nuclei are absent in Ntn1trap/trap animals, indicating that confinement and guidance are differentially affected by the loss of Ntn1, possibly due to differences in the availability or localization of Ntn1 in vivo.
In the developing hindbrain, Ntn1 is present both in the FP and in the SPR (Figure 1) (Dominici et al., 2017; MacLennan et al., 1997). We wondered whether the role in confinement might be attributed specifically to Ntn1 in the SPR, which is primarily supplied by progenitors in the ventricular zone (Dominici et al., 2017; Varadarajan et al., 2017). Using NestinCre/+ (Zimmerman et al., 1994) and a floxed allele of Ntn1, we significantly reduced Ntn1 in the SPR of NestinCre/+; Ntn1fl/− (Nestin conditional knockout [cKO]) animals (Figures 6A–6D; n = 2 controls and 3 Nestin cKO). Despite the presence of residual Ntn1 protein (Figures 6C and 6D) and transcript (Figures 6E and 6F) at the FP, PNs migrated ectopically into the ear (Figures 6G and 6H; n = 2 control and 3 Nestin cKO), partially phenocopying Ntn1−/− animals and fully phenocopying the hypomorphs (Figure 6I; n = 6 ears per genotype), which showed a similar distribution of Ntn1 protein, i.e., a severe decrease in the SPR (Figure 6D) with residual Ntn1 present at the midline (Figures 6C and S4; n ≥ 3 animals per genotype). These results support two conclusions. First, Ntn1 derived from the ventricular zone—which provides most of the Ntn1 in the SPR—ensures the compartmentalization of the CNS and PNS. Second, residual Ntn1 in hypomorphs and from the FP of Nestin cKO animals is sufficient to reduce the departure of CNS neurons into the periphery, but not to guide them reliably to the midline, as the pontine nuclei are missing in the hypomorph (Serafini et al., 1996; Yee et al., 1999).
Overexpression of Ntn1 throughout the CNS Rescues CNS-PNS Boundary Integrity
Our results raise the possibility that Ntn1 serves dual functions in the developing hindbrain, both securing the CNS-PNS boundary and attracting PNs to the ventral midline. In this model, the low levels of Ntn1 that persist in Ntn1 hypomorphs may be sufficient to establish a partially functional boundary, but not to mediate guidance to the midline, thereby explaining phenotypic differences between the hypomorphic and null mutants. Thus, Ntn1 could act instructively in a gradient to direct PNs to the midline and permissively in the SPR to keep them in the CNS.
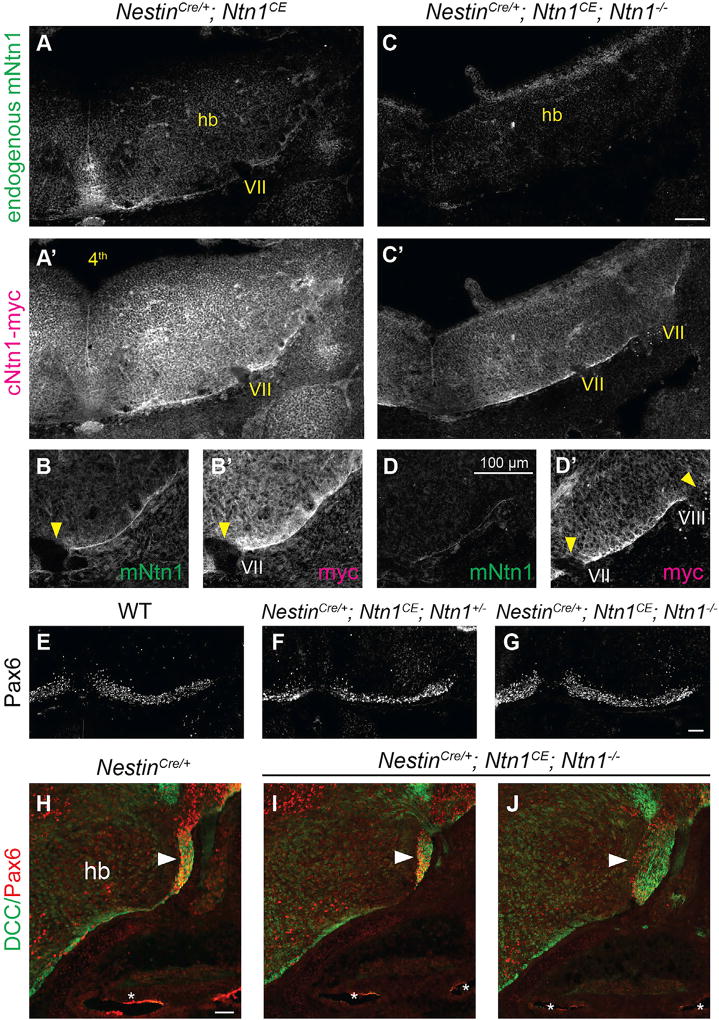
To disambiguate these two possible functions, we disrupted Ntn1’s role as a guidance cue by altering its pattern of distribution using a Cre-dependent Ntn1 conditional expressor (Ntn1CE/+), which produces a myc-tagged chick Ntn1 protein (cNtn1) with the same biological activity as mouse Ntn1 (mNtn1; Serafini et al., 1994). E11.5 NestinCre/+; Ntn1CE/+ animals showed widespread expression of cNtn1-myc throughout the hindbrain, overlaid on top of endogenous mNtn1 protein in the FP and SPR (Figures 7A–7B’; see also Figure S5; n = 4 NestinCre/+; Ntn1CE/+). Despite this clear change in Ntn1 protein distribution, PN migration appeared qualitatively normal: PNs reached the midline (Figures 7E and 7F), and no ectopic neurons were observed in the periphery (data not shown). To determine whether the lack of a phenotype reflected a dominant role for endogenous mNtn1, we crossed the NestinCre/+; Ntn1CE/+ animals onto the Ntn1−/− background. Both the cNtn1 transcript (Figure S6; n = 3 NestinCre/+; Ntn1CE/+; Ntn1−/−) and protein (Figures 7C–7D’; n = 2 NestinCre/+; Ntn1CE/+; Ntn1−/−) were present throughout the hindbrain, with cNtn1-myc enriched in the SPR but reduced at the FP. Thus, we significantly altered Ntn1 localization, thereby distorting any directional information that might be encoded in a gradient while maintaining a rich source of Ntn1 in the SPR.
