Abstract
Background
Chronic heavy alcohol (CHA) use has been associated with perioperative complications. Emergency general surgery (EGS) patients are not routinely screened for CHA. If screened, it is usually for hazardous use of alcohol, using a survey such as the Alcohol Use Disorders Identification Test (AUDIT). This study screened EGS patients for CHA use using serum carbohydrate-deficient transferrin (%dCDT) level, a biomarker that has been validated as an indicator for CHA use, as well as the AUDIT. The purpose of this study was to determine the percent of EGS patients with CHA (as indicated by elevated %dCDT), and the relationship between %dCDT and AUDIT. Secondary aims included comparing the characteristics of EGS patients with and without CHA use, and evaluating the association of CHA use with negative clinical outcomes.
Methods
EGS patients aged 21 and older admitted to the general surgery inpatient service of a tertiary hospital from July 2014 to June 2016 were invited to participate in this study. %dCDT levels above 1.7% were considered positive for CHA use, as were AUDIT scores ≥8.
Results
195 EGS patients were screened for inclusion and 91 (46.7%) agreed to participate. 14 (15.4%) were positive for hazardous alcohol use on AUDIT and 5 (5.5%) were positive for CHA by %dCDT. Positive predictive value of AUDIT for CHA was 21.4%. There was no correlation between positive scores on AUDIT and %dCDT.
Discussion
Identifying at risk patients early on in their hospital course may allow clinicians to institute treatments to mitigate and/or circumvent complications in such patients. This pilot study determined that 17.6% of participating EGS patients were positive for some type of alcohol misuse, but only 5.5% had CHA. Further research is needed to determine whether routine use of %dCDT would be beneficial in reducing perioperative complications in this patient population.
Level of evidence
III (diagnostic test).
Keywords: alcohol use, acute care surgery, Carbohydrate Deficient Transferrin %dCDT, Alcohol Use Disorders Identification Test AUDIT
Background
Alcohol misuse is considered one of the modifiable behaviors that increase the risk of perioperative complications.1 The identification of patients who misuse alcohol remains difficult, likely due to the stigma associated with alcohol misuse and a lack thereof of awareness. An Australian study found that self-reporting surveys regularly underestimated the actual burden of alcohol-related disease due to under-reporting.2 Excessive alcohol use is the third leading preventable cause of mortality in the USA, claiming the lives of more than 88 000 people annually and leading to 2.5 million years of potential life lost.3 4
It is estimated that 4% to 40% of admitted medical and surgical patients suffer complications related to their alcohol use.5 In a study of inpatients at The Johns Hopkins University, 23% of general surgery patients admitted to the hospital screened positive for a Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria of alcohol dependence.6 Patients with trauma who tested positive for alcohol at admission had a twofold increased risk of developing pneumonia and other infections and were 2.5 times more likely to be readmitted to the hospital.7 8 Numerous studies have supported the link between chronic alcohol misuse and perioperative complications.9–13
Although patients with chronic heavy alcohol (CHA) use represent a higher risk category in the perioperative period, routine alcohol screening is still not performed in most centers. Identifying patients with CHA use early on in their admission can empower clinicians to implement strategies for managing these patients, potentially mitigating complications during the acute care process.14 15 The Alcohol Use Disorders Identification Test (AUDIT) developed by the WHO allows clinicians to identify alcohol use disorders in a variety of patient populations.16 17 The AUDIT screening tool is designed to identify hazardous or harmful alcohol use, and has a reported sensitivity and specificity of identifying alcohol dependence in general medical patients of 90% and 80% to 90%, respectively.18 19 Carbohydrate-deficient transferrin (%dCDT) is a serum biomarker and is an objective tool used to identify CHA use.10 20–24 Serum levels of %dCDT rise in patients who consume 50 g to 80 g of alcohol per day for at least a week with a half-life of 15 days; thus, %dCDT can be used to identify moderate to heavy alcohol consumption occurring during a longer period.22 25
Given the increased risk of perioperative morbidity associated with CHA use, the primary aim of this study was to determine the percent of emergency general surgery (EGS) patients with CHA (as indicated by elevated %dCDT), as well as the relationship between CDT and AUDIT scores. The secondary aims were to compare the characteristics of our EGS patients with and without CHA use, and to evaluate the association of CHA use with negative outcomes.
