Abstract
Changing climate scenario has resulted in recent emergence and re-emergence of various arboviral diseases including Chikungunya. This disease is caused by Chikungunya virus (CHIKV), which belongs to Togaviridae family of viruses and spread by Aedes mosquitoes. A resurgence of CHIKV and its rapid global spread has been observed since 2004. The disease reemerged in India in 2005, after a gap of 32 years, causing massive outbreaks in some states and circulating thereafter. In the present paper we analyze CHIKV incidence data from India (2010–2014) with a view to understand association with environmental parameters, if any. Data on country-wide occurrences of CHIKV cases were considered from the National Vector Borne Disease Control Board, India. Meteorological data for different climatic subdivisions of India were obtained and processed mathematically. State-wise association of number of cases with rainfall, if any, were studied by statistical analyses. We observe that prevailing temperature range was favorable for CHIKV propagation and the occurrences were modulated by average rainfall. Most affected states were West Bengal, Maharashtra and Karnataka. Overall for India, favorable climatic conditions have contributed to incidences of CHIKV during the study period. There is strong positive association between rainfall variations and occurrence of CHIKV cases.
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0428-6) contains supplementary material, which is available to authorized users.
Keywords: Aedes, Chikungunya, Climate, Rainfall, Temperature
Introduction
The early years of the twenty-first century has witnessed an alarming increase in vector population and vector borne viral infections across the globe. Arthropods are ectothermic and are highly sensitive to climate change, which affects their growth and reproduction rates. Amidst the many climatic variables, mainly increase in temperature and alterations to rainfall have made major contribution in the geographic expansion and population explosion of arboviral vectors. Temperatures along with changes in rainfall pattern and humidity have influenced the rapid development of the vectors, their survival and vector competency [32]. During the last few decades, spread and establishment of vectors mainly ticks and mosquitoes to higher altitudes in Europe has been observed along with autochthonous transmission of tick-borne virus, dengue, Chikungunya, etc. [34].
Chikungunya virus (CHIKV) virus mostly found in tropical and subtropical regions of Africa, Indian Ocean islands, Indian subcontinent and Southeast Asia. It is an alphavirus of family Togaviridae, which comprises several serogroups of public health importance. CHIKV belongs to the Semliki Forest antigenic complex along with other mosquito-borne alphaviruses viz., Mayaro virus, O’nyong-nyong virus, Ross River virus, Getah, Bebaru and Semliki Forest viruses, etc. [6]. CHIKV is believed to have originated in Africa, where two genetically distinct lineages exists (1) the East, Central, and South African lineage (ECSA) and (2) the West African lineage [30, 36]. A third genotype exists in Asia (Asian genotype) since 1958 and has shown geographic expansion to Pacific Islands, Caribbean Islands and to the Americas recently [29]. Sporadic outbreaks of Chikungunya have been reported in Africa and Southeast Asia since its discovery in 1953 [42]. The ECSA strain of CHIKV re-emerged in an explosive form in 2004 and caused massive outbreaks in Indian Ocean islands (La Reunion, Comoros, Mauritius, Seychelles etc.), India and Southeast Asian countries [33, 40]. The re-emergence has been explosive due to high morbidity and mortality. In comparison to earlier outbreaks, the recent outbreaks in La Reunion and India had symptoms and clinical manifestations, which were unheard of earlier including CHIKV associated mortality [35]. Evolutionary adaptation of the virus for fitness in a new vector (Aedes albopictus) was also detected during the outbreak first in La Reunion Island and subsequently in India and other countries [22, 28, 33].
In India, the re-emergence of CHIKV was reported during 2005–2006 after a gap of 32-year [1, 25]. The outbreak started in certain parts of Andhra Pradesh and Karnataka and spread to other regions of the country, resulting in an estimated 1.4 million cases in 13 different states in 2006 alone [25, 29]. These outbreaks covering Indian Ocean islands and subsequently India has been attributed to the ECSA strain of CHIKV [44].
