Significance
Odor–reward association during appetitive learning is a fundamental process that requires multiple forms of plasticity. In the adult olfactory bulb, the continual production of newborn interneurons contributes to the functional plasticity of the system, placing the newcomers in a key position to participate in olfactory associative learning. Here, we demonstrate that adult-born neurons, but not preexisting ones, contain information about learned positive value. Moreover, specific heightening of this signal improves associative learning and odor value update and is enough in some cases to trigger behavior even without odor stimulus. Collectively, our findings show an important role of this adult-born interneuron population in odor–reward association and unveil the relevance of odor value encoding at early stages of sensory processing.
Keywords: olfactory bulb, adult neurogenesis, associative learning, reinforcement
Abstract
Olfaction is an important sensory modality driving fundamental behaviors. During odor-dependent learning, a positive value is commonly assigned to an odorant, and multiple forms of plasticity are involved when such odor–reward associations are formed. In rodents, one of the mechanisms underlying plasticity in the olfactory bulb consists in recruiting new neurons daily throughout life. However, it is still unknown whether adult-born neurons might participate in encoding odor value. Here, we demonstrate that exposure to reward-associated odors specifically increases activity of adult-born neurons but not preexisting neurons. Remarkably, adult-born neuron activation during rewarded odor presentation heightens discrimination learning and enhances the ability to update the odor value during reversal association. Moreover, in some cases, activation of this interneuron population can trigger olfactory learning without sensory stimulation. Taken together, our results show a specific involvement of adult-born neurons in facilitating odor–reward association during adaptive learning.
In many species, including rodents, the sense of smell is a strong driver of animal behavior. Innate odor responses, observed in animals without prior experience, have been extensively studied (1–3). However, most odors do not trigger any significant behavior in naive mice (2, 3), supporting the idea that odor responses are mainly adaptive and learned following repetitive experiences. Particularly, the assignment of a positive value to an odorant depends on its association with a reward outcome. These odor–reward associations are profoundly flexible processes that require multiple forms of plasticity present in the olfactory system.
In the olfactory bulb (OB), odor information sent from sensory neurons is received by relay neurons, mitral and tufted cells (M/TCs), which are involved in early processing before conveying this information to higher brain regions. Previous studies have reported reward-associated signals following associative learning in structures downstream of the OB, such as the piriform cortex or the olfactory tubercles (4–7). Interestingly, associative learning has also been shown to modify the firing activity of M/TCs (8). However, the cellular mechanisms involved in reward encoding at such an early stage of sensory processing and its relevance for odor-driven behavioral responses remain to be elucidated.
In the adult OB, the continuous production of GABAergic interneurons notably contributes to the functional plasticity of the olfactory system (9, 10). It represents an alternative mechanism of neuronal plasticity that acts in parallel to the conventional molecular, synaptic, and connectivity ones. A small population of periglomerular cells (PGCs) is produced by this process, but the majority of the newborn neurons become granule cells (GCs), which regulate the M/TC activity (10). GCs, and particularly adult-born GCs, are in a key position to promote reward-associated changes in OB output neurons because they exhibit unique features. First, GCs are about 100 times more numerous than M/TCs, and they are the converging endpoint of both widespread top-down inputs to the OB and bottom-up olfactory inputs mediated by M/TCs (11–13), allowing them to bind sensory inputs together with top-down influences. Second, OB interneurons display structural and functional plasticity of their inputs as well as their output synapses (13–15). Third, several studies have revealed distinct synaptic (16–19) and functional (20, 21) properties in adult-born and preexisting neurons. Finally, adult-born GCs are involved in various behavioral responses, such as odorant detection (22) and discrimination (23), olfactory innate behaviors (24–26), and fear memory (27), perceptual learning (28), short-term (22, 29) and long-term olfactory memory (30–33), and olfactory learning during a difficult task (32).
Given their key role in learning, we asked whether adult-born GC activity might be involved in encoding odor value and how manipulation of this signal can drive behavior. We demonstrate that adult-born neurons contain information about learned positive value and that activating these neurons simultaneously with rewarded stimuli could be sufficient to guide goal-directed behaviors. Collectively, our results show the involvement of a specific interneuron population in odor–reward association at early stages of sensory processing.
Results
Adult-Born GCs Overreact to Rewarded Odors.
We investigated the potential involvement of adult-born GCs in odor–reward association by using a go/no-go operant conditioning paradigm. In this task, mice were trained to discriminate between a pair of stimuli: a reinforced odor associated with a water reward (positive stimulus: S+) and an unreinforced odor (negative stimulus: S−) (Fig. 1A). We used only difficult tasks in which mice required extended training to discriminate between the odors correctly (Fig. S1A, Left) (32). During this associative learning, we observed a simultaneous consolidation of a go response only for S+ (hits), and the appearance of no-go responses for S− (correct rejections: CRs) (Fig. S1A, Right). Moreover, as we demonstrated with our odor replacement experiment, after learning, most of the animals had by default a no-go response to any stimulus different from S+ (Fig. S1B). As a consequence, the S+ odor and its associated reward size (Fig. S1C) became key factors to drive correct mouse performance, showing the relevance of rewarded odor perception for learning.
Fig. 1.
