Significance
Tissue injury caused by lack of blood flow results in a series of adaptive responses of the body to ensure survival. Cellular production of carbon monoxide (CO) preserves organ function and promotes healing. How this occurs has remained elusive. Here we demonstrate using a model of ischemia reperfusion injury (IRI) of the kidney, mimicking kidney transplant, that safe administration of CO protects against IRI. Remarkably, this occurs through specific modulation of a gene that regulates energy metabolism (CD39) and one that controls circadian rhythm (Period 2). Collectively, we define here an innovative signaling pathway linking the brain and the kidney vis a vis a gas molecule. These data may have important therapeutic consequences for transplant recipients and victims of stroke.
Keywords: heme oxygenase, circadian rhythm, DAMPS, innate immunity, adenosine
Abstract
Ischemia reperfusion injury (IRI) is the predominant tissue insult associated with organ transplantation. Treatment with carbon monoxide (CO) modulates the innate immune response associated with IRI and accelerates tissue recovery. The mechanism has been primarily descriptive and ascribed to the ability of CO to influence inflammation, cell death, and repair. In a model of bilateral kidney IRI in mice, we elucidate an intricate relationship between CO and purinergic signaling involving increased CD39 ectonucleotidase expression, decreased expression of Adora1, with concomitant increased expression of Adora2a/2b. This response is linked to a >20-fold increase in expression of the circadian rhythm protein Period 2 (Per2) and a fivefold increase in serum erythropoietin (EPO), both of which contribute to abrogation of kidney IRI. CO is ineffective against IRI in Cd39−/− and Per2−/− mice or in the presence of a neutralizing antibody to EPO. Collectively, these data elucidate a cellular signaling mechanism whereby CO modulates purinergic responses and circadian rhythm to protect against injury. Moreover, these effects involve CD39- and adenosinergic-dependent stabilization of Per2. As CO also increases serum EPO levels in human volunteers, these findings continue to support therapeutic use of CO to treat IRI in association with organ transplantation, stroke, and myocardial infarction.
Ischemia reperfusion injury (IRI) is obligatory and unavoidable in patients who undergo an organ transplant. The sudden unavailability of oxygen and glucose initiate a cascade of events including activation of tissue leukocytes and endothelium. Similarly, the reestablishment of blood flow to an ischemic organ elicits a second set of events that include rapid reactive oxygen species (ROS) generation, leukocyte infiltration, and additional mechanical injury. The severity of the IRI and the relative health of the organ is speculated to contribute to long-term graft survival (1–4). While a number of therapeutic approaches have been tested, including a variety of preservation solutions, sophisticated organ transport apparatus, anti-inflammatory agents, live donors, and even ischemic preconditioning, there has been little change in IRI (5–7). What is perhaps even more important is that when a solution to IRI is identified, organs that are otherwise considered too risky to use could be rescued and as such impact the number of transplants that could be performed and decrease an otherwise continuously growing waiting list. Given the impossibility of transplanting an organ without some amount of ischemic time, a focus on interventions that may protect the organ before harvest as well as promoting faster recovery and repair after reperfusion is warranted (8–12).
Heme oxygenase-1 (HO-1) is a member of a stress response gene family and is considered a protective gene. HO-1 catalyzes the breakdown of heme to bilirubin. In so doing, three products are generated and include carbon monoxide (CO), biliverdin, and iron. When HO-1 activity is increased, there is a potent protective phenotype that results. Biliverdin and CO are accepted as the primary underlying bioactive molecules that provide potent protective benefits to the cell by modulating apoptosis, inflammation, and proliferation (13–15). Administration of CO or biliverdin can, in most cases, recapitulate that observed with HO-1 itself. Treatment with CO at low concentrations imparts potent protection in numerous models of disease, including transplantation (16–18), colitis (19), sepsis (20), and lung injury (21). CO has been well-studied and characterized to prevent IRI in small and large animal models (22–24), which in turn resulted in the first clinical trial where CO was administered to kidney transplant recipients intraoperatively (https://clinicaltrials.gov/).
Circadian rhythms are critical determinants of organ function and susceptibility to injury dictated by a family of proteins collectively known as the “clock genes.” Disruption of one or more of these genes increases susceptibility to tissue injury (25). IRI leads to impairment of the circadian clock (26) and is independent of hypoxia. Adenosine-elicited A2b-mediated Period 2 (Per2) stabilization modulates adaptation to ischemic injury of the heart in mice (27). In fact, tolerance to ischemic injury has been shown to be dependent upon the time of day that the injury occurs (28).
Adenosine from the breakdown of extracellular ATP by CD39 and CD73 (ecto-5′-nucleotidase) is generated during tissue hypoxia as an adaptive response. In previous work, we have shown that CO enhances functional expression of adenosine receptors and stabilization of HIF1α in macrophages (29, 30). Employing a murine model of kidney IRI, we have now tested the hypothesis that CO protects against kidney IRI through CD39 and adenosine A2b-mediated receptor stabilization of Per2. Additionally, erythropoietin (EPO), a critical effector protein regulated in a circadian manner, is required for CO-induced renal protection. Collectively, our findings may be related in part to the response of the cellular O2 sensors that drives expression and stabilization of genes that regulate cellular bioenergetics.
Materials and Methods
Animals.
Isogenic male C57BL/6 mice (WT and Per2−/−) were purchased from Charles River or Jackson Labs (at 25–30 g). CD39-knockout mice (Cd39−/−) were bred at the BIDMC as described previously (31). All animals had access to water and food ad libitum. All animal care, housing and procedures were approved by the Beth Israel Deaconess Medical Center (BIDMC) Institutional Animal Care and Use Committee (IACUC) and were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines.
CO Exposure.
CO exposure as an inhaled gas was achieved by placing mice into a sealed Plexiglas chamber at 250 parts per million (ppm) with compressed air (32). Mice were exposed to CO for 1 h before surgery and then returned to room air. Controls received air only for the same time period. BW-101 as a CO prodrug (33) was prepared by solubilizing in solutol and used at a dose of 100 mg/kg, i.p. HBI-002 as a liquid CO formulation was orally dosed at 0.2 mg/kg. HBI-002 was provided by Hillhurst Biopharmaceuticals Inc.
Pharmacologic Agents.
ZM241385 (A2a receptor antagonist) and MRS1754 (A2b receptor antagonist) were obtained from Tocris Bioscience and given in a dose of 5 mg/kg i.p. 30 min before CO treatment and 6 h after reperfusion. Recombinant erythropoietin (rEPO) was obtained from eBioscience, and animals were treated in a dose of 5,000 IU/kg i.p. EPO blocking antibody (R&D) was reconstituted with 1 mL of sterile PBS, and each mouse received 80 μL (40 μg per animal, i.p.) immediately before CO and 6 h after reperfusion. IgG nonspecific antibody (R&D) was used as the control.
Experimental Model of Renal IRI.
Surgery was performed as previously described (34). Briefly, mice were anesthetized with Ketamine-Xylazine, a midline incision was made, and both renal pedicles were cross-clamped for 45 min. During the procedure, animals were kept well hydrated with saline and at a constant temperature (∼37 °C) through a heating pad device. Subsequently, microsurgery clamps were removed, the abdomen closed, and animals placed in single cages, warmed by indirect light until completely recovered from anesthesia. Animals were kept under adjustable conditions until sacrifice—namely, 2, 6 and 24 h after renal reperfusion. For the nephrectomy experiment, mice were anesthetized, and bilateral nephrectomy was performed after renal pedicle occlusion. In this set of experiments, animals were killed 6 h after CO treatment.
Analysis of Renal Function.
Serum creatinine and blood urea nitrogen (BUN) were used for evaluation of renal function. Blood samples were collected at the indicated time points from the heart, immediately before induced death. Serum samples were analyzed on an IDEXX Catalyst DX analyzer (IDEXX Laboratories).