Figure 7. Broadly Expressing cNtn1 in the Hindbrain Can Rescue Confinement Defects in Ntn1−/− Animals.
(A–D’) Transverse sections through E11.5 conditional expressor tissue immunostained for mNtn1 (A–D) and cNtn1-myc (A’–D’). cNtn1-myc is broadly distributed throughout the hindbrain (A’), overlaid on top of endogenous Ntn1 protein (A). Whereas mNtn1 is enriched at the FP, cNtn1 is relatively reduced, but both are enriched in the SPR (B and B’). Low-power images (C, C’) show that a similar distribution of cNtn1 persists in the null background, where despite the absence of mNtn1 (C), cNtn1 is present throughout the hindbrain, with less in the floor plate (C’). High-power images (D, D’) show that without mNtn1 (D), cNtn1 is the only Ntn1 enriched in the SPR (D’). In all cases, note the absence of any Ntn1 near nerve entry roots (yellow arrowheads).
(E–J) E15.5 transverse sections immunostained for Pax6 (red) and DCC (green). Single-channel images of Pax6 (E–G) show PNs accumulating at the midline of all conditional expressors, in both Ntn1+/− (F) and Ntn1−/− (G) backgrounds. Conditional expression of cNtn1-myc rescued confinement in some Ntn1−/− animals (I), as shown by a qualitatively normal AES (white arrowheads). In others, we observed a partial rescue in the form of a misshapen AES and a cluster of ectopic neurons in the nerve (J). Control sections at the midline (E) and AES (H) are provided for comparison.
hb, hindbrain; 4th, fourth ventricle. Roman numerals indicate cranial nerves. Asterisks indicate cochlear duct. All scale bars indicate 100 µm.
See also Figures S5 and S6.
Despite the drastic change in Ntn1 distribution, PN migration was surprisingly normal in all NestinCre/+; Ntn1CE/+; Ntn1−/− embryos (n = 4), as evidenced by the presence of both a well-defined AES and PNs at the midline where the pontine nuclei are normally found (Figure 7G). Although some PNs reached the midline in all animals, the extent of rescue varied, even between the two sides of the animal. We observed a complete rescue in 4 out of 8 cases (two per embryo), as defined by a qualitatively normal AES and no PNs detected in the periphery (Figures 7H and 7I). In the other cases, the AES was misshapen and sometimes accompanied by clusters of Pax6+ neurons in the proximal segment of the vestibulocochlear nerve or sparse Pax6+ neurons in other cranial nerves (Figure 7J). Although PNs appear to be resistant to major disruptions in the pattern of Ntn1 expression, these occasional errors may reflect some requirement for the WT pattern of Ntn1 expression. Alternatively, the degree of rescue may be sensitive to slight variations in the timing or efficiency of NestinCre-mediated recombination. Importantly, none of the embryos contained Pax6+ neurons in the cochlea. Thus, broad expression of Ntn1 is sufficient to restrict PNs from migrating into the periphery, consistent with the model that Ntn1 acts locally to provide a preferred substrate for neuronal migration, thereby keeping neurons confined to the CNS.