Adult-born GCs are differentially activated by S+ and S−. (A) Schematic of the discrimination learning task. In response to S+, licking the water port triggered water delivery. This constitutes a correct response (Go, a hit), whereas not going to lick is considered an error (No-go, a miss). In response to S−, trained mice refrained from licking, thus constituting the correct response (No-go, CR), whereas going to lick constitutes an error [Go, false alarm (FA)]. (B) Experimental timeline. (C, Left) Learning performances, according to age of labeled GCs (P60: adult-born, P6: neonatal-born) and the odorant to which mice are passively exposed at the end of the training session (S+ or S−) (group: F3,31 = 0.85, P = 0.48, repeated-measures two-way ANOVA, n = 8–9; odor pair: P1). (Right) Number of blocks needed to reach the criterion (P60: P = 0.84, n = 8–9; P6: P = 0.88, n = 9, Mann–Whitney test). (D) Schematic of the passive odor exposure. (E, Left) Confocal images showing DAPI (blue), BrdU (green), and c-Fos (red) staining in OB layers (EPL, external plexiform layer; GCL, granule cell layer; IPL, internal plexiform layer; MCL, mitral cell layer). (Scale bars, Left: 100 µm; Right: 2 µm.) (Right) S+ or S− exposure after learning triggered differential activation of adult-born GCs (P = 0.0016, Mann–Whitney test, **P < 0.01, n = 8–9) but not of neonatal-born GCs (P = 0.91, Mann–Whitney test, n = 9). This differential activation was due to an increase of adult-born GCs activated by S+ exposure (P = 0.012, Wilcoxon signed rank test vs. theoretical mean of 1 test, #P < 0.05, n = 9). Data obtained from mice exposed to either S+ or S− after training were normalized with respect to values obtained from mice exposed to the same two odorants without previous training. Data are shown as mean ± SEM and individual data points.
To ascertain the contribution of adult-born and neonatal-born GCs in encoding odor value, they were labeled with BrdU at postnatal day (P)60 and P6, respectively; 2 mo later, a go/no-go task was conducted followed by passive odor exposure (Fig. 1B). P60- and P6-injected mice were divided in two subgroups, the S+ group and the S− group, according to the odorant to which the mice were exposed at the end of the learning. For all groups, performances were similar in terms of the learning rate (Fig. 1C, Left) and the training blocks necessary to reach the learning criterion (85% of correct responses) (Fig. 1C, Right).
Once the task was learned, mice were passively exposed to either S+ or S− without reward delivery (Fig. 1D). Immediate early gene c-Fos expression was then used as a proxy for neuronal activity, and the density of BrdU-labeled cells expressing c-Fos protein was counted in the GC layer (Fig. 1E, Left). To evaluate whether S+ or S− odor exposure alters GC activation, the counted cell densities were normalized with respect to mean densities obtained from naive mice exposed to the same two odors but without previous learning. To avoid odor-specific effects, we used highly similar binary odor mixtures evoking both identical input patterns to the OB (34) and equal GC activation and odor-source approach (Fig. S2 A–C). We found that S+ exposure significantly increased the density of adult-born GCs expressing c-Fos, compared with S− exposure, when animals have previously associated S+ with the reward outcome (Fig. 1E, Right). This differential activation was mainly due to an increase in the density of adult-born GCs activated by S+ exposure with respect to values obtained from naive mice. Remarkably, no difference in the density of activated neonatal-born GCs (Fig. 1E, Right) or PGCs (Fig. S2D) was found between the S+ and S− odor-exposure groups. In summary, our results show that adult-born GCs are specifically prone to react to reward-associated olfactory stimuli.
Activation of Adult-Born GCs Accelerates Learning.
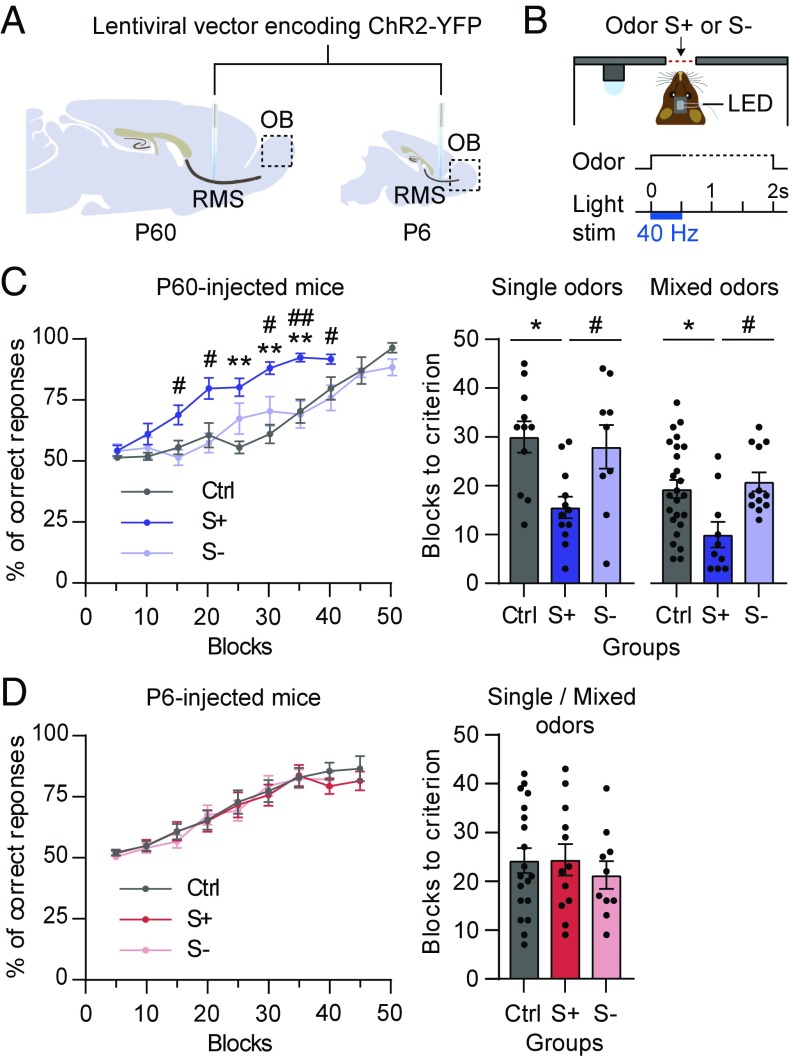