Methods
This prospective observational cohort study evaluated and followed individuals admitted to the EGS inpatient service of a tertiary teaching hospital between July 2014 and June 2016. Eligible subjects were 21 years of age or older, evaluated in the emergency department (ED), and admitted for biliary disease, gastrointestinal hemorrhage, diverticular disease, soft tissue infection, pancreatitis, small bowel obstruction, appendicitis, incarcerated hernia, abscess, hollow viscus perforation, ischemic colitis, volvulus, or other abdominal catastrophes. Individuals who died within 24 hours and those arriving after spending more than 24 hours at another facility were not eligible.
Eligible patients presenting to the EGS service were approached after admission. Consent was obtained from patients or a legally authorized representative if the patient was unable to give consent at that time. Blood drawn within 24 hours of ED admission was tested for %dCDT, medical records were reviewed for demographic and medical data, and an AUDIT was performed prior to discharge. To determine 30-day readmission, the medical record was reviewed to capture postdischarge ED or inpatient admissions to the same facility. To determine admission to other facilities, we attempted to contact each patient 1 month postdischarge. %dCDT level measurements were performed at the Clinical Neurobiology Labs at Medical University of South Carolina with a state-of-the-art, high-performance liquid chromatography assay that is recognized by the International Federation of Clinical Chemistry as a candidate reference method.26 This assay has the advantage of identifying rare genetic transferrin variants as well as significant liver disease variants that lead to false negatives and positives, thereby improving sensitivity and specificity over other methods.
A 2×2 table was created to establish the relationship between AUDIT and %dCDT, and determine sensitivities and specificities. In addition, continuous %dCDT levels and AUDIT scores were evaluated for correlation. A positive AUDIT was defined as a score greater than or equal to 8, and a positive serum %dCDT as greater than 1.7. Due to the few patients with a positive %dCDT, patients with either a positive AUDIT or %dCDT level were compared with the remaining cohort. Demographic characteristics include sex, race (white vs. non-white), age group (21–34, 35–44, 45–54, 55–64, 65–74, and 75+), and primary payer (commercial, Medicare, Medicaid, and uninsured). Participants were further classified as operative or non-operative management. Comorbid conditions were assigned using the Elixhauser et al’s methodology.27 Outcome variables of interest included presence of complications, hospital length of stay, and ED or inpatient readmission within 30 days of discharge.
All statistical analyses were conducted using Stata V.13.28 Differences in distributions by alcohol misuse were evaluated using Χ2 tests of homogeneity for categorical variables and Wilcoxon rank-sum test for continuous variables. The correlation between the continuous values of %dCDT and AUDIT was compared by Spearman rank correlation.
Results
One hundred and ninety-five EGS patients were approached for inclusion in the study, and 91 (46.7%) agreed to participate. At 30-day follow-up, 68 (74.7%) of participants were successfully contacted and participated in the follow-up survey. There were 84 participants with both %dCDT levels and AUDIT scores collected, six with a missing %dCDT and one with a missing AUDIT. Of those with no missing values, three had both an elevated %dCDT level and an elevated AUDIT score (table 1).
Table 1.
2×2 Table for %dCDT and AUDIT scores
| %dCDT >1.7% | ||||
| Yes | No | |||
| AUDIT ≥8 | Yes | 3 (75.0) | 11 (13.8) | 14 |
| No | 1 (25.0) | 69 (86.3) | 70 | |
| 4 | 80 | 84 | ||
%dCDT, carbohydrate-deficient transferrin; AUDIT, Alcohol Use Disorders Identification Test.
When accepting that a positive %dCDT indicates CHA use, we found the sensitivity and specificity of AUDIT for CHA use to be 75.0% and 86.3%, respectively. Figure 1 shows the analytic cohort by %dCDT level and AUDIT score. The correlation coefficient of the two continuous measurements of CHA use was ρ=0.14, which was not statistically significant.
Figure 1.
