Abstract
Over the past 65+ years, most civilian peripheral vascular injuries have been managed by trauma surgeons with training or experience in vascular repair or ligation. This is appropriate as the in-hospital trauma team is immediately available, and there are often other injuries present in the victim.
The pitfall to avoid during evaluation of the patient in the emergency center is a missed diagnosis. In the patient without ‘hard’ signs of a peripheral vascular injury, a careful history (bleeding), physical examination including measurement of ankle–brachial (ABI) or brachial–brachial index and liberal use of CT arteriography depending on an ABI <0.9 should essentially make the diagnosis if an arterial injury is present.
At operation, one pitfall is to limit skin preparation and draping, thereby eliminating the option of removing the greater saphenous vein if needed as a conduit from either the groin or ankle of an uninjured lower extremity. Another pitfall is to make a full longitudinal incision directly over a large pulsatile hematoma. Rather, separate shorter longitudinal incisions should be made to obtain proximal and distal vascular control before entering the hematoma. The failure to recognize patients who should be managed initially with insertion of a temporary intraluminal shunt is a major pitfall as well. Not following time-proven and results-proven ‘fine techniques’ of operative repair is another major pitfall. Such techniques include the following: use of small angioaccess vascular clamps or silastic vessel loops; passage of proximal and distal Fogarty catheters; administration of regional or systemic heparin during complex repairs; an open anastomosis technique; and completion arteriography after a complex arterial repair in a lower extremity.
Avoiding pitfalls should allow for success in peripheral vascular repair, particularly since most patients are young with non-diseased vessels.
Keywords: endovascular stent
There are a number of trends that have significantly impacted the management of peripheral vascular trauma in American trauma centers in recent years. In some areas, the number of gunshot wounds, the most common cause of peripheral vascular trauma in all series, has decreased significantly. There has been a simultaneous decrease in the exposure of general surgery residents, surgical critical care residents and acute care surgery fellows to patients with vascular trauma as well. This is related to the increased number of integrated vascular residents to whom such patients are often directed in certain trauma centers. In addition, there has been an increase in the number of Board-certified vascular surgeons available to operate in many trauma centers. Of interest, the results of operations performed by these specialists are the same as when they are performed by general trauma surgeons.1 The success of non-operative management of non-occlusive arterial injuries in patients presenting with ‘soft’ signs of a peripheral arterial injury is another factor, as these patients almost always had peripheral arterial repairs in the 1980s and early 1990s.2–4 Finally, endovascular stents and stent grafts have now replaced repair in selected peripheral arterial injuries.5 6
This combination of factors means that the general surgery trauma service is now responsible for the management of peripheral vascular trauma only when the vascular surgery service is not available in many trauma centers. Unfortunately, all the issues listed contribute to a lack of recognition of pitfalls when the general surgery trauma team manages peripheral vascular injuries.
Avoiding pitfalls in the preoperative period
In any hemodynamically stable patient with trauma to an extremity, the goal of the general surgery trauma team is to analyze whether a peripheral vascular injury is present (figure 1). This is particularly true when the trauma is in proximity to the brachial artery and veins in the upper extremity and superficial femoral artery and femoral vein in the lower extremity (box 1). When penetrating wounds are the most common mechanism of trauma, these are the most commonly injured vessels in civilian and military reports.
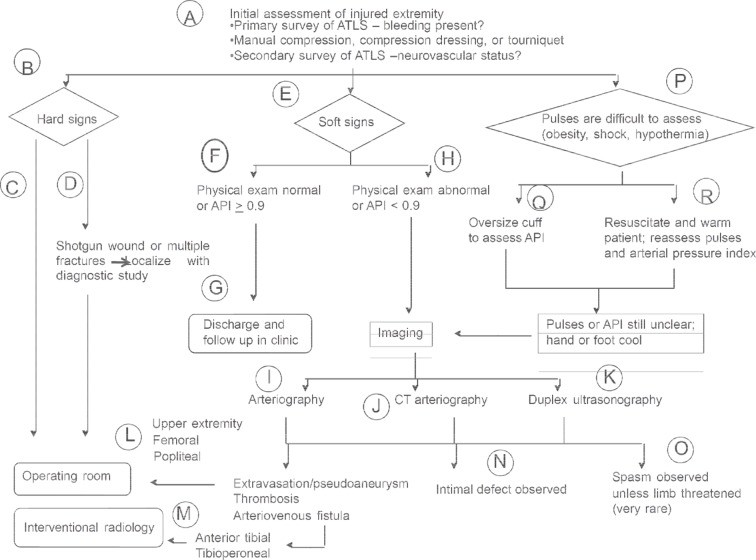
Figure 1.
Algorithm for evaluation of patient with possible peripheral vascular injury (figure reproduced with permission).19 API, arterial pressure index.
Box 1. Pitfalls to be avoided in the management of peripheral vascular injuries: preoperative period. ABI, ankle–brachial index; API, arterial pressure index; BBI, brachial–brachial index.
Avoid missed vascular injury
review field history and physical examination with personnel from emergency medical services
‘hard’ signs except arteriovenous fistula mandate immediate operation
measure ABI, BBI or API as part of physical examination and perform CT arteriogram if <0.9
second team perform arteriogram, if needed, during operation for other life-threatening injury.
Initiate systemic heparinization if major peripheral arterial occlusion and no contraindication.
The first step in avoiding the pitfall of missing a peripheral vascular injury is to question the patient and emergency medical services personnel about the extent of external blood loss from the injured extremity. All physicians recognise that most bleeding is from injuries to soft tissues of the extremity. The trauma team, however, must verify that a peripheral vascular injury is not present by a careful physical examination, including measurement of an ankle–brachial index (ABI) or brachial–brachial index (BBI) (see below), selective imaging and serial physical examinations.
Patients presenting with most of the ‘hard’ signs of arterial injury—occlusion (pulseless, pallor, paresthesia, pain, paralysis and poikilothermy), external bleeding or a rapidly expanding hematoma will have a therapeutic operation 95% to 98% of the time without preoperative diagnostic imaging. Rapid imaging may be helpful if hemorrhage can be controlled by a pressure dressing or tourniquet when the patient has a shotgun wound or injuries at two levels of the extremity.7
In patients presenting with ‘cold’ ischemia of the distal extremity (no Doppler flow and cool extremity) from arterial occlusion with or without a delay in diagnosis, initiating systemic heparinization in the emergency room should be considered. Contraindications obviously include extensive injuries to soft tissues in the extremities, a traumatic brain injury or injury to a solid organ in the abdomen.