Aedes aegypti and A. albopictus are the major vectors for transmission of CHIKV [5]. Both these mosquitoes are abundant in the tropics. A. albopictus, the Asian tiger mosquito, originally indigenous to South-East Asia, has spread during the last decades to the Middle East, Africa, Europe and the Americas [17]. Since 1990, it has become established in Southern Europe especially the eastern Mediterranean region [39]. It is the only invasive mosquito species present since 2004 on the Côte d’Azur region of continental France [12, 23]. It is the principal cause of Chikungunya outbreak in Italy in 2007 [31]. The spread of A. albopictus to newer areas is linked to climate change and is being studied by various approaches [16, 39]. Recent works highlighted the increasing climatic suitability for A. albopictus to establish in Europe and other temperate countries as a consequence of increase in temperature [7, 15, 39]. Climatic scope of CHIKV outbreaks indicate that a mean air temperature of 20 °C or more is required for outbreaks to occur and successful transmission of the virus [38]. Analyses also revealed that average air temperature in the range 20–30 °C is ideal for rapid vector propagation and spread of disease as this temperature range considerably shortens the time-period required between hatching and taking the first blood meal for A. albopictus [41].
In the present paper, we explored the effects of environmental factors i.e., temperature and, rainfall on the CHIKV transmission in India during 2010–2014, based on confirmed cases and All-India meteorological data. To the best of our knowledge, this is the first report on the association of climatological parameters with CHIKV cases from India.
Materials and methods
Data collection
Data on annual confirmed cases of CHIKV from India was obtained from the website of National Vector Borne Disease Control Program, Ministry of Health and Family Welfare, Government of India [19].
A total of 260 weekly reports on disease outbreaks were downloaded from the website of Integrated Disease Surveillance Programme (IDSP) of National Centre for Disease Control, Ministry of Health and Family Welfare, Government of India (http://idsp.nic.in/). Data-mining for information on the rural outbreaks of CHIKV was conducted (from the said reports) and geographical locations (latitude and longitude) of identified villages/places determined with help of Google maps and www.latlong.net/. Plotting of the locations on maps of India and rendering of images were performed using Grids Analyses and Display Systems (GrADS) software package [14].
Climatological parameters like annual average rainfall and maximum and minimum temperature were used. The Area-weighted average rainfall (ARF) and Average maximum Temperature (MXT) and Average minimum Temperature (MNT) were obtained from Government of India websites [20, 21].
Processing and analyses of meteorological data
In India, area covered by each state (province) is divided into different meteorological sub-divisions (according to the Indian Meteorological Department, Ministry of Earth Sciences, Government of India). Sub-divisional annual rainfall for the period 2010–2014 of Maharashtra, Karnataka and West Bengal states and sub-divisional landmass areas are given in Supplementary Table 1. Area weighted average annual rainfall (ARF) for any particular state of India is calculated by using the following formula:
where X(i) is the average annual rainfall for any (ith) Sub-division, A(i) is the area in sq Km for that (ith) sub-division and n is the number of Sub-divisions covering a state or region of India (Thiessen method, [37]).
Softwares
Mathematical calculations were performed in R and graph plotting was carried out using MS Excel software packages.
Results and discussions
All India scenario
Data on total number of confirmed CHIKV cases per year is available from the website [19], Government of India (accessed in March 2017). It was observed that during the study period CHIKV cases occurred in most of the 29 states of India [19]. The highest numbers of cases (from 2010 to 2014) were reported from West Bengal (28,044) followed by Karnataka (25,320) and Maharashtra (17,238). In West Bengal, which had the highest number of cases in 2010, there was a gradual decrease in the number of cases till 2014. There were no reports of Chikungunya from the Himalayan states like Jammu & Kashmir, Himachal Pradesh and Arunachal Pradesh for the said period. However, Uttarakhand (covering a part of the Indian Himalayas and foothills) reported 18 cases in 2011 and no outbreaks thereafter (NVBDCP data). Among the islands of India, Andaman & Nicobar Islands in the Bay of Bengal have reported cases every year during 2010–2014; however, no cases were reported from Lakshadweep Islands (Arabian Sea) during the period.
