Abstract
Chikungunya, a viral fever caused by Aedes mosquito results in extreme morbidity in affected individuals and is a major public health concern in India. Currently, modern vaccines or formulations prescribed by physicians can only provide symptomatic relief for the pyretic and post pyretic phase of the disease. Siddha practitioners follows strict medical regimen based on traditional Indian knowledge/concepts to treat Chikungunya with considerable results. The current study was undertaken to standardize assays for the study of these siddha formulations and to check their efficacy and potential mode of action as antivirals for Chikungunya virus infection in in vitro system. Although, siddha practitioners follow a regime containing 4–6 formulations, of these Brahmanandha bairavam mathirai, a part of the regime for Chikungunya followed at National Institute of Siddha and Vishnu chakram along with Brahmanandha bairavam mathirai, a part of Thiruchergodu Regime were found of have antiviral activities. It was observed that both Vishnu chakram and Brahmanandha bairavam mathirai were equally effective in blocking Chikungunya virus from entering susceptible cells in the concentrations range of 0.0625 and 0.5 mg/ml. Additionally, it was also observed that Brahmanandha bairavam mathirai was more effective than Vishnu chakram against entry of Chikungunya in the cells. The assays used in this study provides insights to the possible mode of action of various formulations used by siddha practitioners for the treatment of Chikungunya infection.
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0421-0) contains supplementary material, which is available to authorized users.
Keywords: Polyherbal, Siddha formulations, Chikungunya, In vitro
Introduction
Chikungunya fever (CHIKF) is a febrile arboviral illness caused by Chikungunya virus (CHIKV), a ssRNA virus that belongs to the alphavirus genus of the family Togaviridae. transmitted by the Aedes mosquito namely Aedes aegypti and Aedes albopictus. ‘Chikungunya’ is a Makonde word that translate to ‘that which bends up’ and refers to the bent posture of infected patients suffering from debilitating joint pain [1]. Chikungunya remerged after a gap of 32 years and since 2005 Chikungunya outbreaks have affected millions in South-Asia, Indian Ocean islands and India [2, 3] and extended to Europe, Central Asia [4, 5] and recently spread further to the Americas [6]. Currently, the disease is well documented in many national health guidelines. Chikungunya is characterized by acute fever of high magnitude (> 39 °C) after a mean incubation period of 5–7 days with headache and severe muscle and joint pain [7] consistent with severe morning stiffness and inflammatory arthritis [8, 9]. While the fever subsides in 7–10 days, the joint pain varies in intensity and can persist for several years post-infection [10, 11] along with severe arthralgia, myalgia and maculopapular rash with ranging severity, [8]. Synovitis or periarticular swelling has been reported in most patients, with large joint effusions occurring in about 15% of individuals infected with Chikungunya [7, 10, 12]. Studies have reported that patients might have relapsing and remitting arthralgia up to 36 months after being diagnosed with acute disease [13, 14]. Risk factors for the development of long-term arthralgia following Chikungunya virus infection include age (> 35 years) and the presence of arthralgia in the first 4 months after onset of symptoms [14]. Interestingly recent studies have reported that initial viral load plays a major role in disease severity both in acute as well as in chronic phase [15], this makes development of antivirals even more important in the present context.
The Indian traditional medicine system, siddha, has been used for centuries to find cure to human diseases based on the concept of Tridosha Theory (Three Humour theory). Siddha system believes that all objects in the universe including human body are composed of five basic primordial elements, namely earth, water, fire, air and space [16]. The human body is a conglomeration of three humors and seven physical components. According to the siddha literature the vitiation of environmental factors such as—water, air, place and season are considered to be responsible for the production of diseases. When contamination and vitiation of these factors takes place, epidemics follow. In the current times, water borne diseases, environmental diseases, epidemiological disorders and seasonal disorders due to infections can be correlated with Janapadodhwamsa Vyadhis, a medical term well defined in the Indian traditional medical system [17]. Though there is no direct reference of Chikungunya in the ayurvedic literature, it can still be equated with the condition where fever is associated with arthritis. The treatment of Chikungunya can be categorized into symptom modifiers and general health promoters; more specifically to say the formulations that improve the quality of life (QoL), provides strength or resistance against the disease and facilitate early recovery. The formulations are classified under General Health Promoters such as Balya (Tonic) or Rasayana (Immunomodulator) and are beneficial in the management of disease. According to the siddha literature, these formulations are defined as QoL as they are administered in several ailments ranging from gastric ulcers, rheumatic pain, insomnia and fever with arthritis [18].
