Abstract
The Exercise Program in Cancer and Cognition (EPICC) Study is a randomized controlled trial designed to test the effects of moderate-intensity aerobic exercise on cognitive function in postmenopausal women with early-stage breast cancer during the first six months of aromatase inhibitor therapy. It is estimated that up to 75% of survivors of breast cancer experience cognitive impairment related to disease and treatment. At present, there are no known interventions to improve or manage cognitive function for women with breast cancer. Here, we describe a single-blinded, randomized controlled trial with allocation of 254 postmenopausal women with early-stage breast cancer to a supervised six-month aerobic exercise intervention or usual care. Prior to beginning aromatase inhibitor (AI) therapy, participants complete baseline assessments of cognitive function, cardiorespiratory fitness, blood-based biomarkers, physical activity and sleep, and symptoms (fatigue, sleep problems, depressive symptoms, anxiety). A random subset of participants (n=150) undergoes neuroimaging procedures that include structural and functional magnetic resonance imaging assessments. All participants maintain an activity diary; physical activity and sleep monitoring is repeated three and seven months post-randomization. The remaining baseline assessments are repeated seven months post-randomization. If successful, exercise could be a low-cost method to improve cognitive function in women with breast cancer that is easily adaptable to the home or community.
Keywords: breast cancer, aerobic exercise, cognitive function, neuroimaging, aromatase inhibitor, randomized controlled trial
1. Introduction
Women with breast cancer are the largest group of cancer survivors in the US. At present, there are more than 3.6 million breast cancer survivors living in the US, and this number is projected to rise to 4.5 million by 2026 owing to advances in early detection and treatment along with the aging of the general population.1,2 Women are increasingly diagnosed with localized (62%) or regional (31%) stage disease which carries 99% and 85% five-year survival rates, respectively.1,3 Most women with breast cancer (80%) have hormone-receptor positive disease4 and are postmenopausal, with a mean age of 61 years, at the time of diagnosis.2 Endocrine therapy, primarily with aromatase inhibitors (AIs), is a mainstay of adjuvant treatment for these women.5 While AIs have improved disease-free and overall survival for postmenopausal women with early stage breast cancer, this therapy is associated with several negative sequelae, among them is a deterioration in cognitive function.
Cognitive function is the information-handling aspect of behavior comprised of multiple, interrelated domains including attention, learning and memory, mental flexibility, psychomotor efficiency, visuospatial ability and executive function.6 It is estimated that up to 75% of breast cancer survivors experience a decline in cognitive function7 which may be short-term or persist years beyond completion of treatment.8,9 While the cognitive decline is typically mild or moderate, and does not meet clinical criteria for dementia, it can negatively impact survivors’ ability to perform everyday tasks as well as long-term quality of life, occupational achievement, development and maintenance of social relationships, appropriate self-care and treatment adherence.10,11
Reductions in concentration, working memory and executive function have been observed with endocrine therapy. Furthermore, there is evidence that, compared to age and education-matched women without breast cancer, some women with breast cancer experience poorer cognitive function prior to beginning systemic adjuvant therapy.12-14 Specifically, results from a large, longitudinal cohort study of postmenopausal women with early-stage breast cancer show decline in working memory and concentration during the first six months of AI therapy in women who received anastrozole with or without chemotherapy. Compared to age- and education-matched controls, women with breast cancer also had poorer executive function prior to beginning systemic adjuvant therapy and at six-months post-initiation of AI treatment.12
In postmenopausal women with breast cancer, multiple disease and treatment-related factors, including estradiol reduction, cytokine dysregulation along with common co-occurring symptoms such as depression, anxiety, fatigue and sleep problems, likely contribute to cognitive changes, although these mechanisms are not well-understood.15,16 While there are no known interventions to manage deteriorations in cognitive function in cancer survivors,17 exercise is a promising, low-cost and highly accessible approach particularly for postmenopausal women with breast cancer. Several randomized controlled trials have demonstrated that six months or more of moderate-intensity exercise can improve cognitive function in older, community-dwelling adults.18,19 Moreover, the greatest effects in these studies appear to be in women20 and in executive function, memory and psychomotor efficiency,17,18 the very cognitive domains that decline with AI therapy in breast cancer.12
Previous studies examining the influence of physical activity on cognitive function in individuals with cancer produced conflicting results. Rogers21 found no change in self-reported cognitive function in a randomized, controlled trial of a 12-week physical activity intervention (n=20) in pre and postmenopausal women with breast cancer about three years post-diagnosis. In unpowered subgroups of two Cochrane reviews, the effects of exercise on self-reported cognitive function were explored during22 and after the completion of cancer therapy.23 They found no significant effect of exercise on self-reported cognitive function at post-treatment but trends in improved self-reported cognitive function during therapy. The authors acknowledged that the heterogeneity of the exercise modes (i.e., aerobic, strength training, Tai Chi, Yoga, Qigong) and the few studies that examined self-reported cognitive function as an outcome, no conclusions could be drawn. More recently, Hartman et al24 found improved processing speed among breast cancer survivors diagnosed within the previous two years following a 12-week physical activity intervention. The bases for the conflicting results of previous studies are likely due to the disparate samples, physical activity interventions and timing of intervention delivery relative to the cancer diagnosis and treatment. Moreover, most studies used self-report and lacked objective, multidimensional measures of cognitive function.
