Significance
Neuregulin3 (NRG3) is a growth factor of the neuregulin (NRG) family and a risk gene of various severe mental illnesses. However, the physiological function of NRG3 remains poorly understood. We showed that, unlike the prototype Nrg1, the effect of which is mediated by activating ErbB4 in interneurons, Nrg3 acts in a cell-autonomous manner in pyramidal neurons. By using a combination of genetic, electrophysiological, biochemical, and molecular biological techniques, we uncovered a function of Nrg3—to regulate glutamate release—and identified Nrg3 as a regulator of the assembly of the SNARE complex. We demonstrate that increasing Nrg3 expression, mimicking higher levels in mental illness patients, impairs glutamate release, revealing a pathophysiological mechanism of hypofunction of glutamatergic function.
Keywords: Nrg3, glutamatergic transmission, SNARE complex, severe mental illness
Abstract
Neuregulin3 (NRG3) is a growth factor of the neuregulin (NRG) family and a risk gene of various severe mental illnesses including schizophrenia, bipolar disorders, and major depression. However, the physiological function of NRG3 remains poorly understood. Here we show that loss of Nrg3 in GFAP-Nrg3f/f mice increased glutamatergic transmission, but had no effect on GABAergic transmission. These phenotypes were observed in Nex-Nrg3f/f mice, where Nrg3 was specifically knocked out in pyramidal neurons, indicating that Nrg3 regulates glutamatergic transmission by a cell-autonomous mechanism. Consequently, in the absence of Nrg3 in pyramidal neurons, mutant mice displayed various behavioral deficits related to mental illnesses. We show that the Nrg3 mutation decreased paired-pulse facilitation, increased decay of NMDAR currents when treated with MK801, and increased minimal stimulation-elicited response, providing evidence that the Nrg3 mutation increases glutamate release probability. Notably, Nrg3 is a presynaptic protein that regulates the SNARE-complex assembly. Finally, increased Nrg3 levels, as observed in patients with severe mental illnesses, suppressed glutamatergic transmission. Together, these observations indicate that, unlike the prototype Nrg1, the effect of which is mediated by activating ErbB4 in interneurons, Nrg3 is critical in controlling glutamatergic transmission by regulating the SNARE complex at the presynaptic terminals, identifying a function of Nrg3 and revealing a pathophysiological mechanism for hypofunction of the glutamatergic pathway in Nrg3-related severe mental illnesses.
Severe mental illnesses including schizophrenia, bipolar disorders, and major depressive disorders affect ∼4% of the adult population in the United States. Patients with severe mental illness suffer psychotic symptoms, negative symptoms, mood alterations, and cognitive deficits. The treatment of severe mental illnesses is far from satisfactory, in part due to limited understanding of underlying pathological mechanisms. Nevertheless, altered activity of glutamatergic and GABAergic pathways has been implicated in severe mental illnesses (1–4).
Neuregulins are a family of EGF domain-containing growth factors that are broadly expressed in the nervous system (5–7). They are encoded by six different genes (NRG1–6) (8), among which Nrg1 is the best characterized. Evidence suggests that Nrg1 and ErbB4 play a critical role in neural development, in particular in GABAergic circuit assembly in the central nervous system, and that Nrg1 and ErbB2/3 promote myelination of peripheral axons (8).
Despite the fact that Nrg3 is highly expressed in the brain (9), much less is known about its function. Nevertheless, single-nucleotide polymorphisms (SNPs) in the NRG3 gene have been associated with several severe illnesses including schizophrenia and mood disorders like depression and bipolar disorder (10–12). The NRG3 SNPs may impact on its expression in the human brain because the mRNA level of NRG3 is increased in patients with schizophrenia and mood disorders (13, 14). Nrg3 null mutant mice display hyperactivity, impaired prepulse inhibition (PPI), deficient fear conditioning, and decreased impulsivity (15, 16). Developmental overexposure of mice to Nrg3 causes an anxiogenic-like phenotype and deficits in social behavior in adulthood (17). Increasing the Nrg3 level in the medial prefrontal cortex enhances the impulsivity (15). However, underlying pathophysiological mechanisms of the Nrg3 mutation or overexpression remain poorly understood. Nrg3 has been implicated in interneuron migration and laminar distribution (18). In vitro studies suggest that Nrg3 may promote neuronal survival, neuritic elongation, and oligodendrocyte survival (19, 20). However, the mechanism of action of Nrg3 is unclear.
