Abstract
Development of drugs for new and persistent diseases will increasingly rely on the expansion of accessible chemical space to allow exploration of novel molecular targets. Here we report the synthesis of a library of novel fused heterobicyclic small molecules based on the 1,4-diazepine and 2,4-pyrrolidinedione scaffolds. Key chemical transformations included a Mannich-type condensation and chemoselective N-acylation reactions. Screening shows anti-cancer activity of several library compounds which suggests translational potential of this novel chemical scaffold.
Keywords: Library, Diazepine, Mannich reaction, Cancer
Graphical Abstract

Introduction
There is a continuous need for the generation of small molecule libraries to identify new lead compounds for drug discovery against novel targets.1 Incorporation of key scaffolds or pharmacophore-based structural features during the design of such small molecules aids in studying diverse biological targets. For instance, molecular frameworks containing the diazepine scaffold have been recognized to have significant medicinal relevance.2 The benzodiazepines motif, for example, is considered a ‘privileged structures’ with derivatives having a broad range of biological activity such as against central nervous system disorders,3 anti-HIV,4–5 anti-malarial and anti-cancer6–7 therapeutics. The benzodiazepine system is thought to have such wide utility in part because of its ability to mimic peptide β- and γ-turn moieties.8
Synthetic agents and natural products containing the 2,4-pyrrolidinedione ring system or tetramic acid core have similarly been shown to have promising biological activity for a number of important indications.9–11
Synthesis of tricyclic chemical compounds containing the 1,4-benzodiazepine and tetramic acid subunits has previously been documented.12 Such tricyclic pyrrolobenzodiazepine derivatives have been synthesized by the condensation reaction of enaminolactams with the corresponding aldehydes.12–13 While some of these compounds produced interesting biological activity,12 the addition of the third ring onto the benzodiazepine core would be expected to produce effects divergent from those of classical bicyclic fused benzodiazepines which mimic β-turn peptide features.8
To the best of our knowledge there have been no reports on the synthesis of bicyclic systems resulting from the fusion of 1,4-diazepine and 2,4-pyrrolidinedione subunits. This is interesting to note since the bicyclic ring system would be expected to possess lower CLogP values than the tricylic system,14 making them potentially more suitable as therapeutics.
Herein, we describe the synthesis of a novel series of bicyclic pyrrolodiazepines comprised of the 1,4-diazepine and 2,4-pyrrolidinedione subunits. This work enables the synthesis of a wide variety of diverse and novel drug-like compounds for potential biomedical applications. They may therefore be of great interest to medicinal chemists looking to prepare molecules that access new chemical space for novel drug targets.
Results
The overall synthetic approach is based on previously reported synthesis of furobenzodiazepines and pyrrolobenzodiazepines by Matsuo and Tanaka.13 Vinylogous urea 6 was synthesized in six steps from commercially available amino acid ester 1 as illustrated in Scheme 1. Reductive amination of amino acid ester 1 with benzaldehyde using NaBH4 as the reducing agent gave the corresponding secondary amine 2 in excellent yield (98%). N-acylation of amine 2 with ethyl 3-chloro-3-oxopropanoate in the presence of triethylamine (TEA) as base gave the intermediate diester. Interestingly, the intermediate acyclic amide could not be isolated as TEA was sufficient to promote the immediate Dieckmann condensation to form 5-membered ring 3 in 60% yield. Hydrolysis-decarboxylation of keto-ester 3 using aqueous AcOH provided pyrrolidinedione 4 in excellent yield (82%). Boc-protected enaminolactam 5 was obtained in 82% yield by treating 4 with tert-butyl (2-aminoethyl)carbamate in the presence of a catalytic amount of pTsOH. Finally, removal of the boc-protecting group afforded the desired vinylogous urea 6 in excellent yield (92%) which could enable further wide structural diversification. This rapid and efficient six step synthetic route furnished vinylogous urea 6 in an overall 36 % yield.
Scheme 1.
Synthesis of vinylogous urea 6.
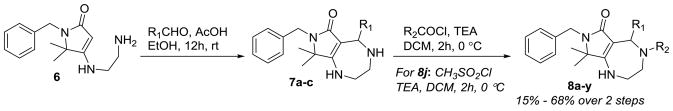
With the key vinylogous urea 6 in hand, we were able to synthesize the final pyrrolodiazepine-based compound library by diversification at two key sites. Scheme 2 depicts the sequence for generation of the desired library of compounds. Mannich-type condensation of 6 with various aldehydes (R1CHO) in the presence of AcOH proceeded smoothly to give the corresponding bicyclic amines 7. These reaction conditions were found to be compatible with various aldehydes including heteroaromatic and aliphatic aldehydes. Amines 7a–c were found to degrade when purification was attempted using normal phase flash chromatography and were therefore used without further purification. The crude amines (7a–c) were immediately subjected to chemoselective N-acylation reactions. Diverse acid chlorides (R2COCl) including aliphatic, heterocyclic, and aromatic were used to provide the final compounds 8a–8y. The sulfonamide could also be formed by using methanesulfonyl chloride and TEA (8j).
