Abstract
Background
Tranexamic acid (TXA) has demonstrated improved mortality among trauma patients. However, recent evidence from urban US trauma centers has failed to show a benefit among the civilian population. TXA in rural states has not been evaluated. This study aimed to evaluate the current use of TXA in the rural trauma population.
Methods
A retrospective observational review at a level 1 trauma center based in a rural environment. Records were reviewed for TXA indications. TXA indication was defined as: systolic blood pressure <90 mm Hg, blood transfusion, or with a clinical concern for ongoing bleeding. Patients were ineligible if the time since injury was >3 hours.
Results
400 patients were evaluated. 54% of patients met indications for TXA. 14% of these received TXA. 30.4% with an indication for TXA were ineligible due to arrival beyond 3 hours from time of injury. 135 patients arrived as transfers, 265 from the scene. There was no difference in TXA indications between scene and transfers (73 vs 144, p=1). Transfers were more likely to arrive beyond the 3-hour window (59 vs 7, p=0.001). Mortality for patients treated with TXA was 12.5%. This was not significantly different from patients not treated with TXA (19%).
Discussion
In a rural system, long transfers exclude most patients from treatment with TXA. A multicenter rural trauma center study will be needed to better define the optimal use of TXA in rural populations.
Level of evidence
Level IV data: therapeutic/care management.
Keywords: rural trauma care, tranexamic acid
Background
Trauma remains a major source of mortality worldwide.1 Hemorrhage is responsible for one-third of all traumatic deaths.2 Recent research has identified the coagulopathy of trauma as a significant component and risk factor for hemorrhagic death.3 Use of technology, specifically thromboelastography (TEG), has allowed new investigations into post-traumatic coagulopathy, including the identification of primary hyperfibrinolysis. Trauma, like major surgery, stimulates clot formation and fibrinolysis pathways within the body. This hyperfibrinolysis, measured by TEG as LY-30, can become pathologic and exacerbate bleeding in these patients. As demonstrated by the Denver group, an LY-30 >3% is associated with a significantly increased risk of hemorrhagic death in trauma patients (45.5% vs 4.8%).4 Tranexamic acid (TXA) is a lysine derivative that binds plasminogen and blocks activation to plasmin. Recent studies have evaluated the use of TXA in the trauma patient to treat hyperfibrinolysis.5–9 The CRASH-2 study7 was an international, randomized, placebo-controlled study investigating the effects of TXA on civilian trauma patients. All-cause mortality was reduced by 1.5% within the TXA group, as was the risk of bleeding specific deaths. Follow-up studies found that this mortality effect was only present when TXA was administered within 3 hours of injury, patients receiving TXA beyond 3 hours from injury had an increase in mortality.10 Additional support for the use of TXA was provided by the Military Application of Tranexamic Acid in Trauma Emergency Resuscitation study (MATTERs).8 This was a retrospective study done by the US military to investigate the effects of TXA in a severely injured warfighter population. These military data also supported the use of TXA for reduction in all-cause and bleeding-specific mortality. It demonstrated the largest improvement in survival for any patient meeting massive transfusion criteria. Given these data, in 2013, a local protocol was developed for the use of TXA. TXA indications were systolic blood pressure (SBP) <90 mm Hg, receiving blood transfusions, on activation of the massive transfusion protocol, or any patient with active bleeding or clinical concern for ongoing bleeding. TXA was contraindicated if the time since injury was >3 hours or in the case of isolated traumatic brain injury.
Large US urban trauma centers had implemented local policies for TXA use as well. Data from Memorial Hermann in Houston,11 and the Ryder Trauma Center in Miami,12 found an increased mortality among the severely injured treated with TXA. These results questioned the efficacy of TXA given the mature trauma systems, and rapid availability of blood products and operating rooms to patients in urban areas of the USA. These data allowed further criticism of the CRASH-2 trial. Critics of the CRASH-2 trial had noted an overall small effect size and the indiscriminate use of TXA with all trauma patients. Additionally, the regions CRASH-2 was performed in were not necessarily applicable to the US population.13 The efficacy of TXA within the US civilian population was therefore called into question. The purpose of this study was to examine the rural experience with TXA. Large urban trauma centers, with extremely short times from injury to definitive care, care for an inherently different population from the rural trauma patient. These trauma centers are afforded a luxury of time, in that urban patients arrive well inside the 3 hours window, allowing time to evaluate patients with TEG before determining the need for TXA treatment. The rural trauma system routinely sees patients arriving for definitive care hours after injury.14–16 With 15% of Americans being served by rural trauma systems, there may still be a large population in which TXA is advantageous. This rural population is likely more similar to the CRASH-2 patient population. This may support a more blanketed approach for TXA treatment and the acceptance of a smaller absolute treatment effect.