The patient with a palpable thrill, audible bruit and presumed arteriovenous fistula is not a surgical emergency, and preoperative imaging may be useful to analyze whether the insertion of an endovascular stent graft is more appropriate than operation. A major pitfall in dealing with a patient with both life-threatening (injury to brain, neck and trunk) injuries and a limb-threatening arterial or venous injury (hard sign on examination) is to delay treatment of the latter (‘life over limb’). Two surgical teams should manage the two separate injuries. Continuing peripheral hemorrhage will aggravate the lethal triad of hypothermia, acidosis and a coagulopathy, whereas a persistent peripheral arterial occlusion will also aggravate a systemic acidosis and lead inevitably to an early reoperation. The simple control of peripheral hemorrhage or relief of a local arterial occlusion followed by insertion of temporary intraluminal shunt will eliminate all of these problems.8–10
Patients presenting with ‘soft’ signs of an arterial injury—history of bleeding at the scene or during transport, proximity of a penetrating wound or contusion/hematoma/fracture, a non-pulsatile hematoma or a neurologic deficit—underwent diagnostic imaging in the past. The historic yield on either emergency center arteriography performed by surgeons or formal arteriography performed in an interventional radiology suite for a ‘soft’ sign was under 4% to 5%.11 Various combinations of soft signs occasionally led to positive arteriograms in up to 13% to25% of patients, but the lesions diagnosed were non-occlusive, as expected, and were managed non-operatively over time as previously noted.2–4 12 13
The pitfall of overusing traditional imaging to rule out non-operative arterial injuries has been minimized in the past 25 years. This has been accomplished by the measurement of the ABI or BBI or arterial pressure index (API) in injured extremities for the past 25 years.14–16 Using the original cut-off of ≥0.9 as described by Johansen and Lynch, a patient with an ABI or API at this level is presumed to have a normal artery or one with a small non-occlusive lesion (intimal injury/intramural hematoma/small traumatic false aneurysm).14 Any lesion such as this is managed with observation and/or repeat imaging but not an operation.
The availability of CT arteriography for patients with an abnormal (<0.9) ABI or API avoids the pitfall of complications related to traditional surgeon or radiology-suite performed arteriography. The advantages include ready availability, need for only intravenous contrast and accuracy comparable with conventional arteriography with the modern generation of multidetector row helical CT machines.17 18 Disadvantages include contrast-induced nephropathy, particularly in patients with blunt trauma and multisystem injuries to be evaluated, and loss of arterial definition secondary to artifacts from metallic fragments or bullets.19
There are circumstances in which the orderly sequence of diagnostic maneuvers described will be compromised. It is difficult to perform an adequate examination of peripheral pulses or measure an ABI or API in patients who are not hemodynamically stable. These can be completed after adequate resuscitation, though this delays diagnosis of a peripheral vascular injury. In the patient who requires an operation to control hemorrhage and/or contamination in the neck or trunk, peripheral pulses should be assessed for symmetry intraoperatively once shock has been reversed. Any injured extremity with diminished distal pulses or an ABI <0.9 can then be studied by a surgeon-performed intraoperative arteriogram, a still valid technique in the modern era in the special circumstance described.
Decision for operation
The insertion of an endostent or endostent graft is appropriate in hemodynamically stable patients with injures to the subclavian artery, a major truncal artery providing flow to the upper extremity. The insertion of a detachable balloon is appropriate in traumatic false aneurysms of the profunda brachii or femoris arteries. Embolization of a single vessel in the shank is appropriate when extravasation or an arteriovenous fistula is demonstrated on a CT arteriogram. Otherwise, operation is appropriate in patients with occlusion of or bleeding from major named peripheral arteries or veins.
Avoiding pitfalls during vascular repair
A common mistake prior to operation for a peripheral vascular injury is to limit skin preparation and draping. For operation on an upper extremity, this should include the area from the chin to the umbilicus and from the opposite nipple to the ipsilateral fingernails. Access to the sternum, supraclavicular and infraclavicular areas and the entire injured extremity is then available. The wrist and hand in the injured upper extremity should be placed in a plastic bag also to allow for observation of color changes in the skin of fair-skinned individuals and to assess palpable pulses and capillary refill after revascularization in all patients.
For operation on a lower extremity, preparation and draping should extend from the umbilicus to both sets of toenails (box 2). This allows for extraperitoneal exposure of the external iliac artery for injuries under or just distal to the inguinal ligament and provides access to the greater saphenous vein in the groin and at the ankle in the uninjured lower extremity. The foot in the injured extremity should be placed in a plastic bag, as well.
Box 2. Pitfalls to be avoided in the management of peripheral vascular injuries: vascular repair.
avoid limiting skin preparation and draping
control external hemorrhage during skin preparation and draping, and then obtain proximal and distal vascular control
avoid straight incisions across axillobrachial area, antecubital fossa and medial or posterior popliteal area
obtain proximal and distal vascular control through short incisions around a large peripheral hematoma
avoid prolonged vascular repair when indication for temporary intraluminal shunt is present.
One pitfall to avoid is excessive loss of blood from the peripheral vascular injury during skin preparation and draping. When rapid external hemorrhage is occurring from a large wound in the proximal thigh, the assistant places sterile gloves on and applies direct pressure to the area of bleeding with the palms of the hands on top of one another. Prepping, draping, proximal incision and proximal arterial control are then performed before the assistant’s hands are removed. If there is a discrete stab or missile track from which the hemorrhage is occurring, the insertion of a sterile finger or 5 mL or 30 mL balloon catheter is performed until hemorrhage is controlled. A blood pressure cuff acting as a proximal tourniquet is used to control external bleeding or expansion of a hematoma from arterial injuries at midthigh level or distally.
Incisions for exposure of peripheral vessels are well described in standard textbooks.20 Longitudinal incisions over the involved vessels are used away from joints. To avoid scar contractures at the anterior shoulder (axillobrachial exposure), elbow (brachial exposure) and knee (medial or posterior popliteal exposure), curvilinear incisions are appropriate.