In the present study polyherbal siddha formulations brahmanandha bairavam mathirai, vishnu chakram, nilavemba kudineer, arumuga chenduram, linga chenduram No. 1 and thirikadugu choornam were evaluated for their antiviral activity using an in vitro assay system in a 96-well format. Our study shows that these assays could be used for any kind of siddha formulation for the evaluation of its antiviral activity.
Brahmanandha bairavam mathirai and Vishnu chakram have been used for the treatment of pyrexic phase of chikungunya [19], and may act as antivirals Nilavembu Kudineer is known for its antipyretic, anti-inflammatory and analgesic properties and thus have a role in immune modulation [20]. Arumuga chenduram is a herbo-metallic formulation [21], known for its role in the treatment of fever and arthritis [19]. Linga chenduram No. is one of the the medicines used in Siddha for treating ‘Azhal Keel Vayu’ which may be correlated with osteo arthritis having the symptoms of Pain and swelling in knee joints [22]. Thirikadugu choornam is used in the treatment of digestive disorders such as indigestion, dyspepsia, flatulence and intermittent fever Development of Ayurvedic and Siddha Medicine through the Application of Modern Pharmaceutical Technique [23] and thus given as a part of the chikungunya treatment regime along with the other polyherbal formulations. Lit of compositions of various formulations employed in the study is present in supplementary Table 1.
Table 1.
Chikungunya medicine regimes followed by siddha practitioners according to AYUSH, government of India, guidelines
| Regime | Formulations followed | Known functions | Reference |
|---|---|---|---|
| Regime for Chikungunya at National Institute of Siddha (Chennai) | Brahmanandha Bairavam Mathirai | Fever | [18] |
| Arumuga chenduram | Post Arthralgia Conditions | ||
| Nilavemba kudineer | Immunomodulator | ||
| Vatakesari thantani | |||
| Laghir visha mushti thailam | Ectopic treatment for swelling | ||
| Thiruchergodu regime | Vishnu chakram | Fever with arthritis | |
| Linga chenduram No. 1 | Fever with immunity booster | ||
| Brahmanandha Bairavam Mathirai | Fever | ||
| Thirikadugu Choornam | Indigestion and intermittent fever |
Materials and methods
Cell maintenance and preparation of virus
Vero (African green monkey kidney) cells used in this study were maintained in Dulbecco’s Modified Eagle’s medium containing 10% inactivated FBS at 37 °C, 5% CO2 and 75% Humidity. formulations. During the time of virus propagation and antiviral assay the FBS concentration of the cell culture medium was reduced to 2%.
Virus amplification and concentration
The CHIKV isolate used in this experiment was a clinical isolate from an outbreak in Delhi in 2010 and belonged to the ECSA strain [24]. The CHIKV strain was propagated in Vero cells and harvested after full cytopathic effect was observed. Virus stock was concentrated using modified PEG concentration methods [6]. Viruses were further characterized by plaque assay [25]; the obtained stock was aliquoted and stored at − 80 °C.
Cell viability assay
MTT 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) assay (Sigma, Cat No: D4540) was performed to evaluate the cytotoxicity of siddha formulations in Vero cells using previously standardized protocols [26]. Briefly, monolayers of Vero cells were grown in 96-well plate and were treated with different concentrations of the formulations in 8 replicates together with negative control (media containing 0.1% DMSO). It was followed by incubation at 37 °C with 5% CO2 for 48 h before the MTT assay was performed. Two days post-treatment, 10 μl of 5 mg/ml MTT solution was added to the cells and incubated for 4 h at 37 °C with 5% CO2 followed by the addition 150ul of DMSO, prior to absorbance detection at 495 nm wavelength using multiplate reader. Percentage survival of cells after treatment with the formulations was determined through this assay using Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA, USA, 2005).