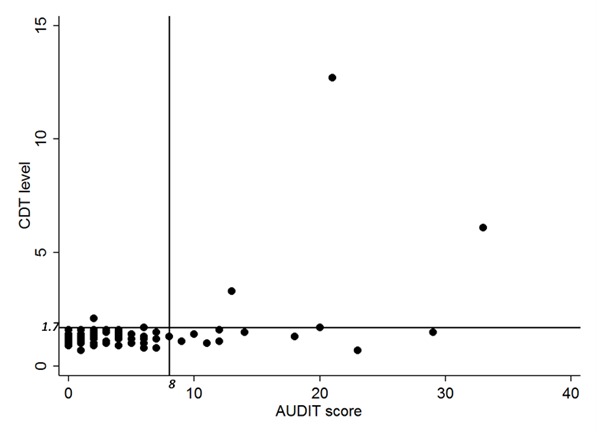
Correlation between carbohydrate-deficient transferrin (%dCDT) and Alcohol Use Disorders Identification Test (AUDIT) scores.
Among the 91 participants with either positive AUDIT or positive CDT, 16 (17.6%) were positive for hazardous or harmful drinking (table 2). The average age was 45.5 (±17.9) years and 50.5% were male. All participants were discharged alive and the median length of stay was 2 days (IQR=1–4 days). Seven participants (7.7%) experienced complications during their hospital stay, which consisted of urinary retention, arrhythmia, kidney injury, altered mental status, and colostomy necrosis. Nineteen (20.9%) participants were admitted to an ED or hospital within 30 days postdischarge. Those positive for hazardous or harmful drinking were less likely to have documented comorbidities. Readmission, complications, length of stay, race, age group, and payer type did not significantly differ by alcohol use category.
Table 2.
Demographic and clinical characteristics of study cohort
| n=91 | AUDIT or CDT | |||
| Positive | Negative | |||
| n=16 (17.6) | n=75 (82.4) | |||
| Readmitted within 30 days (ED or inpatient) | ||||
| No | 72 (79.1) | 13 (81.3) | 59 (78.7) | 0.817 |
| Yes | 19 (20.9) | 3 (18.8) | 16 (21.3) | |
| Complication noted | ||||
| No | 76 (83.5) | 11 (68.8) | 65 (86.7) | 0.301 |
| Yes | 7 (7.7) | 2 (12.5) | 5 (6.7) | |
| Surgery done? | ||||
| No | 16 (17.6) | 5 (31.3) | 11 (14.7) | 0.147 |
| Yes | 75 (82.4) | 11 (68.8) | 64 (85.3) | |
| Length of stay | ||||
| Mean±SD | 4.01± 6.44 | 4.13± 4.13 | 3.99± 6.85 | 0.9914 |
| Median (IQR) | 2.0 (3.0) | 3.0 (5.0) | 2.0 (3.0) | |
| Sex | ||||
| Female | 45 (49.5) | 4 (25.0) | 41 (54.7) | 0.052 |
| Male | 46 (50.5) | 12 (75.0) | 34 (45.3) | |
| Race | ||||
| White | 66 (72.5) | 10 (62.5) | 56 (74.7) | 0.361 |
| Non-white | 24 (26.4) | 6 (37.5) | 18 (24.0) | |
| Age | ||||
| 21–34 | 33 (36.3) | 5 (31.3) | 28 (37.3) | 0.801 |
| 35–44 | 14 (15.4) | 4 (25.0) | 10 (13.3) | |
| 45–54 | 14 (15.4) | 2 (12.5) | 12 (16.0) | |
| 55–64 | 14 (15.4) | 2 (12.5) | 12 (16.0) | |
| 65–74 | 9 (9.9) | 1 (6.3) | 8 (10.7) | |
| 75 and over | 7 (7.7) | 2(12.5) | 5(6.7) | |
| Primary payer | ||||
| Commercial | 51 (56.0) | 10 (62.5) | 41 (54.7) | 0.913 |
| Medicare | 16 (17.6) | 3 (18.8) | 13 (17.3) | |
| Medicaid | 6 (6.6) | 1 (6.3) | 5 (6.7) | |
| Uninsured | 18 (19.8) | 2 (12.5) | 16 (21.3) | |
| Number of comorbidities | ||||
| 0 | 42 (46.2) | 10 (62.5) | 32 (42.7) | 0.175 |
| 1 or more | 49 (53.8) | 6 (37.5) | 43 (57.3) | |
AUDIT, Alcohol Use Disorders Identification Test; CDT, carbohydrate-deficient transferrin; ED, emergency department.