Therefore, the aims of this study were to evaluate the current use of TXA in the rural trauma population. First, to identify how many of the patients within a rural trauma system would meet indications for TXA treatment. Second, to determine if long transport times within a rural system would deem many patients ineligible. Our hypothesis was that many patients would be made ineligible for treatment based on time in transport. Our third aim was to report on this early implementation in rural trauma system. Lastly to evaluate if TXA did improve survival in those patients who received it.
Methods
With IRB approval, a retrospective review was performed of the trauma registry for the John Michael Moore Trauma Center at West Virginia University. This is a level 1 trauma center based in a rural environment. The registry was queried from July 1 2013 through October 31 2014. Inclusion criteria were all adult full trauma team activations. Full trauma team activations were selected, as this population is more likely to be in shock or receiving blood transfusions, therefore more likely to be candidates for TXA treatments. Full team activation was indicated for SBP <90 mm Hg, respiratory compromise, patients receiving blood en route, patients requiring intravenous fluids to maintain vital signs, penetrating trauma to the neck or torso and Glasgow coma scale (GCS) <8 with mechanism associated with trauma.
Registry data and charts were first reviewed to determine if TXA administration was indicated. TXA indication was defined as any of the following: hypotension (SBP <90 mm Hg), receiving blood transfusions, on initiation of the massive transfusion protocol, actively bleeding or with a clinical concern for ongoing bleeding. In the case of isolated traumatic brain injury, patients were marked as not indicated. Patients were ineligible if the time since injury was >3 hours. Records were then reviewed to determine if the patient arrived within 3 hours of injury time. Emergency medical services (EMS) records and registry data determined time from injury. If the researchers were unable to determine a reliable time of injury after review of EMS and transfer records the patient was considered ineligible due to time (figure 1).
Figure 1.
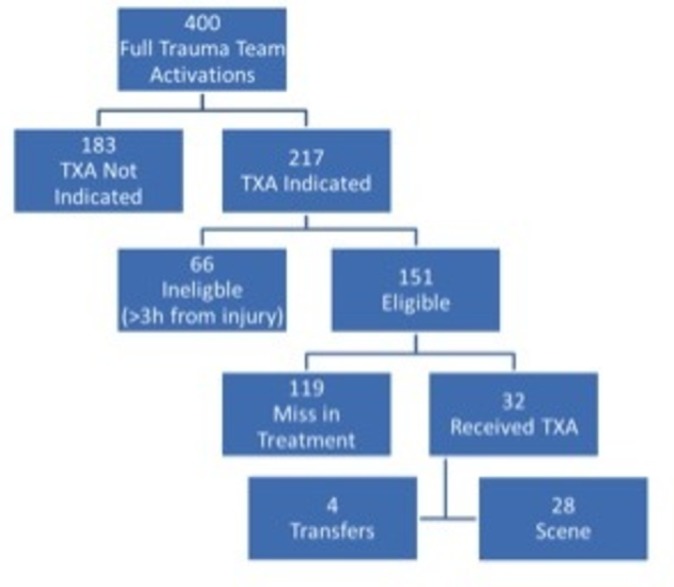
Patient population. TXA, tranexamic acid.