In Vitro Hypoxia-Reoxygenation Model.
LLCPK-1 cells were grown to subconfluency, serum-starved overnight, and exposed to hypoxia (0.1% O2/5% CO2, balance N) for 16 h followed by reoxygenation (21% O2/5% CO2/balance N2 and/or 21% O2/5% CO/250 ppm CO/balance N2) for 8 h. A group of cells received 250 ppm CO for 4 h before hypoxia. Control cells were exposed to either normoxia or 250 ppm CO for the same duration.
Cell Death.
LLCPK-1 were subjected to a regimen of 16-h anoxia and 8-h reoxygenation (±250 ppm CO) to determine cell death. At each time point, both adherent and suspended cells were harvested and labeled with propidium iodide as a marker of cell death and to determine cell cycle and DNA content by FACS.
Cytotoxicity Assay.
Crystal violet staining was used to assess the number of surviving cells under either normoxic or hypoxic conditions, with or without CO pretreatment. At the end of treatment, the number of surviving cells was determined by the crystal violet staining method. Briefly, surviving cells in six-well plates were washed twice with PBS, and then crystal violet solution (Sigma-Aldrich) was added to the cells. After washing with water, plates were left to dry overnight. On the following day, a solution of 10% acetic acid was added to each well, and the number of surviving cells was determined by measuring absorbance at 562 nm.
ROS Measurement.
Intracellular ROS generation was assessed using 2′,7′-dichlorofluorescin diacetate (DCF-DA; 10 mM), and microscopy was performed. Cells on coverslips were perfused under controlled O2 and CO conditions in a flow-through chamber at 37 °C on an inverted fluorescent microscope. Images were acquired with an Olympus camera (excitation, 488 nm; emission, 535 nm).
Gene Expression.
Total RNA was extracted from homogenized tissues using RNeasy Mini Kit (Qiagen). For cDNA synthesis, 2 μg of total RNA was used and transcribed with SuperScript II Reverse Transcriptase (Invitrogen) and random primers (Invitrogen). The real-time PCR was performed in duplicate with the Cyber Green PCR Master Mix (BioRad) with the following primers:
Beta-Actin: forward (F)-ACTGGCATTGTCATGGACTC, revers (R)-GCACAGCTTCTCCTTGATGT;
TNF: F-CCTCCCTCTCATCAGTTCTATGG, R-TGTCCCTTGAAGAGACCTGG;
IL-10: F-CCAAGCCTTATCGGAAATGA, R-TTTTCACAGGGGAGAAAT;
PER2: F-GGTGGACAGCCTTTCGATTA, R-AGGGCCTACCCTGACTTTGT;
Adora1 (A1): F-AGAACCACCTCCACCCTTCT, R-TACTCTGGGTGGTGGTCACA;
Adora2a (A2a): F-GAAGCAGATGGAGAGCCAAC, R-GAGAGGATGATGGCCAGGTA;
Adora2b (A2b): F-TGCATGCCATCAACTGTATC, R-TGGAAACTGTAGCGGAAGTC.
The cycling conditions were as following: 95 °C for 15 s, 56 °C for 40 s.
TNF, cGMP, and EPO Measurement.
Protein levels of cAMP, TNF, and EPO were determined by ELISA (Enzo and R&D) according to the manufacturer’s instructions. cAMP content was normalized to protein concentration and expressed as picomole of cAMP/milligram of total protein.
ATP Measurements.
ATP levels were determined using a fluorimetric reaction assay (Biovision) according to the manufacturer’s instructions.
Western Blotting.
Kidney samples were sonicated in tissue lysis buffer (5 M NaCl, 5 mM EDTA, 1% Triton, 10 mM Tris∙Cl, pH 7.4) and clarified by centrifugation for 10 min at 17,709 × g. The supernatants were aliquoted and stored at −80 °C. Protein concentration was measured using the bicinchoninic acid (BCA) method following the manufacturer’s protocol, as described in Pierce BCA protein assay kit (Pierce). Thirty micrograms of protein extract were added on SDS/PAGE, transferred to nitrocellulose, and detected using SuperSignal (Thermo). HIF-1α antibody was purchased from Novus (CO).
Histologic Analysis.
During animal sacrifice, one of the kidneys was removed, and after sectioning and removing the renal capsule, it was cut sagittally in two approximately symmetrical fragments. The material was fixed in 10% buffered formalin until paraffin blocks were assembled. After processing, at least one cut of each fragment was studied in hematoxylin and eosin (H&E) staining. Immunohistochemical staining for infiltrating cells was performed on 5-μm sections using the HRP method with primary antibodies specific for macrophages (F4/80) or neutrophils/granulocytes (GR1; BD Bioscience). Antigenic recovery was initially performed with 10 mM citrate buffer, pH 6.0. Then blocking solution (horse serum, 7%) was added to for 30 min. Primary validated antibodies were then added (dilution 1:100 of F4/80 and 1:400 of GR1), and the slides were incubated overnight. The slides were then washed, and a solution of H2O2 was added for 10 min, followed by further washing. Secondary antibody (with 1 h incubation) was then added, followed by application of the avidin/biotin complex. After these steps, hematoxylin counterstaining was performed. For analysis, the slides were observed in a blinded fashion under light microscopy, and 25 randomly selected fields per slide were evaluated and enumerated for positively stained cells (100–200 cells counted per field) and compared to naïve control kidney staining and secondary antibody controls. This area of interest covered nearly the entire outer medullary regions of each section and thus is highly representative of the extent of positive-stained cells. We used this method of quantitative analyses previously (32).
Hypoxia Staining.
Hypoxia levels were evaluated in kidney tissue and cells. Animals and cells were treated with hypoxyprobe (Hypoxyprobe Inc.) at 60 mg/kg and 400 mM, respectively, 1 h before harvesting. For tissue sections, slides were stained following the protocol described above. The detection of hypoxia in the cells was done by immunofluorescence. Briefly, the cells were grown on slides and washed 2× with PBS, and a 2% paraformaldehyde solution was added. Thereafter, a further wash was performed, and a 0.5% triton solution was added. After further washing, blocking solution (7% horse serum) was added for 30 min, followed by addition of the primary antibody (diluted 1:100 in PBS) with overnight incubation. The sections were then incubated for 1 h in secondary antibody and labeled with the TexasRed fluorophore (Abcam). The nuclear dye (Hoechst-Life Technologies) was then added, and the slides were analyzed on a fluorescence microscope (Zeiss).
Statistical Analyses.
Results are expressed at means ± SD. Statistical analyses were performed using the Student’s t test and ANOVA. P values less than 0.05 were considered significant.
Results
CO Attenuates Renal Dysfunction Caused by IRI.