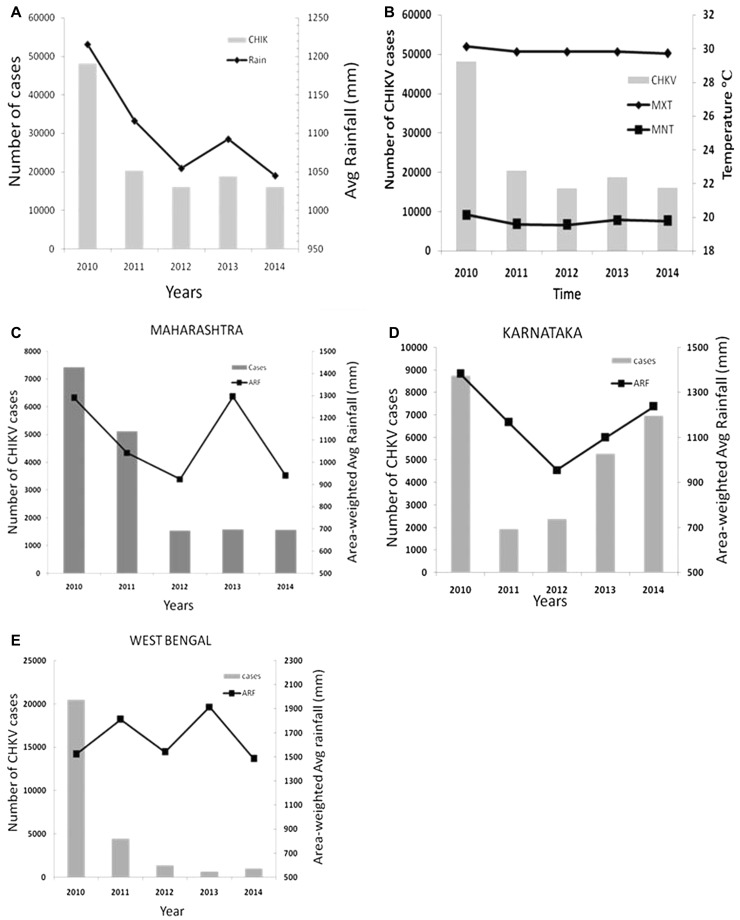
The effect of rainfall on CHIKV occurrences was also examined by comparing the area-weighted average annual rainfall (ARF) for India with annual number of CHIKV confirmed cases (Fig. 1a). We found that the number of CHIKV occurrences (confirmed cases) decreased over the years with decreasing rainfall during 2010 and 2014. Correlation analyses revealed that there is good positive association between rainfall and occurrences of CHIKV cases (Pearson correlation coefficient, r = 0.9559548, p value = 0.11007 and 95% confidence).
Fig. 1.
All India scenario of Chikungunya: a CHIKV confirmed cases (grey bar) versus Area-weighted avg rainfall (black line) from 2010 to 2014. Pearson correlation Coefficient, r = 0.95595; b Chikungunya confirmed cases with average maximum (MXT) and minimum temperatures (MNT) for India. Positive correlation between cases versus MXT (r = 0.97835) and cases versus MNT (r = 0.85494); c Occurrences of CHKV over the years along with the Area-weighted Average Annual Rainfall (ARF) in the state of Maharashtra; d Occurrences of CHKV over the years along with the ARF in the state of Karnataka; e Occurrences of CHKV over the years along with the ARF in the state of West Bengal
In order to investigate the impact of surface air temperature we compared the number of cases with annual average maximum temperature (MXT) and annual average minimum temperature (MNT) (Fig. 1b). It was observed that the number of cases increased with increase in both MXT (Pearson correlation coefficient, r = 0.97835) and MNT (Pearson correlation r = 0.85494). The average mean temperature (MMT) was found to vary between 24.5 and 25.1 °C.
Analyses for the most affected Indian states
Available data indicate that West Bengal, Karnataka and Maharashtra were the most affected Indian states during 2010–2014 (Supplementary Figure S1). We made an attempt to analyze the association of rainfall (area-weighted average annual rainfall, ARF) with the number of CHIKV cases in these states. Figure 1c shows a positive correlation of rainfall (ARF) on the number of CHKV cases in the case of Maharashtra (Pearson Correlation coefficient, r = 0.4703, p value = 0.4241 and confidence 95% (− 0.704 0.955)), which indicates that increased rainfall modulated CHIKV transmission during the period.