Scheme 2.
Synthesis of a library of bicyclic pyrrolodiazepine derivatives.
A subset of the compound library was submitted to the National Cancer Institute (NCI) for screening in their NCI60 panel for anti-cancer activity.15–17 Assay details are provided in the supplemental. Notably, compound 8f was found to reduce cell growth of A549 non-small cell lung cancer cells by 32% at a concentration of 10 μM. Compounds 8c and 8m each reduced of A549 cell growth by 24% at 10 μM. While the potency is less than optimal, we anticipate that the general synthetic route will facilitate further optimization and exploration of these polycyclic scaffolds.
Discussion
The diazepine and tetramic acid rings have proven to be privileged structures in biology and medicinal chemistry; however, there are remarkably few reports of heterocyclic scaffolds containing these two ring systems fused together. Here we report an efficient synthesis of a series of novel fused bicyclic heterocycles incorporating these motifs. The synthesis allows for extensive diversification of substituents on the unique 5,6-fused pyrrolodiazepinone ring system. We have demonstrated that this chemistry is wide in scope and can allow access to a large number of structurally diverse analogs. Yields for the final two-step sequence which allows wide diversification were highly variable and ranged from low (15% for 8d) to good (68% for 8k). There was no obvious pattern which explained the wide-ranging yields. However, it was observed that many of the final acylation reactions produced a large number of impurities (as assessed by LC/MS). As the intermediate free amines (7a–c) showed degradation to normal phase flash chromatography, it is possible that some of the amine degraded before the final acylation reaction, resulting in lower yields. Also, acylation of compounds 7a–c is likely not entirely chemoselective. For instance, besides the desired path to final compounds 8, the intermediate 7a–c has another amine and an enamine-like motif, both of which could contribute to impurities.
New potential drug targets are constantly being uncovered in the search for ways to treat existing diseases. In particular, there is increasing interest in developing small molecules that can modulate protein-protein18–19 and protein-DNA/RNA20–21 interactions. However, reliance on existing molecular space to pursue these challenging targets has led to frustratingly little success in the development of drugs against new target classes. One reason for this is that current compound libraries that are used for high-throughput screening and which define the chemotypes pursued in subsequent lead optimization cover very little chemical space.22 In addition, they often over-represent scaffolds with highly aromatic and flat structures which may be unsuitable for targeting new types of drug targets.1 Many of the new fused heterocycles we describe in this report have very high fractions of sp3 (Fsp3) character. For example, compound 8e has very favorable physiochemical properties, with a Fsp3 = 0.56 and a CLogP = 3.1.14 Future efforts that replace the 7-benzyl group with other less hydrophobic groups would enable the generation of even more diverse structures with lower CLogP values and potentially higher Fsp3 and solubility. Given the unique nature of this fused scaffold, the privileged structural character from which it was derived, and its highly 3-dimensional architecture, this may be a useful chemotype for many current and future drug discovery efforts.
To demonstrate the utility of this drug-like scaffold, several library members were screened in the NCI-60 cell line panel to identify anti-cancer activity.15–17 Four compounds were chosen that represented a diverse spectrum of structures: 8e, 8f, 8i, and 8m. These compounds had both aryl and alkyl groups at C-5, and at N-5, they had small cycloalkyl and heteroaryl groups, thereby providing reasonable chemical space coverage from this set of available compounds. Notably, compound 8f showed significant cytotoxicity against the non-small cell lung cancer line A549. Cell proliferation was reduced 32% at a single concentration of 10 μM. In addition, compounds 8c and 8m also showed substantial cell growth inhibition as each reduced viability of A549 cells by 24% at 10 μM. Compound 8e had essentially no effect on the proliferation of A549 cells (< 5% growth reduction). These results are especially significant since these three compounds are all closely structurally-related members of this drug-like bicylic system. This strongly suggests that these compounds share a common molecular target that may be exploitable for future development as a new anti-cancer strategy.
In conclusion, we have described the synthesis of a library of novel fused heterocyclic compounds. We expect that the synthetic routes we have described will allow the generation of many new diverse molecules from these potentially bioactive chemotypes. These molecules will enable chemical biology and drug discovery researchers to expand into new chemical space and explore new avenues for both basic and translational research.
Supplementary Material
Figure 1.
Examples of pharmacologically relevant diazepine-containing compounds.
Figure 2.