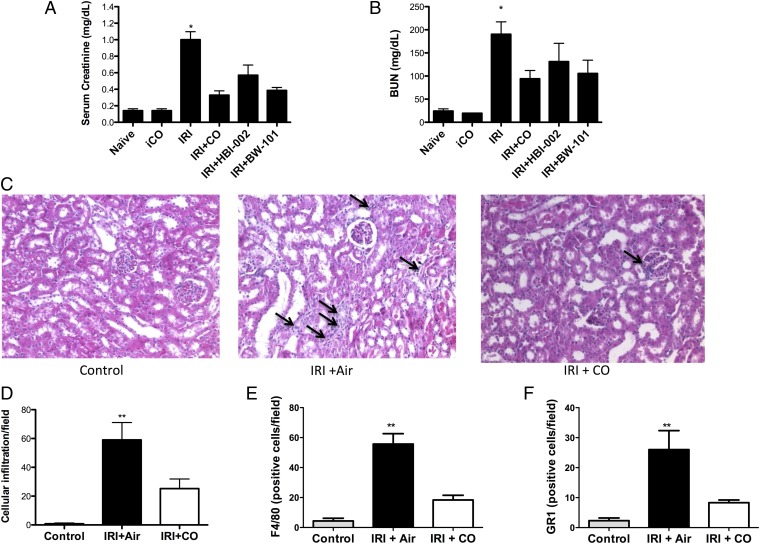
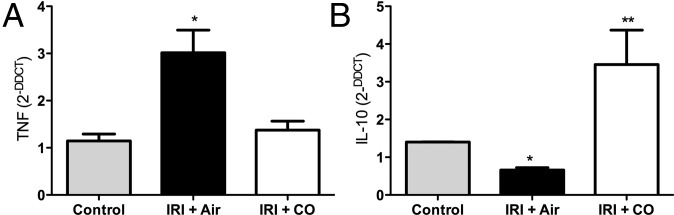
Others and we have demonstrated in rat and pig models that inhaled CO given before ischemia and reperfusion is protective. Therefore, our first experiment was to validate the model. Pretreatment with CO (inhaled, orally, or via a CO prodrug) before IRI surgery prevented kidney injury as determined by decreased levels in serum creatinine and BUN, which correlated with less inflammatory cell infiltrate and preservation of kidney architecture (Fig. 1 A–C). IRI-induced monocyte and neutrophil infiltration measured by staining with F4/80 and GR1, respectively, was markedly reduced in mice treated with CO (Fig. 1 D–F). Inflammatory cytokines are elevated as a hallmark of IRI. We observed a significant increase in the proinflammatory mediator TNF in air-treated controls, which was significantly abrogated in the presence of CO (Fig. 2A, P < 0.05). As has been shown in other models (35–37), CO exposure simultaneously increased expression of the anti-inflammatory cytokine IL-10 (Fig. 2B). These results suggest that protection afforded by CO is in part attributable to modulation of the inflammatory response. Whether these data suggest an overall mechanism by which CO protects against IRI or if they simply reflect the health of the kidney is unclear. Testing treatment with CO beginning either 1 h or 4 h after reperfusion did not offer significant benefit. This is not concerning since pretreating the donor or recipient are feasible options. Vera et al. (38) showed similar protective effects against IRI-induced kidney injury with a CO releasing molecule (CORM-3). Taken together, these data show that exposure to CO gas modulated cytokine expression to favor a protective phenotype with decreased infiltration of granulocytes and macrophages and maintenance of renal function otherwise lost in air-treated controls.
Fig. 1.
CO prevents kidney IRI in mice. (A and B) Mice were treated with inhaled CO (iCO), oral CO (HBI-002), or a CO-prodrug (BW-101) 1 h before a 45-min bilateral kidney ischemia. Serum creatinine (A) and BUN (B) were measured 24 h after reperfusion. Results represent mean ± SD of 5–10 mice per group. *P < 0.001 vs. iCO, P < 0.05 vs. HBI-002, and P < 0.01 vs. BW-101. (C) Representative H&E-stained sections from control kidney and from mice subjected to IRI treated with Air or iCO as above. Images are representative of 10 sections from five mice. Arrows indicate leukocyte infiltration. (D–F) Quantitation of F4/80- and GR1-positive staining for macrophage and neutrophils, respectively. Results represent mean ± SD of 6–8 sections from five mice in each group. **P < 0.05 vs. CO.
Fig. 2.
CO modulates cytokine expression in the kidney after IRI. (A and B) Tissue expression of TNF and IL-10 mRNA in the kidney in naïve mice or kidneys from mice 24 h after IRI treated with either Air or CO. CO blocked TNF and simultaneously enhanced IL-10 expression. Results represent mean ± SD of 5 per group. *P < 0.05 vs. CO; **P < 0.01 vs. control and IRI + Air.
CO Prevents Hypoxia/Reoxygenation (H/R)-Induced Cellular Stress.
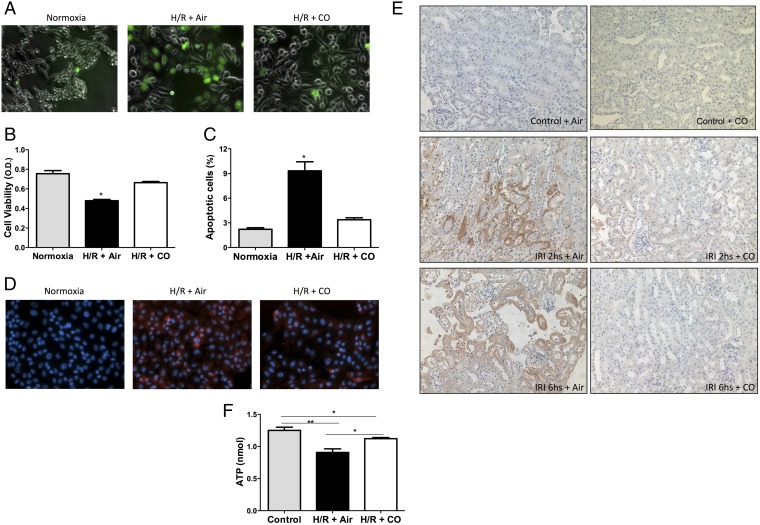
Exposure of cells to a period of hypoxia followed by transfer to a normoxia atmosphere results in a very similar cellular response to that observed with IRI cytokine expression, ROS generation and cell death. Parallel experiments in vitro in kidney epithelial cells showed that exposure to CO reduced (H/R)-induced elevations in ROS generation and completely prevented cell death (Fig. 3 A–C). As observed in the kidney, CO also reduced elevations in TNF, MCP-1, and IL-1β expression (Fig. S1).
Fig. 3.
Effects of CO on promoting kidney epithelial cell bioenergetics and viability in response to H/R conditions. (A) Exposure to CO (250 ppm) prevented H/R-induced elevations in DCF fluorescence, as a marker for reactive oxygen species production. Images are representative of at least three independent experiments in triplicate. (B and C) LLCPK-1 kidney epithelial cell viability measured by crystal violet (B) or propidium iodine incorporation (C) was determined in response to H/R in the presence and absence of CO (250 ppm). Results represent mean ± SD of 4–6 samples in triplicate. *P < 0.02 vs. normoxia or H/R + CO. (D and E) CO reduced tissue hypoxia measured with the O2-sensitive hypoxyprobe. Cells and animals were treated with hypoxyprobe at 400 mM and 60 mg/kg 1 h before harvesting, and cells and tissue sections from H/R and IRI, respectively, were stained as described in Materials and Methods. (F) ATP levels in LLCPK-1 cells shows CO reversing an H/R-induced decrease in ATP at 24 h of H/R. Results represent mean ± SD of three independent experiments in triplicate. *P < 0.05, **P < 0.02.
Previous studies in the liver and lung showed that exposure to CO can paradoxically reduce tissue hypoxia, likely involving the ability of CO to displace cellular O2 stores (39–41). We therefore tested the effects of CO on kidney epithelial cells exposed to H/R and in vivo in a kidney subjected to ischemia/reperfusion (I/R). CO effectively reduced cell and tissue hypoxia measured with the O2-sensitive hypoxyprobe (Fig. 3 D and E). The lack of hypoxia observed in H/R and I/R corresponded to the reduction in ROS shown in Fig. 3A. Further, we measured ATP in H/R samples and observed a significant decrease in ATP (0.9 ± 0.14 nmol) by H/R versus normoxic controls (1.25 ± 0.10 nmol, P < 0.01; Fig. 3F). CO restored ATP levels to 1.12 ± 0.04 nmol, which could be explained by enhancing oxidative phosphorylation given the results of the hypoxyprobe experiments. CO may act to reduce oxygen consumption, thereby making more available; generate more ATP via regulation of cytochrome oxidase activity; or influence O2 availability by displacement from intracellular stores. Work is ongoing toward delineating the biochemical effects low concentrations of CO impart on overall cellular function and metabolism. Control animals treated with CO or air in the absence of H/R or I/R showed no increase in hypoxyprobe positivity, ROS generation, or cell death. These data suggest that CO acts in part to reduce the severity of cellular and tissue hypoxia that ultimately contributes in part to attenuation of kidney damage that occurs at the time of reoxygenation.