The purpose of this paper is to describe the protocol of a randomized controlled trial designed to examine whether moderate-intensity aerobic exercise, initiated before beginning AI therapy, improves cognitive function in postmenopausal women with early-stage breast cancer.
2. Methods/design
2.1 Study design
Exercise Program in Cancer and Cognition (EPICC) is a single-blinded randomized controlled trial (RCT) with allocation of 254 postmenopausal women with early-stage breast cancer to a supervised six-month moderate intensity aerobic exercise intervention or usual care (Figure 1), NCT02793921. The design, conduct and reporting of this RCT adheres to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for trials. Human subjects’ approval was obtained from the University of Pittsburgh Human Research Protection Office (PRO15120433) and the Carnegie Mellon University Institutional Review Board (study2016_00000197).
Figure 1.
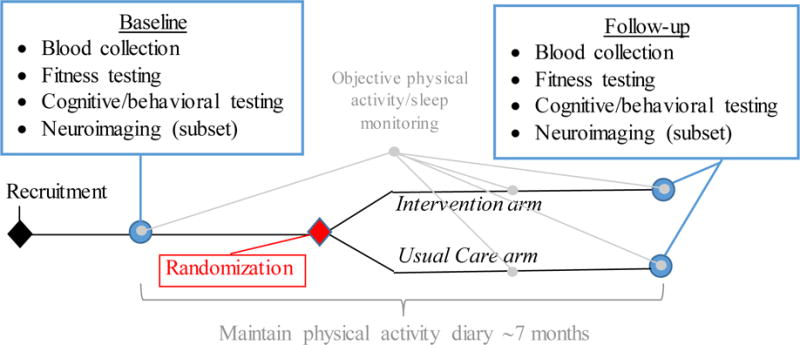
Study schema
2.2 Specific aims
The specific aims include
Compared to usual care, examine whether the 6-month exercise intervention improves cognitive function over the first six months of AI therapy in postmenopausal women with early stage breast cancer. Hypothesis 1. Exercise will improve cognitive function in women receiving AI therapy in a domain specific fashion such that attention, executive and memory functions will be influenced more than other domains.
Compared to usual care, examine the direct effects of exercise on (a) neuroimaging metrics of brain health including regional gray matter volume, white matter architecture and functional dynamics of the brain and (b) the pro-inflammatory biomarkers [Interleukin-6 (IL-6) and C-reactive protein (CRP) as primary outcomes; tumor necrosis factor-alpha (TNF-α) as a secondary outcome, and explore the direct effects of exercise on symptoms (fatigue, sleep problems, depressive symptoms, anxiety). Hypothesis 2. Exercise will improve neuroimaging metrics of brain health and reduce circulating levels of pro-inflammatory biomarkers.
Compared to usual care, explore whether the effects of exercise on cognitive function are mediated by a) neuroimaging metrics of cognitive function, b) IL-6, CRP and TNF-α levels and c) symptoms (fatigue, sleep problems, depressive symptoms, anxiety), and moderated by estradiol (E2) levels over the first six months of AI therapy.
2.3 Setting
Participants are recruited from the Comprehensive Breast Care Program of the University of Pittsburgh Cancer Institute and UPMC Cancer Centers. Neurocognitive testing takes place in a private conference room at the University of Pittsburgh and participant homes. Neuroimaging is conducted in a subgroup of participants at the Scientific Brain Imaging Center (SIBR) at Carnegie Mellon University. The exercise intervention is delivered at the University of Pittsburgh (Brain Aging and Cognitive Health Lab; Director, Co-PI: Erickson) and several neighborhood-based sites (e.g., YMCAs, Jewish Community Centers) which have been strategically selected to represent the main geographic areas surrounding Pittsburgh. All non-University of Pittsburgh intervention sites have partnered with EPICC to facilitate the implementation of the intervention and obtained Federal Wide Assurance for the protection of human subjects.