Antiviral activity of siddha formulations
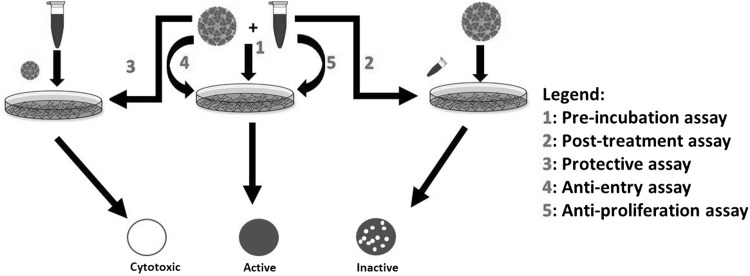
Schematic representation of the in vitro bioassays developed to evaluate antiviral activity of amukkara choornam is given in Fig. 1. Screening of activity of these siddha formulations was done using the screening assays designed using variation of plaque reduction neutralization assay (PRNT). In PRNT assay, Vero cells were infected with CHIKV strains at a multiplicity of infection (MOI) of 1 in the presence of siddha formulations at non-cytotoxic concentrations. Following incubation at 37 °C for 1 h, the virus was removed. The infected cells were covered with 150ul of overlay medium (1% CMC (Sigma, catalog # C5678) prepared in 2% DMEM). Cells were then incubated at 37 °C for 2 days. Finally, cells were fixed and stained using 0.25% crystal violet solution prepared in 30% methanol to count plaques and determine virus titers, which are presented as plaque-forming units per 100 μl. PRNT was performed only after the screening assay steps have been performed. The half-maximal inhibitory concentration (PRNT50 value) for each concentration of the polyherbal formulation against CHIKV, with reference to the virus control (which represented 100% infection or 0% inhibition), was defined as the concentration of polyherbal formulations, in μg/ml, resulting in atleast 50% inhibition of the plaque count.
Preincubation with polyherbal formulations
Vero cells were seeded in 96-well plates (20,000 cells/well), a day in advance. CHIKV (MOI 1) were separately pre-incubated with serial dilutions of polyherbal formulations at non-cytotoxic concentrations in 100 μl volume, at 37 °C for 2 h and at 4 °C overnight (12 h). The preincubation mixture was diluted with an equal volume of medium (DMEM + 2% FBS) and used to infect Vero cells (3 wells for each concentration at 200 μl/well) in the 96-well plate. After 2 h of adsorption in the incubator (37 °C, 5% CO2), infected cells were overlaid with 150 μl of methylcellulose-containing growth medium and processed thereafter as described for the plaque assay above. To assess any potential cytotoxicity, cells were exposed to the herbal formulations (in the same concentration range) in the absence of CHIKV infection. Additional control experiments, run in parallel, included cells which were either mock-infected (negative control) or infected with CHIKV in the absence of polyherbal formulations (positive control).
-
2.
Post treatment with polyherbal formulations
Vero cells in 96-well plates (20,000 cells/well) were infected with CHIKV (MOI = 1) without pre-incubating with the polyherbal formulations. After 2 h of adsorption, the virus inoculum was aspirated, the monolayer rinsed with 1X PBS, and then fed with complete medium containing the siddha formulations (corresponding non-cytotoxic concentrations). After 2 h of exposure to the formulations, the monolayer was aspirated and overlaid with growth medium containing methylcellulose and plaques were developed 48 days later as above.
-
3.
Protective effect of Polyherbal formulations
Vero cells in 96-well plates (20,000 cells/well) were treated with the various non-cytotoxic concentrations of siddha formulations for 12 h. Post treatment, the cells were washed twice with 1X PBS and infected with CHIKV (MOI = 1) for 2 h. After 2 h of adsorption, the mix was aspirated, the monolayer was rinsed with 1X PBS and overlaid with growth medium containing methylcellulose and plaques were developed 48 days later as above.
-
4.