When a large pulsatile hematoma is present, there is no need to make the entire incision immediately. Two separate short longitudinal incisions are made to obtain proximal and distal vascular control prior to completing the middle aspect of the incision and entering the hematoma.
Role of intraluminal shunts
A major pitfall in management of patients with peripheral vascular injuries is the failure to recognize the select groups of patients who should have insertion of a temporary intraluminal shunt rather than definitive vascular repair. In the extremity with a significant vascular injury, the indications for inserting a temporary intraluminal vascular shunt are as follows: (1) Gustilo IIIC open fracture; (2) need for distal perfusion as a complex revascularization (ie, extra-anatomic bypass) is performed; (3) ‘damage control’ for the patient with near-exsanguination; and (4) perfusion of an amputated part of an upper extremity prior to replantation.9 Much as in prior reviews, the most recent multicenter review noted that the most common indications continue to be ‘physiologic exhaustion’ mandating damage control and Gustilo IIIC open fractures.8 10
To avoid the pitfall of having a temporary intraluminal shunt thrombose after insertion, there are technical points that need to be emphasized. The largest possible arterial intraluminal shunt should be inserted, and this was a #14-FR Argyle Carotid Artery Shunt (Kendall, Mansfield, MA) in the largest civilian single-center review reported.8 When the distal end of the transected artery is in spasm, the topical application of papaverine combined with gentle manual dilation of the end of the artery by a mosquito hemostat may allow for insertion of a larger shunt. The shunt should be 4 cm longer than the gap between the two ends of the vessel, with a 2–0 silk tie marking the midpoint and an occluding hemostat placed at the same point. After the shunt is inserted 1.5 cm into the proximal end of the artery, a 2–0 silk tie is used to compress the wall of the vessel onto the shunt 1 cm from the end. The hemostat is removed to verify pulsatile flow and then reapplied. The shunt is then inserted 1.5 cm into the distal end of the vessel, and the hemostat is released as a 2–0 silk tie is once again used to compress the distal artery down onto the shunt. Pulsatile flow distal to the shunt is verified visually, by palpation and/or by application of a Doppler device.
Ligation of a major vein such as the popliteal, femoral or common femoral in the same extremity in which an intraluminal arterial shunt has been inserted may have a detrimental effect on flow through the shunt.21 Therefore, none of these veins should be ligated in the same extremity. The exception might be that the patient is in advanced physiologic exhaustion with secondary cardiac arrhythmias or cardiovascular failure. Otherwise, thoracostomy tubes sized #16–24 FR are used to shunt the large veins of the lower extremity.
The role of systemic heparinization to minimize thrombosis of intraluminal shunts in non-coagulopathic patients is unclear based on available data. Thrombosis rates for shunts inserted in peripheral arteries in civilian series have ranged from 4% to 5%, certainly an acceptable figure considering the magnitude of physiologic exhaustion or injuries to the extremity mandating shunting.8 10
One of the major disadvantages to insertion of an intraluminal shunt into a peripheral vessel is the need for later definitive repair with a substitute vascular conduit. This is due to the magnitude of the original injury, debridement of injured ends and the further debridement needed to remove crushed vessel when the shunt is removed. A second disadvantage is that defects in soft tissue associated with the original trauma often lead to exposure of the injured vessel with shunt after the damage control shunting procedure. Every attempt should be made to cover the injured vessel with shunt with a porcine xenograft or temporary approximation of adjacent muscle.22 An operative field felt to be heavily contaminated at the time of removal of the shunt (necrotic muscle, seropurulent drainage, unable to maintain coverage of the shunt) should prompt the insertion of an extra-anatomic bypass graft.23
Simple lateral repair
Once proximal and distal vascular control is attained with DeBakey angled clamps, bulldog clamps, silastic vessel loops or balloon catheters (at bifurcations), an area of perforation or laceration in a peripheral artery or vein is debrided. In vascular trauma, this means removal of cracked intima, any intramural hematoma and frayed adventitia. If the defect can be closed in a transverse fashion, this is performed with interrupted full-thickness 6–0 polypropylene sutures in children. In adults, either interrupted or a continuous suture line can be used. With a rapid repair as described performed soon after injury and obvious back-bleeding from the distal end of the injured artery before application of clamps, neither passage of Fogarty balloon catheters nor administration of regional or systemic heparin is indicated.
Complex repair after segmental resection
A variety of other options for repair are available for larger or more complex lesions (box 3).24
Box 3. Options for operative peripheral vascular repair*.
Laterally arteriorrhaphy or venorrhaphy
Patch angioplasty
Resection
End-to-end anastomosis
Interposition graft
Bypass graft
Extra-anatomic bypass graft
*Adapted from ref 24.
The basic principles for repair of these are summarized in box 4 and illustrated in figure 2.
Box 4. Fine techniques of peripheral vascular repair for major injuries.
Vascular control with small angioaccess clamps or silastic vessel loops
Passage of an appropriately sized Fogarty balloon catheter proximally and distally (arteries only; peroneal artery nearly 90% of the time)
Either systemic (100 u/kg) or regional heparinization (12 500 units/250 mL normal saline or 50 units/mL with injection of 15–20 mL/end of vessel)
Open technique (no posterior knot tied and ends approximated after 1/3 anastomosis completed) for either end-to-end anastomosis or insertion of interposition graft
Appropriate flushing sequence before removal of vascular clamps
Completion arteriogram (fluoroscopy or hard copy) for either end-to-end anastomosis or insertion of interposition graft in lower extremity.
Figure 2.