CO Increases CD39 and Modulates Adenosine Receptor Expression in the Kidney.
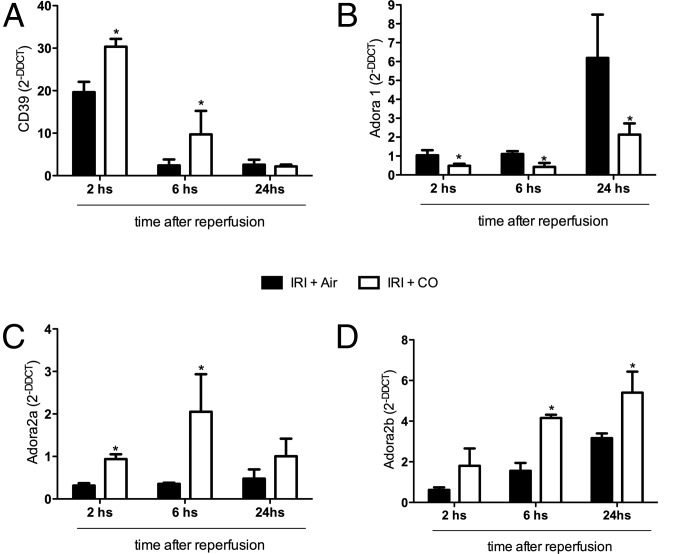
We previously described the effects of CO on adenosine receptor expression in endotoxin-stimulated macrophages yet had no in vivo correlate (29). Adenosine levels are increased in response to tissue hypoxia and are important in renal cytoprotection (42, 43). The abrogation of cellular and tissue hypoxia by CO described above directed us to next look at the effects of CO on tissue levels of the 5′ectonucleotidase CD39 and the adenosine receptors A1 and A2 in the kidney subjected to IRI. CD39 is known to be up-regulated in response to changes in tissue O2 levels dictated by oxygen sensors (44). Following reperfusion, we observed a 20-fold up-regulation of CD39 by 2 h that returned to baseline by 6 h. Animals treated with CO showed enhanced expression of CD39 50 ± 3% over IRI alone at 2 h, which remained nearly eightfold higher at 6 h before returning to baseline at 24 h (Fig. 4A). The increase in CD39 expression correlated with a threefold down-regulation in the expression of A1 in the kidney following reperfusion at 2, 6, and 24 h and conversely a twofold up-regulation of A2a and A2b receptor expression at these same time points (Fig. 4 B–D). Kidneys harvested from control naïve mice exposed to CO showed up-regulation of A2b, no change in A2a, and a down-regulation of A1 expression over kidneys from naïve air controls (Fig. S2). Collectively, these data suggest that exposure to CO by modulating adenosine receptor expression levels influences susceptibility to injury by differentially altering sensitivity to adenosine.
Fig. 4.
Effects of IRI and CO on CD39 and A2 receptor expression. (A–D) Expression levels of CD39, A1, A2a, and A2b were measured by PCR over time after reperfusion. Animals treated with CO increased expression of CD39 and the A2 receptors but inhibited IRI-induced increases in A1 receptor expression. Results represent mean ± SD of 4–6 mice per group at each time point. *P < 0.02 vs. Air.
CO Requires CD39 to Prevent Kidney IRI.
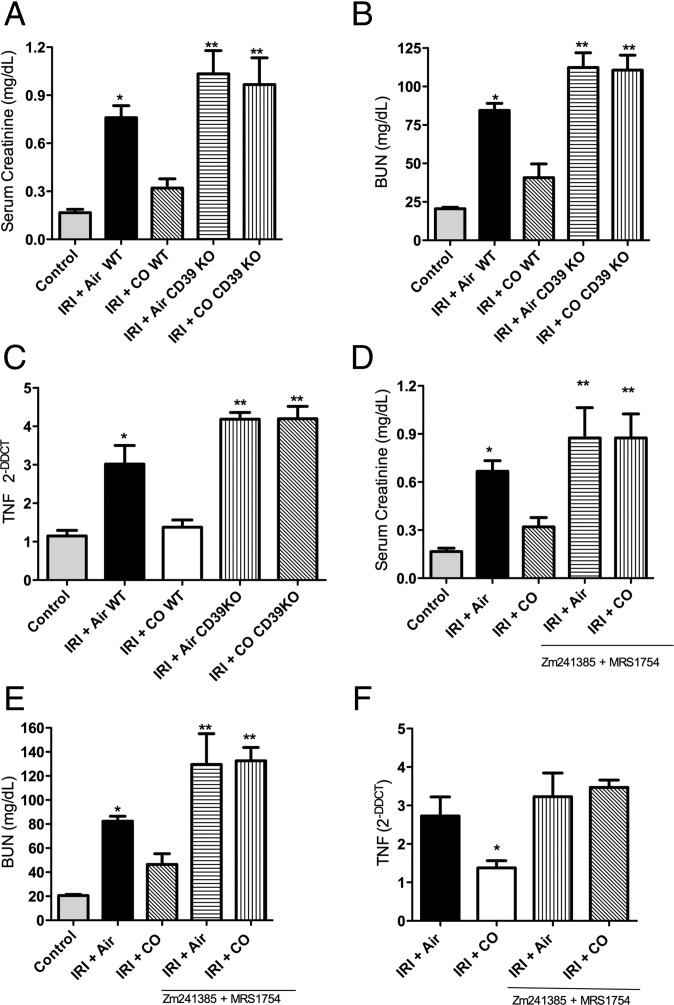
Up-regulation of CD39 and the A2b receptor by both IRI and CO suggested an important role in the injury response. We next tested the ability of CO to prevent IRI in Cd39−/− mice. The IRI injury in the absence of CD39 was modest yet significantly elevated over wild-type controls (P < 0.05). However, unlike the protection observed by CO in WT mice, CO was completely ineffective in Cd39−/− mice in reducing IRI-induced elevations in serum creatinine, BUN, and TNF levels (Fig. 5 A–C). Pharmacologic inhibition of A2 receptors using Zm241385 plus MRS1754 mirrored the results observed in the Cd39−/− mice. The protection afforded by CO to prevent IRI, as measured by serum creatinine, BUN, and TNF, was lost in these mice with decreased adenosine generation with Cd39 deletion or A2b/A2a inhibition (Fig. 5 D–F).
Fig. 5.
CD39 activity and A2 receptor signaling are required for CO to prevent kidney IRI in mice. (A–C) CO blocks IRI-induced elevations in creatinine and BUN and tissue levels of TNF, but these effects are lost in Cd39−/− mice. Results represent mean ± SD of 4–6 mice per group. Moreover, animals lacking CD39 and subjected to IRI showed enhanced severity of injury compared with WT mice with IRI regardless of CO. (D–F) Similar to Cd39−/− mice, WT mice treated with selective inhibitors of A2a and A2b receptors, Zm241385 and MRS1754, respectively, were also nonresponsive to the protection afforded by CO against IRI. Serum creatinine, BUN, and tissue TNF levels were similar to that observed in Cd39−/− mice. Results represent mean ± SD of 4–6 mice per group. *P < 0.05 vs. control and CO-WT; **P < 0.001 vs. other groups.
CO Regulates Per2 via CD39 and A2 Receptor Signaling.