Anti-entry assay for polyherbal formulations
The procedure of anti-entry assay was modified and performed as mentioned in earlier studies [27]. Monolayers of Vero cells were grown in 96-well plate with DMEM supplemented with 10% inactivated FBS. The Vero cells were then infected with CHIKV at MOI 1 and the plate was incubated for 1 h at 4 °C. Non-adsorbed virus was washed with 1X PBS. Polyherbal formulations were added in different concentrations and incubated at 37 °C with 5% CO2 for 2 h. The plates were again washed with 1X PBS and treated with citrate buffer (pH = 3) to inactivate the non-internalized virus, before washing with 1X PBS. PRNT overlay media was added into every well and the plate was incubated for 48 h at 37 °C with 5% CO2. PRNT assay was performed as aforementioned.
-
5.
Anti-penetration assay for polyherbal formulations
For anti-penetration assay monolayers of Vero cells were grown in 96-well plate with DMEM supplemented with 10% inactivated FBS. The Vero cells were then infected with CHIKV at MOI 1 and the plate was incubated for 1 h at 37 °C. Non-adsorbed virus was then washed with 1X PBS. Siddha formulations were added in different non-cytotoxic concentrations and incubated at 37 °C with 5% CO2 for 2 h. The plates were again washed with 1X PBS to remove non-adsorbed virus. PRNT overlay media was added into every well and the plate was incubated for 48 h at 37 °C with 5% CO2. PRNT assay was performed as aforementioned.
Fig. 1.
Schematic representation of in vitro bioassays developed for the understand of antiviral effect of the polyherbal formulations. 5 types of anti-viral assays were developed to evaluate the anti-viral activity of siddha formulations. All the assays were based on modified plaque reduction neutralization assay. Type 1 assay: Pre-incubation of CHIKV and siddha formulations at 4 °C overnight followed by PRNT assay with Vero cells. Type 2 assay: Post treatment of the CHIKV infected cells with siddha formulations at 37 °C followed by PRNT assays to evaluate the role of siddha formulations. Type 3 assay: to evaluate protective effect of polyherbal siddha formulations preceding CHIKV infection. Type4 assay: Anti-entry assay of CHIKV in the presence of siddha formulations at 4 °C followed by PRNT assay with Vero cells. Type 5 assay: Anti-proliferative assay of CHIKV in the presence of siddha formulations at 37 °C followed by PRNT assay with Vero cells
Siddha medica materia
In case of Chikungunya there are two major regimes that are followed by the siddha practitioners i.e. Regime for Chikungunya prescribed by National Institute of Siddha (Chennai) and Thiruchergodu regime. The individual formulations with their known functions against Chikungunya disease are given in Table 1. Of these formulations, brahmanandha bairavam mathirai, vishnu chakram, nilavemba kudineer, arumuga chenduram, linga chenduram No. 1 and thirikadugu Choornam were studied for their antiviral effect against Chikungunya virus in in vitro conditions.
Results and discussions
Cell cytotoxicity
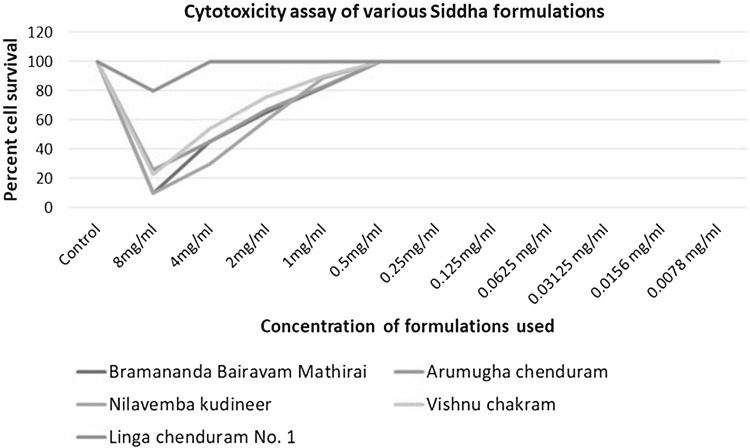
Cell cytotoxicity of various siddha formulations was determined using MTT assay in Vero cells. Percent cell survival cells was determined and non-cytotoxic concentrations of different formulations were evaluated. It was observed that all the formulations were non-cytotoxic below 0.5 mg/ml (Fig. 2).
Fig. 2.