Next, we sought to manipulate the differential activation of adult-born GCs during S+ and S− odor presentation to probe whether this dissimilarity is sufficient to trigger appropriate behaviors. To do this, we used channelrhodopsin-2 (ChR2) to control neuronal activity exclusively in neurons born either at P60 or at P6 (32). We injected a lentiviral vector encoding ChR2-YFP into the rostral migratory stream (RMS) of mice, resulting 10 wk later in comparable cohorts of light-sensitive fully mature GCs (Fig. 2A and Fig. S3 A–C). To stimulate these neurons in vivo, an LED (465 nm) was implanted over the OB, allowing 40-Hz light stimulation during the discrimination learning task (Fig. 2B) (32).
Fig. 2.
Activation of adult-born GCs facilitates learning during S+ presentation. (A) Lentiviral vectors encoding ChR2-YFP were injected in the RMS during adulthood (P60) (Left) or at the neonatal stage (P6) (Right). (B) The LED implanted above the OB allowed in vivo 40-Hz photoactivation during odor delivery in the discrimination learning task. (C, Left) Adult-born GC photoactivation during S+ or S− presentation (group: F2,24 = 9.6, P = 0.0009, repeated-measures two-way ANOVA followed by Bonferroni post hoc test; **P < 0.01 for S+ vs. control (Ctrl), #P < 0.05, ##P < 0.01 for S+ vs. S−, n = 9–12; odor pair: P2). Ctrl mice stimulated during S+ vs. S− odor presentations showed identical performances and were pooled together (group: F1,9 = 1.06, P = 0.32, repeated-measures two-way ANOVA, n = 5–7). (Right) The number of blocks needed to reach the criterion for P60-injected mice for tasks with single odors (odor pair: P2) or mixed odors (odor pair: P1) (P = 0.007, n = 9–12 and P = 0.009, n = 10–25, respectively, Kruskal–Wallis test followed by post hoc Dunn’s test, *P < 0.05, #P < 0.05). (D, Left) Neonatal-born GC photoactivation during S+ or S− presentation (group: F2,39 = 0.068, P = 0.93, repeated-measures two-way ANOVA, n = 9–19; odor pairs: P1 and P2). Control mice stimulated during S+ vs. S− odor presentations showed identical performances and were pooled together (group: F1,17 = 0.06, P = 0.81, repeated-measures two-way ANOVA, n = 8–11). (Right) The number of blocks needed to reach the criterion for P6-injected mice (P = 0.80, Kruskal–Wallis test, n = 9–19; odor pairs: P1 and P2). Data are shown as mean ± SEM and individual data points.
In a first initiation task without light stimulation, controls and animals injected with ChR2 performed similarly (Fig. S3 D and E). Then, a difficult task was conducted, with 40-Hz photoactivation delivered for 500 ms during either S+ or S− presentations (S+ and S− groups, respectively). In mice expressing ChR2, discrimination learning was facilitated by simultaneous photoactivation of adult-born neurons with S+ but not with S− presentations (Fig. 2C, Left). As expected for a go/no-go task, this facilitation was reflected by an accelerated appearance of CRs during S− trials (Fig. S4A). Animals required fewer training blocks to learn to discriminate for both single and mixed odors when light was simultaneously provided with S+ (Fig. 2C, Right). Despite this change in learning rate, all groups eventually reached the same number of correct responses (last two blocks: P = 0.69, Kruskal–Wallis test, n = 9–12). Interestingly, this differential effect was specific to adult-born neurons, as there was no improvement in learning during photoactivation of neonatal-born neurons (Fig. 2D and Fig. S4 B–D). P6-injected groups learned at comparable rates and reached a similar number of correct responses (last two blocks: P = 0.49, Kruskal–Wallis test, n = 9–19). Importantly, in previous work we showed that ChR2+ GCs born at P60 had an optical threshold to spike similar to those born at P6, and thus the two groups of neurons were activated by light with a similar regimen (18). Finally, we observed that movement and discrimination times remained the same for all groups (Fig. S5). Taken together, our data showed that activating adult-born GCs specifically during rewarded odor presentation is sufficient to alter behavioral responses.
The Mere Activation of Adult-Born GCs Is Sufficient to Trigger Behaviors.