In this study, we demonstrated that Nrg3 expression is developmentally regulated and is specific in pyramidal neurons. We discovered an increase in the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in Nrg3 mutant mice. To investigate the underlying cellular mechanisms, we characterized the effect of the Nrg3 mutation on the paired-pulse ratio, MK801-sensitive currents mediated by NMDA receptors, and responses of pyramidal neurons to minimal stimulation. We characterized the subcellular expression of Nrg3 and studied molecular mechanisms of Nrg3 in regulating glutamate release. Together, our results demonstrate that Nrg3 is a presynaptic protein that maintains the soluble NSF attachment protein receptor (SNARE) complex, a complex critical for glutamate release (21, 22), thus identifying a function of Nrg3. Increasing Nrg3 expression, mimicking higher levels in mental illness patients (13, 14), impairs glutamate release, revealing a pathophysiological mechanism of hypofunction of glutamatergic transmission.
Results
Increased sEPSC Frequency by GFAP-Mediated Nrg3 Mutation.
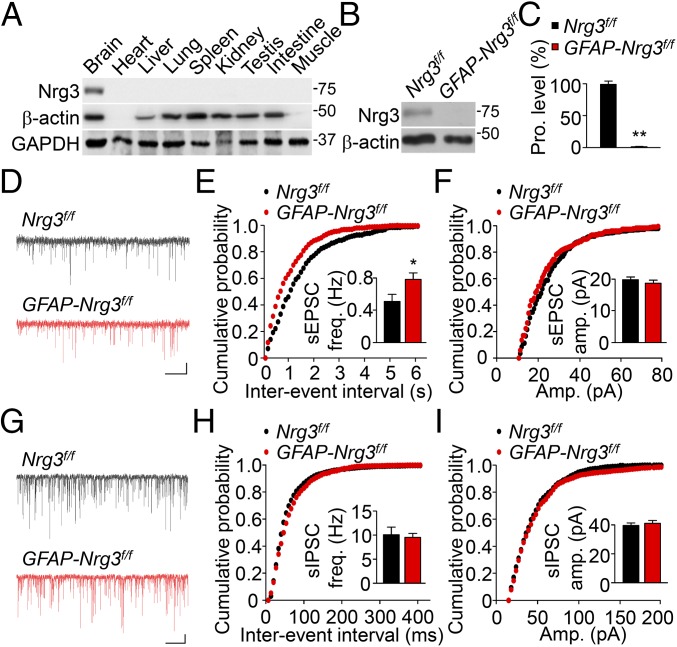
Using an antibody against Nrg3’s EGF domain, we detected a single band at the predicted molecular weight (∼75 kDa) in brain homogenates (Fig. 1A and Fig. S1A). Its intensity was diminished by the Nrg3 mutation, indicating the specificity of the antibody (Fig. 1B). Nrg3 expression was brain-specific (Fig. 1A), in agreement with its mRNA pattern (9). In the brain, Nrg3 was at higher levels in the hippocampus and cortex and at lower levels in the cerebellum (Fig. S1 B and C). Nrg3 in the cortex and hippocampus increased during development and peaked at postnatal day 10 (P10) (Fig. S1 D–F).
Fig. 1.
Increased spontaneous EPSC frequency of CA1 pyramidal neurons in GFAP-Nrg3f/f mice. (A) Specific Nrg3 expression in the brain. Homogenates of indicated organs or tissues were subjected to Western blotting with the anti-Nrg3 antibody. β-Actin and GAPDH served as loading controls. (B) Ablation of Nrg3 in GFAP-Nrg3f/f mice. Homogenates of hippocampus and cortex were subjected to Western blotting. (C) Quantitative analysis of data in B. n = 3 mice per genotype; t test. (D) Representative traces of sEPSCs in CA1 pyramidal neurons. (Scale bars: 2 s, 10 pA.) (E and F) Cumulative probability plots of sEPSC interevent intervals (E) and amplitudes (F). (Insets) A summary of frequency and amplitudes. n = 18 neurons of four Nrg3f/f mice and n = 17 neurons of four GFAP-Nrg3f/f mice; t test. (G) Representative traces of sIPSCs in CA1 pyramidal neurons. (Scale bars: 2 s, 20 pA.) (H and I) Cumulative probability plots of sIPSCs interevent intervals (H) and amplitudes (I). (Insets) A summary of frequency and amplitudes. n = 13 neurons of four Nrg3f/f mice and n = 13 neurons of four GFAP-Nrg3f/f mice; t test. *P < 0.05; **P < 0.01.