Discussion
CHA use continues to plague our society,5 with surgical patients having higher rates of alcohol misuse and dependence than the general public.29 In our EGS patients, 16 of the 91 (17.6%) were positive on either the AUDIT or CDT, indicating some level of hazardous or harmful drinking. Our findings are lower than that reported by Moore et al,6 who found self-reported alcohol abuse rates to be 23% in their general surgery population, but within reported ranges in other studies.30 Only 47% of eligible patients approached for inclusion in this study agreed to participate. This high rate of rejection may have led to a biased sample, selecting out those that have a history of CHA or other hazardous use behavior. Self-reporting surveys regularly underestimate the true burden of this disease by up to 40% to 50%, as pointed out by Livingston and Callinan,2 and our estimates of those truly affected by heavy alcohol use could be low since half of the eligible population opted out of the study after being informed that it was evaluating alcohol use. It is this very reluctance to report alcohol misuse that motivated us to compare %dCDT and the AUDIT, as %dCDT would be a more objective screening tool for CHA use. It could then be implied that the actual burden of disease is greater than previously anticipated, further highlighting the need for this investigation.
The AUDIT screening tool was chosen as it has a reported sensitivity and specificity of identifying alcohol use disorder in general medical patients of 90% and 80% to 90%, respectively.18 The AUDIT screening tool has also been tested and validated in multiple settings with a diverse population including surgical patients,17 19–21 has proven to be superior to most biochemical markers for detecting alcohol misuse,31 and excelled over other screening tools such as the CAGE (Cut down, Annoyed, Guiltly, Eye-Opener) Questionnaire.18 32 Its limitations arise from the need for patients to cooperate and divulge the information required in the questionnaire, hence a lack of objectivity. Also, it assesses alcohol-related problems during a longer prehospitalization period and thus identifies those potentially providing false-positive indications of recent heavy drinking. The inability of some patients to participate in self-assessment questionnaires due to the severity and acuity of their disease process at admission also adds to its limitations.
Numerous biochemical markers have been used in the clinical setting to help clinicians identify patients with varying degrees of alcohol use disorders. Gamma glutamyl transferase (GGT), aspartate aminotransferase, alanine aminotransferase, and mean corpuscular volume have all been proposed as markers of liver injury that increase in the serum due to chronic alcohol use, but have suffered from poor accuracy due to low sensitivities or specificities, whereas serum %dCDT levels have reported improved reliability.24 33 In a systematic review, %CDT’s performance (including older, less specific assays) in the clinical setting elucidated its higher specificity and in some cases sensitivity at detecting alcohol misuse compared with GGT.34 %dCDT’s levels are not impacted by diet, common drugs, or comorbid diseases such as hypertension, diabetes, lipid metabolism disorders, or disorders of the gastrointestinal tract; however, older assays can be less sensitive in women than in men.35 36
When examining the relationship between %dCDT and AUDIT, the correlation was low (ρ=0.14) but also limited to low numbers, and is analogous to a study by Hermansson et al,37 who saw the correlation between serum %dCDT levels and AUDIT scores to be between 0.15 and 0.2. Of the four patients with positive %dCDT levels, three had positive AUDIT scores, but most patients with positive AUDIT scores did not have elevated %dCDT levels. This makes sense, since %dCDT specifically measures heavy alcohol use in the recent past, whereas AUDIT is designed to capture a wider range of hazardous or harmful alcohol behaviors during a longer period.