DISCUSSION
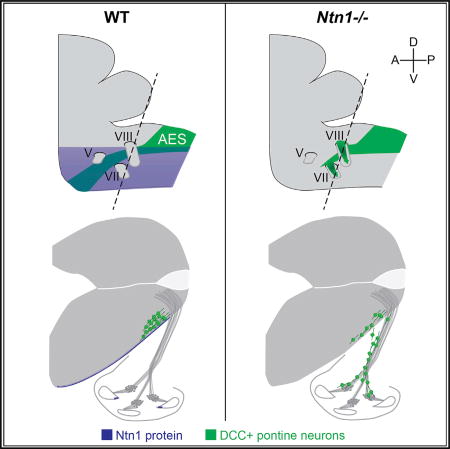
In the developing hindbrain, rhombic lip-derived neurons migrate long distances to form brainstem nuclei amidst a crowded network of nerves linking the CNS and PNS. We show here that SPR-localized Ntn1 maintains the CNS-PNS divide by preventing these highly motile neurons from straying into cranial nerves and entering the periphery. Our findings point to a model in which Ntn1 in the SPR acts as a preferred substrate for migrating neurons, thereby keeping them away from nerve roots devoid of Ntn1. Like flags marking a hiking trail, Ntn1 facilitates the successful migration of rhombic lip-derived neurons by establishing a preferred corridor for growth. Without this corridor, the neurons wander off trail, losing track of and failing to reach their destination.
In support of the idea that Ntn1 acts in the SPR to keep migrating neurons on track, migrating PNs express the Ntn1 receptor DCC and respond to Ntn1 in vitro (Yee et al., 1999). Ntn1 protein is also notably enriched in the SPR but absent at cranial nerve roots, which rhombic lip derivatives avoid. Thus, DCC+ PNs may prefer the Ntn1-rich environment surrounding the nerve roots so much that they reliably migrate around them, with the Ntn1-negative gap discouraging their entry. In agreement with this interpretation, the amount of Ntn1 in the SPR correlates with the strength of the confinement phenotype. For instance, using NestinCre to selectively reduce Ntn1 in the SPR but not the FP caused many PNs to enter the periphery. More strikingly, no ectopic PNs were observed in the cochlea when NestinCre was used to restore cNtn1-myc only to the SPR in the null background, where Ntn1 is never produced by the FP and the broad ectopic distribution of Ntn1 throughout the hindbrain obscures any positional information normally encoded by localized Ntn1. Thus, the pattern of Ntn1 expression does not seem to matter for the confinement of migrating neurons, as long as Ntn1 protein accumulates in the SPR.
Although a direct role for Ntn1 seems most likely, indirect effects might also contribute to the overall phenotype. For example, the departure of PNs could be facilitated by the presence of errant axons from earlier born neurons that breached the CNS-PNS border. However, such a mechanism is unlikely to account for the entire phenotype, as both neuronal cell bodies and processes have already entered the periphery at E11.5 (arrows in Figure S1B’’), the earliest point when we can detect a phenotype. Likewise, PNs appear segregated from other DCC+ ectopic axons in the VIIIth nerve, and these ectopias can arise independently (Figure S2). Thus, the departure of neurons does not seem to depend, a priori, on the presence of a pre-existing ectopic axon tract. It is, of course, possible that PNs occasionally migrate along earlier born ectopic processes as they escape the CNS, similar to the fasciculation of PN leading processes within the normal AES (Ono and Kawamura, 1990) and of later born axons that follow pioneer axons toward their targets. However, this would not rule out or diminish the role of Ntn1 in the confinement of rhombic lip-derived neurons overall.
In another scenario, SPR-localized Ntn1 could promote or maintain a physically sound CNS-PNS boundary, in addition to affecting neuronal migration. While we cannot rule out subtle changes, the overall organization of the CNS-PNS boundary appeared intact in Ntn1−/− animals. BCCs were present at nerve roots, and there were no obvious changes in the integrity of the BM surrounding the hindbrain, consistent with the fact that Ntn1 has no effect on BM assembly in vitro (Schneiders et al., 2007). These data suggest that Ntn1 acts directly on PNs to corral them within the SPR, thereby preventing them from leaving the CNS altogether. Our findings add to a growing body of evidence supporting a permissive role for Ntn1 (Dominici et al., 2017; Varadarajan et al., 2017; Yamauchi et al., 2017), and they expand the repertoire of Ntn1 functions in the developing nervous system.
Distinct Functions for Ntn1 in Confinement along the Rostrocaudal Axis
Compared to other aspects of neural development, little is known about the initiation or maintenance of the CNS-PNS boundary, which selectively permits the passage of neural processes— but not cell bodies—into peripheral nerves. Studies in the spinal cord have highlighted the importance of BCCs (Vermeren et al., 2003) and chemorepellents such as Ntn5 (Garrett et al., 2016), Sema3B, Sema3G, and Sema6A (Bron et al., 2007; Mauti et al., 2007) in retaining motor neuron cell bodies inside the CNS, even as they extend their axons out to the periphery. In stark contrast, confinement phenotypes have not been reported in the hindbrain, although BCCs express similar repellents at cranial nerve roots. For example, we found no evidence of ectopic Pax6+ neurons in the cochlea of Ntn5−/− mice (data not shown), despite the presence of ectopic motor neurons in the spinal cord (Garrett et al., 2016). This discrepancy underscores two points. First, the molecular mechanisms that define the CNS-PNS boundary in the vertebrate brainstem remain unknown; and second, the hindbrain and the spinal cord may have evolved unique ways of maintaining the CNS-PNS boundary.