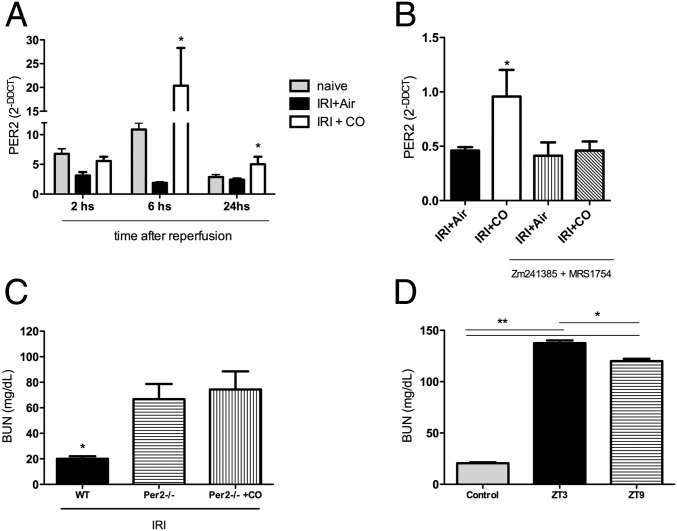
Adenosine binding to the A2b receptor has been shown to increase expression of the circadian rhythm molecule Per2, which directs a protective response to cardiac ischemia (27). It is also known that CO can regulate circadian rhythm gene expression, specifically NPAS2 (45), which regulates the period genes (46). Given the above, we next tested if CO modulated Per2 expression via A2 receptor signaling. Data presented in Fig. 6A show that animals subjected to kidney IRI and exposed to CO have a significant increase in Per2 expression at 6 and 24 h versus no change in air-exposed IRI alone-treated mice. This expression required A2 receptor activity, as inhibition with Zm241385 plus MRS1754 completely abrogated Per2 expression in CO-treated mice subjected to kidney IRI (Fig. 6B). The increase in A2 receptor expression by CO has been demonstrated in macrophages where A2 receptors were increased on the cell membrane. This resulted in potent activation of intracellular signal transduction that resulted in a more tolerogenic profile and lower production of TNF (22, 29).
Fig. 6.
Expression of the circadian rhythm gene Per2 is required for CO to protect the kidney against IRI and is dependent on A2 receptor activation. (A) Expression levels of Per2 over time in the kidney in response to IRI ± CO. CO markedly increases Per2 in conjunction with IRI versus no change observed in IRI+Air-treated mice. *P < 0.05 vs. Air. (B) Blockade of A2 receptors prevents the CO-induced increase in Per2 expression. *P < 0.05 vs. Air. (C) CO is unable to prevent IRI-induced elevations in serum BUN in Per2−/− mice. *P < 0.05 vs. other groups. (D) Since Per2 cycles throughout the day and peaks at ZT9, we tested susceptibility to kidney IRI compared with ZT3 when Per2 expression is low (Fig. S3). Mice that underwent IRI at ZT9 showed less severe IRI measured by elevations in BUN versus those subjected to IRI at ZT3. Results represent mean ± SD of 4–6 mice per group. *P < 0.05, **P < 0.001.
The increased expression of Per2 led us to test whether absence of Per2 would abrogate the reno-protective effects of CO. Per2−/− mice subjected to the standard 45-min IRI did not survive the insult, and CO was unable to provide protection. We therefore reduced the IRI to 30 min, which was not sufficient to induce injury in WT mice but resulted in significant injury in the Per2−/− mice with elevations in serum BUN without mortality. The increased BUN in the Per2−/− was unaffected by CO treatment (Fig. 6C). Characterization of the Per2 circadian expression profile showed that Per2 expression in naïve mice peaked in the kidney in the late afternoon (Zeitgeber 9, ZT9; Fig. S3). All studies described above were performed in the morning (ZT3) when Per2 expression was lowest. To further validate a role for Per2 in IRI, we subjected a cohort of WT mice to IRI (45 min) at ZT9 and observed significantly less IRI versus IRI performed at ZT3 (Fig. 6D). Collectively, we identify Per2 as a cytoprotective gene important in protecting against kidney IRI. Such modulation, known as “immunochronotherapy,” aids in tissue recovery and elimination of potentially harmful intracellular molecules (47).
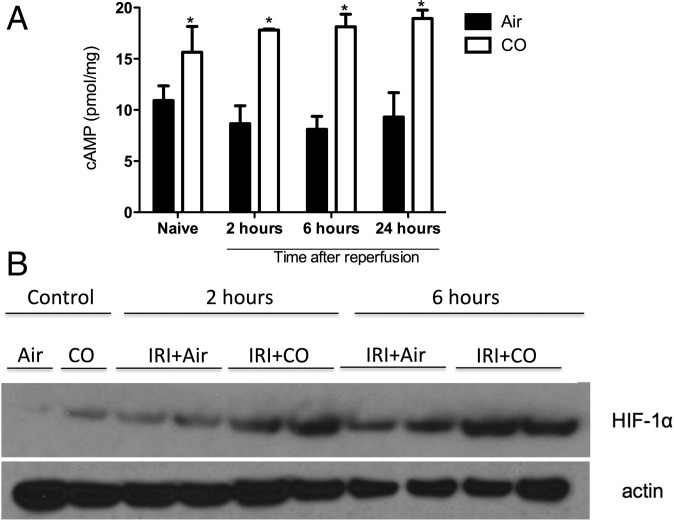
Adenosine A2 receptors are coupled to stimulatory G protein, and their activation leads to increased levels of cAMP and regulation of Per2. With the increase in A2 receptor expression and elevated levels of CD39 and ATP by CO, we next looked at A2-linked cAMP activation. Exposure to CO resulted in significantly increased levels of cAMP and HIF1α levels in the kidney after IRI (Fig. 7). The elevation in cAMP, HIF1α, and Per2 is important in protection against IRI (27). Eckle and coworkers demonstrated that A2b-dependent Per2 stabilization promotes HIF-dependent gene regulation; Per2 is also known to stabilize HIF-1α expression, as does CO (27, 30, 39). The protective role of HIF-1α in renal ischemia has been described previously and thought to be important for a faster recovery of the injured tissue (39, 46). Indeed, Hill and coworkers (48, 49) showed that renal IRI in HIF-1α+/− animals showed significant worsening of renal dysfunction, while pharmacologically increasing the half-life of HIF-1α led to improved renal function and reduced inflammation.
Fig. 7.
A2 receptor signaling is activated in CO-treated mice and associated with increased expression of HIF-1α. (A) Mice subjected to kidney IRI ± CO were killed at different times after reperfusion, and cAMP levels were measured by ELISA. *P < 0.01 vs. Air. (B) Kidney lysates were evaluated at different time points after reoxygenation for HIF-1α expression as indicated. Results represent mean ± SD of 4–6 mice per group.
EPO Mediates CO-Induced Protection Against IRI.
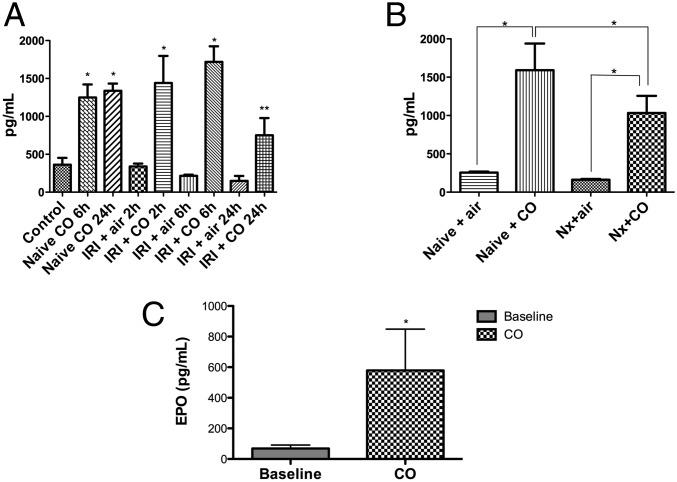
Stabilization of HIF-1α results in translocation to the nucleus to promote transcription of a number of target genes including endothelin and EPO. The remarkable effects of CO on HIF1α described above and in previous reports and the fact that increased carboxyhemoglobin leads to elevations in EPO led us to next test the hypothesis that EPO was the penultimate target gene involved in the protective effects observed with CO against IRI. Exposure of naïve mice to CO led to increased serum EPO levels within 2 h (Fig. 8A). The increased serum EPO partially originated from the kidney as CO-exposed binephrectomized animals also exhibited significant elevations in serum EPO, which we posit is liver-derived (Fig. 8B). Assessment of EPO pharmacodynamics showed that administration of recombinant mouse EPO was similar with or without CO exposure (Fig. S4). The CO-induced increase in serum EPO was also observed in animals subjected to IRI compared with air-treated mice subjected to IRI (Fig. 8A). Interestingly, CO-treated Per2−/− mice showed an up-regulation of serum EPO, compared with nontreated littermates (Fig. 8C), suggesting that CO could increase EPO through an indirect manner, or perhaps Per2 lies downstream of EPO signaling.