Representative natural products containing the tetramic acid scaffold.
Table 1.
Synthesis of bicyclic pyrrolodiazepines
Highlights.
A library combining features of diazepines and pyrrolidinediones was prepared
Highly efficient Mannich-type intramolecular cyclization is described
Library explore new chemical space with highly 3D and Sp3-rich molecules
Several compounds have significant anti-cancer activity
Acknowledgments
Financial support has been provided by the Dr. Scholl Foundation and by the National Cancer Institute of the National Institutes of Health under Award Number R01CA189074. Part of this work was performed by the Northwestern University Medicinal and Synthetic Chemistry Core (ChemCore) at the Center for Molecular Innovation and Drug Discovery (CMIDD), which is funded by the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust and Cancer Center Support Grant P30 CA060553 from the National Cancer Institute awarded to the Robert H. Lurie Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J Med Chem. 2009;52(21):6752–6. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 2.Horton DA, Bourne GT, Smythe ML. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chemical reviews. 2003;103(3):893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 3.Welsch ME, Snyder SA, Stockwell BR. Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol. 2010;14(3):347–61. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Braccio M, Grossi G, Roma G, Vargiu L, Mura M, Marongiu ME. 15-Benzodiazepines PartXII. Synthesis and biological evaluation of tricyclic and tetracyclic 1,5-benzodiazepine derivatives as nevirapine analogues. European journal of medicinal chemistry. 2001;36(11–12):935–49. doi: 10.1016/s0223-5234(01)01283-1. [DOI] [PubMed] [Google Scholar]
- 5.Merluzzi VJ, Hargrave KD, Labadia M, Grozinger K, Skoog M, Wu JC, Shih CK, Eckner K, Hattox S, Adams J, et al. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990;250(4986):1411–3. doi: 10.1126/science.1701568. [DOI] [PubMed] [Google Scholar]
- 6.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, White J, Kirilovsky J, Rice CM, Lora JM, Prinjha RK, Lee K, Tarakhovsky A. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468(7327):1119–23. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenstrom U, Skold C, Lindeberg G, Botros M, Nyberg F, Karlen A, Hallberg A. Design, synthesis, and incorporation of a beta-turn mimetic in angiotensin II forming novel pseudopeptides with affinity for AT1 and AT2 receptors. J Med Chem. 2006;49(20):6133–7. doi: 10.1021/jm051222g. [DOI] [PubMed] [Google Scholar]
- 9.Jeong Y-C, Moloney MG. Tetramic Acids as Scaffolds: Synthesis, Tautomeric and Antibacterial Behaviour. Synlett. 2009;2009(15):2487–2491. [Google Scholar]
- 10.Holloway CA, Matthews CJ, Jeong YC, Moloney MG, Roberts CF, Yaqoob M. Novel chiral skeletons for drug discovery: antibacterial tetramic acids. Chemical biology & drug design. 2011;78(2):229–35. doi: 10.1111/j.1747-0285.2011.01133.x. [DOI] [PubMed] [Google Scholar]
- 11.Royles BJL. Naturally Occurring Tetramic Acids: Structure, Isolation, and Synthesis. Chemical reviews. 1995;95(6):1981–2001. [Google Scholar]
- 12.Cui J, Matsumoto K, Wang CY, Peter ME, Kozmin SA. Synthesis of a high-purity chemical library reveals a potent inducer of oxidative stress. Chembiochem: a European journal of chemical biology. 2010;11(9):1224–7. doi: 10.1002/cbic.201000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo K, Tanaka K. Syntheses of the Novel Furo [3, 4-b] [1, 5] benzodiazepinone and Pyrrolo [3, 4-b] [1, 5] benzodiazepinone Systems. Chem Pharm Bull. 1984;32(9):3724–3729. [Google Scholar]
- 14.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 15.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6(10):813–23. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 16.Boyd MR, Paull KD. Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Development Research. 1995;34(2):91–109. [Google Scholar]
- 17.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 18.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3(4):301–17. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 19.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013;19(2):202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 20.Rzuczek SG, Colgan LA, Nakai Y, Cameron MD, Furling D, Yasuda R, Disney MD. Precise small-molecule recognition of a toxic CUG RNA repeat expansion. Nat Chem Biol. 2017;13(2):188–193. doi: 10.1038/nchembio.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velagapudi SP, Cameron MD, Haga CL, Rosenberg LH, Lafitte M, Duckett DR, Phinney DG, Disney MD. Design of a small molecule against an oncogenic noncoding RNA. Proc Natl Acad Sci U S A. 2016;113(21):5898–903. doi: 10.1073/pnas.1523975113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipinski C, Hopkins A. Navigating chemical space for biology and medicine. Nature. 2004;432(7019):855–61. doi: 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.