MTT based cell cytotoxicity assay for the siddha formulations used in the study. It was observed that all formulations were non-cytotoxic between 0.5 mg/ml till 0.078 mg/ml, these concentrations were used for all the further in vitro bioassay
Anti-viral activity of various siddha formulations
Further, five types of bioassays were developed to identify the concentration of these siddha polyherbal formulations for potential CHIKV inhibitory activity. For all experiments viruses were prepared as aforementioned in the material and method sections and were checked using PRNT to determine no. of plaques formed at MOI 1. It was found that the virus used for the study was 2 × 108 pfu/100 μl in concentration (data not shown).
Effect of pre-incubation of virus and drug on its anti-viral activity
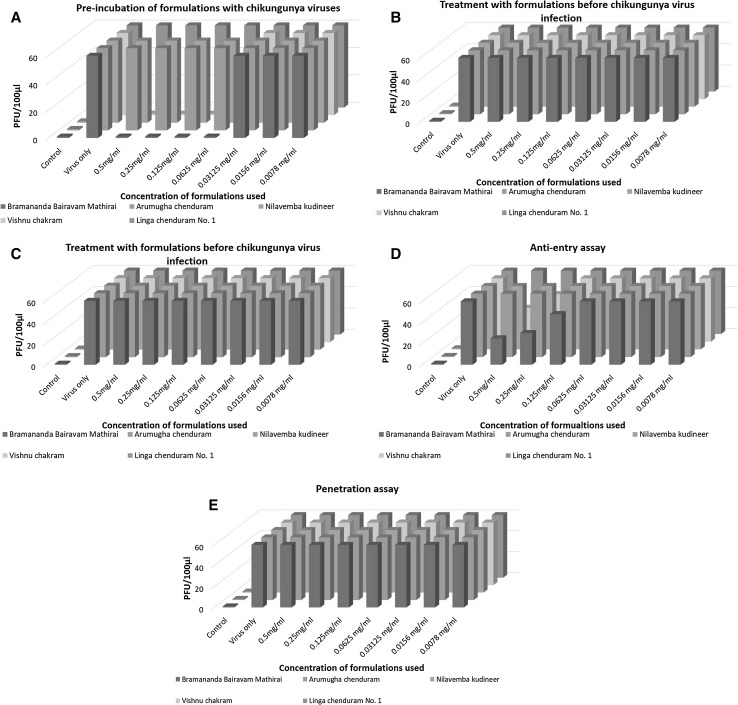
The type-1 assay was designed to identify the ability of these siddha formulations to block CHIKV from entering susceptible cells. It was observed that brahmanandha bairavam mathirai and vishnu chakram were able to resist the development of plaques when used in the concentrations between 0.5 and 0.0625 mg/ml. For all other formulations, the number of plaques formed was more than 50 at all concentrations which is equivalent to the number of plaques formed by the concentrated virus at MOI 1. Based on these observations, it was concluded that brahmanandha bairavam mathirai and vishnu chakram could inhibit CHIKV entry into the host cells (Fig. 3a). However, type-1 assays did not necessarily reveal if the formulations possessed the ability to inhibit the post entry steps in the CHIKV replication cycle.
Fig. 3.
5 types of bioassays developed to evaluate anti-viral activity of the siddha formulations. a Preincubation of siddha formulations with Chikungunya virus: Brahmanandha bairavam mathirai and Vishnu chakram were found to have inhibit CHIKV entry into the susceptible cells and may affect post entry replication steps at concentrations range of 0.0625 and 0.5 mg/ml. b Effect of treatment with siddha formulations on CHIKV infected cells. The results showed that none of the formulations could inhibit CHIKV entry into the host cells. c Prophylaxis effect of siddha formulations before CHIKV infections. The results depicted that none of the formulations showed any protective effect against CHIKV infections. d Anti-entry effect of siddha formulations upon CHIKV infections: Brahmanandha Bairavam Mathirai and Vishnu chakram could prevent entry of CHIKV into the cells/adsorption of CHIKV onto the cells. e Anti-penetration effect of siddha formulations upon CHIKV infections: None of the formulations prevented penetration of CHIKV into the cells
Effect of treatment with siddha formulation on CHIKV infected cells
The type-2 assay was designed to assess the capacity of siddha formulations to inhibit CHIKV within the infected cell. Therefore, CHIKV infection preceded exposure to the herbal formulations. All the siddha formulations used in the study neither inhibited CHIKV replication post entry nor reduced the number of plaques formed (Fig. 3b). For all the concentrations, the number of plaques formed was more than 50 which is equivalent to the number of plaques formed by the concentrated virus at MOI 1.