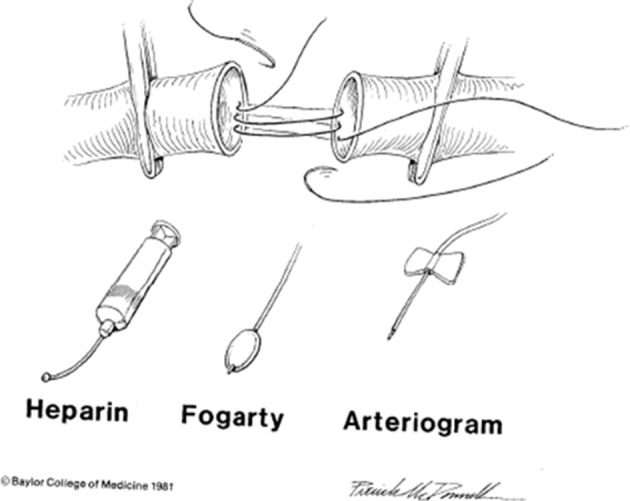
Top: open anastomosis technique using 5–0 or 6–0 polypropylene suture for end-to-end anastomosis or for insertion of an interposition graft. Bottom (left to right): regional heparin at 50 units/mL is administered directly into the proximal and distal artery (15–20 mL/end) after passage of Fogarty balloon catheter to clear distal emboli or distal in situ thrombosis. Completion arteriography using 35 mL of diatrizoate meglumine dye is mandatory in the lower extremity after an end-to-end arterial anastomosis or insertion of an interposition graft.
It is technically awkward to apply an appropriately sized venous patch to an injured artery in an extremity that has been clamped. For this reason, the rarely used technique of venous patch angioplasty using a segment of greater saphenous vein is usually restricted to repair of large veins of the extremities—popliteal, femoral, common femoral and axillary.
Segmental resection of an injured peripheral artery or vein is appropriate when there are through-and-through perforations, a longitudinal jagged laceration or extensive loss of a portion of the vessel wall. The major pitfall after segmental resection is to ‘force’ an end-to-end anastomosis to avoid having two fresh graft anastomoses in close proximity. An end-to-end anastomosis can be performed when only modest tension is needed to bring the ends of the vessel together. Also, this should not require sacrifice of excessive arterial branches in the area, particularly the medial and lateral superior and inferior genicular arteries around the knee. An end-to-end anastomosis performed under tension will increase the risk of bleeding from suture holes after release of vascular clamps as well as an increased risk of early postoperative thrombosis. The ‘open anastomosis’ technique listed in box 4 and illustrated in figure 2 allows for precise placement of posterior wall sutures and should eliminate leaks from this area. A modification of this standard technique favored by the author is to place the first out-in-in-out untied suture at the 3 o’clock position.25 The loose suture ends are then pulled tight as the vascular clamps are pushed toward one another when the suture line has reached the 7 o’clock position (figure 3). After the entire posterior suture line (3 to 9 o’clock) is completed, the surgeon can choose any technique (interrupted sutures vs continuous) to complete the anterior aspect of the anastomosis.
Figure 3.
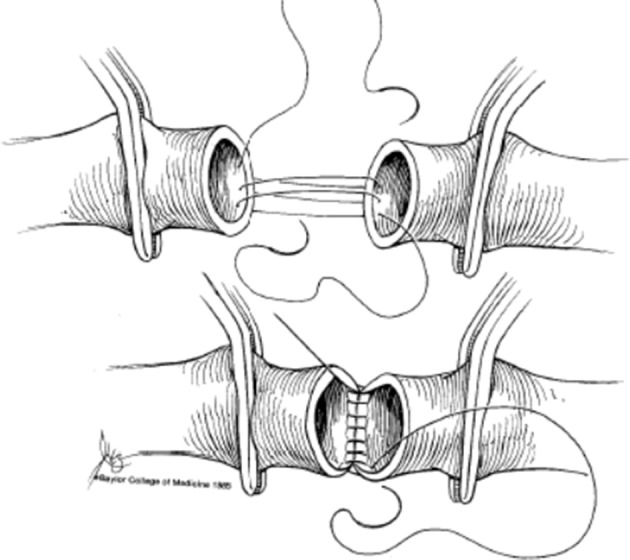
After completion of the posterior 1/3 of the end-to-end anastomosis, the loose suture ends are pulled tight as the vascular clamps are pushed toward one another.
The last few sutures on the anterior walls of the anastomosis are always left loose to allow for proper flushing before reestablishing vascular inflow or outflow from the injured extremity (figure 4). Flushing of the proximal end of the anastomosis, reapplication of the proximal vascular clamp and then removal of the distal vascular clamp are performed in sequence. Leaving the distal clamp off as the first knot is tied down allows for evacuation of air between the clamps. Finally, the proximal clamp is removed as the remaining knots are tied.
Figure 4.
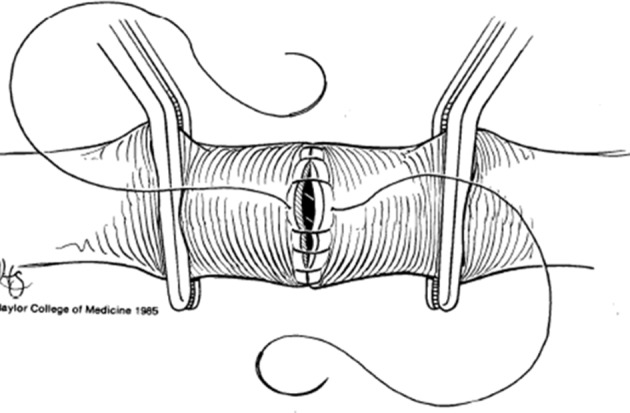
Leaving the last few continuous (or interrupted) sutures loose allows for flushing of both ends of the vessel before reestablishing flow.
The ideal sequence when choosing an interposition graft for segmental replacement of a vessel should be as follows: (1) greater saphenous vein in groin or at ankle; (2) lesser saphenous vein on the posterior leg and lateral ankle; (3) basilic or cephalic vein of forearm; and (4) plastic conduit. The clinical reality, however, is that trauma vascular teams find the lesser saphenous vein difficult to expose and excise and rarely prepare and drape the upper extremity when injuries are confined to a lower extremity.
The greater saphenous vein remains the ideal substitute vascular conduit for the following reasons: (1) ready availability as an intima-lined conduit; (2) a size appropriate to replace most major arteries and some veins in the extremities; (3) resistance to infection when covered by healthy soft tissue; and (4) excellent long-term patency.26 Contraindications to the use of the saphenous vein are obvious and include a diseased vein (stenosis), too small luminal diameter, size mismatch as compared with injured vessel or the vein may become the sole venous outflow for an injured lower extremity (severe injury to popliteal or femoral vein in same extremity).27
It is often obvious that a substitute vascular conduit of greater saphenous vein will be needed as soon as the magnitude of the injury to a peripheral artery or vein is assessed after exposure. A fellow or surgical resident should be directed immediately to start retrieval of the appropriate length of the greater saphenous vein from an uninjured lower extremity as the attending surgeon continues to prepare the injured vessel for repair. Cardiac surgical teams have long described the appropriate operative techniques to minimize damage to the greater saphenous vein during retrieval. These include gentle dissection, avoiding overdistension during flushing for leaks and keeping the graft moist before insertion.