Figure 1d shows the positive correlation between CHIKV cases and with rainfall (ARF) in Karnataka. The Pearson Correlation coefficient, r = 0.7981 [p value = 0.1055, 95% confidence (− 0.284 0.980)], indicates positive association between rainfall (ARF) and CHIKV cases.
West Bengal had 20,503 confirmed CHIKV cases in 2010 (Fig. 1e). However, the number of cases decreased with time though rainfall had increased. Analyses shows a negative impact of rainfall on CHKV cases with Pearson Correlation coefficient, r = − 0.33101 (p value = 0.5864; confidence 95% (− 0.939 0.778)). This implies that the number of cases and transmission of CHIKV is not directly related to rainfall.
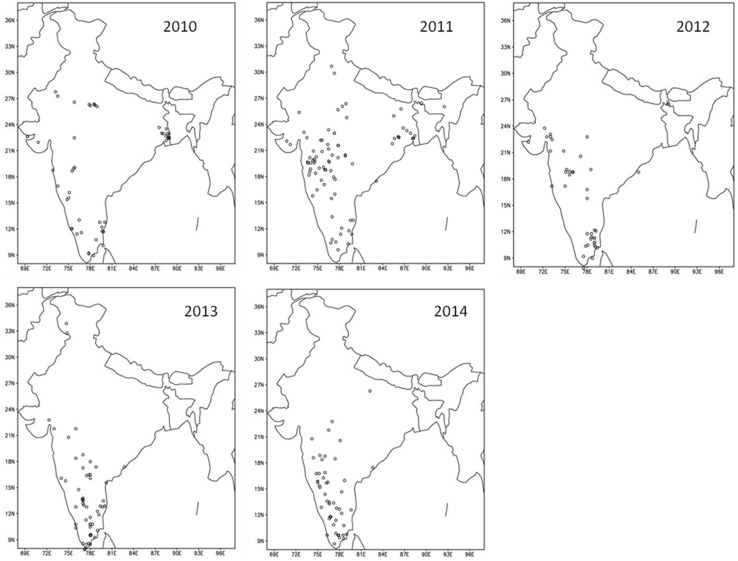
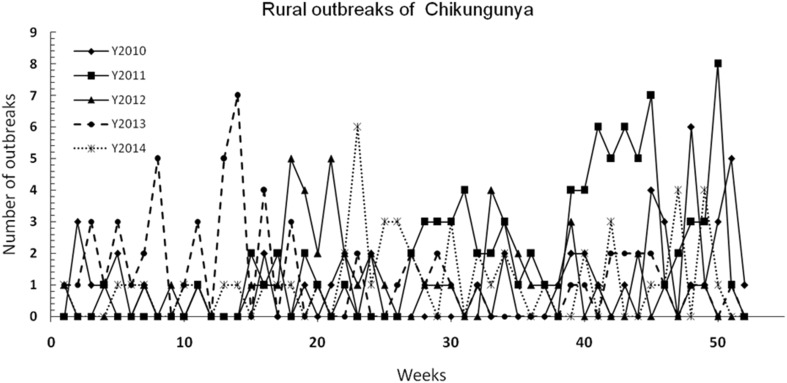
Figure 2 describes the locations of rural outbreaks of CHIKV based on IDSP reports. It is observed that in 2010, majority of outbreaks occurred in regions of Gangetic West Bengal in eastern India, Tamil Nadu in southern India with few places across the western part of the peninsular India. In 2011, large number of rural outbreaks were reported from Maharashtra-Karnataka and Tamil Nadu regions of peninsular India and a few in eastern India (West Bengal-Odisha) and very few in northern India. In 2012, the rural outbreaks were mostly in the western part of peninsular India and Tamil Nadu with a few in Gujarat. In 2013, majority of such outbreaks occurred in southern part of the peninsular India covering (Tamil Nadu, Kerala and Karnataka) with a few in Andhra Pradesh and Maharashtra regions. In 2014, majority of such outbreaks occurred in the western part of peninsular India covering interior Tamil Nadu, Kerala, Karnataka and a few across Maharashtra region. The occurrence of weekly rural outbreaks have been compared in Fig. 3. It should be noted that most of the outbreaks occurring between 38th and 52nd weeks are from Tamil Nadu and Kerala regions, which receive rainfall during this period every year.
Fig. 2.