To study the function of Nrg3, we generated a Nrg3-floxed mouse (Nrg3f/f) (Fig. S2A). Nrg3f/f mice were crossed with hGFAP::Cre mice where Cre is expressed in neural progenitor cells at embryonic day 13.5 (E13.5) and in all forebrain neurons and astrocytes (23). Resulting hGFAP::Cre; Nrg3f/f (hereafter referred to as GFAP-Nrg3f/f) mice did not express Nrg3 in the brain (Fig. 1 B and C). Because Nrg3 mRNA was detectable as early as at E14 (16), we determined whether the Nrg3 mutation alters neural development. As shown in Fig. S3A, GFAP-Nrg3f/f mice displayed a similar brain weight to that of control (Nrg3f/f) mice and showed no global morphologic deficits (Fig. S3B). The number of neurons in different layers of the somatosensory cortex was similar between control and mutant mice (Fig. S3 C and D). The mutant mice had a similar number of spines in basal dendrites of hippocampal CA1 neurons (Fig. S3 E and F).
To determine whether the Nrg3 mutation alters neurotransmission in the brain, we first measured sEPSCs in CA1 pyramidal neurons. Notably, GFAP-Nrg3f/f slices showed an increase in sEPSC frequency, compared with control slices (Fig. 1 D–F). However, there was no change in sEPSC amplitude in GFAP-Nrg3f/f neurons. Next, we measured spontaneous inhibitory postsynaptic currents (sIPSCs) in CA1 neurons. As shown in Fig. 1 G–I, sIPSC frequency and amplitude were similar between GFAP-Nrg3f/f and control slices. Together, these results indicate that the Nrg3 mutation specifically increases sEPSC frequency, but not amplitude, and has no effect on GABA transmission onto CA1 pyramidal neurons.
Enhanced Glutamatergic Transmission by the Nrg3 Mutation in Pyramidal Neurons.
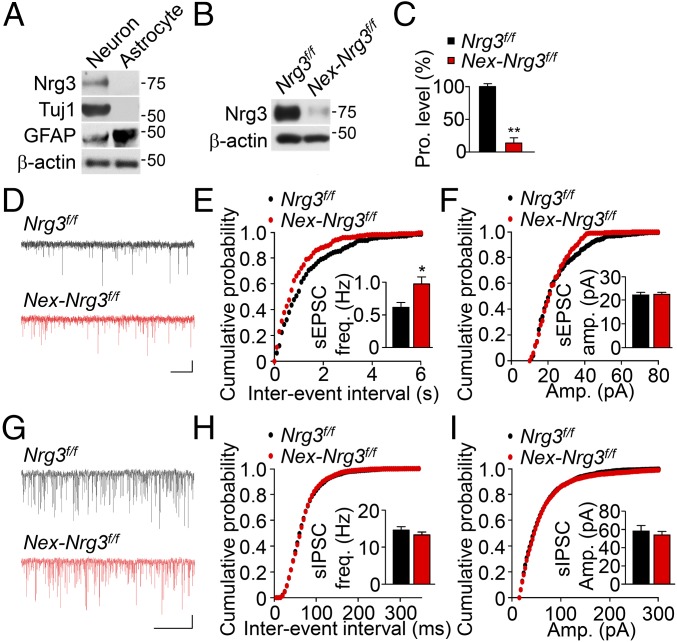
Because the hGFAP promoter is expressed in both neurons and astrocytes (23), it was unclear whether the enhanced glutamatergic transmission is due to the Nrg3 mutation in astrocytes or neurons. Astrocytes have been shown to regulate glutamatergic transmission (24). To this end, we cultured neurons and astrocytes from mouse embryonic brain. As shown in Fig. 2A, Nrg3 was abundantly expressed in cultured neurons, but almost undetectable in astrocytes. This result suggests that Nrg3’s effect is cell-autonomous, that is, that Nrg3 in pyramidal neurons regulates glutamatergic transmission. To test this hypothesis, we crossed Nrg3f/f mice with Nex::Cre mice where Cre is expressed exclusively in pyramidal neurons, not in interneurons or astrocytes (25). Nrg3 levels in the hippocampus and cortex of Nex::Cre; Nrg3f/f (Nex-Nrg3f/f) mice were reduced to 13% of control (Fig. 2 B and C), indicating that Nrg3 is expressed mainly in pyramidal neurons, in agreement with a recent report (18).
Fig. 2.