2.4 Eligibility screening and recruitment
Participants are primarily recruited at the time of their initial post-operative appointment which typically takes place 2-3 weeks following breast surgery and 3-4 weeks before commencing radiation therapy. At the time of this appointment, a clinician provides a brief introduction to the study and if the patient is interested in learning more, a research assistant is present to provide more detail about the study and further assess eligibility. Eligibility is confirmed once an interested participant has received her treatment plan which can take place up to two weeks after the initial meeting at the post-operative visit.
Eligible women are a) postmenopausal, b) younger than 80 years of age, c) diagnosed with stage 0, 1, 2 or 3a breast cancer, d) eligible to receive, but have not yet begun, AI therapy, and e) English-speaking with a minimum of 8 years of education.
Women who meet the following criteria are excluded if they report: a) prior diagnoses of any type of cancer (excluding some skin cancers), b) clinical evidence of distant metastases, c) self-report of hospitalization for psychiatric illness within the past two years, d) history of neurological illness, e) breast cancer surgery complications (e.g., persistent seroma requiring aspiration, wound dehiscence, infection, prolonged drain output, lymphedema as confirmed by participant’s surgical oncologist), f) reconstructive surgery within study period, g) eating disorders, h) history of substance abuse, i) use of assisted walking device, j) history of falls or balance problems, and k) any significant medical condition that would make the participant unsafe to exercise.
A subgroup of participants (n=150) is randomly selected for neuroimaging. Participants who a) have a presence of metal implants or b) self-reported claustrophobia are not included in the random selection process for the neuroimaging subgroup. Additionally, participants whose estimated VO2max is in the upper 50th percentile for women of similar age are excluded from neuroimaging and randomization in order to focus the intervention on those participants that are likely not already relatively active.
2.5 Baseline assessments
Prior to beginning AI therapy, all participants complete a two-visit baseline assessment. These visits typically take place toward the end of a participant’s 4-week radiation therapy. Because AI therapy is frequently prescribed to begin 1-2 weeks following radiation, this timeframe ensures that participants may complete baseline visits prior to beginning their AI and begin their group assignment (described below) within 2 weeks of beginning their AI. The first visit consists of blood collection and fitness testing. The second visit consists of cognitive testing and a collection of other behavioral measures. Objective physical activity and sleep data are collected. A subset of participants (n=150) is randomly selected to undergo neuroimaging during a third study visit. Written informed consent is obtained at the time of the first baseline visit prior to participating in any data-related activities.
2.5.1 Blood collection
Approximately 30cc or six teaspoons of blood is drawn for assessment of proinflammatory cytokines and estradiol (E2) levels prior to beginning the fitness test. Blood sampling is postponed in subjects experiencing symptoms of an acute infection via an 8-item screening tool. Following blood collection, samples are immediately processed and stored in accordance with set protocols for future analyses of IL-6, TNF-α, CRP and E2.
2.5.2 Fitness Testing
To monitor the fidelity of the intervention, cardiorespiratory fitness is measured in all participants via a submaximal graded exercise test which is often used in at-risk populations as a safer yet highly correlated (r > 0.80) alternative to a maximal VO2 test.25 This test requires walking on a treadmill between 2.0–4.0 mph as the grade increases 1% each minute. Test speed is self-selected by the participant at increments of 0.5 mph within the 2.0-4.0 mph range. Prior to the test, height and body mass are recorded and the participant is fitted with a mouthpiece and nose clip to collect expired air. Resting heart rate and resting blood pressure values are also obtained to ensure they are within a safe range to begin testing. Heart rate, blood pressure, respiratory gases and ratings of perceived exertion are continuously monitored during the test. The test is terminated when the participant reaches 85% of her age-predicted maximal heart rate (220–age), a Borg26 rating of perceived exertion of 15 or greater in participants taking a beta blocker, or volitional exhaustion. Peak oxygen consumption is analyzed using a ParvoMedics metabolic cart and data is extrapolated to estimate maximal oxygen consumption (VO2 max). Participants with an estimated VO2 max above the upper 50th percentile complete all baseline assessments, but are not selected for randomization (described below). For randomized participants, changes in aerobic capacity between baseline and follow-up testing are a marker of the efficacy of the aerobic intervention.
2.5.3 Cognitive and behavioral testing
To assess multiple domains of cognitive function, a comprehensive neuropsychological battery of computerized and paper-and-pencil tasks is administered to participants (Table 1).27–34 Our battery includes 4 tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB) Connect, a computerized battery that consists of measures documented to be sensitive to changes in cognitive function in women with breast cancer35 and that provides immediate data and detailed accuracy and response speed recording. The primary endpoint, changes in cognitive function, will be derived from normed (for age, education) composites of neuropsychological test scores (expressed as mean Z-scores), with the composite scores for the targeted cognitive function domains empirically derived via exploratory factor analysis.
Table 1.