The greater saphenous vein is a ‘floppy’ conduit and fixation sutures or knots 120° (Carrel-Guthrie triangulation technique) or 180° apart maintain stability and open ends as the anastomosis is completed.28 One pitfall that is easily avoided when inserting a graft in awkward locations—axillary artery posterior to the pectoralis major muscle, popliteal artery posterior to the knee joint, tibioperoneal trunk near its bifurcation—is to complete the distal or, more difficult, anastomosis first. This allows for free movement of the entire graft and better visualization of what is always a smaller anastomosis. As the second or proximal anastomosis is completed, the previously described flushing sequence is completed.
The basilic and cephalic veins are thin-walled as compared with the greater and lesser saphenous veins in the lower extremities. The dilation that occurs when either of these veins is placed as an interposition graft in a peripheral artery is a pitfall to be aware of. To avoid ties on the side branches from falling off or creating intimal defects, ties on branches should be further away from the main basilic or cephalic vein during retrieval than with the greater or lesser saphenous vein.
The major concerns about using plastic interposition grafts for peripheral vascular repair are increased postoperative infection and decreased long-term patency. Only the latter is true, as polytetrafluoroethylene (PTFE or Teflon) grafts in civilian centers appear to have a postoperative infection rate equivalent to autologous vein when not exposed or lying next to infection in soft tissue or bone.29 The major pitfall in dealing with PTFE grafts is to avoid making the graft too long. The rigidity of a PTFE graft can cause kinking of the native artery, particularly if adjacent to a major joint (figures 5 and 6).
Figure 5.
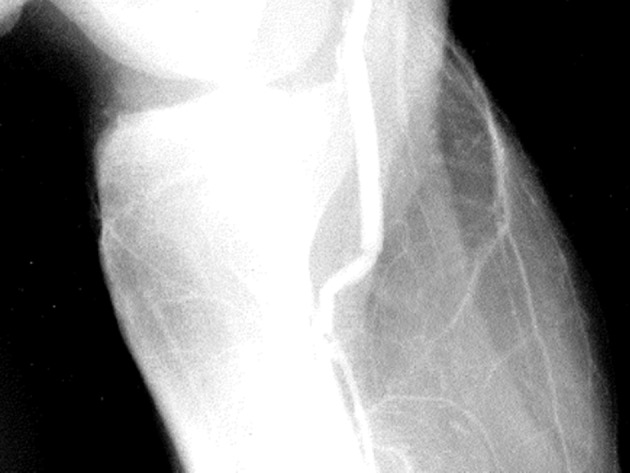
6 mm PTFE graft is too long on completion arteriography, and the distal artery is kinked. No revision was made, and the graft thrombosed 3 months after surgery (from Feliciano DV. Pitfalls in the management of peripheral vascular injuries. Gen Surg 1986;3:101–13. Reproduced with permission). PTFE, polytetrafluoroethylene.
Figure 6.
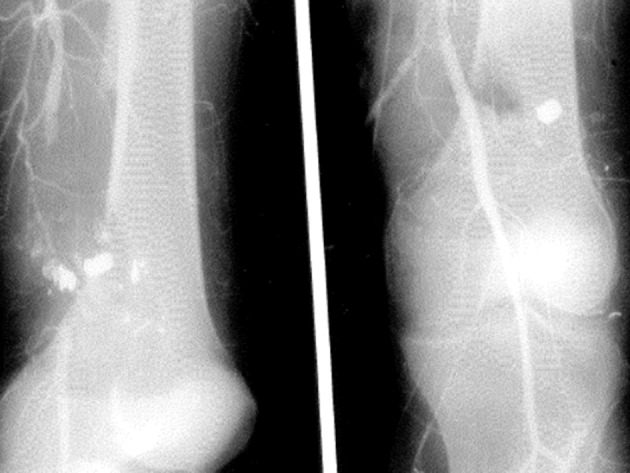
Preoperative and intraoperative completion arteriogram in a 40-year-old man with a gunshot wound to the proximal popliteal artery. Completion arteriogram after insertion of a PTFE interposition graft (contralateral greater saphenous vein was stenotic) demonstrated patent anastomosis and proximal 3-vessel runoff. PTFE, polytetrafluoroethylene.
Extra-anatomic bypass
In trauma vascular repair, the indications for inserting an extra-anatomic bypass are as follows: (1) loss of soft tissue over injured vessel(s); (2) incisional infection with blowout of an underlying vascular repair; and (3) simultaneous infections in soft tissue and underlying native vessel secondary to injection of illicit drugs.23 The fundamental principle is to move the peripheral vascular repair as there is inadequate or infected tissue for coverage if the repair is performed in situ.
The major pitfall when dealing with these complex injuries is to assume that there will always be a plastic surgery technique to deal with all the issues involved. This is simply not true, and shotgun wounds of the extremities or complex infections related to injections of illicit drugs are the classical examples. In addition to loss of long segment of vessel and overlying soft tissue, shotgun wounds often create a large cavity in the muscles of the extremity. A rotation muscular flap to fill the cavity, to maintain contact with a saphenous vein graft and to cover the graft may not be available secondary to scatter of pellets, local edema and the size of the cavity. With simultaneous infections (soft tissue and artery) in patients who abuse intravenous drugs, the extent of local infection precludes an in situ vascular repair. The other major pitfall/assumption is that the trauma vascular surgeon thinks that advanced vascular training is necessary to perform the extra-anatomic bypass. In truth, this is a technically simple operation for any experienced general or trauma surgeon.