Increased sEPSC frequency of CA1 pyramidal neurons in Nex-Nrg3f/f mice. (A) Expression of Nrg3 in neurons, but not astrocytes. Tuj1 served as a neuronal marker and GFAP served as an astrocyte marker. (B) Ablation of Nrg3 in Nex-Nrg3f/f mice. (C) Quantitative analysis of data in B. n = 4 mice per genotype; t test. (D) Representative traces of sEPSCs in CA1 pyramidal neurons. (Scale bars: 2 s, 10 pA.) (E and F) Cumulative probability plots of sEPSC interevent intervals (E) and amplitudes (F). (Insets) A summary of frequency and amplitudes. n = 16 neurons of four Nrg3f/f mice and n = 15 neurons of four Nex-Nrg3f/f mice; t test. (G) Representative traces of sIPSCs in CA1 pyramidal neurons. (Scale bars: 2 s, 20 pA.) (H and I) Cumulative probability plots of sIPSC interevent intervals (H) and amplitudes (I). (Insets) Summary of frequency and amplitudes. n = 14 neurons of four Nrg3f/f mice and n = 13 neurons of four Nex-Nrg3f/f mice; t test. *P < 0.05; **P < 0.01.
Our hypothesis predicts that Nex-Nrg3f/f mice should display similar phenotypes observed in GFAP-Nrg3f/f mice. Indeed, Nex-Nrg3f/f slices showed an increase in sEPSC frequency (Fig. 2 D–F), but not amplitude. In addition, sIPSC frequency and amplitude were similar between control and Nex-Nrg3f/f mice (Fig. 2 G–I). These results demonstrate that the Nrg3 mutation specifically in pyramidal neurons has a similar effect on glutamatergic transmission, suggesting a cell-autonomous regulatory mechanism. As observed in GFAP-Nrg3f/f mice, the Nrg3 mutation by Nex-Cre had no effect on brain weight (Fig. S4A) or laminar structure and neuron numbers in the hippocampus and somatosensory cortex (Fig. S4 B–E).
Behavioral Deficits of Nex-Nrg3f/f Mice.
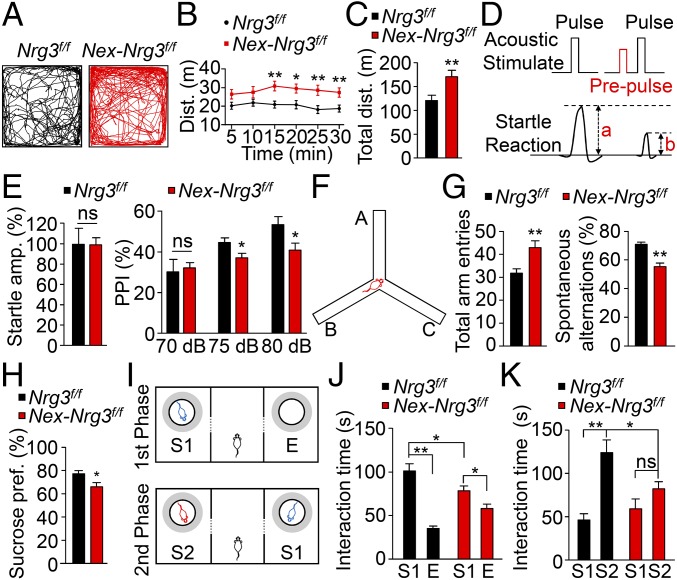
Abnormal locomotor activity and impaired PPI in rodents are thought to correspond to psychomotor agitations of schizophrenic patients (26, 27). Intriguingly, both hyperactivity and impaired PPI have been reported in Nrg3 null mice (15, 16). However, it was unclear in which neurons or cells Nrg3 ablation is sufficient to induce such a phenotype. Notably, Nex-Nrg3f/f mice displayed enhanced locomotor activity in the open field test, compared with control mice (Fig. 3 A–C). The mutant mice showed similar startle responses (Fig. 3D and Fig. 3E, Left), suggesting normal hearing and acoustic startle reflex. However, PPI was substantially lower in Nex-Nrg3f/f mice than in control mice (Fig. 3E, Right). Together, these observations indicate that Nrg3 in pyramidal neurons is necessary for normal locomotor activity and PPI. Mental illnesses such as schizophrenia usually show cognitive deficits including impaired working memory (28). In a Y-maze test (Fig. 3F), Nex-Nrg3f/f mice had more arm entries, compared with control mice, which was consistent with increased locomotor activity (Fig. 3G, Left). However, the numbers of spontaneous alterations were reduced in Nex-Nrg3f/f mice, compared with controls (Fig. 3G, Right), suggesting an impairment of spatial working memory.
Fig. 3.