Comprehensive neuropsychological battery
| Cognitive domain | Task |
|---|---|
| Attention | CANTAB* Connect Rapid Visual Processing |
| Digit Vigilance Test | |
| Learning and memory | CANTAB Connect Paired Associates Learning |
| CANTAB Connect Spatial Working Memory | |
| Rivermead Behavioral Memory Test | |
| Rey Auditory Verbal Learning Test | |
| Rey Complex Figure Test | |
| Executive function | CANTAB Connect One-touch Stockings of Cambridge |
| Delis Kaplan Verbal Fluency Test | |
| Delis Kaplan Color Word Interference | |
| Mental flexibility | Trail Making Test - B |
| Psychomotor efficiency | Digit Symbol Substitution |
| Grooved Pegboard | |
| Visuospatial ability | Rey Complex Figure Test |
Cambridge Neuropsychological Test Automated Battery
Participants also complete measures of perceived cognitive functioning, mood, fatigue and sleep problems. Perceived cognitive functioning is measured using the Patient Assessment of Own Functioning.36,37 Mood is measured using the Beck Depression Inventory II38 and the Patient-Reported Outcomes Measurement Information System® (PROMIS®) 8-item Emotional Distress – Anxiety questionnaire.39 Fatigue is measured using the PROMIS® 8-item Fatigue questionnaire.39 Sleep problems are measured using the Pittsburgh Sleep Quality Index,40 Epworth Sleepiness Scale41 and monitoring devices (described below). General intelligence is estimated using the National Adult Reading Test–Revised.42 Information related to participants’ demographics, disease and treatment, personal and family health history and lifetime exposure to hormones are also collected.
2.5.4 Objective physical activity and sleep monitoring
Physical activity and sleep are measured objectively using the SenseWear Armband (BodyMedia, Inc.).43 The device is placed on the participant’s upper arm and she is instructed to wear it at all times (other than showering, swimming, or bathing) for 7 days. Minute-by-minute data are used to quantify her energy expenditure and sleep efficiency. This device is worn by all participants, regardless of group assignment, at baseline, the 3-month midpoint, and at the 6-month post-intervention assessment.
2.5.5 Neuroimaging
Participants whose estimated VO2max is within the lower 50th percentile for age and meet the additional eligibility criteria for neuroimaging may be randomly selected for neuroimaging based on group assignment, age and executive function composite score. Our aim was to randomize a representative sample (n=150) of the larger group (n=254) for neuroimaging.
Neuroimaging assessments include the measure of brain morphology using high-resolution T1 weighted images, hippocampal subfields using a T2-weighted sequence localized to the hippocampus, a diffusion-weighted sequence for white matter imaging and tractography, a FLAIR image for assessing white matter hyperintensities, a resting state BOLD sequence for examining intrinsic connectivity, arterial spin labeling for measuring cerebral blood flow, and task-evoked activation using a n-back working memory paradigm.
2.6 Randomization
Following completion of the baseline assessments, participants who meet the eligibility criteria for randomization (i.e., estimated VO2 max < 50%) are randomly assigned via Study360, a web-accessible software system, which uses a minimization algorithm with equal allocation to one of two arms: 1) exercise intervention, or 2) usual care. Use of a computerized minimization algorithm insured treatment balance on two factors: 1) age at study entry (<60, ≥60 to <70, ≥70 years), and 2) baseline levels of cognitive function, particularly executive function (based on the composite mean Z-score, < −0.5, ≥ −0.5 to ≤ +0.5, > +0.5). In this study, the treatment assignment probability is adjusted in response to the marginal distributions of categories of age at entry and baseline executive function such that the marginal distributions of these categories will be approximately equal between treatment groups.
2.7 Blinding
All investigators and staff responsible for conducting cognitive and behavioral or neuroimaging assessments are blinded to group assignment. The study project director and exercise physiologists who administer the intervention are not blinded.
2.8 Follow-up
Following baseline testing, all participants are asked to maintain a weekly log of aerobic exercise via an automated REDCap44 survey. Following completion of group assignment, participants are asked to return for follow-up assessments which include the measures collected at baseline as described above. To limit practice effects, alternative cognitive battery test forms are used during follow-up assessments. Objective physical activity and sleep monitoring for a period of one week is repeated at midpoint (approximately 3 months after beginning group assignment as described below) and follow-up. Return for follow-up is encouraged through participant remuneration (up to $340), regular mailings including thank you notes, biannual study newsletters, and birthday cards as well as a phone call from the project director at midpoint. Additionally, participants who do not complete their weekly log of aerobic exercise are promptly contacted by an exercise physiologist in order to obtain this information; this also ensures participation remains ongoing.