Locations for the rural outbreaks of Chikungunya based on the IDSP reports from 2010 to 2014
Fig. 3.
Number of rural outbreaks per week in India from 2010 to 2014. Note: Y2010 denotes year 2010 AD and so on
Global perspective and Indian scenario 2010–2014
Though considerable advancements have been made in the management of major vector borne diseases i.e. malaria, filaria, Kala-azar, schistosomiasis, etc., vector borne viral infections have shown a surge in the last few decades. Among the several factors, increase in temperature associated with rainfall plays an important role in the explosion of vector population and spread of arboviral infections. Climate change has direct impact in survival rates, generation time, abundance, dispersion ability of arthropods to establish in newer habitats especially in the temperate regions more efficiently [24]. This also results in the autochthonous transmission of arboviral infections and outbreaks. The introduction of West Nile virus to New York in 1999 and its rapid spread to other states of United States and Canada subsequently with more than a thousand deaths has drawn global attention [2, 18]. The list of emerging and re-emerging arboviruses causing infections in naive populations included the explosive outbreaks by CHIKV in Indian ocean Islands, India, Italy and the Caribbean Islands [8, 9, 29, 42] the spread of dengue in Hawaii and Texas in the US and the introduction of Crimean Congo hemorrhagic fever virus to India [11, 26]. Recently, during 2010–2014, Chikungunya has spread to new areas of Europe especially France (2014 and 2010) whereas, in the USA, ~ 3000 cases were reported in 2013–2015, most of which being imported, but 11 autochthonous cases were also confirmed [43]. In Nepal, Chikungunya outbreaks occurred in the terai regions in the Himalayan foot hills (2014 and beyond) [27] and domain of Aedes mosquitoes have been found to expand from the low valleys and up the mountains till 2100 m above sealevel due to warming climate [13].
In the present study, we tried to establish an association between climatic factors such as temperature and area-weighted average rainfall (ARF) with confirmed cases of Chikungunya in India. It is observed that the All-India number of CHIKV cases was modulated by annual rainfall. This is consistent with the findings elsewhere that enhanced rainfall results in better vector propagation, vector competence [41] and hence enhanced transmission of Chikungunya [3].
In Maharashtra and Karnataka, positive association of rainfall was observed with number of CHIKV cases which is consistent with findings elsewhere [3]. Geographically both these states are located in the Deccan plateau of peninsular India (Suppl Fig. 2). Except the narrow coastal belt bordering the Arabian Sea, most of the land area for both these states are covered with mountainous terrain (Western Ghats mountain range), grasslands, highlands and some river basins of rain-fed rivers with a semi-arid grassland type of climate [10]. These states get maximum rainfall only during the South West Monsoon (June to October).
In West Bengal, a densely populated state with abundance of water bodies, dense vegetation and mostly hot and humid climate (except the Himalayan district of Darjeeling) [10], the decrease in CHIKV cases with time (irrespective of rainfall variations) may be attributed to the control measures undertaken. Enhanced personal protection against mosquito bites, source reduction and vector control efforts might have resulted in restricting the transmission despite favorable environmental factors for Aedes mosquito propagation [4], the principal vector for CHIKV. However, no official record is available regarding vector management from the state (during the period of study).
Occurrences of rural outbreaks of CHIKV (based on the IDSP reports) have been mapped year-wise after identification of geographical locations. While many outbreaks occurred in northern and eastern India and western peninsular India during the South West Monsoon season (June–September), many such outbreaks reported during 39th week and 52nd week every year occurred mostly in the extreme southern part of peninsular India covering Tamil Nadu and parts of Kerala, which receive rainfall under the influence of North East Monsoon winds (October–December/January). It should be noted that the large number of cases reported from various states consists of both rural and urban centres. Since urban centres in India are huge and densely populated, with variable population (migrant labourers, migrant workforce, tourists, etc.), it would be difficult to associate annual incidences with specific ‘outbreak’ scenarios. Hence, while mapping outbreaks we have considered only registered and recorded rural outbreaks and neglected the urban clusters.
Also, please note that there is no existing mechanism in India to undertake entomological studies at outbreak sites during the outbreak period. Hence, no mosquito population data was available for studying the possible correlation with incidences of CHIKV. This can be taken up as future research.