Prophylaxis activity of siddha formulations
The type-3 assay was designed to monitor protective effect of siddha formulations against CHIKV. For same, the number of plaque forming units were measured to gauge the magnitude of inhibition. It was observed that none of the formulation was not able to inhibit or reduce the number of plaque forming units. For all the concentrations, the number of plaques formed was more than 50 which is equivalent to the number of plaques formed by the concentrated virus at MOI 1 (Fig. 3c). Thus, none of the siddha formulations used for the study showed protective role.
Effect of siddha formulations on CHIKV adherence on susceptible cells
The type-4 assays, this assay was performed to detect the ability of siddha formulations to block the CHIKV adherence into the susceptible cells. the result clearly indicates that none of the formulations was 100% effective in blocking entry of CHIKV in the cells. But, brahmanandha bairavam mathirai and vishnu chakram could inhibit CHIKV entry into the cells to by reducing the number of plaque forming units. It was found that brahmanandha bairavam mathirai was able to reduce viral replication by 66.67% at 0.5 mg/ml whereas, vishnu chakram was able to reduce viral replication by 33.33% % at 0.5 mg/ml. For all concentrations, the number of plaques formed was more than 50 which is equivalent to the number of plaques formed by the concentrated virus at MOI 1 (Fig. 3d).
Effect of siddha formulations on CHIKV entry into susceptible cells
The type-5 assays, this assay was done to evaluate the penetration capacity of CHIKV in the cells after the treatment with siddha formulations. Anti-penetration assays were performed wherein cell infection with CHIKV at MOI 1 was followed by the treatment of the cell with the polyherbal formulations at various concentration as mentioned earlier. It was observed that number of plaques formed even after the treatment with these formulations were more than 50 or more which is equivalent to the number of plaques formed by the concentrated virus at MOI 1 hence it was established that none of the siddha formulations used in the regime exhibited anti-penetration phase post entry (Figure 3e).
These assays were designed and standardized to analyze the effectiveness of siddha polyherbal formulations and to understand the effectiveness of the regimes followed by siddha practitioners. It was found that in both the regimes siddha doctors use at least one CHIKV antiviral to curtail the effect of the pathogen and hence the regimes are effective as also depicted in the study by central council for research in Ayurveda and Siddha, Department of AYUSH [19], wherein most patients recover 100% after 10 days of treatment.
Siddha formulations though effective against viral infections lacks experimental proofs and thus is unable to compete with modern medicine system. Keeping in mind the holistic concept of this medicine system researcher/doctors need to provide biologically relevant data and the assays developed in this study could be used to understand the anti-viral activity of the siddha formulations in in vitro system.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful to Dr. Sathya Rajeshwaran, Siddha Central Research Institute, Chennai, for his inputs regarding approved siddha regimes. We acknowledge Dr. Vimal Narayanan’s assistance in the preparation of supplementary table. Financial support for the study was received from the Ministry of Ayush, (Grant No: ND/AYU/15/019), Government of India and their support is duly acknowledged.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0421-0) contains supplementary material, which is available to authorized users.