2.9. Experimental Design
Upon completion of baseline testing and randomization, participants are notified of group assignment via telephone by the unblinded project director. At that time, a participant’s group assignment, exercise or usual care, is scheduled to begin within 2 weeks of beginning her AI therapy.
2.9.1 Intervention Arm
The intervention is consistent with the current American College of Sports Medicine and American Cancer Society recommendations for cancer patients and survivors and adheres to basic principles for exercise programming which advocate a minimum of 150 minutes of moderate-intensity or 75 minutes of vigorous exercise per week.45,46 Participants randomized to the intervention arm begin with exercising 10-15 minutes, three times per week during the first two weeks. The duration gradually increases during the following four weeks until the participant reaches 45-60 minutes of exercise three days per week. This level is then maintained for the remainder of the six months. The rate of increase is tailored to each participant based on her baseline fitness test and response to exercise.
Research staff trained in exercise physiology and supervision are present at each exercise session to monitor adherence, intensity and safety. Sessions may be one-on-one or held in small groups of two to four participants at a time. Each exercise session begins and ends with a vital sign check and 5-10 minutes of warm-up and cool-down. Participants perform aerobic exercise on treadmills, cycling and elliptical machines, and indoor walking tracks. Exercise intensity is measured continuously via a Polar heart rate monitor and every 15 minutes via a rating of perceived exertion using the unmodified BORG scale (6-20). Participants are encouraged to maintain moderate-level intensity that is equivalent to 60-75% of age-predicted maximal heart rate.
2.9.2 Usual Care Arm
Participants randomized to the usual care arm are instructed to continue their usual activities for six months. No intervention or exercise recommendations are delivered; self-initiated exercise is neither withheld nor limited.
2.10 Statistical methods
2.10.1 Preliminary Analyses
Exploratory analyses will be first performed for data description and screening for anomalies (e.g., outliers, nonnormality) that may invalidate the results based on the planned analysis. This will be done to: 1) describe univariate and bivariate distributions; 2) identify group imbalances and associations between dependent variables and suspected covariates/ confounders; 3) evaluate missing data; and 4) check for violation of statistical assumptions. If assumptions are violated, data transformations or more statistically robust procedures will be considered. Covariates/ confounders will be included in models secondarily and their effects on primary predictors/factors will be evaluated. The randomness of missing data will be investigated using available information on subject characteristics to help discern patterns in missing data, identify possible missing data mechanisms and inform strategies to handle missing data. If the data are ignorably missing, the estimation procedures to be used will produce unbiased estimates while allowing us to retain observations with missing values on the outcome variables. If needed, multiple imputation would be used to impute missing values on covariates. If the data are not ignorably missing, we may use selection or pattern mixture modeling to investigate the sensitivity of results.
2.10.2 Analysis Plan for Aim 1
An “intent-to-treat” (ITT) approach will be used for data analysis such that all participants will be invited to return for follow-up assessments and included in the groups to which they were assigned, regardless of adherence to protocol, treatment received, withdrawal or protocol deviation. Although this approach is recommended for efficacy analyses in RCTs, the sensitivity of the results assuming ITT will be explored using information collected regarding participant’s adherence to the exercise intervention (e.g., per protocol, amount of treatment received) as well as the amount of activity engaged in by the usual care group.
Linear mixed-effects modeling with linear contrasts will be used to examine the effect of treatment assignment on cognitive function over time. When fitting models, time will be a repeated within-subjects factor, while treatment assignment will be a between-subjects factor, with an interaction between time and group. Each derived cognitive domain composite will be modeled separately and interaction terms will be included to test our hypothesis that executive, memory, and attention domains will be more affected than other domains. We will consider baseline cognitive function values both as an assessment point and as a possible covariate in analyses. Fixed and/or time-dependent covariates may be included to adjust for group imbalances or variables related to the dependent variables. Standard fit indices will also be used to identify the most appropriate covariance structure for the repeated measures. F-tests will test the main and interaction effects included in the model. Individual regression parameters will be computed and reported with standard errors to yield confidence intervals. For each model, residual analysis will be conducted to identify sources of model misspecification, outliers and influential observations. Sensitivity analyses will also be performed to discern the impact of influential cases on results. To test specific hypotheses, linear contrasts will be specified and estimated in the repeated measures model to compare changes from baseline to follow-up values between groups. Wald t-statistics will be used to test each contrast. Marginal modeling with generalized estimating equations will also be used as it tends to be more robust to misspecification of the covariance structure and violations in normality assumptions. Results from each modeling approach will be compared via sensitivity analyses.