Schizophrenia-associated behavioral deficits of Nrg3 conditional mutant mice. (A) Increased locomotor activity of Nex-Nrg3f/f mice. Representative traces of locomotor activity in first 5 min of open field test. (B and C) Increased distance at 5-min intervals over a 30-min test (B) and increased total distance traveled for 30 min (C); n = 17 Nrg3f/f mice and n = 15 Nex-Nrg3f/f mice; t test. (D) Diagram of PPI test. PPI (%) = 100 × (a − b)/a. (E) Similar baseline startle responses of Nrg3f/f mice and Nex-Nrg3f/f mice (Left) and impaired PPI in Nex-Nrg3f/f mice (Right); n = 11 per genotype; t test for baseline startle responses analysis and two-way ANOVA for PPI analysis. (F) Diagram of Y-maze test. (G) Increased total arm entries in Nex-Nrg3f/f mice (Left) and reduced spontaneous alternations in Nex-Nrg3f/f mice (Right). n = 14 from Nrg3f/f mice and n = 12 from Nex-Nrg3f/f mice; t test. (H) Reduced sucrose consumption in Nex-Nrg3f/f mice. n = 13 per genotype; t test. (I) Diagram of social interaction test. Upper and Lower illustrate the first and second phases, respectively. See SI Materials and Methods for details. Gray area indicates the interaction region. (J) There was reduced interaction time in Nex-Nrg3f/f mice, compared with Nrg3f/f mice, although both Nrg3f/f and Nex-Nrg3f/f mice spent more time with the S1 mouse, compared with the empty cage (E), which served as an inanimate object with no social valence; n = 12 per genotype; two-way ANOVA. (K) Inability to distinguish S1 and S2 mice by Nex-Nrg3f/f mice; n = 12 per genotype; two-way ANOVA. *P < 0.05; **P < 0.01. ns, no significant difference.
To determine whether the Nrg3 mutation causes behavioral deficits related to negative symptoms of mental illnesses, we conducted sucrose preference and social interaction tests (29, 30). As shown in Fig. 3H, sucrose consumption by Nex-Nrg3f/f mice was reduced, compared with Nrg3f/f mice. In the social interaction test, Nrg3 mutant mice were able to recognize the initial stranger mouse (S1), but the time spent with it was reduced, compared with control mice (Fig. 3 I and J). When a second stranger mouse (S2) was introduced, control mice spent more time with the latter (Fig. 3K). In contrast, Nex-Nrg3f/f mice spent relatively equal amounts of time with S1 and S2 mice, indicating an impairment in social interaction. Together, these behavioral studies demonstrate that Nrg3 in pyramidal neurons is critical for locomotor activity, working memory, and social interaction. In the absence of Nrg3 in pyramidal neurons, mice displayed schizophrenia-related behavioral deficits.
Higher Glutamate Release Probability in Nex-Nrg3f/f Mutants.
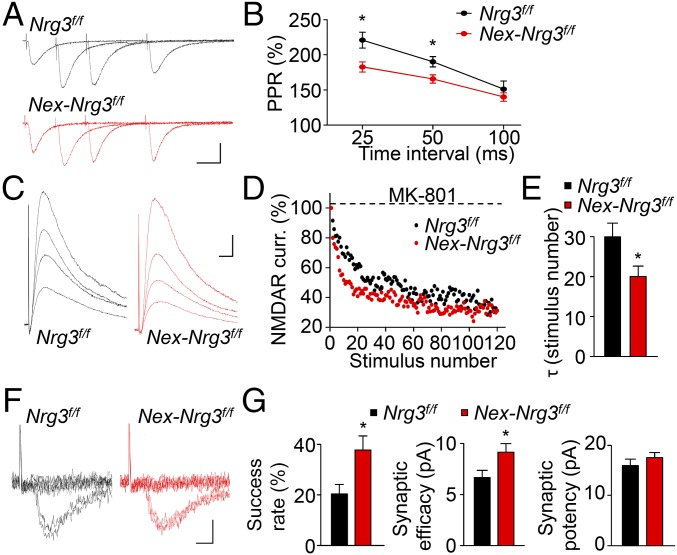
To investigate how the Nrg3 mutation alters glutamatergic transmission, we determined whether Nrg3 alters spine development. We crossed Nrg3f/f mice with Thy1-GFP (GFP-M line) mice (31) and examined GFP-labeled spines of basal dendrites of CA1 neurons. There was no difference in spine morphology or number (Fig. S5 A and B). In addition, the Nrg3 mutation did not alter the electrophysiological properties of pyramidal neurons such as resting membrane potentials and input resistance (Fig. S5 C and D) or firing frequency in response to injected currents (Fig. S5 E and F), indicating similar excitability. Next, we performed three sets of experiments to investigate how Nrg3 regulates glutamate release. First, when hippocampal CA1 pyramidal neurons are stimulated with two consecutive stimulations, the second stimulation generates larger evoked EPSC (eEPSC) because of residual calcium concentration of the first eEPSC (32). As shown in Fig. 4 A and B, paired-pulse ratios (PPRs) in Nex-Nrg3f/f slices were reduced, compared with those in control slices. Second, eEPSC amplitudes of CA1 pyramidal neurons in the presence of MK801 are progressively reduced during repetitive stimulation of the Schaffer collateral pathway (33). Notably, the usage-dependent decay of NMDAR currents was faster in Nex-Nrg3f/f slices than in control slices (Fig. 4 C–E). Finally, we characterized eEPSCs in CA1 pyramidal neurons in response to minimal stimulation (34). The rate of successful response was higher in Nex-Nrg3f/f mice, compared with control (Fig. 4F and Fig. 4G, Left). Accordingly, Nex-Nrg3f/f mice showed increased synaptic efficacy (amplitudes of failed and successful eEPSCs) (Fig. 4G, Middle), but no change in synaptic potency (amplitudes of successful eEPSCs) (Fig. 4G, Right). Together, these results indicate that sEPSC frequency increase in Nex-Nrg3f/f mice may be due to an increased probability of glutamate release.