Indeed, our work illustrates that the same molecule may have distinct functions in hindbrain versus spinal cord confinement. In the spinal cord, Ntn1 plays a relatively limited role, preventing the axons of a single population of neurons from straying into the periphery. Notably, the cell bodies do not follow in Ntn1 mutants (Laumonnerie et al., 2014). Thus, in this context, the misrouting of CNS axons into the PNS is much like other axon guidance phenotypes. In contrast, in the hindbrain, Ntn1 signaling appears to play an integral role in defining the CNS-PNS boundary, as evidenced by both the sheer number of neurons exiting the CNS and, most importantly, the departure of cell bodies, which an effective CNS-PNS boundary absolutely forbids. These differences in Ntn1 function may reflect the distinct developmental demands of the two brain regions: whereas migration is limited in the spinal cord, there is extensive migration of multiple populations of neurons over long distances and past multiple nerve roots in the hindbrain. As such, having a centrally derived cue play a weightier role in confinement may offer the greater fidelity and robustness needed for rhombic-lip derivatives to complete their migratory routes successfully. It remains to be seen whether another centrally derived cue might play a more prominent role in confinement in the spinal cord, particularly since most motor neurons stay within the CNS, even when all BCCs are ablated (Vermeren et al., 2003).
Cell-Autonomous and Non-Cell-Autonomous Functions for Multiple Ntn1 Receptors in Confinement
Our findings provide additional evidence for Ntn1’s multifunctionality, which likely depends on its diverse repertoire of receptors. PNs express receptors mediating both attraction, such as DCC, and repulsion, such as Unc5B/C. However, Ntn1-mediated confinement does not seem to depend on repulsion, since PNs remain within the CNS in Unc5b and Unc5c mutants (Di Meglio et al., 2013; Kim and Ackerman, 2011). Moreover, both HoxB4+ (Unc5B-low) and HoxB4− (Unc5B-high) PNs (Di Meglio et al., 2013) escape into the periphery in Ntn1 hypomorphs (Figure S7), indicating that differential responsiveness to Ntn1 cannot account for the partial phenotype. Thus, Unc5B/Cs appear to influence only the later stages of Ntn1-mediated PN migration, comparable to the way Unc5A/Cs position commissural neuron cell bodies in the spinal cord but are not required for confinement of their axons (Laumonnerie et al., 2014).
In contrast, as an obligate receptor expressed on commissural axons and PNs, DCC mediates confinement in both the spinal cord and hindbrain. We also discovered a surprising role for Neogenin in trans, as Neo1 is neither expressed nor required in migrating PNs, though it is present at low levels throughout the neuroepithelium and at higher levels on cranial nerves and in the surrounding mesenchyme. These data suggest that complete containment depends both on Ntn1-DCC signaling within PNs and also on Ntn1-receptor interactions in the environment. Since Ntn1-Neogenin interactions mediate adhesion in the developing mammary gland (Srinivasan et al., 2003), similar interactions in the BM around the VIIIth nerve could prevent movement into the nerve root, providing an additional safeguard for CNS-PNS segregation. However, any effects of Ntn1 signaling on the structural integrity of the CNS-PNS border are likely to be subtle, as the BM did not appear strikingly different in Ntn1−/− mutants by electron microscopy (EM) or immunostaining. Moreover, PNs stay confined to the CNS in ISPD mutants (data not shown), which have fragmented BMs (Wright et al., 2012). Hence, disrupting boundaries alone is not sufficient to induce the departure of CNS neurons, indicating that Ntn1 plays an active signaling role across multiple cell types in confining migrating neurons to the CNS.
Finding Unity in Ntn1’s Diverse Functions: a Role for Locally Produced Ntn1 in Neural Development
In addition to being the archetype of diffusible guidance cues, much of Ntn1’s prominence can be attributed to its versatility. Beyond its role in axon guidance, cell migration, and confinement, Ntn1 modulates angiogenesis and tissue morphogenesis, cell adhesion, synapse formation, and cell survival in cancer (reviewed in Cirulli and Yebra, 2007; Lai Wing Sun et al., 2011). Historically, a large emphasis has been placed on the division between long- and short-range functions, which are categorized based on where Ntn1 acts relative to the source of its expression. For instance, textbook models of Ntn1 as a long-range attractant depict commissural axons navigating along an increasing gradient of FP-derived Ntn1 in the spinal cord. The situation in vivo, however, is more complicated. Although Ntn1 is distributed in a gradient in the spinal cord (Kennedy et al., 2006) and can act over a distance in vitro (Kennedy et al., 1994; Yee et al., 1999), it was purified as a heparin-binding protein (Serafini et al., 1994) and found to interact with BM components, including type IV collagen and heparin sulfate proteoglycans (Geisbrecht et al., 2003; Geisen et al., 2008; Kappler et al., 2000). This had raised the possibility that it might function at both short- and long-range (Serafini et al., 1994; Kennedy et al., 1994), and a local role in short-range guidance was soon demonstrated at the optic nerve head (Deiner et al., 1997). Short-range functions have also been demonstrated during tissue morphogenesis, such as BM breakdown in the inner ear (Nishitani et al., 2017; Salminen et al., 2000) or adhesion between two cell layers in the mammary gland (Srinivasan et al., 2003).