2.10.3 Analysis Plan for Aim 2
We will use the same analytical approach described for Aim 1 to fit models for intermediate outcomes (neuroimaging metrics of brain heath, inflammatory markers, and symptoms) and to test Aim 2 hypotheses regarding the effects of exercise on primary intermediate outcomes (neuroimaging metrics and inflammatory markers of IL-6 and CRP). The only difference will be that initial neuroimaging analyses will be conducted on a voxel-wise basis using FSL, SPM, or FreeSurfer, depending on the imaging modality and analysis (e.g., volumetric). In addition to voxel-wise analyses we may apply regions-of-interest (ROI) analyses since our primary hypotheses focus on prefrontal and hippocampal regions. These ROIs can be generated using anatomical templates from normalized MNI space or from functionally-derived estimates of activation or connectivity. We will use standard cluster-based corrections for multiple comparisons of fMRI results, threshold-free cluster enhancement, and a false discovery rate (FDR) of p<.01 for volumetric and white matter analyses.
2.10.4 Analysis Plan for Aim 3
We will explore neuroimaging metrics of brain health, biomarkers, and symptoms as variables that may mediate the effects of the exercise intervention on cognitive function and E2 as a possible moderator. Initially, we will fit simple mediational models (predictor, single mediator, single outcome) applying the change score method and estimate whether effects of the exercise intervention (predictor) on changes in cognitive function outcomes are mediated through changes in the suspected mediators. Goodness-of-fit will be assessed through standard summary indices (e.g., RMSEA, CFI) and residual analyses. Total, indirect and direct effects will be estimated and reported with confidence intervals. Additionally, we will explore mediation applying the half-longitudinal approach proposed by Cole and Maxwell47 as well as an autoregressive mediational approach. Depending on the results from simple mediational modeling, we may also combine mediators into a multiple or serial mediator models to develop a more comprehensive picture of these pathways. Voxel-based mediation of neuroimaging results will be conducted using BRAVO by employing the same approach described above. To explore E2 as a moderator of exercise effects on cognitive function, the models developed in Aim 1 will be expanded to also include E2 as main effect as well as interactions of E2 with the other fixed effects.
2.10.5 Exploratory analysis
We will also explore whether physical activity diary data are related to objective physical activity data. If validated, we will explore the physical activity pattern over 6 months and its relationship to change in outcomes.
3. Discussion
The main purpose of EPICC is to examine whether moderate-intensity aerobic exercise, initiated before beginning AI therapy, can improve cognitive function in postmenopausal women with early-stage breast cancer. If successful, exercise would be a low-cost, self-supportive method of care to improve cognitive function in women with breast cancer that is easily adaptable to the home or community.
Historically, patients with cancer were advised to rest and avoid activity; however, this recommendation has been challenged by numerous studies which have demonstrated the beneficial effects of exercise during cancer treatment including improved physical functioning, quality of life and fatigue and possibly improved prognosis and survival.22,23,48–50 In a 2010 roundtable consensus report,45 the American College of Sports Medicine published evidence-based, site-specific guidelines for exercise testing and programming for survivors of cancer. In accordance with the 2008 Physical Activity Guidelines for Americans51 and the American Cancer Society,46 these guidelines include a recommended volume of at least 150 minutes per week of moderate-intensity exercise or 75 minutes of vigorous intensity exercise or an equivalent combination. Yet, few survivors meet these guidelines52,53 and moreover, there is evidence that physical activity levels often reduce significantly following a diagnosis of breast cancer.54,55
Knowledge gleaned from our study may help cancer care providers to make specific recommendations to women with breast cancer receiving AI therapy or inform the development of health promotion materials for patients. In a recent qualitative study, Smaradottir et. al56 reported that patients with cancer believe that exercise is important and want to receive exercise prescription from there oncology providers, yet few receive any guidance or support regarding exercise from any health care provider. On the other hand, oncologists feel reluctant to include exercise as a routine part of a comprehensive treatment plan due to a variety of reasons ranging from lack of specialized knowledge of exercise to clinic visit time constraints.
Results from this study may also help to elucidate the multiple behavioral and biological mechanisms underlying the changes in cognitive function experienced by women with cancer as well as the effects of exercise on cognitive function, both of which are not well understood. Among the biological pathways involved with cognitive changes with cancer and therapy, there is evidence that inflammation with corresponding changes in levels of pro-inflammatory cytokines may be involved. Higher levels of IL-6 were related to greater self-reported cognitive problems in women with breast cancer receiving chemotherapy and higher TNF-R2 levels were found in pre and postmenopausal women with breast cancer who had completed chemotherapy over 8 months before evaluation compared to women who had not received chemotherapy.57,58 Increases in inflammatory cytokines are also associated with symptoms commonly experienced by women with breast cancer including depression, anxiety, fatigue and sleep problems, also referred to as “sickness behavior.”59
Neuroimaging techniques including fMRI, diffusion tensor imaging (DTI) and positron emission tomography (PET), have been used to study the effects of cancer and cancer therapy on cognitive function.60 These same techniques are useful in elucidating the effects of exercise on cognitive function in adults. For example, Erickson et al.61 demonstrated that exercise was capable of increasing hippocampal volume and improving spatial memory in healthy older adults.