Fig. 4.
Enhanced probability of glutamate release in Nex-Nrg3f/f mice. (A) Representative superimposed sweeps with three different interstimulus intervals of pair-pulse stimulations of indicated mice. (Scale bars: 20 ms, 100 pA.) (B) PPRs plotted against interstimulus intervals; n = nine neurons from three mice per genotype; two-way ANOVA. (C) Representative traces of NMDAR currents in the presence of MK-801. (Scale bars: 10 ms, 50 pA.) (D) Normalized NMDAR currents plotted against stimulus number. (E) Decreased τ values in Nex-Nrg3f/f mice; n = 8 neurons from three mice per genotype; t test. (F) Representative 10 successive individual sweeps of EPSCs evoked by minimal stimulation. (Scale bars: 5 ms, 10 pA.) (G) Increased success rate, synaptic efficacy, and no change in synaptic potency in Nex-Nrg3f/f mice; n = 9 neurons from three mice per genotype; t test. *P < 0.05.
Nrg3 Localization at Presynaptic Terminals and Regulation of the SNARE Complex.
Nrg3 was thought to act by stimulating ErbB4 (9). ErbB4 is specifically expressed in GABAergic neurons and is necessary for maintaining GABAergic transmission (35–38). GABA release is reduced in ErbB4 null mice and PV+ interneuron-specific mutant mice (37–39). However, the Nrg3 mutation had no effect on sIPSCs (Figs. 1 and 2), excluding a possible secondary effect of altered GABAergic transmission. Next, we determined the subcellular domain where Nrg3 is expressed by discontinuous sucrose density centrifugation. Nrg3 was enriched in the presynaptic fraction that was labeled by an anti-synaptophysin (SYP) antibody, not in the postsynaptic density (PSD) fraction that was labeled by anti-PSD95 antibody (Fig. 5A). This result indicates that Nrg3 is specifically localized in presynaptic terminals, consistent with a recent report (40). Next, we determined whether the Nrg3 mutation alters the levels of SNARE proteins that are critical for vesicle fusion (21, 22). As shown in Fig. S6 A and B, Nex-Nrg3f/f mice showed comparable levels of v-SNARE protein VAMP2; t-SNARE proteins (syntaxin1 and SNAP25); vesicle protein SYP; the calcium sensor synaptotagmin1 (SYT1); RIM1α, a protein critical for vesicle release; Sec1/Munc18-1 protein (Munc18-1 and Munc13-1); and Complexin 1/2 in both hippocampal homogenates and synaptosomes. This result suggests that the Nrg3 mutation does not change the levels of proteins key to synaptic vesicle fusion. In addition, the protein levels of APMA receptors (GluA1 and GluA2) and NMDA receptors (GluN1, GluN2A, and GluN2B) were not changed in both hippocampal homogenates and the PSD fraction between Nex-Nrg3f/f mice and Nrg3f/f mice (Fig. S6 C and D).