TXA was administered as a 1 g bolus over 10 min, followed by a 1 g infusion over 8 hours. TEG was routinely performed on these patients. However in contrast to protocols published from urban trauma centers, TXA treatment was initiated prior to LY-30 results. It was felt that awaiting these results would cause a greater number of patients to fall outside of the 3-hour treatment window. Of note, no referring hospital or EMS agency was administering TXA during the study period. All TXA administered was given at the study institution. Patients were considered a ‘miss’ if they arrived within 3 hours of injury, had TXA indications, but did not receive treatment. Data were analyzed using SPSS Statistics V.23 (IBM, Armonk, New York, USA). Two-tailed, Fischer’s exact test was used to compare outcomes, and multivariate regression performed. All p values considered significant for <0.05.
Results
For the study period, 400 patients were evaluated. Mean age was 43.9 years (range 18–94). Ninety patients (22.5%) were female. Mean injury severity score (ISS) was 19 (range 1–75). Mean ISS for those receiving TXA was 24 (range 2–50). Two hundred and twenty-four (56%) patients had an ISS ≥15. Eighty-three (20.8%) patients suffered penetrating injuries, the rest were blunt trauma. Overall mortality was 16.25% (65 patients) (table 1).
Table 1.
Demographics baseline
| TXA indicated (n=217) | TXA not indicated (n=183) | p Value | |
| Age | 45.5 | 42.1 | 0.57 |
| Male (%) | 73.3% | 82.5% | 0.27 |
| Blunt mechanism (%) | 70.0% | 89.1% | 0.0001 |
| Penetrating mechanism (n) | 30.0% | 10.9% | 0.0001 |
| % Patients receiving blood product | 65.9% | 7.1% | 0.0001 |
| Mean PRBC units (if received blood product) | 6.9 U | 3.1 U | 0.13 |
| Mean ISS | 21 | 14 | >0.0001 |
| ISS ≥15 (%) | 65.90% | 44.30% | >0.0001 |
| Mean arrival SBP (mm Hg) | 109.8 | 140.6 | >0.001 |
| Arrival lactate* | 3.2 | 2.4 | >0.001 |
*Arrival lactate available for 337 patients.
ISS, injury severity score; PRBC, packed red blood cell; TXA, tranexamic acid.
Of the patients evaluated, 217 (54.2%) met indications for TXA administration. These patients were further analyzed. TXA was administered to 32 (14.7%) of those patients. On retrospective analysis, four (1%) patients treated with TXA were given a dose outside of the 3-hour window from time of injury. They were analyzed as a portion of the TXA cohort. No mortalities occurred within these four patients. Within the TXA indicated cohort, 66 (30.4%) of patients were deemed ineligible for treatment due to arrival beyond 3 hours from time of injury; the remaining 151 (69.6%) arrived within the 3-hour window. Referring hospitals transferred 135 (33.7%) patients, 265 (66.3%) patients arrived from the scene. Scene patients and transfer patients were just as likely to meet indications for TXA (144 scene patients vs 73 transfer patients, p=1). Transfer patients were also statistically more likely to arrive outside the 3-hour window (59 transfers vs 7 scene, p=0.001). Scene and transfer patients were not statistically different in receiving TXA when indicated and eligible (28 scene vs 4 transfers, p=0.497) (Table 2). Among the four transfer patients who received TXA, ground crew transported only one. Of the transfer patients, 59 patients (43.7% of transfers) were transported by aeromedical services to our center, 24 of these patients (17.8% of transfers) would arrive more than 3 hours from time of injury. Ground crew transferred the remaining 76 patients (56.3% of transfers), with 32 (23.7% of transfers) of them arriving >3 hours from injury. There was no significant difference between aeromedical and ground transport and the likelihood of arriving outside the treatment window.
Table 2.
Comparison of scene vs transfer patients
| Scene (n=265) | Transfer (n=135) | p Value | |
| TXA indicated | 144 | 73 | 1 |
| TXA eligible | 137 | 14 | 0.001 |
| TXA not eligible (>3 hour since injury) | 7 | 59 | 0.001 |
| TXA received | 28 | 4 | 0.497 |
TXA, tranexamic acid.
Overall, in the TXA treatment group, there were four deaths, a mortality rate of 12.5%; 119 (29.8%) patients were considered a miss. Mortality within the patients who missed being treated with TXA was 19.5%. The mortality difference between the TXA group and those that missed treatment, was not statistically significant, p=0.45. Of those with an indication for TXA but were ineligible due to time, mortality was 19%. This was also not statistically significant when compared with patients treated with TXA, p=0.56.