Our findings add to a growing body of work that suggest that many of Ntn1’s other functions in the nervous system may be grounded in local signaling, a shared mechanism that may provide a foundation for its diverse roles. Membrane-tethered versions of Ntn, for example, can rescue guidance defects in the Drosophila nerve cord and visual system that were previously ascribed to soluble Ntn (Brankatschk and Dickson, 2006; Timofeev et al., 2012). More recently, several groups have demonstrated that commissural guidance depends on ventricular- zone-derived Ntn1 accumulating in the SPR and along the commissural axons (Dominici et al., 2017; Varadarajan et al., 2017; Yamauchi et al., 2017), expanding on related observations (Charron et al., 2003; Kennedy et al., 2006). We have similarly revealed a role for SPR-localized Ntn1 in cellular confinement, providing an alternative explanation for both the reduced number of PNs in Ntn1trap/trap mice, which was thought to reflect Ntn1’s tropic and trophic roles (Yee et al., 1999), and the presence of ectopic neurons in DCC−/− cochleae, which was attributed to a mis-positioning of SGNs (Kim et al., 2016). Thus, across multiple species and in multiple regions of the nervous system, Ntn1 appears to act locally to mediate its purported long-range functions.
Given the clear importance of Ntn1 for nervous system wiring, the possibility that Ntn1 may not act as a long-range instructive cue for PNs raises the question of where the directional information comes from. One idea is that a gradient of Ntn1 activity is achieved through interactions with other cues in the environment. Indeed, every confirmed Ntn1 receptor also interacts with additional ligands (Ahmed et al., 2011; Karaulanov et al., 2009; Rajagopalan et al., 2004; Yamagishi et al., 2011), raising the possibility that Ntn1 is a crucial collaborator for many guidance pathways, perhaps mediating short-range interactions that are necessary for axons to grow reliably toward other ligands. This could occur either directly, i.e., by binding to the same receptors, or indirectly, i.e., by attaching migrating neurons to the BM, where they may be steered by other cues such as Slits. This may explain Ntn1’s ability to augment the effect of other guidance molecules synergistically (reviewed in Morales and Kania, 2017). Thus, even 20 years after its discovery, Ntn1 continues to inform new models for how the complex networks of the nervous system are constructed reliably and accurately using relatively few guidance cues.
EXPERIMENTAL PROCEDURES
Further details and an outline of resources used in this work can be found in the Supplemental Experimental Procedures.
Animal Models
The following mouse strains were used and genotyped as described previously: Ntn1fl/fl, Ntn1+/− (Yung et al., 2015), Ntn1CE/+ (Nishitani et al., 2017), DCC+/−; Neo1Gt/+ (Fazeli et al., 1997; Leighton et al., 2001; Xu et al., 2014), Neo1−/− and Neo1fl/fl (Kam et al., 2016), Atoh1CreERT2 (Machold and Fishell, 2005), Six3Cre (Furuta et al., 2000), NestinCre (Tronche et al., 1999), MafBGFP (Moriguchi et al., 2006), and Ai14 Cre-dependent tdTomato (Madisen et al., 2010).
Mice were maintained on a C57BL/6 background. Noon on the day of the plug was considered E0.5. Tamoxifen (Sigma-Aldrich) injections were carried out at 20 mg/mL in sunflower oil at 1 mg/10 g of body weight. Since all experiments were performed on embryonic mice, whole litters, which included both male and female mice, were used for experiments. We did not detect any sex-based differences in our phenotype. The ages used in each experiment are included in the relevant text, figures, and figure legends. Experiments were performed with the observer blind to genotype, though the ensuing image analyses were not due to the obvious nature of the phenotypes. All animal experiments were approved by the Institutional Animal Use Care Committee at Harvard Medical School.
Statistical Analysis
All statistical comparisons were done using Prism software (GraphPad, La Jolla, CA, USA) and presented as mean ± SD. Statistical significance was determined by a Student’s t test when comparing between two groups. If more than two groups were being considered, a one-way ANOVA was performed with Tukey’s multiple comparisons test. In cases of the latter, the multiplicity adjusted p values were included in the figures, and the p value of the ANOVA was reported in the figure legends. Sample size for all experiments was determined empirically based on standards in the field. Specific details for each experiment are included in the text (n values and their meanings) or in the figure legends (statistical tests used).
Supplementary Material
Highlights.