Fig. 8.
CO exposure increases serum EPO levels. (A) Serum EPO levels were evaluated in naive, Air-, and CO-treated mice ± IRI. Blood samples were collected at different times after reoxygenation. *P < 0.05 vs. Air; **P < 0.001 vs. Air. (B) EPO levels were measured in naive and bilateral nephrectomized mice. *P < 0.05. (C) EPO levels were measured in per2−/− mice. *P < 0.05 vs. baseline. Results represent mean ± SD of 4–6 mice per group.
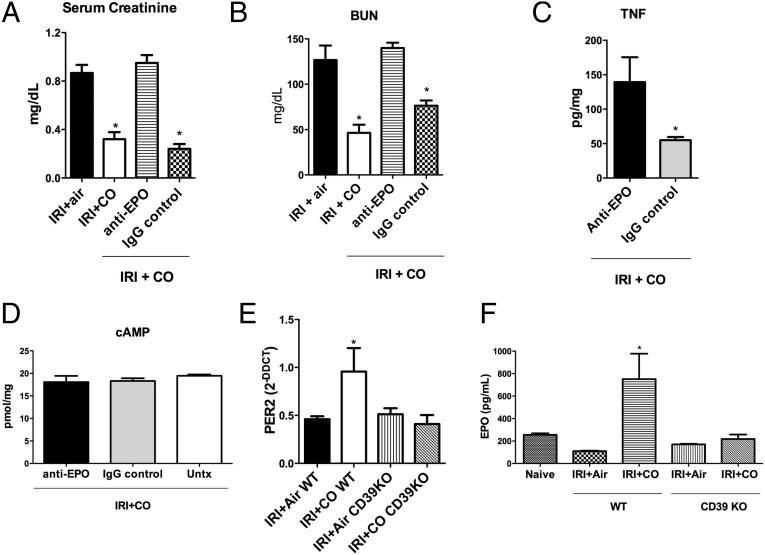
Neutralization of EPO with an anti-mouse EPO antibody completely abrogated the effects of CO to protect against kidney IRI versus IgG control (Fig. 9 A–C). CO-induced increases in cAMP levels remained unchanged in the antibody-treated animals, suggesting that the upstream A2–HIF1α/Per2 signaling was still operational (Fig. 9D). Finally, we tested the effects of CD39 on the signaling cascade that resulted in EPO up-regulation. CD39-null mice exposed to CO showed no increase in Per2 and EPO expression (Fig. 9 E and F). Collectively we delineate a signaling cascade for CO in the kidney that involves increases in ATP and CD39 that ultimately leads to Per2-dependent up-regulation of EPO and protection against IRI.
Fig. 9.
EPO is associated with a better outcome after CO treatment, and CD39 is an important mediator of the CO-induced protection in the IRI model. Mice were subjected to IRI ± CO in the presence or absence of anti-EPO blocking antibody or an IgG control antibody and were evaluated by Serum Creatinine (A), BUN (B), kidney TNF (C), and cAMP levels (D). *P < 0.05 vs. the other groups. (E and F) Per2 mRNA and serum EPO levels in WT and CD39 KO mice, subjected to IRI ± CO. *P < 0.02 vs. all of the other groups. Results represent mean ± SD of 5 mice per group.
Discussion
Results obtained in this study demonstrate that CO protects the kidney against IRI, at least in part by increasing circulating levels of EPO. Further mechanistic investigation of the increase in EPO found expression to be regulated and completely dependent on purinergic signaling initiated by stabilization of ATP production and increased expression of CD39, an ecto-nucleoside triphosphate diphosphohydrolase/ectonucleotidase. The resulting generation of adenosine correlated with a marked increase in CD39 and CD73. Downstream of the A2 receptor, we find increased cAMP and Per2 expression and stabilization of HIF1α and likely other O2 sensors that in large part would regulate EPO expression. Curiously, both extracellular ATP (eATP) and hypoxia, in the setting of inflammation might trigger aryl hydrocarbon receptor (AHR) inactivation by HIF1α. In a reciprocal manner, the presence of CD39, which might be induced by AHR, would deplete eATP and suppress HIF1α- mediated activity by generating adenosine in tandem with CD73.
Several studies have tried to unravel the cytoprotective role of EPO in models of renal injury. The kidneys are particularly sensitive to high glucose levels, and EPO administration prevented recurrent dysfunction during transient hyperglycemia. While unable to prevent tubular necrosis, treatment with EPO was able to attenuate apoptosis and glomerular dysfunction (50). Perhaps the best described mechanism supporting the beneficial properties of EPO is its potent anti-inflammatory effects. In a model of acute kidney injury (AKI) in response to sepsis, treatment with EPO resulted in lower renal expression of TLR4, NF-κB, CD68, and a battery of proinflammatory molecules compared with the vehicle controls. In contrast, levels of EPOR were elevated after treatment (51–53). Hu and colleagues have shown that treatment with EPO before ischemic injury promoted an improvement in renal function with a reduction in tubular necrosis, which correlated to decreased neutrophil infiltration, lower expression of proinflammatory chemokines, and decreased translocation of NF-κB into the nucleus (54). As to Per and A2A versus A2B functionality, it has been shown that A2A and A2B interact to promote expression of a chimeric receptor. A2B can only be expressed with A2A and would thus explain the Per2 data (55).
There are several studies that have demonstrated that EPO regulates the expression of HO-1. In a model of experimental autoimmune encephalomyelitis (EAE), EPO administration led to increased expression of HO-1 and modulation of adaptive immunity, leading to a suppression of the inflammatory response (56). Another study showed that the increased expression of HO-1 by EPO occurs through the activation of PI3K pathways, MAPK, and Nrf2 (57). Finally, Burger et al. (58) showed that EPO-induced up-regulation of HO-1 was responsible for the reduction of apoptosis of cardiomyocytes after IRI because blockade of HO-1 or administration of EPO to Hmox1−/− mice resulted in loss of EPO protection. Based on these data, we propose a cycle or feed-forward loop amplification in the kidney where HO-1, likely via CO generation, leads to increased expression of EPO as defined by the signaling described above. The EPO, in turn, imparts protection but requires the presence of HO-1, perhaps as continuous protection.