A multivariate regression was performed to evaluate the effect TXA may have had on mortality. Lactate, ISS, SBP, transfer status and TXA administration were evaluated. Lactate (p<0.001) and ISS (p<0.001) were associated with mortality. Transfer status (p=0.35) and SBP (p=0.39) were not associated with mortality. TXA administration was not statistically significant for association with mortality (p=0.10), but shows a trend towards treatment effect.
Subgroup analysis was then performed for patients undergoing massive transfusion. Thirty (7.5%) patients met criteria for massive transfusion, 24 scene patients and 6 transfer patients. Mean arrival SBP was 84 mm Hg (range 0–138). Mean packed red blood cell transfusion (PRBC) requirements were 20.8 units (range 10–62). Mean ISS among these patients was 31 (range 16–57). TXA was administered to 13 of 24 (54.2%) scene patients. No scene patient in the massive transfusion cohort was ineligible due to time. Of the six transfer patients, four (66.7%) of the patients were ineligible due to time, and neither of the remaining patients received TXA treatment. Mortality was 33% among scene patients and 50% among transfer patients. Of the eight deaths in the scene cohort, three patients received TXA, five did not. In the survivor cohort, 10 patients received TXA, 6 did not. There was no statistically significant effect of TXA in this group, p=0.39.
Conclusion
The use of TXA in the US civilian population is still being evaluated. Initial review of a large international, randomized control trial and a well-constructed military retrospective suggested both a bleeding specific, and all-cause mortality benefit to the injured patient. However, recent evidence from two major urban trauma centers11 12 has failed to show a mortality benefit when TXA is administered. This brings into questions the applicability of the CRASH-2 and MATTERs trials to the US civilian population. These urban center authors have suggested that the rapid availability of EMS, blood products and operating rooms may make TXA use less efficacious in this setting.
This study aimed to evaluate the experience of the rural trauma patient. These patients encompass 15% or nearly 47 million potential patients with the USA. This is a significant portion of the population and inherently different from those injured in the urban environment. Long transport times, lack of widely available blood products and the need for transfer from critical access hospitals cause delays in definitive treatment. These rural patients may be more similar to the CRASH-2 patient population than previously considered. These data illustrate that a large portion of this rural patient population would be a candidate for TXA treatment. Long transport times cause a significant delay in the administration of TXA, regardless of the robust use of aeromedical transport within our rural system. Often this delay is so extreme that the patient is deemed ineligible for treatment. While 30.4% of all patients with an indication for TXA arrived outside the treatment window, transfer patients encompassed the majority. The vast majority of scene patients (137 of 144, 95%) arrive while still eligible for treatment with TXA. In contrast, transfer patients met indications for TXA 73 times (54.1% of transfers), 59 (80.8%) of these arrived outside the treatment window. Regardless of transport type, the severely injured rural trauma patient is likely to arrive for definitive care >3 hours since the time of injury. That this phenomenon occurs in both air and ground transport highlights time spent at referring facilities. This highlights the need for TXA use by local EMS, aeromedical units and at the critical access hospitals in these rural areas. If patients undergo transfer within a rural trauma setting without receiving TXA, they are likely to miss their treatment eligibility window. This is meant to again shed light on the challenges faced in the rural trauma environment. And while TXA has been shown to have a reduction in bleeding specific mortality, there has also been an all-cause mortality reduction as well. This has been attributed to anti-inflammatory effects of treatment. These rural patients may be missing additional benefits as this aspect of TXA treatment is still investigated.