Ntn1 is enriched in the sub-pial region (SPR) but absent at cranial nerve roots
Pontine neurons exit the CNS along nerves when SPR-localized Ntn1 is removed
DCC and Neogenin act in distinct cell types to confine pontine neurons to the CNS
Restoring Ntn1 to the SPR rescues the CNS-PNS boundary and pontine neuron migration
Acknowledgments
We thank the Neurobiology Department; the Neurobiology Imaging Facility (NINDS P30 NS072030); the HMS Electron Microscopy Core Facility; Drs. Kevin Wright (OHSU) and Rob Burgess (The Jackson Laboratory) for embryonic tissue; Jocelyn Curran and Emilie Dumontier for genotyping assistance; and Dr. Elio Raviola for help with EM analysis. We are also grateful to Dr. Alain Chédotal for sharing unpublished results. This work was supported by NIH grant R21 DC014916 (to L.V.G.), by NIDCD training grants F31 DC014603 and T32 DC000038 (to A.R.Y.), and by CIHR grants MOP136872 and MOP130387 (to J.-F.C.). Z.W. and M.T.L. were supported by the Rockefeller University and Stanford University.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.068.
AUTHOR CONTRIBUTIONS
A.R.Y. and L.V.G. designed the research, analyzed the results, and wrote the manuscript. N.R.D. discovered the phenotype and A.R.Y. performed all the experiments. J.-F.C. provided the Wnt1Cre; DCC−/−; Neo1fl/fl, and Neo1−/− tissue. Z.W. and M.T.-L. provided the DCC+/−; Neo1Gt/+ mice and tissue. J.-F.C., Z.W., and M.T.-L. also shaped the course of the study and provided input on the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Abraira VE, Del Rio T, Tucker AF, Slonimsky J, Keirnes HL, Goodrich LV. Cross-repressive interactions between Lrig3 and netrin 1 shape the architecture of the inner ear. Development. 2008;135:4091–4099. doi: 10.1242/dev.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed G, Shinmyo Y, Ohta K, Islam SM, Hossain M, Naser IB, Riyadh MA, Su Y, Zhang S, Tessier-Lavigne M, Tanaka H. Draxin inhibits axonal outgrowth through the netrin receptor DCC. J. Neurosci. 2011;31:14018–14023. doi: 10.1523/JNEUROSCI.0943-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcántara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. doi: 10.1242/dev.127.7.1359. [DOI] [PubMed] [Google Scholar]
- Bae G-U, Yang Y-J, Jiang G, Hong M, Lee H-J, Tessier-Lavigne M, Kang J-S, Krauss RS. Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro. Mol. Biol. Cell. 2009;20:4920–4931. doi: 10.1091/mbc.E09-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin JM, Han D, Lai Wing Sun K, Croteau L-P, Dumontier E, Cloutier J-F, Kania A, Kennedy TE. Complete loss of netrin-1 results in embryonic lethality and severe axon guidance defects without increased neural cell death. Cell Rep. 2015;12:1099–1106. doi: 10.1016/j.celrep.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Bloch-Gallego E, Ezan F, Tessier-Lavigne M, Sotelo C. Floor plate and netrin-1 are involved in the migration and survival of inferior olivary neurons. J. Neurosci. 1999;19:4407–4420. doi: 10.1523/JNEUROSCI.19-11-04407.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat. Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugeaud A, Tong M, Luo L, Edge ASB. Inhibition of repulsive guidance molecule, RGMa, increases afferent synapse formation with auditory hair cells. Dev. Neurobiol. 2014;74:457–466. doi: 10.1002/dneu.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causeret F, Danne F, Ezan F, Sotelo C, Bloch-Gallego E. Slit antagonizes netrin-1 attractive effects during the migration of inferior olivary neurons. Dev. Biol. 2002;246:429–440. doi: 10.1006/dbio.2002.0681. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Cirulli V, Yebra M. Netrins: beyond the brain. Nat. Rev. Mol. Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- de Diego I, Kyriakopoulou K, Karagogeos D, Wassef M. Multiple influences on the migration of precerebellar neurons in the caudal medulla. Development. 2002;129:297–306. doi: 10.1242/dev.129.2.297. [DOI] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Di Meglio T, Kratochwil CF, Vilain N, Loche A, Vitobello A, Yonehara K, Hrycaj SM, Roska B, Peters AHFM, Eichmann A, et al. Ezh2 orchestrates topographic migration and connectivity of mouse precerebellar neurons. Science. 2013;339:204–207. doi: 10.1126/science.1229326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P, Chédotal A. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature. 2017;545:350–354. doi: 10.1038/nature22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DP, Bradford D, Cooper HM. Neogenin is expressed on neurogenic and gliogenic progenitors in the embryonic and adult central nervous system. Gene Expr. Patterns. 2007;7:784–792. doi: 10.1016/j.modgep.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BLM, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–132. [PubMed] [Google Scholar]