Although well documented in the literature, our study failed to show any statistically significant association between drinking behaviors and complication rates, readmission rates, or length of stay. This may be attributed to the rarity of those complications and the relatively small cohort of only 91 participants, of whom only 5 had a positive %dCDT. Patients who do not have CHA use but other risky alcohol-related behaviors (such as occasional binge drinking, or drinking and driving) may be captured by the AUDIT but will not be detected by elevated %dCDT values. These behaviors, although increasing the risk of injury, are less likely to cause the pathophysiologic changes associated with CHA use. As Stibler indicates, %dCDT may be a marker of drinking habits associated with CHA use-related pathologic findings.23 36 Examining for the assignment of any diagnosis codes included under Elixhauser et al’s27 comorbidity grouping for alcohol abuse among the participants, we found that only one patient had been assigned such a diagnosis. That patient was positive on the AUDIT, but not %dCDT, and none of the four participants with positive %dCDT had diagnoses codes indicating alcohol abuse, suggesting that %dCDT can identify people missed during clinical assessment.
Aside from the potential selection bias discussed earlier, the 47% patient participation rate underpowered our study, limiting our ability to make inferential conclusions about alcohol use and this patient population.
There is a large body of literature supporting the view that CHA use leads to increased morbidity and mortality in patients admitted to the hospital for trauma or elective surgery.7–13 38 39 Identifying at risk patients early on in their hospital course may allow clinicians to institute treatments to mitigate and/or circumvent complications in such patients. This pilot study determined that 17.6% of our participating EGS patients had either a positive %CDT or AUDIT score, indicating the presence of some level of alcohol misuse. The AUDIT, however, is neither objective nor has the sensitivity to identify patients with increased risk of the physiologic impacts of CHA use. While we were unable to show a difference in outcomes, this could be due to the relatively small number of patients we were able to recruit, as well as the smaller subset that was specifically positive for CHA use. Additional research is needed to examine the impact of CHA use in larger cohorts of EGS patients, as well as the utility of routine %dCDT testing.
Footnotes
Contributors: Study design: JVS, SMF, PLF, RFA, BJ. Literature search: MMM, BJ, JVS, PLF. Data collection/analysis/interpretation: MMM, BJ, SMF, DAW, PLF, RFA, JVS. Writing: MMM, JVS, SMF, PLF, DAW, RFA.
Funding: Charleston Alcohol Research Center - Medical University of South Carolina (Grant# P50AA010761).
Competing interests: None declared.
Ethics approval: Medical University of South Carolina’s Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bradley KA, Rubinsky AD, Sun H, Blough DK, Tønnesen H, Hughes G, Beste LA, Bishop MJ, Hawn MT, Maynard C et al. Prevalence of alcohol misuse among men and women undergoing major noncardiac surgery in the Veterans Affairs health care system. Surgery 2012;152:69–81. doi:10.1016/j.surg.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 2.Livingston M, Callinan S. Underreporting in alcohol surveys: whose drinking is underestimated? J Stud Alcohol Drugs 2015;76:158–64. doi:10.15288/jsad.2015.76.158 [PubMed] [Google Scholar]
- 3.Harwood H. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. Rockville, MD: National institute on drug abuse and the national institute on alcohol abuse and alcoholism, national institutes of health, department of health and human services, national institute on drug abuse and the national institute on alcohol abuse and alcoholism NIoH, department of health and human services, 1998. Report No: NIH Publication No. 98-4327. [Google Scholar]