The first step is to make longitudinal incisions over the underlying artery to be resected just outside the area of tissue loss or infection. Several centimeters of normal artery and/or vein are then exposed and controlled proximally and distally. In addition, the segments of the artery where the extra-anatomic anastomoses are to be located are mobilized so that they can be moved in a subcutaneous direction away from the area of tissue loss or infection. A saphenous vein graft of appropriate length is excised from an uninjured and not infected lower extremity. After administration of systemic heparinization (or regional heparinization if a large defect in soft tissue), vascular clamps are applied around the segment of exposed normal proximal artery and it is divided. The distal end of this is ligated, and the reversed saphenous vein graft is sewn in an end-to-end fashion to the proximal end of the artery. A dilator used during explorations of the common bile duct or a long Kelly clamp is then passed through normal subcutaneous tissue around the area of injury or infection down to the exposed normal distal artery. After the tunnel has been dilated, the distal-end of the saphenous vein graft is pulled through the tunnel by tying it to the dilator or grasping a suture at the end of a Kelly clamp. Vascular clamps are then applied around the segment of exposed normal distal artery, and it is divided. The proximal end is ligated, flow through the extra-anatomic saphenous vein graft confirmed, and it is then sewn in an end-to-end fashion to the distal end (figure 7). After arterial inflow (and, if needed, venous outflow) is established, a completion arteriogram is performed (see below). The longitudinal incisions over the anastomoses are closed to separate the entire graft from the area of injury, any blast cavity or any area of infection. The injured or infected artery is excised, followed by wide debridement of soft tissue. With an open wound separate from the extra-anatomic bypass, either daily wet-to-dry dressing changes or application of a vacuum-assist device can be used till the wound is ready for a split-thickness skin graft.
Figure 7.

Proximal anastomosis of extra-anatomic saphenous vein bypass graft replacing right superficial femoral artery and distal anastomosis of extra-anatomic 8 mm externally supported PTFE graft replacing right femoral vein. The blast cavity from the shotgun wound is at the bottom of the figure (from Feliciano DV. Vascular trauma. In: Levine BA, Copeland EM III, Howard RJ, Sugerman JH, Warshaw AL, eds. Current practice of surgery. New York: Churchill Livingstone; 1993. pp. 1–18. Reproduced with permission). PTFE, polytetrafluoroethylene.
Completion arteriography
Distal in situ thrombosis beyond a complex arterial repair in the upper extremity is almost unheard of unless a tourniquet has been in place for several hours. Therefore, the return of bounding radial and ulnar pulses at the wrist after completion of an arterial repair proximally is presumptive evidence of a technically appropriate repair. In such a situation, the author does not perform a completion arteriogram.
In contrast, an end-to-end anastomosis, interposition graft or extra-anatomic bypass in an artery of the lower extremity should be evaluated by completion arteriography.27 This will detect narrowing of an anastomosis, an intimal flap, distal arterial emboli or in situ thrombosis or distal spasm.
Completion arteriography is performed through a 20-gauge Teflon-over-needle catheter. The needle is used to puncture the artery 3 cm proximal to the anastomosis, and the catheter is advanced into the lumen. During this first step, the artery proximal to the anastomosis is stabilized with a vascular forceps to prevent perforation of the posterior wall. After attaching the catheter to a short intravenous extension tube, a stopcock and a 50 mL syringe filled with heparinized saline, an injection is made to ensure proper placement of the Teflon catheter. A second syringe containing 35 mL of diatrizoate meglumine dye is attached to the stopcock directly in line with the intravenous tubing. After opening the stopcock to the syringe containing the dye, the dye is injected rapidly. Either real-time fluoroscopic images throughout the injection or a one shot hard copy x-ray after completion of the 35 mL injection can be used.
Should any of the defects previously listed be present on the completion arteriogram, heparinization is reinstituted, if appropriate. An anastomotic problem mandates takedown and reanastomosis. If distal in situ thrombosis or an embolus is present, a decision must be made on where to insert Fogarty balloon catheters. This could be through a small venotomy in a recently inserted saphenous vein graft or in the normal artery beyond the distal anastomosis. The author favors the former as repair of this small venotomy is less likely to narrow the lumen. One previously mentioned pitfall is the known tendency of Fogarty catheters placed through the femoral or popliteal artery to pass into the peroneal artery of the shank. Therefore, an embolus or thrombus in the anterior or posterior tibial artery would have to be approached through a ‘trifurcation embolectomy’ via the below knee popliteal artery.
Injury to peripheral veins
Although any major peripheral vein can be ligated with rare loss of an extremity, there is significant morbidity in some patients. This includes the following: (1) increased venous hemorrhage from injuries to soft tissues or distal fasciotomy sites; (2) an increased need for below knee fasciotomy after ligation of popliteal or femoral vein; (3) a transient adverse effect on arterial inflow; and (4) an increase in postoperative swelling of the injured extremity.
Options for peripheral venous repair are somewhat different from those used in peripheral arterial repairs. These include the previously mentioned thoracostomy tubes as intraluminal venous shunts and use of venous patch angioplasty instead of segmental resection. When an interposition graft is needed in the popliteal or proximal femoral vein, the contralateral greater saphenous vein from the groin may be an appropriate size match. Panel or spiral venous grafts have been suggested as interposition grafts in the more distal femoral vein, common femoral vein or axillary vein. Once a trauma vascular surgeon creates one of these suture-laden grafts early in his or her career, there is often an internal vow never to perform the procedure again.
Externally supported (ringed) PTFE grafts in large sizes are available for insertion in the large veins mentioned above. The combination of elevation of the injured extremity whenever possible in the first 4 to 6 weeks after repair, administration of 81 mg or 162 mg aspirin daily, cessation of smoking and a daily walking program contribute to a surprisingly long patency of many of these grafts despite non-pulsatile flow.30
Compartment syndrome and fasciotomy
The history, causes, pathophysiology, indications for and operative techniques of fasciotomy in the extremities are described in available literature,31 but a brief summary is included here. A compartment syndrome is increased pressure within a closed fascial space that reduces capillary perfusion to a level less than required for tissue viability. It occurs when there is increased content of the compartment (ischemia–reperfusion, contusion/fractures, venous outflow obstruction and capillary ‘leak’) or decreased volume (external compression and closure of fascia).