- Garrett AM, Jucius TJ, Sigaud LPR, Tang F-L, Xiong W-C, Ackerman SL, Burgess RW. Analysis of expression pattern and genetic deletion of Netrin5 in the developing mouse. Front. Mol. Neurosci. 2016;9:3. doi: 10.3389/fnmol.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbrecht BV, Dowd KA, Barfield RW, Longo PA, Leahy DJ. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. J. Biol. Chem. 2003;278:32561–32568. doi: 10.1074/jbc.M302943200. [DOI] [PubMed] [Google Scholar]
- Geisen MJ, Di Meglio T, Pasqualetti M, Ducret S, Brunet J-F, Chedotal A, Rijli FM. Hox paralog group 2 genes control the migration of mouse pontine neurons through slit-robo signaling. PLoS Biol. 2008;6:e142. doi: 10.1371/journal.pbio.0060142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip Y-P, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DM, Morgan WJ, Jarjour AA, Spirou GA, Berrebi AS, Kennedy TE, Mathers PH. Molecular guidance cues necessary for axon pathfinding from the ventral cochlear nucleus. J. Comp. Neurol. 2007;504:533–549. doi: 10.1002/cne.21443. [DOI] [PubMed] [Google Scholar]
- Kam JWK, Dumontier E, Baim C, Brignall AC, Mendes da Silva D, Cowan M, Kennedy TE, Cloutier J-F. RGMB and neogenin control cell differentiation in the developing olfactory epithelium. Development. 2016;143:1534–1546. doi: 10.1242/dev.118638. [DOI] [PubMed] [Google Scholar]
- Kappler J, Franken S, Junghans U, Hoffmann R, Linke T, Müller HW, Koch KW. Glycosaminoglycan-binding properties and secondary structure of the C-terminus of netrin-1. Biochem. Biophys. Res. Commun. 2000;271:287–291. doi: 10.1006/bbrc.2000.2583. [DOI] [PubMed] [Google Scholar]
- Karaulanov E, Böttcher RT, Stannek P, Wu W, Rau M, Ogata S, Cho KWY, Niehrs C. Unc5B interacts with FLRT3 and Rnd1 to modulate cell adhesion in Xenopus embryos. PLoS ONE. 2009;4:e5742. doi: 10.1371/journal.pone.0005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J. Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Ackerman SL. The UNC5C netrin receptor regulates dorsal guidance of mouse hindbrain axons. J. Neurosci. 2011;31:2167–2179. doi: 10.1523/JNEUROSCI.5254-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Wang S-Z, Tymanskyj S, Ma L, Tao HW, Zhang LI. Dcc mediates functional assembly of peripheral auditory circuits. Sci. Rep. 2016;6:23799. doi: 10.1038/srep23799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil CF, Maheshwari U, Rijli FM. The long journey of pontine nuclei neurons: from rhombic lip to cortico-ponto-cerebellar circuitry. Front. Neural Circuits. 2017;11:33. doi: 10.3389/fncir.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- Laumonnerie C, Da Silva RV, Kania A, Wilson SI. Netrin 1 and Dcc signalling are required for confinement of central axons within the central nervous system. Development. 2014;141:594–603. doi: 10.1242/dev.099606. [DOI] [PubMed] [Google Scholar]
- Lee H, Song M-R. The structural role of radial glial endfeet in confining spinal motor neuron somata is controlled by the Reelin and Notch pathways. Exp. Neurol. 2013;249:83–94. doi: 10.1016/j.expneurol.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, Tessier-Lavigne M, Hogan BLM. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr. Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, McLaurin DL, Marks L, Vinson EN, Pfeifer M, Szulc SV, Heaton MB, Lee N. Immunohistochemical localization of netrin-1 in the embryonic chick nervous system. J. Neurosci. 1997;17:5466–5479. doi: 10.1523/JNEUROSCI.17-14-05466.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos S, Backer S, Causeret F, Tessier-Lavigne M, Bloch-Gallego E. Differential roles of Netrin-1 and its receptor DCC in inferior olivary neuron migration. Mol. Cell. Neurosci. 2009;41:429–439. doi: 10.1016/j.mcn.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev. 2007;2:28. doi: 10.1186/1749-8104-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Morales D, Kania A. Cooperation and crosstalk in axon guidance cue integration: additivity, synergy, and fine-tuning in combinatorial signaling. Dev. Neurobiol. 2017;77:891–904. doi: 10.1002/dneu.22463. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 2006;26:5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa N, Yagi H, Kato K, Takematsu H, Oka S. Ectopic clustering of Cajal-Retzius and subplate cells is an initial pathological feature in Pomgnt2-knockout mice, a model of dystroglycanopathy. Sci. Rep. 2015;5:11163. doi: 10.1038/srep11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DH, Bruce LL. Migratory routes and fates of cells transcribing the Wnt-1 gene in the murine hindbrain. Dev. Dyn. 2006;235:285–300. doi: 10.1002/dvdy.20611. [DOI] [PubMed] [Google Scholar]
- Niederländer C, Lumsden A. Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development. 1996;122:2367–2374. doi: 10.1242/dev.122.8.2367. [DOI] [PubMed] [Google Scholar]