Then, we investigated if activating adult-born GCs before reward delivery, even in the absence of an odor stimulus, was sufficient to drive appropriate behavioral responses. To address this question, 40-Hz photostimulation without odor presentation was associated with reward delivery for “S+” trials but not for “S−” trials (Fig. 3A, Upper). We found that GC photoactivation was sufficient to generate a reinforceable stimulus in a subgroup of P60-injected mice (three out of nine mice), whereas all P6-injected (n = 11) and control (n = 14) mice performed at the 50% chance level (Fig. 3A, Lower). This learning in the P60-injected group is again reflected by an appearance of CRs during “S−” trials (Fig. S6A). Differences between groups persisted if all P60-injected mice were pooled together (Fig. S6B), but variability in this group cannot be explain by ChR2-YFP+ cell density (Fig. S6C). P60 learners still succeeded in learning the task even when the “S−” stimulus was ambiguously photoactivated by a low-frequency stimulus (“S+”: 40 Hz vs. “S−”: 10 Hz). However, their performance did not differ from that of the control group if the difference between GC activation during “S+” and “S−” trials was too small (40 Hz vs. 25 Hz) (Fig. 3A).
Fig. 3.
The mere activation of adult-born GCs is enough to trigger reward-oriented behavior. (A, Upper) Experimental design of the learning task without odor delivery. For “S+” trials, 40-Hz photostimulation was associated with reward delivery. No light, 10 Hz, or 25 Hz photostimulation was used as a stimulus for “S−” trials (unrewarded). (Lower) For the 40-Hz vs. no-light task, some P60-injected mice associate the photostimulation with reward delivery, whereas the other groups kept performing at chance level (50% of correct responses; group: F3,30 = 268.9, P < 0.0001, repeated-measures two-way ANOVA followed by Bonferroni post hoc test; **P < 0.01, ****P < 0.0001 for P60-learners vs. the other groups, n = 3–14). P60 learners succeeded in discriminating between 40 Hz and 10 Hz (F1,5 = 38.55, P = 0.0016) but not between 40 Hz and 25 Hz (F1,5 = 3.90, P = 0.11). P6 and P60 control mice showed identical performances and were pooled together (group: F1,12 = 0.38, P = 0.55, repeated-measures two-way ANOVA, n = 7). (B, Upper) Experimental design of the learning task without odors, with no photostimulation for “S+” trials (rewarded) and 40-Hz photostimulation for “S−” trials (unrewarded). (Lower) Performances were similar for all groups, and no mice managed to learn the task (group: F2,26 = 0.15, P = 0.86, repeated-measures two-way ANOVA, n = 8–12). Data are shown as mean ± SEM.
In a similar experiment, 40-Hz photostimulation associated with “S−” unrewarded trials did not drive appropriate behavioral responses in any of the groups (controls: n = 8; P60: n = 9; P6: n = 12) (Fig. 3B). These results suggest that activation of adult-born GCs in association with a reward signal can drive the behavioral response. An alternative explanation could be that GC activation is itself rewarding. However, GC photostimulation without or with only a partial water reinforcement did not trigger learning (Fig. S7). In sum, our data support the idea that adult-born neuron activation during rewarded odor presentation and preceding complete reward delivery might be sufficient for triggering the learning process.
Adult-Born GC Activation Improves Reversal Learning.
We next asked if GC activation could differentially modify acuity in S+ or S− trials. However, once the learning criterion was reached without photostimulation (Fig. S8A), we observed that adult-born GC activation did not further improve discrimination acuity of an already established odor–reward association (Fig. S8B). This result suggests a predominant role of adult-born GCs in the early phases of the odor–reward association. To challenge this idea, animals were trained in a reversal version of the go/no-go task. Mice had to reverse the values associated with an odor pair that they had already learned. Without light stimulation all mice reached similar learning performances (Fig. 4A, Left). Then, the odorant previously associated with a reward was unreinforced and vice versa (Fig. 4A, Right). As expected, during the first few blocks of the reversal task (reset), all animals performed below the chance level, as they kept following the previous odor–reward association. However, the adjustment to the new odor–reward association was facilitated by photoactivation of adult-born neurons but not of neonatal-born neurons. This increased learning flexibility was demonstrated by those animals requiring fewer trials before switching to a go response to the newly rewarded odor (Fig. S9A). Full learning of the new task was also accelerated; however, the effect was smaller (Fig. 4B, Left). Despite this improvement in reversal learning, all groups eventually reached the same number of correct responses (last two blocks: P = 0.76, Kruskal–Wallis test, n = 6–10). Similar results were found when analyzing the number of blocks needed to reach the criterion with the new task rules (Fig. 4B, Right). It is noteworthy that the dissociation between odor and reward outcome using an extinction protocol, in which no reward was delivered, was not affected by GC photoactivation (Fig. 4C and Fig. S9B). Reacquisition of the task after extinction was fast, indicating that the odor–reward association was still partially stored (Fig. 4D). Collectively, these results point to the specific involvement of adult-born GC activity in the initial positive value attribution to rewarded stimuli.
Fig. 4.