Patients at ‘high risk’ for developing a compartment syndrome in an extremity based on trauma history are the following: (1) hypotension in the field; (2) delay in treatment, especially if no arterial inflow into the injured extremity for 4–6 hours; and (3) disproportionate pain in the injured extremity. In unstable patients with ongoing hemorrhage in whom a comprehensive physical examination of the injured extremity is not possible, ‘high risk’ situations would include the following: (1) ongoing hypotension and continuing resuscitation; (2) crush injury; and (3) significant swelling of the extremity without much local injury. Finally, patients at ‘high risk’ based on operative management would include the following: (1) combined arterial and venous injuries mandating simultaneous clamping, especially at the popliteal level; and (2) need for arterial or venous ligation or early thrombosis of repair.
There are trauma vascular surgeons who routinely perform ‘prophylactic fasciotomy’ when any of the high-risk scenarios or physical findings listed above are present. This approach ignores the pain after the procedure, possible unnecessary conversion of a closed to an open fracture, need for closure or coverage of the open wound and the impressive long-term sequelae. In the British series of 60 patients with extremity fasciotomies reported by Fitzgerald et al 32 in 2000, chronic limb pain was present in 54% (limited to fasciotomy site in 10%). Also, altered sensation was present in 95% overall, and this was limited to the fasciotomy site in 100% of the patients who had primary closure.
The author agrees that there are some patients in whom fasciotomy should be performed prophylactically (ie, without measurement of compartment pressure). As noted above, examples would be patients with long ischemic times, significant swelling of the calf or forearm after injury or need for ligation of the popliteal or femoral vein. All other patients should have measurement of the compartment pressure using a commercially available pressure monitor system or a transduced pressure on the monitor in the operating room. Most experienced trauma vascular surgeons perform a fasciotomy if the patient is ‘high risk’ and the compartment pressure is >35 Hg (away from the site of a fracture after blunt trauma). Orthopedic surgeons are more conservative for the reason listed above and often use the Whitesides differential, that is, a compartment pressure within 20 mm Hg of the patient’s diastolic blood pressure as the indication to perform a fasciotomy.33 34
The ideal operative approach to performing a below knee fasciotomy is the two-skin incision four-compartment technique described by Mubarak and Owen in 1977.35 The ideal operative approach to performing a below-elbow fasciotomy is the two-skin three-compartment technique described by many authors during the past 35 years; however, the Henry approach with the curved skin incision on the volar forearm is confusing to residents and should be abandoned. The volar-ulnar incision approach (transverse antecubital incision from radial to ulnar side with right angle turn and straight line angling from the volar ulnar forearm to mid-wrist) is easy to remember and perform. It allows for decompression of the mobile wad and flexor compartments (superficial and deep), and release of the forearm extensor compartment may then not be necessary.
Footnotes
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it was published Online First. We have made changes in the first two paragraphs of the ‘Avoiding pitfalls in the preoperative period’ section in th paper.
References
- 1.Shackford SR, Kahl JE, Calvo RY, Shackford MC, Danos LA, Davis JW, Vercruysse GA, Feliciano DV, Moore EE, Moore HB, et al. Limb salvage after complex repairs of extremity arterial injuries is independent of surgical specialty training. J Trauma Acute Care Surg 2013;74:716–24. doi:10.1097/TA.0b013e3182827035 [DOI] [PubMed] [Google Scholar]
- 2.Frykberg ER, Dennis JW, Bishop K, Laneve L, Alexander RH. The reliability of physical examination in the evaluation of penetrating extremity trauma for vascular injury: results at one year. J Trauma 1991;31:502–11. doi:10.1097/00005373-199104000-00009 [DOI] [PubMed] [Google Scholar]
- 3.Dennis JW, Frykberg ER, Veldenz HC, Huffman S, Menawat SS. Validation of nonoperative management of occult vascular injuries and accuracy of physical examination alone in penetrating extremity trauma: 5- to 10-year follow-up. J Trauma 1998;44:243–53. doi:10.1097/00005373-199802000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Stain SC, Yellin AE, Weaver FA, Pentecost MJ. Selective management of nonocclusive arterial injuries. Arch Surg 1989;124:1136–41. doi:10.1001/archsurg.1989.01410100034007 [DOI] [PubMed] [Google Scholar]
- 5.White R, Krajcer Z, Johnson M, Williams D, Bacharach M, O’Malley E. Results of a multicenter trial for the treatment of traumatic vascular injury with a covered stent. J Trauma 2006;60:1189–96. doi:10.1097/01.ta.0000220372.85575.e2 [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen TE, Clouse WD, Peck MA, Bowser AN, Eliason JL, Cox MW, Woodward EB, Jones WT, Jenkins DH. Development and implementation of endovascular capabilities in wartime. J Trauma 2008;64:1169–76. doi:10.1097/TA.0b013e31816b6564 [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen TE, DuBose JJ, Asensio JA, Feliciano DV, Fox CJ, Nuñez TC, Sise MJ. Military Liaison Committee of the American Association for the Surgery of Trauma. Tourniquets, vascular shunts, and endovascular technologies: esoteric or essential? A report from the 2011 AAST Military Liaison Panel. J Trauma Acute Care Surg 2012;73:282–5. doi:10.1097/TA.0b013e3182569df4 [DOI] [PubMed] [Google Scholar]
- 8.Subramanian A, Vercruysse G, Dente C, Wyrzykowski A, King E, Feliciano DV. A decade’s experience with temporary intravascular shunts at a civilian level I trauma center. J Trauma 2008;65:316–26. doi:10.1097/TA.0b013e31817e5132 [DOI] [PubMed] [Google Scholar]