We have published previously that exposure of macrophages to CO resulted in increased expression of the A2a receptor and a significant increase in sensitivity to adenosine (29). Given the role of CD39 in the generation of adenosine, we tested Cd39−/− animals to examine whether CO-mediated ectonucleotidase signaling was important in the protection afforded by CO and therein the effects on EPO expression. Indeed, lack of CD39 resulted in worse renal function after ischemia and reperfusion, with increased serum levels of creatinine and BUN and expression of TNF. CO was unable to rescue Cd39−/− animals, likely explained by a poor EPO response. Finally, with decreased pericellular adenosine levels, we observed no increase in cAMP or Per2 compared with wild-type littermates exposed to Air. HIF1α has been shown to affect circadian expression of Per2 in kidney cells, and inhibition in HIF1α decreased the amplitude of the circadian rhythm of the Per2 promoter (59). Adamovich et al. (60) recently reported that oxygen, via HIF1α, is a resetting cue for circadian clocks and includes regulation of transcript levels of Rev-erbα and Per2. The requisite competition that CO has for similar binding sites also bound by oxygen in numerous heme-containing proteins such as Rev-erbα, it is plausible that CO targets similar signaling pathways directly or indirectly via HIF1α activity. Perhaps the most compelling and elegant data linking HIF1α and Per2 were reported by Eckle et al. (27), who showed that stabilization of Per2 is controlled by HIF1α and that this effect limited ischemic damage of the heart. Further, they show that Per2 could be stabilized with exposure of the mouse to intense light. With regard to organ transplantation, perhaps the recipient or the organ itself, during the preservation time and while in transit, could be exposed to light to improve function in conjunction with optimal protective rhythms. Whether AKI has a circadian rhythmicity is unclear, but studies in the heart and lung have clearly demonstrated that the dyssynchrony or reorganization of the central clock in the brain can impact susceptibility to cardiovascular disease and lung injury (61, 62). The association between AKI and circadian rhythm has not been well studied and validated. However, it has been substantiated by literature evidence showing the clear impact of the central and local circadian clocks on sodium regulation and susceptibility to organ injury in individuals with low nocturnal blood pressures versus those with high nocturnal pressures. Understanding how and when an organ might be most sensitive to injury may offer innovative therapeutic opportunities. Methodologies for continuously measuring real-time kidney function in animals has been described and will be useful in models of IRI and AKI.
Due to its physicochemical characteristics, CO easily crosses the plasma membrane and acts as a potent intracellular messenger. The genetic regulation of CD39 appears to be exerted by the transcription factor Sp1, a member of a family referred to as Sp/XKLF, which also regulates VEGF and cystathionine-β-synthase (44). Furthermore, ischemic injury leads to a reduction in expression of Sp1 in the renal tissue (63). There is one report showing that treatment with CO leads to increased expression of Sp1 and overexpression of a mutant form of this factor abrogated its transcriptional activity induced by CO (64). Liao et al. (65) showed that CD39 expression is also regulated by increased cytoplasmic levels of cAMP, which involves activation of the PKA/CREB, PKA/PI3K/ATF2, and PKA/ERK/ATF2 pathways. Because we also observed an increase in cAMP levels in the kidneys of our treated animals, we conclude that the regulation of CD39 may occur in part via activation of this second messenger (65). Both ATP and adenosine are important in protection against renal injury (66). It is also worth noting that renal tubular, vascular endothelial, as well as mesangial cells express ectonucleotidases on their surfaces with enzymatic properties that have high similarity to CD39. Finally, during an ischemic injury, the enzymatic capacity to convert ATP to ADP and then to AMP is reduced by up to 71% (67–69) Thus, these results support our findings that CO-induced up-regulation of CD39 on renal cells is a critical event necessary in protecting the kidney from injury.
Cell regeneration after an ischemic insult is favored by the presence of energy sources capable of accelerating such reparative process. Thus, the availability of ATP is crucial for an efficient recovery. In this light, CO exposure leads to an increase in protein levels of PGC-1α and mitochondrial biogenesis (70–72), which has been observed by others and both of which are regulated by adenosine signaling (73, 74). The greater number of mitochondria in the animals exposed to CO could explain the continued presence of ATP that is otherwise lost in response to IR. Mitochondrial dysfunction is a mediator of a variety of cellular insults and a common element in the initiation of various diseases. The importance of mitochondrial biogenesis is reflected in its ability to increase the activity of metabolic pathways such as fatty acid oxidation, in addition to increasing the antioxidant defense mechanisms, mitigating damage from aging, tissue hypoxia, excess glucose, or fatty acids, which contribute to the pathogenesis of acute and chronic renal injuries (70, 71). A recently published study showed that the use of compounds that may promote an increased capacity for mitochondrial biogenesis in the cell could be a promising therapeutic target in the future (72). Without the loss in ATP, cells can proliferate, as evidenced by increased ERK expression, better viability, and normal cell cycle pattern, suggesting that the presence of CO favors tissue homeostasis, promoting faster recovery when necessary.
Based on our findings, we conclude that CO promotes a beneficial effect on ischemic renal injury by a mechanism dependent on purinergic signaling and Per2 expression. Treatment with CO generates an increased expression of CD39 and type 2 adenosine receptors, which once activated lead to a cascade of events that stabilizes HIF-1α via Per2, allowing its transcriptional activity to be more sustainable. One of the target genes for HIF1α is EPO, which is important in protection of the kidney. Collectively, our work provides a critical mechanism by which CO is able to protect the kidney in a model of IRI. Translation to organ transplant utilizing a pretreatment regimen is certainly viable when considering treating the donor and/or recipients before harvest or implantation, as has been demonstrated in large and small animal models (23, 24). In pigs, having COHb peaking at the time the renal vessels are unclamped is very effective at reducing delayed graft function (23). Additional postoperative dosing with CO as well as treatment of the donor and graft itself during preservation may offer additional benefits. Given that clinical trials for CO are ongoing, these findings lend insight into the cellular and molecular mechanisms of action. We further conclude that the protective effects of CO treatment also extend to cellular metabolic changes, which improve energy charge, functional capacity, and as such, favor tissue recovery.
Supplementary Material
Acknowledgments
The studies were supported by NIH Award R44 DK111260-01 and Department of Defense Award W81XWH-16-0464 (to L.E.O.) and the Sao Paulo Research Foundation (FAPESP) Grant 2011/19581-8 (to M.C.-C.).
Footnotes
Conflict of interest statement: L.E.O. is a scientific consultant for Hillhurst Biopharmaceuticals and has stock options. E.G. is a founder of Hillhurst Biopharmaceuticals and owns stock.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716747115/-/DCSupplemental.