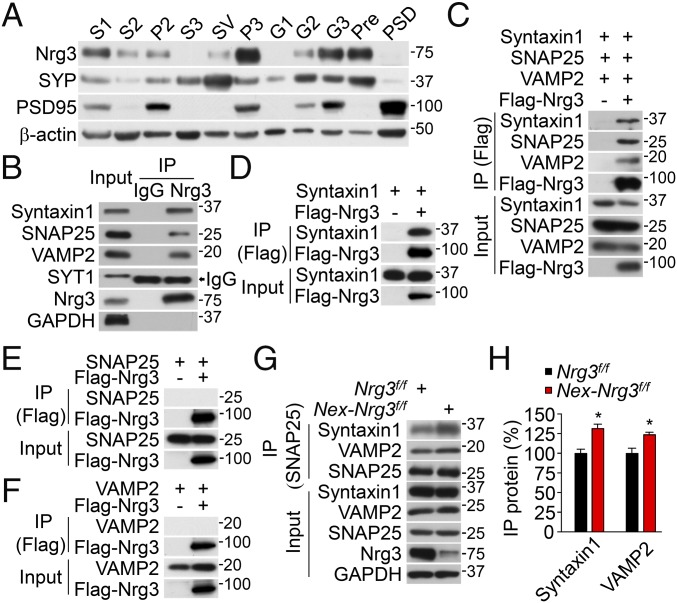
Fig. 5.
Nrg3 expression in presynaptic fractions and interaction with syntaxin1. (A) Enriched expression of Nrg3 in the presynaptic fraction. Aliquots of subcellular fractions (SI Materials and Methods) were probed for Nrg3, PSD95, SYP, and β-actin. (B) Association of Nrg3 with the in vivo SNARE complex. Presynaptic fractions were incubated with mouse anti-Nrg3 antibody or mouse IgG (as control), and the resulting immunocomplex was probed with antibodies against Nrg3, syntaxin1, SNAP25, VAMP2, synaptotagmin (SYT1), and GAPDH. Arrow indicates the heavy chain of mouse IgG used in co-IP, which migrated close to the band of SYT1. (C) Nrg3 association with the SNARE complex in HEK293T cells. Cells were transfected with syntaxin1, SNAP25, VAMP2, and Flag-Nrg3. Resulting complexes were probed with antibodies to Flag, syntaxin1, SNAP25, and VAMP2. (D–F) Nrg3 interaction with synatxin1, but not SNAP25 or VAMP2. Resulting complexes were probed for Flag and syntaxin1 (D), SNAP25 (E), or VAMP2 (F). (G) Increased association of SNAP25 with SNARE proteins in Nex-Nrg3f/f mice, compared with Nrg3f/f mice. Hippocampus lysates were incubated with mouse SNAP25 antibody, and resulting immunoprecipitates were probed for indicated SNARE proteins. (H) Quantitative analysis of data in G; n = 3 independent experiments; t test; *P < 0.05.
We reasoned that, being strategically localized at nerve terminals, Nrg3 may interact with a SNARE protein(s). We precipitated Nrg3 from the presynaptic fraction of hippocampus with the anti-Nrg3 antibody. Interestingly, both v-SNARE and t-SNARE proteins were coprecipitated with Nrg3, suggesting a potential interaction (Fig. 5B). This interaction was specific because SNARE proteins were not detectable in the complex of control IgG. Moreover, SYT1 was not detectable in the Nrg3 complex. To determine which SNARE protein interacts with Nrg3, HEK293T cells were transfected with Flag-tagged Nrg3 and SNARE proteins (syntaxin1, SNAP25, and VAMP2). As shown in Fig. 5C, Flag-Nrg3 could coprecipitate with syntaxin1, SNAP25, and VAMP2, which further confirmed the in vivo interaction. Further experiments with HEK293T cells transfected with individual SNARE proteins demonstrate that Nrg3 was able to coprecipitate syntaxin1 (Fig. 5D), but not SNAP25 or VAMP2 (Fig. 5 E and F, respectively). Syntaxin1 has three domains: Habc, SNARE, and transmembrane (41). As shown in Fig. S7 A and B, deletion of the SNARE domain prevented syntaxin1 from interacting with Nrg3. Together, these results indicate that Nrg3 interacts with the SNARE complex in vivo, likely by interacting with the SNARE domain of syntaxin1. The SNARE-complex assembly is critical for vesicle release (21, 22, 42). Considering increased glutamate release in Nrg3 mutant mice, we posit that Nrg3 may regulate the assembly of the SNARE complex. To this end, we precipitated SNAP25 from hippocampal homogenates and probed for syntaxin1 and VAMP2. As shown in Fig. 5 G and H, the amount of syntaxin1 and VAMP2 coprecipitated with SNAP25 was increased in Nex-Nrg3f/f mice, compared with Nrg3f/f mice. This result demonstrates an enhanced interaction of SNAP25 with syntaxin1 and VAMP2 in Nex-Nrg3f/f mice and suggests that Nrg3 may maintain the SNARE-complex assembly. The enhanced interaction of SNAP25 with synatxin1 and VAMP2 was further strengthened by treating neurons with KCl, indicating a role of neuronal activity (Fig. S7 C and D).