- 9.Feliciano DV, Subramanian A. Temporary vascular shunts. Eur J Trauma Emerg Surg 2013;39:553–60. doi:10.1007/s00068-011-0171-9 [DOI] [PubMed] [Google Scholar]
- 10.Inaba K, Aksoy H, Seamon MJ, Marks JA, Duchesne J, Schroll R, Fox CJ, Pieracci FM, Moore EE, Joseph B, et al. Multicenter evaluation of temporary intravascular shunt use in vascular trauma. J Trauma Acute Care Surg 2016;80:359–65. doi:10.1097/TA.0000000000000949 [DOI] [PubMed] [Google Scholar]
- 11.Reid JD, Weigelt JA, Thal ER, Francis H. Assessment of proximity of a wound to major vascular structures as an indication for arteriography. Arch Surg 1988;123:942–6. doi:10.1001/archsurg.1988.01400320028004 [DOI] [PubMed] [Google Scholar]
- 12.O’Gorman RB, Feliciano DV, Bitondo CG, Mattox KL, Burch JM, Jordan GL. Emergency center arteriography in the evaluation of suspected peripheral vascular injuries. Arch Surg 1984;119:568–73. doi:10.1001/archsurg.1984.01390170064013 [DOI] [PubMed] [Google Scholar]
- 13.O’Gorman RB, Feliciano DV. Arteriography performed in the emergency center. Am J Surg 1986;152:323–5. doi:10.1016/0002-9610(86)90267-9 [DOI] [PubMed] [Google Scholar]
- 14.Johansen K, Lynch K, Paun M, Copass M. Non-invasive vascular tests reliably exclude occult arterial trauma in injured extremities. J Trauma 1991;31:515–22. doi:10.1097/00005373-199104000-00011 [PubMed] [Google Scholar]
- 15.Lynch K, Johansen K. Can Doppler pressure measurement replace “exclusion” arteriography in the diagnosis of occult extremity arterial trauma? Ann Surg 1991;214:737–42. doi:10.1097/00000658-199112000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadjadi J, Cureton EL, Dozier KC, Kwan RO, Victorino GP. Expedited treatment of lower extremity gunshot wounds. J Am Coll Surg 2009;209:740–5. doi:10.1016/j.jamcollsurg.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 17.Seamon MJ, Smoger D, Torres DM, Pathak AS, Gaughan JP, Santora TA, Cohen G, Goldberg AJ. A prospective validation of a current practice: the detection of extremity vascular injury with CT angiography. J Trauma 2009;67:238–44. doi:10.1097/TA.0b013e3181a51bf9 [DOI] [PubMed] [Google Scholar]
- 18.White PW, Gillespie DL, Feurstein I, Aidinian G, Phinney S, Cox MW, Adams E, Fox CJ. Sixty-four slice multidetector computed tomographic angiography in the evaluation of vascular trauma. J Trauma 2010;68:96–102. doi:10.1097/TA.0b013e318190c4ca [DOI] [PubMed] [Google Scholar]
- 19.Feliciano DV, Moore FA, Moore EE, West MA, Davis JW, Cocanour CS, Kozar RA, McIntyre RC. Western Trauma Association critical decisions in trauma: evaluation and management of peripheral vascular injury, part I. J Trauma 2011;70:1551–6. [DOI] [PubMed] [Google Scholar]
- 20.Wind GG, Valentine RJ. Anatomic exposures in vascular surgery. 3rd edn Philadelphia: Wolters Kluwer, 2013. [Google Scholar]
- 21.Hobson RW, Howard EW, Wright CB, Collins GJ, Rich NM. Hemodynamics of canine femoral venous ligation: significance in combined arterial and venous injuries. Surgery 1973;74:824–9. [PubMed] [Google Scholar]
- 22.Feliciano DV, Rasmussen TE. Evaluation and treatment of vascular injuries : Browner BD, Jupiter JB, Kretteck C, Skeletal trauma. Basic science, management and reconstruction. Philadelphia: Elsevier Saunders, 2015:423–35. [Google Scholar]
- 23.Feliciano DV. Heroic procedures in vascular injury management: the role of extra-anatomic bypasses. Surg Clin North Am 2002;82:115–24. doi:10.1016/S0039-6109(03)00144-0 [DOI] [PubMed] [Google Scholar]
- 24.Feliciano DV. Vascular injuries : Maull KI, Cleveland HC, Straugh GO, Advances in trauma. Volume 2 Chicago: Year Book Medical Publishers, 1987:179–206. [Google Scholar]
- 25.Ball CG, Feliciano DV. A simple and rapid vascular anastomosis for emergency surgery: a technical case report. World J Emerg Surg 2009;4:30–2. doi:10.1186/1749-7922-4-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell FL, Thal ER. Results of venous interposition grafts in arterial injuries. J Trauma 1990;30:336–9. doi:10.1097/00005373-199003000-00014 [DOI] [PubMed] [Google Scholar]
- 27.Feliciano DV, Moore EE, West MA, Moore FA, Davis JW, Cocanour CS, Scalea TM, McIntyre RC. Western Trauma Association critical decisions in trauma: evaluation and management of peripheral vascular injury, part II. J Trauma Acute Care Surg 2013;75:391–7. doi:10.1097/TA.0b013e3182994b48 [DOI] [PubMed] [Google Scholar]
- 28.Carrel A. The surgery of blood vessels. Johns Hopkins Hosp Bull 1907;18:18–28. [Google Scholar]
- 29.Feliciano DV, Mattox KL, Graham JM, Bitondo CG. Five-year experience with PTFE grafts in vascular wounds. J Trauma 1985;25:71–82. doi:10.1097/00005373-198501000-00012 [DOI] [PubMed] [Google Scholar]
- 30.Parry NG, Feliciano DV, Burke RM, Cava RA, Nicholas JM, Dente CJ, Rozycki GS. Management and short-term patency of lower extremity venous injuries with various repairs. Am J Surg 2003;186:631–5. doi:10.1016/j.amjsurg.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 31.Dente CJ, Wyrzykowski AD, Feliciano DV. Fasciotomy. Curr Probl Surg 2009;46:779–839. doi:10.1067/j.cpsurg.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald AM, Gaston P, Wilson Y, Quaba A, McQueen MM. Long-term sequelae of fasciotomy wounds. Br J Plast Surg 2000;53:690–3. doi:10.1054/bjps.2000.3444 [DOI] [PubMed] [Google Scholar]
- 33.Whitesides TE, Haney TC, Morimoto K, Harada H. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res 1975;113:43–51. doi:10.1097/00003086-197511000-00007 [DOI] [PubMed] [Google Scholar]
- 34.Whitesides TE, Heckman MM. Acute compartment syndrome: update on diagnosis and treatment. J Am Acad Orthop Surg 1996;4:209–18. doi:10.5435/00124635-199607000-00005 [DOI] [PubMed] [Google Scholar]
- 35.Mubarak SJ, Owen CA. Double-incision fasciotomy of the leg for decompression in compartment syndromes. J Bone Joint Surg Am 1977;59:184–7. doi:10.2106/00004623-197759020-00008 [PubMed] [Google Scholar]