Adult-born GC activation improves reversal learning. (A, Left) Mouse performances before reversal learning and without photostimulation (group: F2,25 = 0.26, P = 0.77, repeated-measures two-way ANOVA, n = 7–11; odor pair: P1). P6 and P60 control mice showed identical performances and were pooled together (group: F1,9 = 3.09, P = 0.11, repeated-measures two-way ANOVA, n = 5–6). (Right) Mouse performances for the first 10 blocks of reversal learning (reset) with photoactivated adult-born or neonatal-born GCs (group: F2,25 = 4.88, P = 0.016, repeated-measures two-way ANOVA followed by Bonferroni post hoc test; *P < 0.05, **P < 0.01 for P60 vs. Ctrl, #P < 0.05, ###P < 0.001 for P60 vs. P6, n = 7–11). (B, Left) Mouse performances in reversal of full learning following reset (group: F2,21 = 3.54, P = 0.047, repeated-measures two-way ANOVA followed by Bonferroni post hoc test; *P < 0.05 for P60 vs. Ctrl, #P < 0.05 for P60 vs. P6, n = 6–10). (Right) The number of blocks needed to reach the criterion (P = 0.025, Kruskal–Wallis test followed by post hoc Dunn’s test, *P < 0.05, n = 6–10). (C, Left) Mouse performances before extinction and without light delivery (group: F2,42 = 1.07, P = 0.35, repeated-measures two-way ANOVA, n = 10–19; odor pair: P2). (C) Performances during the extinction protocol, in which the reward was removed and light stimulation was delivered simultaneously with previous “S+” presentations (group: F2,42 = 0.32, P = 0.72, repeated-measures two-way ANOVA, n = 10–19). The animals’ performance level was reduced until the percentage of correct responses was not different from chance level. (D, Left) Mouse performances during reacquisition of the task, with light stimulation delivered with “S+” presentations reassociated with the water reward (group: F2,38 = 1.72, P = 0.19, repeated-measures two-way ANOVA, n = 10–19). (Right) The number of blocks needed to reach the criterion (P = 0.23, Kruskal–Wallis test, n = 10–19). Data are shown as mean ± SEM and individual data points.
Discussion
The present study demonstrates that in the OB the activity of adult-born GCs, but not of neonatal-born GCs, contains information about learned positive value. Concomitant stimulation of ChR2-expressing adult-born neurons with rewarded stimulus presentation accelerates discrimination learning and improves the odor valence update that occurs during reversal learning. Moreover, activation of this interneuron population, in the absence of odor stimulus, is enough to trigger reward-motivated behavior in some mice. Collectively, our study demonstrates that adult neurogenesis endows the olfactory system with the capacity to enhance odor–reward association at early stages of sensory processing.
Contribution of Adult-Born Neurons in Olfactory Behavior.
It is well established that adult-born neurons are involved in several behavioral processes (22–33). However, reducing adult neurogenesis has produced mixed results, frequently not preventing odor learning (27, 28, 30, 31, 33, 35). In general, previous studies used approaches that were not completely selective and/or induce long-term ablation of adult neurogenesis, which may recruit homeostatic mechanisms and thereby introduce confounding factors. Using an optogenetic approach, our recent study showed an immediate causal relationship between adult-born neuron activity and difficult (but not easy), odor discrimination learning (32). These results indicate that the role of adult neurogenesis is extremely dependent on the behavioral task, as previously discussed (36). However, how newborn neurons exactly impact the functioning of preexisting circuits is still unclear (10, 37).
Adding further complexity to this panel of adult neurogenesis-dependent behavioral responses, the present investigation describes a role of adult-born neurons in odor–reward association. In our experiments, we found that adult-born neurons activation can boost olfactory learning but only when it occurs during rewarded odor presentations. Interestingly, we observed that facilitated learning produced by photostimulation during S+ presentations did not modify the percentage of hits, but it was reflected by an accelerated appearance of CRs during S− trials (Fig. S4). A possible explanation of this apparent paradox may be the switch from programmed go to no-go responses observed in this task. During pretraining sessions, animals learn to exhibit by default a go response to obtain a reward. However, once odor stimuli have been presented during the discrimination task, the mismatch detected between S− presentation and reward outcome triggers a consolidation of a go response only for the rewarded odor. As a result, at criterion level, S+ became the key signal, and most of the animals exhibited by default a no-go response for any other stimulus, as demonstrated with our odor-replacement experiment (Fig. S1B). We therefore hypothesize that adult-born neurons improve learning by facilitating the association of S+ with the reward, a process required to change the default response. These results support the idea that adult-born GCs mostly participate in the associative learning process per se and not only in the ability to identify and distinguish between the two different odorants.
Although adult neurogenesis produces both GCs and PGCs, our present results indicate that PGCs are not involved in odor–reward coding. As photostimulation of ChR2-positive neurons led to selective activation of GCs (32), future experiments will be required to elucidate the specific role of PGCs on associative learning.
Finally, our gain-of-function approach reveals a role of adult-born GCs in odor–reward association, but inhibition of GC activity would be essential to investigate their necessity for this process. An inducible genetic approach for loss-of-function analysis will be required to target a large number of GCs. In addition, an inhibitory opsin with higher conductance and a low-intensity level for activation will be critical to reach deeper regions of the OB using fiber optics or noninvasive illumination (38).
Different Functions for Newly Generated and Preexisting GCs.
Recent studies demonstrated that predominant manipulation of preexisting GCs alters innate odor attractiveness (3), and their inactivation disrupts responses to innately aversive odors (26). These experiments therefore support the notion that preexisting GCs are typically involved in innate responses to odors. On the other hand, our present findings unveil the unexpected role of adult-born neurons, but not neonatal-born neurons, in attributing early positive value to odorants that do not elicit reliable innate attraction or avoidance behavior (2). Given this context, we propose that adult-born GCs could be more prone to participate in adaptive odor responses, while their preexisting counterparts would be dedicated to innate behaviors.