- 4.Gonzales K, Roeber J, Kanny D, Tran A, Saiki C, Johnson H, Yeoman K, Safranek T, Creppage K, Lepp A, et al. Alcohol-attributable deaths and years of potential life lost--11 States, 2006-2010. MMWR Morb Mortal Wkly Rep 2014;63:213–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis 2014;11:E109 doi:10.5888/pcd11.130293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore RD, Bone LR, Geller G, Mamon JA, Stokes EJ, Levine DM. Prevalence, detection, and treatment of alcoholism in hospitalized patients. JAMA 1989;261:403–7. doi:10.1001/jama.1989.03420030077033 [PubMed] [Google Scholar]
- 7.Rivara FP, Koepsell TD, Jurkovich GJ, Gurney JG, Soderberg R. The effects of alcohol abuse on readmission for trauma. JAMA 1993;270:1962–4. doi:10.1001/jama.1993.03510160080033 [PubMed] [Google Scholar]
- 8.Jurkovich GJ. The effect of acute alcohol intoxication and chronic alcohol abuse on outcome from trauma. JAMA: The Journal of the American Medical Association 1993;270:51–6. doi:10.1001/jama.1993.03510010057029 [PubMed] [Google Scholar]
- 9.Spies CD, Neuner B, Neumann T, Blum S, Müller C, Rommelspacher H, Rieger A, Sanft C, Specht M, Hannemann L, et al. Intercurrent complications in chronic alcoholic men admitted to the intensive care unit following trauma. Intensive Care Med 1996;22:286–93. doi:10.1007/BF01700448 [DOI] [PubMed] [Google Scholar]
- 10.Spies CD, Kissner M, Neumann T, Blum S, Voigt C, Funk T, Runkel N, Pragst F. Elevated carbohydrate-deficient transferrin predicts prolonged intensive care unit stay in traumatized men. Alcohol Alcohol 1998;33:661–9. doi:10.1093/alcalc/33.6.661 [DOI] [PubMed] [Google Scholar]
- 11.Tønnesen H. Alcohol abuse and postoperative morbidity. Dan Med Bull 2003;50:139–60. [PubMed] [Google Scholar]
- 12.Rubinsky AD, Bishop MJ, Maynard C, Henderson WG, Hawn MT, Harris AH, Beste LA, Tønnesen H, Bradley KA. Postoperative risks associated with alcohol screening depend on documented drinking at the time of surgery. Drug Alcohol Depend 2013;132:521–7. doi:10.1016/j.drugalcdep.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 13.Eliasen M, Grønkjær M, Skov-Ettrup LS, Mikkelsen SS, Becker U, Tolstrup JS, Flensborg-Madsen T. Preoperative alcohol consumption and postoperative complications: a systematic review and meta-analysis. Ann Surg 2013;258:930–42. doi:10.1097/SLA.0b013e3182988d59 [DOI] [PubMed] [Google Scholar]
- 14.Tonnesen H, Rosenberg J, Nielsen HJ, Rasmussen V, Hauge C, Pedersen IK, Kehlet H. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ 1999;318:1311–6. doi:10.1136/bmj.318.7194.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oppedal K, Møller AM, Pedersen B, Tønnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev 2012;7:CD008343 doi:10.1002/14651858.CD008343.pub2 [DOI] [PubMed] [Google Scholar]
- 16.Lundin A, Hallgren M, Balliu N, Forsell Y. The use of alcohol use disorders identification test (AUDIT) in detecting alcohol use disorder and risk drinking in the general population: validation of AUDIT using schedules for clinical assessment in neuropsychiatry. Alcohol Clin Exp Res 2015;39:158–65. doi:10.1111/acer.12593 [DOI] [PubMed] [Google Scholar]
- 17.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction 1993;88:791–804. doi:10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 18.Soderstrom CA, Smith GS, Kufera JA, Dischinger PC, Hebel JR, McDuff DR, Gorelick DA, Ho SM, Kerns TJ, Read KM, et al. The accuracy of the CAGE, the Brief michigan alcoholism screening test, and the alcohol use disorders identification test in screening trauma center patients for alcoholism. J Trauma 1997;43:962–9. doi:10.1097/00005373-199712000-00017 [DOI] [PubMed] [Google Scholar]
- 19.Babor TF, Higgins-Biddle JC, Saunders JB. The alcohol use disorders identification test: guidelines for use in primary care: Dependence DoMHaS, 2001. Report No.: WHO/MSD/MSB/01.6a. [Google Scholar]