In this paper we made an attempt to study the association, if any, between rainfall and occurrences of CHIKV cases in India. Meteorological data was analyzed and possible correlation with confirmed cases of CHIKV was obtained. We conclude that recent spread of Chikungunya in India is aided by favorable climatic conditions, where rainfall played a prominent role in modulation of occurrences of CHIKV cases. There is positive association between occurrence of CHIKV cases and average rainfall in Maharashtra and Karnataka states. However, in case of West Bengal, no such association was observed probably due to interventions. Recorded rural outbreaks occurred mostly during the rainy seasons in different parts of the country.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors would like to thank Dr. D. T. Mourya, Director, National Institute of Virology, Pune and Dr. Krishna Kumar, Director, Indian Institute of Tropical Meteorology, Pune, India for their encouragement and support in facilitating the collaboration.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0428-6) contains supplementary material, which is available to authorized users.
References
- 1.Arankulle VA, Shrivastava S, Chrian S, Gunjikar RS, Walimbe AM, Jadhav SM, et al. Genetic divergence of chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J Gen Virol. 2007;88:1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 2.Barrett ADT. Economic burden of West Nile virus in the United States. Am J Trop Med Hyg. 2014;90(3):389–390. doi: 10.4269/ajtmh.14-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia R, Narain JP. Re-emerging Chikungunya fever: some lessons from Asia. Trop Med Int Health. 2009;14(8):940–946. doi: 10.1111/j.1365-3156.2009.02312.x. [DOI] [PubMed] [Google Scholar]
- 4.Biswas DK, Bhunia R, Basu M. Dengue fever in a rural area of West Bengal, India, 2012: an outbreak investigation. WHO South-East Asia J Public Health. 2014 doi: 10.4103/2224-3151.206883. [DOI] [PubMed] [Google Scholar]
- 5.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 6.Calisher CH, Karabatsos N. Arbovirus serogroups: definition and geographic distribution. In: Monath TP, editor. The arboviruses: epidemiology and ecology. Boca Raton: CRC Press; 1988. pp. 19–58. [Google Scholar]
- 7.Caminade C, Medlock JM, Duchyne E, McIntyre KM, Leach S, Baylis M, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface. 2012;9:2708–2717. doi: 10.1098/rsif.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell LP, Luther C, Moo-Llanes D, et al. Climate change influences on global distributions of dengue and chikungunya virus vectors. Phil Trans R Soc B. 2015;370:20140135. doi: 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks—the globalization ofvector borne diseases. New Engl J Med. 2007;356:769. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 10.Climate Atlases (2005):”Climate of Maharashtra”, “Climate of Karnataka” and “Climate of West Bengal” issued by National Climate Centre, Office of Additional Director General of Meteorology (research), Indian Meteorology Department HQ, Pune, India.
- 11.Dash AP, Bhatia R, Sunyoto T, Mourya DT. Emerging and re-emerging arboviral diseases in Southeast Asia. J Vector Borne Dis. 2013;50(2):77–84. [PubMed] [Google Scholar]
- 12.Delaunay P, Mathieu B, Marty P, Fauran P, Schaffner F. Chronology of the development of Aedes albopictus in the Alpes-Maritimes Department of France, from 2002 to 2005. Med Trop. 2007;67:310–311. [PubMed] [Google Scholar]
- 13.Dhimal M, Gautam I, Joshi HD, O’Hara RB, Ahrens B, Kuch U. Risk factors for the presence of chikungunya and dengue vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in Central Nepal. PLoS Negl Trop Dis. 2015;9(3):e0003545. doi: 10.1371/journal.pntd.0003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doty BE, Kinter JK., III . Geophysical Data Analysis and Visualization using GrADS. In: Szuszczewicz EP, Bredekamp JH, editors. Visualization techniques in space and atmospheric sciences. Washington: NASA; 1995. pp. 209–219. [Google Scholar]
- 15.Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Glob Planet Change. 2011;78:54–64. doi: 10.1016/j.gloplacha.2011.05.008. [DOI] [Google Scholar]
- 16.Fischer D, Thomas SM, Suk JE, Hess A, Tjaden NB, Beierkuhnlein C, et al. Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int J Health Geogr. 2013;12:51. doi: 10.1186/1476-072X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 18.Hayes EB, Sejvar JJ, Zaki SR, Zaki SR, Lanciotti RS, Bode AV, et al. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.http://nvbdcp.gov.in/chik-cd.html. Accessed on 1 March 2017.