How can these functional differences between the two GC populations be explained? Several studies have revealed distinct functional properties between adult-born and neonatal-born neurons. For instance, young adult-born OB interneurons have been reported to be more excitable than preexisting ones (16) and to undergo different experience-dependent synaptic modifications (17) before losing these features once mature. In addition, adult-generated neurons respond differently to novel odors (20) and are more prone to cell death compared with their neonatal counterparts (21). In our study, newly generated GCs were more than 10 wk old at the time the mice learned the task; therefore they had already reached their final excitability (39). Interestingly, it has been shown that OB adult-born neurons undergo experience-dependent plasticity long after maturation and integration, suggesting that the potential time window for plasticity of these neurons extends well into maturity (14, 19). In the same line, we recently showed matching, robust structural plasticity in GCs and M/TCs of the OB, and computational modeling demonstrated this plasticity to be compatible with stable memory (15). Finally, we have shown that mature adult-born neurons express unique features in their synaptic outputs that are distinctly sensitive to GABAB receptor modulation, leading to their escape from autoinhibition (18). This last property could explain in part why photoactivation of adult-born neurons is more effective in accelerating learning than activation of preexisting ones.
Top-Down Control of GC Activity.
To be efficient, sensory processing requires a combination of external sensory inputs and top-down feedback provided by higher-order brain areas, which assign meaning to the sensory cue. In the olfactory system, the OB is not a mere relay for olfactory information. In addition to receiving sensory inputs from the olfactory epithelium, it receives numerous centrifugal inputs from different brain areas, such as regions of the olfactory cortex and limbic and neuromodulatory subcortical regions (10). GCs are therefore perfectly located to integrate both sensory and top-down information, adapting sensory processing to the behavioral and/or internal context, which may include valence, sleep/arousal, stress, nutrition status, attention, and motivation. Coarse lesioning of centrifugal afferents to the OB impairs the animal’s ability to perform reward-associated olfactory discrimination tasks or perceptual learning (40). Along these lines, we recently found that olfactory associative learning promotes a selective synaptic plasticity of cortico-bulbar inputs to young adult-born GCs, resulting in the strengthening of top-down inputs (13). Future experiments will be required to decipher how top-down inputs could affect adult-born neuron activity during the olfactory learning process. In addition to the already identified discrepancies between the two GC subpopulations, the possibility of a unique functional connectivity within the OB circuit and/or with higher brain regions remains to be explored.
Relevance of Coding Odor Value at Early Stages of Information Processing.
The associative features of reward outcome and the expectation following learning occur at early stages in several primary sensory cortices (41–43). In the olfactory system, reward-outcome encoding has been characterized in different regions (4–7). Our present results show that odor value information is present in GC interneurons, specifically in those produced in the adult brain. Remarkably, light-induced activity of adult-born GCs, without odor stimulus, is enough to trigger reward-motivated behavior in a few blocks for a subgroup of animals. The difficulty with using GC photoactivation as the only stimulus could explain why only some animals are able to learn this task. Because mere adult-born GC activation is able to drive behavioral responses, we propose that adult-born GCs could target spontaneous M/TC activity to generate relevant “virtual olfactory information.” Then this information, instead of odor-evoked signals, could be conveyed to the cortex. We demonstrated previously that in vivo GC 40-Hz photoactivation shapes spontaneous M/TC responses in favor of highly active M/TCs, thus enhancing the contrast between M/TC firing rates (32). Here, as no odor stimulus was present, we hypothesized that M/TC spontaneous activity might be adjusted by anticipatory information, potentially mediated by top-down fibers. This anticipatory activity may thus help to produce regularity of the virtual olfactory information throughout learning. However, intertrial variability of this anticipatory information could also be one of the reasons why this learning is extremely difficult. Additionally, even if odor stimuli could be replaced in some cases by GC photoactivation to learn this task, the absence of reward cannot be replaced by light stimulation, indicating that GC activation is not itself rewarding and that a real reward is necessary to trigger this behavior (Fig. S7). Further experiments will be necessary to decipher which features of GC activity acting on M/TCs are used by downstream brain structures to trigger an adequate reward-motivated behavior.
Reward-outcome encoding in adult-born GCs, probably mediated by top-down inputs, might improve stimulus selection and information extraction, two processes required to cope with ambiguous sensory inputs. As proposed in other sensory modalities, odor-reward coding in adult-born neurons might change the state of the OB circuit to achieve rapid processing of olfactory stimuli during learning. We and others have previously demonstrated that GC activation shapes M/TC firing, increasing the signal-to-noise ratio (32, 34). Furthermore, adult-born neurons might play a role in supporting coincident activity detection necessary to associate sensory inputs with reward values. Although odor and reward are never coincident in our task, both M/TC and GC activity could be modulated in the long term by local and/or external inputs (44, 45). Recent results suggest the presence of strong sensory-evoked feedback projections from the anterior olfactory nucleus to the OB that mediate different brain states (46). We hypothesize that preferential control of adult-born neurons by the top-down fibers carrying information about reward outcome could explain the differences between GC subpopulations (Fig. S10). The odor-activated GCs might be endowed with a sensory experience-dependent tag that could be potentiated by top-down fibers containing reward information. As mentioned before, long-lasting GC activity could be the potential neural substrate of this tag. For instance, glomerulus-specific long-term potentiation (LTP) at GABAergic GC-M/TC synapses, requiring activity-dependent IGF1 signaling, was recently described to be essential for social memory formation in the OB (47). Alternatively, LTP at the synapses between centrifugal top-down fibers and adult-born GCs could be a potential mechanism capturing this sensory experience-dependent tag (13, 17).