- 20.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 2007;31:185–99. doi:10.1111/j.1530-0277.2006.00295.x [DOI] [PubMed] [Google Scholar]
- 21.Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613–9. doi:10.1111/j.1530-0277.1997.tb03811.x [PubMed] [Google Scholar]
- 22.Sharpe PC. Biochemical detection and monitoring of alcohol abuse and abstinence. Ann Clin Biochem 2001;38(Pt 6):652–64. doi:10.1258/0004563011901064 [DOI] [PubMed] [Google Scholar]
- 23.Stibler H. Carbohydrate-deficient transferrin in serum: a new marker of potentially harmful alcohol consumption reviewed. Clin Chem 1991;37:2029–37. [PubMed] [Google Scholar]
- 24.Hock B, Schwarz M, Domke I, Grunert VP, Wuertemberger M, Schiemann U, Horster S, Limmer C, Stecker G, Soyka M, et al. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: a study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction 2005;100:1477–86. doi:10.1111/j.1360-0443.2005.01216.x [DOI] [PubMed] [Google Scholar]
- 25.Nanau RM, Neuman MG. Biomolecules and Biomarkers Used in Diagnosis of Alcohol Drinking and in Monitoring Therapeutic Interventions. Biomolecules 2015;5:1339–85. doi:10.3390/biom5031339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schellenberg F, Wielders J, Anton R, Bianchi V, Deenmamode J, Weykamp C, Whitfield J, Jeppsson JO, Helander A. IFCC approved HPLC reference measurement procedure for the alcohol consumption biomarker carbohydrate-deficient transferrin (CDT): Its validation and use. Clin Chim Acta 2017;465:91–100. doi:10.1016/j.cca.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. doi:10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 28.Statacorp. Stata statistical software: release 13.1. College station, TX: Statacorp LP, 2013. [Google Scholar]
- 29.Craft PP, Foil MB, Cunningham PR, Patselas PC, Long-Snyder BM, Collier MS. Intravenous ethanol for alcohol detoxification in trauma patients. South Med J 1994;87:47–54. doi:10.1097/00007611-199401000-00011 [DOI] [PubMed] [Google Scholar]
- 30.Magruder-Habib K, Durand AM, Frey KA. Alcohol abuse and alcoholism in primary health care settings. J Fam Pract 1991;32:406–13. [PubMed] [Google Scholar]
- 31.Neumann T, Gentilello LM, Neuner B, Weiss-Gerlach E, Schürmann H, Schröder T, Müller C, Haas NP, Spies CD. Screening trauma patients with the alcohol use disorders identification test and biomarkers of alcohol use. Alcohol Clin Exp Res 2009;33:970–6. doi:10.1111/j.1530-0277.2009.00917.x [DOI] [PubMed] [Google Scholar]
- 32.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131:1121–3. doi:10.1176/ajp.131.10.1121 [DOI] [PubMed] [Google Scholar]
- 33.Peterson K. Biomarkers for alcohol use and abuse--a summary. Alcohol Res Health 2004;28:30–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999;19:261–71. doi:10.1016/S0741-8329(99)00044-0 [DOI] [PubMed] [Google Scholar]
- 35.Bean P, Harasymiw J, Peterson CM, Javors M. Innovative technologies for the diagnosis of alcohol abuse and monitoring abstinence. Alcohol Clin Exp Res 2001;25:309–16. doi:10.1111/j.1530-0277.2001.tb02214.x [PubMed] [Google Scholar]
- 36.Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem 2001;47:13–27. [PubMed] [Google Scholar]
- 37.Hermansson U, Helander A, Brandt L, Huss A, Rönnberg S. The Alcohol Use Disorders Identification test and carbohydrate-deficient transferrin in alcohol-related sickness absence. Alcohol Clin Exp Res 2002;26:28–35. doi:10.1111/j.1530-0277.2002.tb02428.x [PubMed] [Google Scholar]
- 38.Tønnesen H, Schütten BT, Jørgensen BB. Influence of alcohol on morbidity after colonic surgery. Dis Colon Rectum 1987;30:549–51. doi:10.1007/BF02554788 [DOI] [PubMed] [Google Scholar]
- 39.Spies C, Tønnesen H, Andreasson S, Helander A, Conigrave K. Perioperative morbidity and mortality in chronic alcoholic patients. Alcohol Clin Exp Res 2001;25(5 Suppl ISBRA):164S–70. doi:10.1111/j.1530-0277.2001.tb02392.x [DOI] [PubMed] [Google Scholar]