References
- 1.Eltzschig HK, Eckle T. Ischemia and reperfusion–From mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 2009;87:859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 4.Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC Med. 2011;9:11. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 6.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ysebaert DK, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 8.Hwang HS, et al. Pretreatment with paricalcitol attenuates inflammation in ischemia-reperfusion injury via the up-regulation of cyclooxygenase-2 and prostaglandin E2. Nephrol Dial Transplant. 2013;28:1156–1166. doi: 10.1093/ndt/gfs540. [DOI] [PubMed] [Google Scholar]
- 9.Kinsey GR, et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko GJ, et al. Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:732–742. doi: 10.1681/ASN.2010010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang PH, et al. Bradykinin [corrected] B1 receptor antagonism is beneficial in renal ischemia-reperfusion injury. PLoS One. 2008;3:e3050. doi: 10.1371/journal.pone.0003050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SH, et al. Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:1305–1314. doi: 10.1681/ASN.2010080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wegiel B, Nemeth Z, Correa-Costa M, Bulmer AC, Otterbein LE. Heme oxygenase-1: A metabolic nike. Antioxid Redox Signal. 2014;20:1709–1722. doi: 10.1089/ars.2013.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep. 2009;11:56–62. doi: 10.1007/s11906-009-0011-z. [DOI] [PubMed] [Google Scholar]
- 15.Wagener FA, et al. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J Pharmacol Exp Ther. 1999;291:416–423. [PubMed] [Google Scholar]
- 16.Amano MT, Camara NO. The immunomodulatory role of carbon monoxide during transplantation. Med Gas Res. 2013;3:1–15. doi: 10.1186/2045-9912-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozaki KS, et al. Carbon monoxide inhibits apoptosis during cold storage and protects kidney grafts donated after cardiac death. Transplant Int. 2012;25:107–117. doi: 10.1111/j.1432-2277.2011.01363.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, et al. Activation of peroxisome proliferator-activated receptor γ prolongs islet allograft survival. Cell Transplant. 2012;21:2111–2118. doi: 10.3727/096368911X637399. [DOI] [PubMed] [Google Scholar]
- 19.Onyiah JC, et al. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology. 2013;144:789–798. doi: 10.1053/j.gastro.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacGarvey NC, et al. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2012;185:851–861. doi: 10.1164/rccm.201106-1152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuckerbraun BS, et al. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006;203:2109–2119. doi: 10.1084/jem.20052267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou J, et al. 5-Aminolevulinic acid combined with ferrous iron induces carbon monoxide generation in mouse kidneys and protects from renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2013;305:F1149–F1157. doi: 10.1152/ajprenal.00275.2013. [DOI] [PubMed] [Google Scholar]
- 23.Hanto DW, et al. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant. 2010;10:2421–2430. doi: 10.1111/j.1600-6143.2010.03289.x. [DOI] [PubMed] [Google Scholar]
- 24.Caumartin Y, et al. Carbon monoxide-releasing molecules protect against ischemia-reperfusion injury during kidney transplantation. Kidney Int. 2011;79:1080–1089. doi: 10.1038/ki.2010.542. [DOI] [PubMed] [Google Scholar]
- 25.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Kung TA, et al. Rapid attenuation of circadian clock gene oscillations in the rat heart following ischemia-reperfusion. J Mol Cell Cardiol. 2007;43:744–753. doi: 10.1016/j.yjmcc.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durgan DJ, et al. Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haschemi A, et al. Cross-regulation of carbon monoxide and the adenosine A2a receptor in macrophages. J Immunol. 2007;178:5921–5929. doi: 10.4049/jimmunol.178.9.5921. [DOI] [PubMed] [Google Scholar]
- 30.Chin BY, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beldi G, et al. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otterbein LE, et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 33.Ji X, et al. Toward carbon monoxide-based therapeutics: Critical drug delivery and developability issues. J Pharm Sci. 2016;105:406–416. doi: 10.1016/j.xphs.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correa-Costa M, et al. Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS One. 2012;7:e49569. doi: 10.1371/journal.pone.0049569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawanishi S, et al. Inhalation of carbon monoxide following resuscitation ameliorates hemorrhagic shock-induced lung injury. Mol Med Rep. 2013;7:3–10. doi: 10.3892/mmr.2012.1173. [DOI] [PubMed] [Google Scholar]
- 36.Piantadosi CA, et al. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem. 2011;286:16374–16385. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulido JN, et al. Inhaled carbon monoxide attenuates myocardial inflammatory cytokine expression in a rat model of cardiopulmonary bypass. J Extra Corpor Technol. 2011;43:137–143. [PMC free article] [PubMed] [Google Scholar]
- 38.Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- 39.Faleo G, et al. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- 40.Fujita T, et al. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 41.Zuckerbraun BS, et al. Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resuscitation. Shock. 2005;23:527–532. [PubMed] [Google Scholar]
- 42.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 43.Vallon V, Mühlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 44.Eltzschig HK, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilun P, et al. Carbon monoxide-mediated humoral pathway for the transmission of light signal to the hypothalamus. J Physiol Pharmacol. 2013;64:761–772. [PubMed] [Google Scholar]
- 46.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill P, et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19:39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hochhauser E, et al. Recombinant human erythropoietin attenuates hepatic injury induced by ischemia/reperfusion in an isolated mouse liver model. Apoptosis. 2008;13:77–86. doi: 10.1007/s10495-007-0155-8. [DOI] [PubMed] [Google Scholar]
- 51.Caetano AM, et al. Erythropoietin attenuates apoptosis after ischemia-reperfusion-induced renal injury in transiently hyperglycemic Wister rats. Transplant Proc. 2011;43:3618–3621. doi: 10.1016/j.transproceed.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 52.Souza AC, et al. Erythropoietin prevents sepsis-related acute kidney injury in rats by inhibiting NF-κB and upregulating endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2012;302:F1045–F1054. doi: 10.1152/ajprenal.00148.2011. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues CE, et al. Effects of continuous erythropoietin receptor activator in sepsis-induced acute kidney injury and multi-organ dysfunction. PLoS One. 2012;7:e29893. doi: 10.1371/journal.pone.0029893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu L, et al. Erythropoietin ameliorates renal ischemia and reperfusion injury via inhibiting tubulointerstitial inflammation. J Surg Res. 2012;176:260–266. doi: 10.1016/j.jss.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 55.Moriyama K, Sitkovsky MV. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem. 2010;285:39271–39288. doi: 10.1074/jbc.M109.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen SJ, et al. Erythropoietin enhances endogenous haem oxygenase-1 and represses immune responses to ameliorate experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:210–223. doi: 10.1111/j.1365-2249.2010.04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Genc K, Egrilmez MY, Genc S. Erythropoietin induces nuclear translocation of Nrf2 and heme oxygenase-1 expression in SH-SY5Y cells. Cell Biochem Funct. 2010;28:197–201. doi: 10.1002/cbf.1639. [DOI] [PubMed] [Google Scholar]
- 58.Burger D, Xiang F, Hammoud L, Lu X, Feng Q. Role of heme oxygenase-1 in the cardioprotective effects of erythropoietin during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2009;296:H84–H93. doi: 10.1152/ajpheart.00372.2008. [DOI] [PubMed] [Google Scholar]
- 59.Okabe T, et al. The impact of HIF1α on the Per2 circadian rhythm in renal cancer cell lines. PLoS One. 2014;9:e109693. doi: 10.1371/journal.pone.0109693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab. 2017;25:93–101. doi: 10.1016/j.cmet.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Haspel JA, et al. Circadian rhythm reprogramming during lung inflammation. Nat Commun. 2014;5:4753. doi: 10.1038/ncomms5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khaper N, et al. 2017. Implications of disturbances in circadian rhythms for cardiovascular health: A new frontier in free radical biology. Free Radic Biol Med, S0891-5849(17)31199-1.
- 63.Wu N, Siow YL, O K. Ischemia/reperfusion reduces transcription factor Sp1-mediated cystathionine beta-synthase expression in the kidney. J Biol Chem. 2010;285:18225–18233. doi: 10.1074/jbc.M110.132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin HH, Lai SC, Chau LY. Heme oxygenase-1/carbon monoxide induces vascular endothelial growth factor expression via p38 kinase-dependent activation of Sp1. J Biol Chem. 2011;286:3829–3838. doi: 10.1074/jbc.M110.168831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao H, Hyman MC, Baek AE, Fukase K, Pinsky DJ. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. J Biol Chem. 2010;285:14791–14805. doi: 10.1074/jbc.M110.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szoleczky P, et al. Identification of agents that reduce renal hypoxia-reoxygenation injury using cell-based screening: Purine nucleosides are alternative energy sources in LLC-PK1 cells during hypoxia. Arch Biochem Biophys. 2012;517:53–70. doi: 10.1016/j.abb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ribeiro MC, et al. Characterization of ecto-ATPase activity in the surface of LLC-PK1 cells and its modulation by ischemic conditions. Biochim Biophys Acta. 2012;1820:2030–2036. doi: 10.1016/j.bbagen.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Candinas D, et al. Loss of rat glomerular ATP diphosphohydrolase activity during reperfusion injury is associated with oxidative stress reactions. Thromb Haemost. 1996;76:807–812. [PubMed] [Google Scholar]
- 69.Kishore BK, et al. Expression of NTPDase1 and NTPDase2 in murine kidney: Relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol. 2005;288:F1032–F1043. doi: 10.1152/ajprenal.00108.2004. [DOI] [PubMed] [Google Scholar]
- 70.Lancel S, et al. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther. 2009;329:641–648. doi: 10.1124/jpet.108.148049. [DOI] [PubMed] [Google Scholar]
- 71.Tran M, et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med. 2012;53:2043–2053. doi: 10.1016/j.freeradbiomed.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wills LP, et al. The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther. 2012;342:106–118. doi: 10.1124/jpet.112.191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.