Strengths of this study include use of a single-blinded, randomized controlled design with initiation of the intervention within two weeks of women beginning their AI therapy. This is based upon our prior work demonstrating poorer executive function at this time point. Another strength of the intervention is that it takes place in a community setting facilitating easy access for participants. In order to accommodate the busy lives of participants, the option for neurocognitive testing to take place in their homes is offered.
Although restricting our sample strengthens the methodology of the study, generalizability may be somewhat limited to postmenopausal women with endocrine receptor positive cancer. Future studies may extend the intervention to women who receive chemotherapy, premenopausal women with breast cancer, and in patients with other types of cancers that are known to be associated with cognitive deterioration. This study does not include postmenopausal women with early-stage breast cancer who receive chemotherapy. According to a 2016 Cochrane review62, few exercise trials have evaluated exercise during chemotherapy or radiation, and there are no studies that have examined the use of exercise to improve cognitive function specifically during AI therapy.
With only two time points, we will be unable to examine non-linear change in cognitive or brain health nor will we be able to identify an inflection point where exercise loses its efficacy. It will be important for future research to address the long-term effects of aerobic exercise on neurocognitive function in women with breast cancer, as well as the dose-response. Moreover, it will be important to determine whether a booster of some type is needed to promote sustainability of the effects of exercise.
We have chosen to use a usual care group to compare to the exercise intervention because an attention control group at this stage would be premature and cost-restrictive. Nonetheless, we recognize the benefits of an attention-control group and believe that future studies would warrant such a group if the results from this study were positive.
Other than skin cancer, breast cancer is the most prevalent cancer among women in the US.63 This randomized controlled trial should provide important data to support an easily accessible intervention to improve cognitive function in women with the disease. Findings may also enhance our understanding of the mechanisms underlying the changes in cognitive function experienced by these women as well as the mechanisms underlying the effects of exercise on cognitive function. Ultimately, the results of this study may also extend to patients who experience deterioration in cognitive function with other types of cancer and cancer therapies.
Acknowledgments
National Cancer Institute
Funding: National Cancer Institute [R01CA196762].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: Clinicaltrials.gov NCT02793921. Registered 20 May 2016.
Contributor Information
Amanda L. Gentry, University of Pittsburgh School of Nursing.
Susan M. Sereika, University of Pittsburgh School of Nursing and Graduate School of Public Health.
Frances E. Casillo, University of Pittsburgh School of Nursing.
Mary E. Crisafio, University of Pittsburgh Department of Psychology.
Patrick T. Donahue, University of Pittsburgh Department of Psychology.
George A. Grove, University of Pittsburgh Department of Psychology.
Anna L. Marsland, University of Pittsburgh Department of Psychology.
Jennifer C. Watt, University of Pittsburgh Department of Psychology.
Catherine M. Bender, University of Pittsburgh School of Nursing and Graduate School of Public Health.
References
- 1.SEER Cancer Stat Facts: Female Breast Cancer. Bethesda, MD: National Cancer Institute; [Google Scholar]
- 2.Miller KD, Siegel R, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Breast Cancer Facts & Figures 2017-2018. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 4.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011 featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. Journal of the National Cancer Institute. 2015;107(6):dvj048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology practice guideline focused update. Journal of Clinical Oncology. 2014;32(21):2255–2270. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lezak MDHDB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th. New York: Oxford University Press; 2004. [Google Scholar]
- 7.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. Journal of Clinical Oncology. 2008;26(5):768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. Journal of Clinical Oncology. 2002;20(2):485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 9.Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology. 2012;30(10):1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 10.Bender C, Merriman J. Cancer- and treatment-related cognitive changes: What can we do now? What lies ahead? Oncology. 2014;18(9):1–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Bender CM, Gentry AL, Brufsky AM, et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncology nursing forum. 2014 May 1;41(3):274–285. doi: 10.1188/14.ONF.274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender C, Merriman J, Gentry A, et al. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121(15):2627–2636. doi: 10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess LM, Insel KC. Chemotherapy-related change in cognitive function: a conceptual model. Oncology Nursing Forum. 2007;34:981–984. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- 14.Ganz PA. Doctor, will the treatment you are reccomending cause chemobrain? Journal of Clinical Oncology. 2012;30:229–31. doi: 10.1200/JCO.2011.39.4288. [DOI] [PubMed] [Google Scholar]