As was classically described for basal ganglia/midbrain dopamine neurons (48), after the association between a sensory cue and the presence of a reward, reward-predicting cues become capable of triggering reward expectation. These reward signals in the adult-born GC population may be boosted by photoactivation, thereby increasing the likelihood of top-down–mediated synaptic plasticity specifically in these neurons. Further characterization of reward-signaling dynamics in the OB during the learning process as well as the top-down circuits implicated in their generation will be required to fully understand the circuit basis of olfactory-associative learning.
Materials and Methods
Animals.
The mice used in this study were young adult (>2 mo old, n = 160) C57BL/6JRj males, except for those injected at P6, which were either male (n = 58) or female (n = 55). There was no significant difference between males and females in learning performance (first initiation task, group: F1,80 = 0.33, P = 0.57, repeated-measures two-way ANOVA, n = 41). Mice were housed under a 12-h light/dark cycle with dry food and water available ad libitum except during behavioral experiments. All procedures were consistent with the European Communities Council Directive of 22 September 2010 (2010/63/EU) and the European Union guidelines and were reviewed and approved by the Animal Welfare Committee of Institut Pasteur. We used the minimum number of animals, estimated from our previous knowledge in performing the same type of experiments.
Lentiviral Vectors and Stereotaxic Injections.
LV-ChR2(H134R)-YFP was injected into the RMS as described previously (32).
Behavior, in Vivo Light Stimulation, and Odorants.
Olfactory stimulation was performed as described previously (32). Odor-exposure sessions were conducted in modified olfactometers. The difficult pairs of odorants and the dilutions used in the experiments were 0.6% ethyl butyrate + 0.4% amyl acetate vs. 0.4% ethyl butyrate + 0.6% amyl acetate (P1) and 1% (+)-limonene vs. 1% (−)-limonene (P2).
BrdU Injection, Immunohistochemistry, and Image Analysis.
Optimal detection and quantification of c-Fos+/BrdU+ cells were obtained as described previously (32). See SI Materials and Methods for more detailed information.
Supplementary Material
Acknowledgments
We thank Gabriel Lepousez and Kurt Sailor for helpful discussions, Aleksandra Polosukhina and Christoph Schmidt-Hieber for critical reading of the manuscript, and Natacha Menezes, Romane Manceau, and Benoit Theuil for assistance with preliminary behavioral experiments. This work was supported by Agence Nationale de la Recherche Grants ANR-15-CE37-0004-01 “SmellBrain” and ANR-15-NEUC-0004-02 “Circuit-OPL,” Laboratory for Excellence Programme “Revive” Grant ANR-10-LABX-73, and the Life Insurance Company AG2R-La Mondiale.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All relevant data are available from Zenodo, https://doi.org/10.5281/zenodo.1173612.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716400115/-/DCSupplemental.
References
- 1.Li Q, Liberles SD. Review aversion and attraction through olfaction. Curr Biol. 2015;25:120–129. doi: 10.1016/j.cub.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Root CM, Denny CA, Hen R, Axel R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515:269–273. doi: 10.1038/nature13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kermen F, et al. Topographical representation of odor hedonics in the olfactory bulb. Nat Neurosci. 2016;19:876–878. doi: 10.1038/nn.4317. [DOI] [PubMed] [Google Scholar]
- 4.Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:643–652. doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calu DJ, Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in posterior piriform cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:1342–1349. doi: 10.1093/cercor/bhl045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gire DH, Whitesell JD, Doucette W, Restrepo D. Information for decision-making and stimulus identification is multiplexed in sensory cortex. Nat Neurosci. 2013;16:991–993. doi: 10.1038/nn.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadziola MA, Tylicki KA, Christian DL, Wesson DW. The olfactory tubercle encodes odor valence in behaving mice. J Neurosci. 2015;35:4515–4527. doi: 10.1523/JNEUROSCI.4750-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doucette W, et al. Associative cortex features in the first olfactory brain relay station. Neuron. 2011;69:1176–1187. doi: 10.1016/j.neuron.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepousez G, Valley MT, Lledo P-M. The impact of adult neurogenesis on olfactory bulb circuits and computations. Annu Rev Physiol. 2013;75:339–363. doi: 10.1146/annurev-physiol-030212-183731. [DOI] [PubMed] [Google Scholar]
- 10.Lepousez G, Nissant A, Lledo P-M. Adult neurogenesis and the future of the rejuvenating brain circuits. Neuron. 2015;86:387–401. doi: 10.1016/j.neuron.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits. Neuron. 2012;76:1161–1174. doi: 10.1016/j.neuron.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76:1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepousez G, et al. Olfactory learning promotes input-specific synaptic plasticity in adult-born neurons. Proc Natl Acad Sci USA. 2014;111:13984–13989. doi: 10.1073/pnas.1404991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livneh Y, Mizrahi A. Experience-dependent plasticity of mature adult-born neurons. Nat Neurosci. 2011;15:26–28. doi: 10.1038/nn.2980. [DOI] [PubMed] [Google Scholar]