This study does have its limitations. First as a retrospective study, the authors were limited to data available in the medical registry. The authors also recognize the low compliance with their institutions own TXA protocol, only 32 patients receiving treatment as indicated. One hundred and nineteen (29.8%) patients missed treatment with TXA. TEG data were not available for all patients at the initiation of TXA treatment; this was to avoid missing the treatment window while awaiting results. Some patients with a TXA indication may have had normal levels of fibrinolysis and would not have benefited from the drug, except for its reported anti-inflammatory effects. This may have introduced a bias into the results. Some of these misses were inevitably identified only by the retrospective review of the charts, with patients experiencing occult bleeding not recognized until later in the patients care. However, this rate of compliance is similar to studies published from urban centers.11 12 The authors also had access to additional EMS records to evaluate time since injury. Some of this dispatch data would not have been available to clinical providers, and may have led to TXA being withheld due to uncertainty of time since injury. Additionally, some patients likely missed treatment during protocol roll out and education. While no mortality benefit was shown, this study is limited as a single institution experience with small numbers of mortalities and low compliance with its own TXA protocols.
A multicenter rural trauma center study will be needed to better define the use of TXA in this population. Additionally, integration and study of TXA protocols with critical access hospitals and EMS agencies may better define its place in rural trauma care. Further study with system-wide TXA administration may yield the greatest benefit.
Footnotes
Contributors: Study design was performed by JMB, AP, GS and AW. Data collection was performed by JMB, AP and JC. The manuscript was written by JMB, JC and GS. All authors participated in critical revisions and resubmission.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.World Health Organization (WHO). Injuries Violence. Causes of Injuies Death 2014. [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 2006;60(6 Suppl):S3–S11. doi:10.1097/01.ta.0000199961.02677.19 [DOI] [PubMed] [Google Scholar]
- 3.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T et al. The coagulopathy of trauma: a review of mechanisms. J Trauma 2008;65:748–54. doi:10.1097/TA.0b013e3181877a9c [DOI] [PubMed] [Google Scholar]
- 4.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg 2013;75:961–7. doi:10.1097/TA.0b013e3182aa9c9f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg 2015;261:390–4. doi:10.1097/SLA.0000000000000717 [DOI] [PubMed] [Google Scholar]
- 6.Guerriero C, Cairns J, Perel P, Shakur H, Roberts I. CRASH 2 trial collaborators. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One 2011;6:e18987 doi:10.1371/journal.pone.0018987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. doi:10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 8.Ghazi S, Pierson R, Torlinski T. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation Study. J Intensive Care Soc 2013;14:86–8. doi:10.1177/175114371301400118 [Google Scholar]
- 9.Ker K, Kiriya J, Perel P, Edwards P, Shakur H, Roberts I. Avoidable mortality from giving tranexamic acid to bleeding trauma patients: an estimation based on WHO mortality data, a systematic literature review and data from the CRASH-2 trial. BMC Emerg Med 2012;12:3 doi:10.1186/1471-227X-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011;377 doi:10.1016/S0140-6736(11)60278-X [DOI] [PubMed] [Google Scholar]
- 11.Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, Wade CE, Holcomb JB, Cotton BA. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg 2015;78:905–11. doi:10.1097/TA.0000000000000612 [DOI] [PubMed] [Google Scholar]
- 12.Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, Livingstone AS, Namias N, Schulman CI, Proctor KG. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg 2014;76:1373–8. doi:10.1097/TA.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 13.Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE, et al. Tranexamic acid in trauma. J Trauma Acute Care Surg 2013;74:1575–86. doi:10.1097/TA.0b013e318292cc54 [DOI] [PubMed] [Google Scholar]
- 14.Grossman DC, Kim A, Macdonald SC, Klein P, Copass MK, Maier RV. Urban-rural differences in prehospital care of major trauma. J Trauma 1997;42:723–9. doi:10.1097/00005373-199704000-00024 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez RP, Cummings G, Mulekar M, Rodning CB. Increased mortality in rural vehicular trauma: identifying contributing factors through data linkage. J Trauma 2006;61:404–9. doi:10.1097/01.ta.0000229816.16305.94 [DOI] [PubMed] [Google Scholar]
- 16.Zwerling C, Peek-Asa C, Whitten PS, Choi SW, Sprince NL, Jones MP. Fatal motor vehicle crashes in rural and urban areas: decomposing rates into contributing factors. Inj Prev 2005;11:24–8. doi:10.1136/ip.2004.005959 [DOI] [PMC free article] [PubMed] [Google Scholar]