- 15.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandelblatt JS, Hurria A, McDonald BC, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Seminars in oncology. 2013 Dec;40(6):709–725. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Systematic Review. 2016;8 doi: 10.1002/14651858.CD011325.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003 Mar;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 19.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neutrophicfactor mRNA and protein expression in the hippocampus. European Journal of Neuroscience. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integrative cancer therapies. 2013 Jul;12(4):323–335. doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. The Cochrane Library. 2012;2012(8):1–459. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. The Cochrane Library. 2012;2012(8):1–379. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer cytopathology. 2018;124(1):192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitchett MA. Predictability of VO2 max from submaximal cycle ergometer and bench stepping tests. British Journal of Sports Medicine. 1985;19(2):85–88. doi: 10.1136/bjsm.19.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borg GA. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 27.CANTAB®. Cognitive assessment software. Cambridge Cognition; 2017. All rights reserved. www.cantab.com. [Google Scholar]
- 28.Lafayette clinical repeatable neuropsychological test battery. Sagamore: Lafayette Clinical Instrument Company; 1989. [Google Scholar]
- 29.Wilson B, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. Journal of Clinical & Experimental Neuropsychology. 1989;11(6):855–870. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- 30.Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie. 1964;122:382–340. [Google Scholar]
- 31.Osterrieth PA. Test of copying a complex figure; contribution to the study of perception and memory. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- 32.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan (D-KEFS) Executive Function System, Examiners Manual. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 33.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 34.Wechsler D. The Wechsler Memory-Scale-Revised. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 35.Bender CM, Sereika SM, Ryan CM, Brufsky AM, Puhalla S, Berga SJ. Does lifetime exposure to hormones predict pretreatment cognitive function in women before adjuvant therapy for breast cancer? Menopause. 2013;20(9):905–913. doi: 10.1097/GME.0b013e3182843eff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell MJ, Terhorst L, Bender CM. Psychometric analysis of the Patient Assessment of Own Functioning Inventory in women with breast cancer. J Nurs Meas. 2013;21(2):320–334. doi: 10.1891/1061-3749.21.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability. In: Goldstein G, Tarter RE, editors. Advances in Clinical Neuropsychology. Vol. 3. New York: Plenum Press; 1986. pp. 95–126. [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 39.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buysee DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 42.Nelson HE. Nelson Adult Reading Test (NART) manual. Windsor: NFER-Nelson; 1981. [Google Scholar]
- 43.Liden CB, Wolowicz M, Stivoric JM, et al. Accuracy and reliability of the Sensewear™ armband as an energy expenditure assessment device. BodyMedia Inc White Papers. 2002;12:1–15. [Google Scholar]
- 44.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 46.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012 Jul-Aug;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 47.Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- 48.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. Journal of Clinical Oncology. 2003 May 1;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 49.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Journal of Clinical Oncology. 2007 Oct 1;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 50.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Canadian Medical Association Journal. 2006 Jul 4;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.USDHHS. 2008 Physical Activity Guidelines for Americans. Washington D.C.: 2008. [Google Scholar]
- 52.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations With health-related quality of life: results from the American Cancer Society’s SCS-II. Journal of CLinical Oncology. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 53.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Medicine and Science in Sports and Exercise. 2004;36(9):1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 54.Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014 Jul 15;120(14):2174–2182. doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1756–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smaradottir A, Smith AL, Borgert AJ, Oettel KO. Are We on the Same Page? Patient and Provider Perceptions About Exercise in Cancer Care: A Focus Group Study. Journal of the National Comprehensive Cancer Network. 2017;(15):588–594. doi: 10.6004/jnccn.2017.0061. [DOI] [PubMed] [Google Scholar]
- 57.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013 Mar;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janelsins MC, Mustian KM, Palesh OG, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Support Care Cancer. 2012;20(4):831–839. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith LB, Leo MC, Anderson C, Wright TJ, Weymann KB, Wood LJ. The role of IL-1beta and TNF-alpha signaling in the genesis of cancer treatment related symptoms (CTRS): a study using cytokine receptor-deficient mice. Brain Behav Immun. 2014 May;38:66–76. doi: 10.1016/j.bbi.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saykin AJ, de Ruiter MB, McDonald BC, Deprez S, Silverman DH. Neuroimaging biomarkers and cognitive function in non-CNS cancer and its treatment: current status and recommendations for future research. Brain Imaging and Behavior. 2013 Dec;7(4):363–373. doi: 10.1007/s11682-013-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer (Review) Cochrane Database of Systematic Reviews. 2016;(9) doi: 10.1002/14651858.CD005001.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breast Cancer Facts & Figures 2015-2016. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]