- 15.Sailor KA, et al. Persistent structural plasticity optimizes sensory information processing in the olfactory bulb. Neuron. 2016;91:384–396. doi: 10.1016/j.neuron.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo P-M. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 17.Nissant A, Bardy C, Katagiri H, Murray K, Lledo P-M. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 18.Valley MT, Henderson LG, Inverso SA, Lledo P-M. Adult neurogenesis produces neurons with unique GABAergic synapses in the olfactory bulb. J Neurosci. 2013;33:14660–14665. doi: 10.1523/JNEUROSCI.2845-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breton-Provencher V, et al. Principal cell activity induces spine relocation of adult-born interneurons in the olfactory bulb. Nat Commun. 2016;7:12659. doi: 10.1038/ncomms12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magavi SS, Mitchell BD, Szentirmai O, Carter BS, Macklis JD. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci. 2005;25:10729–10739. doi: 10.1523/JNEUROSCI.2250-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemasson M, Saghatelyan A, Olivo-Marin J-C, Lledo P-M. Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J Neurosci. 2005;25:6816–6825. doi: 10.1523/JNEUROSCI.1114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto M, et al. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc Natl Acad Sci USA. 2011;108:8479–8484. doi: 10.1073/pnas.1018782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrett L, et al. Conditional reduction of adult born doublecortin-positive neurons reversibly impairs selective behaviors. Front Behav Neurosci. 2015;9:302. doi: 10.3389/fnbeh.2015.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muthusamy N, Zhang X, Johnson CA, Yadav PN, Ghashghaei HT. Developmentally defined forebrain circuits regulate appetitive and aversive olfactory learning. Nat Neurosci. 2017;20:20–23. doi: 10.1038/nn.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno MM, et al. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci USA. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochefort C, Gheusi G, Vincent J-D, Lledo P-M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarini F, et al. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4:e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sultan S, et al. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 2010;24:2355–2363. doi: 10.1096/fj.09-151456. [DOI] [PubMed] [Google Scholar]
- 32.Alonso M, et al. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci. 2012;15:897–904. doi: 10.1038/nn.3108. [DOI] [PubMed] [Google Scholar]
- 33.Arruda-Carvalho M, et al. Posttraining ablation of adult-generated olfactory granule cells degrades odor-reward memories. J Neurosci. 2014;34:15793–15803. doi: 10.1523/JNEUROSCI.2336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gschwend O, et al. Neuronal pattern separation in the olfactory bulb improves odor discrimination learning. Nat Neurosci. 2015;18:1474–1482. doi: 10.1038/nn.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 36.Lazarini F, Lledo P-M. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34:20–30. doi: 10.1016/j.tins.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Sailor KA, Schinder AF, Lledo P-M. Adult neurogenesis beyond the niche: Its potential for driving brain plasticity. Curr Opin Neurobiol. 2017;42:111–117. doi: 10.1016/j.conb.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O. Silencing neurons: Tools, applications, and experimental constraints. Neuron. 2017;95:504–529. doi: 10.1016/j.neuron.2017.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardy C, Alonso M, Bouthour W, Lledo P-M. How, when, and where new inhibitory neurons release neurotransmitters in the adult olfactory bulb. J Neurosci. 2010;30:17023–17034. doi: 10.1523/JNEUROSCI.4543-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiselycznyk CL, Zhang S, Linster C. Role of centrifugal projections to the olfactory bulb in olfactory processing. Learn Mem. 2006;13:575–579. doi: 10.1101/lm.285706. [DOI] [PubMed] [Google Scholar]
- 41.Samuelsen CL, Gardner MP, Fontanini A. Effects of cue-triggered expectation on cortical processing of taste. Neuron. 2012;74:410–422. doi: 10.1016/j.neuron.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaramillo S, Zador AM. The auditory cortex mediates the perceptual effects of acoustic temporal expectation. Nat Neurosci. 2011;14:246–251. doi: 10.1038/nn.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiffer AM, Muller T, Yeung N, Waszak F. Reward activates stimulus-specific and task-dependent representations in visual association cortices. J Neurosci. 2014;34:15610–15620. doi: 10.1523/JNEUROSCI.1640-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoor V, Urban NN. Glomerulus-specific, long-latency activity in the olfactory bulb granule cell network. J Neurosci. 2006;26:11709–11719. doi: 10.1523/JNEUROSCI.3371-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cazakoff BN, Lau BY, Crump KL, Demmer HS, Shea SD. Broadly tuned and respiration-independent inhibition in the olfactory bulb of awake mice. Nat Neurosci. 2014;17:569–576. doi: 10.1038/nn.3669. [DOI] [PubMed] [Google Scholar]
- 46.Rothermel M, Wachowiak M. Functional imaging of cortical feedback projections to the olfactory bulb. Front Neural Circuits. 2014;8:73. doi: 10.3389/fncir.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, et al. IGF1-dependent synaptic plasticity of mitral cells in olfactory memory during social learning. Neuron. 2017;95:106–122.e5. doi: 10.1016/j.neuron.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.