References
- 1.Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49(1):28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 2.Gerardin P, Guernier V, Perrau J, Fianu A, Le Roux K, Grivard P, et al. Estimating Chikungunya prevalence in La Reunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis. 2008;8:99. doi: 10.1186/1471-2334-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mavalankar D, Shastri P, Raman P. Chikungunya epidemic in India: a major public-health disaster. Lancet Infect Dis. 2007;7(5):306–307. doi: 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 4.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 5.Zheng K, Li J, Zhang Q, Liang M, Li C, Lin M, et al. Genetic analysis of chikungunya viruses imported to mainland China in 2008. Virol J. 2010;7:8. doi: 10.1186/1743-422X-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84(13):6497–6504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochedez P, Jaureguiberry S, Debruyne M, Bossi P, Hausfater P, Brucker G, et al. Chikungunya infection in travelers. Emerg Infect Dis. 2006;12(10):1565–1567. doi: 10.3201/eid1210.060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miner JJ, Aw-Yeang HX, Fox JM, Taffner S, Malkova ON, Oh ST, et al. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67(5):1214–1220. doi: 10.1002/art.39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain J, Nayak K, Tanwar N, Gaind R, Gupta B, Shastri JS, et al. Clinical, serological and virological analysis of 572 chikungunya patients during the years 2010–2013 from India. Clin Infect Dis. 2017 doi: 10.1093/cid/cix283. [DOI] [PubMed] [Google Scholar]
- 10.Brighton SW, Prozesky OW, de la Harpe AL. Chikungunya virus infection. A retrospective study of 107 cases. S Afr Med J. 1983;63(9):313–315. [PubMed] [Google Scholar]
- 11.Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49(6):942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 12.Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, et al. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis. 2007;44(11):1401–1407. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- 13.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, et al. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clin Infect Dis. 2008;47(4):469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- 14.Schilte C, Staikowsky F, Couderc T, Madec Y, Carpentier F, Kassab S, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7(3):e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain J, Dubey SK, Shrinet J, Sunil S. Dengue Chikungunya co-infection: a live-in relationship? Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Thirunarayanan T. An introduction to siddha medicine. Tiruchendur: Thirukumaran Publishers; 1981. [Google Scholar]
- 17.Subbarayappa B. Siddha medicine: an overview. The Lancet. 1997;350(9094):1841. doi: 10.1016/S0140-6736(97)04223-2. [DOI] [PubMed] [Google Scholar]
- 18.Lavekar G, Padhi M. Management of chikungunya through ayurveda and siddha a technical_report. New Delhi: Central Council for Research in Ayurveda and Siddha; 2009. [Google Scholar]
- 19.Lavekar G, Padhi M. Management of chikungunya through ayurveda and siddha: a technical report. New Delhi: Central Council for Research in Ayurveda and Siddha; 2007. pp. 28–44. [Google Scholar]
- 20.Anbarasu K, Manisenthil KK, Ramachandran S. Antipyretic, anti-inflammatory and analgesic properties of nilavembu kudineer choornam: a classical preparation used in the treatment of chikungunya fever. Asian Pac J Trop Med. 2011;4(10):819–823. doi: 10.1016/S1995-7645(11)60201-0. [DOI] [PubMed] [Google Scholar]
- 21.Murugan R, Vembu T, Kumarswamy M. Toxicological study of a siddha sastric formulation arumuga chendhuram in rat model. J Appl Pharm Sci. 2016;6(3):81–87. doi: 10.7324/JAPS.2016.60314. [DOI] [Google Scholar]
- 22.Manickavasakam K. Acute and sub acute toxicity studies on Sarva Noi Linga Chenduram in rodents. Int J Curr Res Chem Pharm Sci. 2017;4(2):13–18. doi: 10.22192/ijcrcps.2017.04.02.004. [DOI] [Google Scholar]
- 23.Ramya Devi D, Sandhya P, Malarvizhi K, Subashri S, Abinaya M, Vedha Hari B. Development of ayurvedic and siddha medicine through the application of modern pharmaceutical technique. Curr Trends Biotechnol Pharm. 673
- 24.Shrinet J, Jain S, Sharma A, Singh SS, Mathur K, Rana V, et al. Genetic characterization of Chikungunya virus from New Delhi reveal emergence of a new molecular signature in Indian isolates. Virol J. 2012;9(1):100. doi: 10.1186/1743-422X-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemm JA, Durbin RK, Stollar V, Rice CM. Mutations which alter the level or structure of nsP4 can affect the efficiency of Sindbis virus replication in a host-dependent manner. J Virol. 1990;64(6):3001–3011. doi: 10.1128/jvi.64.6.3001-3011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, et al. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007;3(6):e89. doi: 10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur P, Thiruchelvan M, Lee RC, Chen H, Chen KC, Ng ML, et al. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob Agents Chemother. 2013;57(1):155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.