- 20.https://data.gov.in/. Accessed on 1st April 2017.
- 21.http://www.tropmet.res.in/. Accessed on 1 April 2017.
- 22.Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R, et al. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills JN, Gage KL, Khan AS. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ Health Perspect. 2010;118(11):1507–1514. doi: 10.1289/ehp.0901389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mourya DT, Thakare JP, Gokhale MD, Powers AM, Hundekar SL, Jayakumar PC, et al. Isolation of Chikungunya virus from Aedes aegypti mosquitoes collected in the town of Yawat, Pune district, Maharashtra state, India. Acta Virol. 2001;45:305–309. [PubMed] [Google Scholar]
- 26.Mourya DT, Thakare JP, Gokhale MD, Powers AM, Hundekar SL, Jayakumar PC, et al. Isolation of Chikungunya virus from Aedes aegypti mosquitoes collected in the town of Yawat, Pune district, Maharashtra state, India. Acta Virol. 2001;45:305–309. [PubMed] [Google Scholar]
- 27.Pandey K, Pandey BD, Chaurasiya RR, Thakur M, Neupane B, Shah Y, et al. Evidence of Chikungunya virus circulation in the Terai region of Nepal in 2014 and 2015. Trans R Soc Trop Med Hyg. 2017;111(7):294–299. doi: 10.1093/trstmh/trx059. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino B, Muyembe-Tamfum JJ, Bessaud M, Tock F, Tolou H, Durand JP, et al. Epidemic resurgence of Chikungunya virus in Democratic Republic of the Congo: identification of a new central Africa strain. J Med Virol. 2004;74:277–282. doi: 10.1002/jmv.20168. [DOI] [PubMed] [Google Scholar]
- 29.Petersen LR, Powers AM. Chikungunya: epidemiology [version 1; referees: 2 approved] F1000 Research 2016, 5(F1000 Faculty Rev):82.
- 30.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 31.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 32.Rogers DJ, Randolph SE. Climate change and vector-borne disease. Adv Parasitol. 2006;62:345–381. doi: 10.1016/S0065-308X(05)62010-6. [DOI] [PubMed] [Google Scholar]
- 33.Schuffenecker I, Iteman I, Michault A, Michault A, Murri S, Frangeul L, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:1058–1070. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenza JC, Menne B. Climate change and infectious diseases in Europe. Lancet ID. 2009;9:365–375. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- 35.Sudeep AB, Parashar D. Chikungunya: an overview. J Biosci. 2008;33(4):443–449. doi: 10.1007/s12038-008-0063-2. [DOI] [PubMed] [Google Scholar]
- 36.Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nouquerede Gould EA, Roques P, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antivir Res. 2013;99:345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiessen AH, Alter JC. Climatological data for July 1911—District No. 10, Great Basin. Mon Weather Rev. 1911;39(7):1082–1084. [Google Scholar]
- 38.Tilston N, Skelly C, Weinstein P. Pan-European Chikungunya surveillance: designing risk stratified surveillance zones. Int J Health Geogr. 2009;8:61. doi: 10.1186/1476-072X-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran A, L’Ambert G, LacourG Benoît R, Demarchi M, Cros M, et al. A rainfall- and temperature-driven abundance model for Aedes albopictus populations. Int J Environ Res Public Health. 2013;10:1698–1719. doi: 10.3390/ijerph10051698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specifi city and epidemic potential. PLoS Pathog. 2007;3:1895–1906. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldock J, Chandra NL, Lelieveld J, Proestos Y, Micheal E, Christophides G, et al. The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathog Glob Health. 2013;107(5):224–241. doi: 10.1179/2047773213Y.0000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines. 2012;11(9):1087–1101. doi: 10.1586/erv.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO Chikungunya disease: gaps and opportunities in public health and research in the Americas. Wkly Epidemiol Rec. 2015;42:571–576. [PubMed] [Google Scholar]
- 44.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12(10):1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.