- Nishitani AM, Ohta S, Yung AR, Del Rio T, Gordon MI, Abraira VE, Avilés EC, Schoenwolf GC, Fekete DM, Goodrich LV. Distinct functions for netrin 1 in chicken and murine semicircular canal morphogenesis. Development. 2017;144:3349–3360. doi: 10.1242/dev.144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kawamura K. Mode of neuronal migration of the pontine stream in fetal mice. Anat. Embryol. (Berl.) 1990;182:11–19. doi: 10.1007/BF00187523. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, Mueller BK, Strittmatter SM. Neogenin mediates the action of repulsive guidance molecule. Nat. Cell Biol. 2004;6:756–762. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- Ray RS, Dymecki SM. Rautenlippe redux – toward a unified view of the precerebellar rhombic lip. Curr. Opin. Cell Biol. 2009;21:741–747. doi: 10.1016/j.ceb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riyadh MA, Shinmyo Y, Ohta K, Tanaka H. Inhibitory effects of draxin on axonal outgrowth and migration of precerebellar neurons. Biochem. Biophys. Res. Commun. 2014;449:169–174. doi: 10.1016/j.bbrc.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Salminen M, Meyer BI, Bober E, Gruss P. Netrin 1 is required for semicircular canal formation in the mouse inner ear. Development. 2000;127:13–22. doi: 10.1242/dev.127.1.13. [DOI] [PubMed] [Google Scholar]
- Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, Turk R, Nguyen H, Ross-Barta SE, Westra S, et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J. Neurosci. 2010;30:14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiders FI, Maertens B, Böse K, Li Y, Brunken WJ, Paulsson M, Smyth N, Koch M. Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J. Biol. Chem. 2007;282:23750–23758. doi: 10.1074/jbc.M703137200. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Timofeev K, Joly W, Hadjieconomou D, Salecker I. Localized netrins act as positional cues to control layer-specific targeting of photoreceptor axons in Drosophila. Neuron. 2012;75:80–93. doi: 10.1016/j.neuron.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- van den Heuvel DMA, Hellemons AJCGM, Pasterkamp RJ. Spatiotemporal expression of repulsive guidance molecules (RGMs) and their receptor neogenin in the mouse brain. PLoS ONE. 2013;8:e55828. doi: 10.1371/journal.pone.0055828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan SG, Kong JH, Phan KD, Kao T-J, Panaitof SC, Cardin J, Eltzschig H, Kania A, Novitch BG, Butler SJ. Netrin1 produced by neural progenitors, not floor plate cells, is required for axon guidance in the spinal cord. Neuron. 2017;94:790–799.e3. doi: 10.1016/j.neuron.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeren M, Maro GS, Bron R, McGonnell IM, Charnay P, Topilko P, Cohen J. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron. 2003;37:403–415. doi: 10.1016/s0896-6273(02)01188-1. [DOI] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron. 2012;76:931–944. doi: 10.1016/j.neuron.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Wu Z, Renier N, Antipenko A, Tzvetkova-Robev D, Xu Y, Minchenko M, Nardi-Dei V, Rajashankar KR, Himanen J, et al. Neural migration. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science. 2014;344:1275–1279. doi: 10.1126/science.1255149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Hampel F, Hata K, Del Toro D, Schwark M, Kvachnina E, Bastmeyer M, Yamashita T, Tarabykin V, Klein R, Egea J. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. EMBO J. 2011;30:2920–2933. doi: 10.1038/emboj.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Yamazaki M, Abe M, Sakimura K, Lickert H, Kawasaki T, Murakami F, Hirata T. Netrin-1 derived from the ventricular zone, but not the floor plate, directs hindbrain commissural axons to the ventral midline. Sci. Rep. 2017;7:11992. doi: 10.1038/s41598-017-12269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yebra M, Montgomery AMP, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev. Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Yee KT, Simon HH, Tessier-Lavigne M, O’Leary DM. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24:607–622. doi: 10.1016/s0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]
- Yung AR, Nishitani AM, Goodrich LV. Phenotypic analysis of mice completely lacking netrin 1. Development. 2015;142:3686–3691. doi: 10.1242/dev.128942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelina P, Blockus H, Zagar Y, Péres A, Friocourt F, Wu Z, Rama N, Fouquet C, Hohenester E, Tessier-Lavigne M, et al. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron. 2014;84:1258–1272. doi: 10.1016/j.neuron.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Matsumoto T, Mikami S, Nagasawa T, Murakami F. SDF1/CXCR4 signalling regulates two distinct processes of precerebellar neuronal migration and its depletion leads to abnormal pontine nuclei formation. Development. 2009;136:1919–1928. doi: 10.1242/dev.032276. [DOI] [PubMed] [Google Scholar]
- Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat. Cell Biol. 2009;11:183–189. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.