Impaired Glutamate Release by Increasing Nrg3.
Nrg3 levels were higher in patients with schizophrenia (13, 14) although Nrg3’s pathogenic mechanisms were unclear. Therefore, we studied the effects of elevating Nrg3 levels on glutamatergic transmission. Neurons were cultured from the hippocampus of mouse embryos and recorded for sEPSCs. Remarkably, when control (Nrg3f/f) neurons were infected with lentivirus that overexpressed Nrg3, sEPSC frequency was reduced, indicating that elevating Nrg3 suppresses glutamate release. On the other hand, compared with Nrg3f/f neurons, sEPSC frequency, but not amplitude, was increased in Nex-Nrg3f/f neurons (Fig. 6 A–C), in agreement with results from slice recordings (Figs. 1 and 2). This increase was diminished in Nex-Nrg3f/f neurons that were infected by lentivirus expressing Nrg3, demonstrating that restoring Nrg3 would rescue the phenotypes of increased glutamate transmission. Together with results from slice recordings (Figs. 1 and 2), these data demonstrate that Nrg3 controls glutamate release, suggesting that it has to be maintained at a proper level at nerve terminals for glutamate transmission.
Fig. 6.
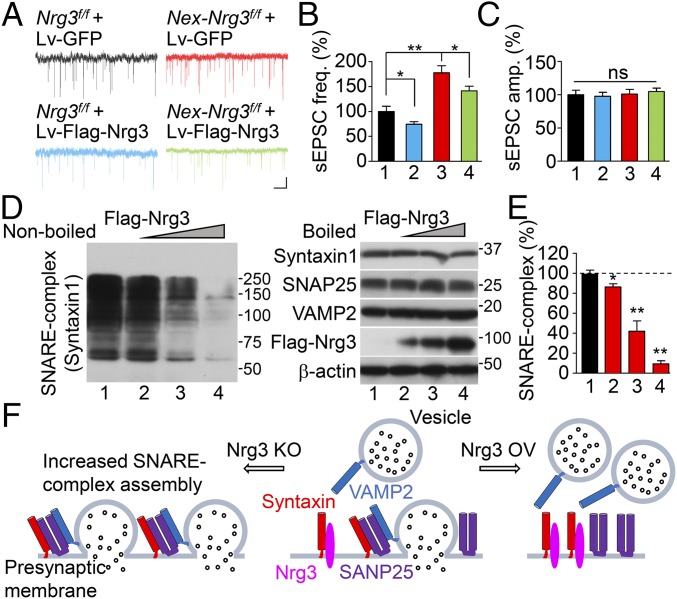
Suppressing glutamate release by enhanced Nrg3 levels via inhibiting SNARE-complex assembly. (A–C) Reduced sEPSC frequency in neurons expressing exogenous Nrg3. (A) Representative sEPSC traces in cultured hippocampal neurons infected with indicated lentivirus. (Scale bars: 2 s, 10 pA.) (B and C) Quantitative data of frequency (B) and amplitude (C). Lane 1, Nrg3f/f + Lv-GFP; Lane 2, Nrg3f/f + Lv-Flag-Nrg3; Lane 3, Nex-Nrg3f/f + Lv-GFP; Lane 4, Nex-Nrg3f/f + Lv-Flag-Nrg3; n = 10 neurons from three independent experiments; one-way ANOVA; (D) Dose-dependent reduction in SNARE-complex assembly by Nrg3. HEK293T cells were cotransfected with syntaxin1, VAMP2, and SNAP25 and increasing amounts of Flag-Nrg3. Cell lysates were immunoblotted without (Left) and with boiling (Right); SNARE complexes above 40 kDa were probed with antibody to syntaxin1, and the boiled sample was probed with antibodies to syntaxin1, SNAP25, VAMP2, and Flag. (E) SNARE-complex assembly was quantitated and normalized to β-actin; n = 3 independent experiments; one-way ANOVA. (F) A working model shows that Nrg3 regulates the SNARE-complex assembly for glutamate release. *P < 0.05; **P < 0.01. ns, no significant difference.
To investigate mechanisms by which elevated Nrg3 suppresses glutamate release, we assayed the SNARE complex in HEK293T cells in the absence and the presence, respectively, of Nrg3, using a previously developed method (43). When homogenates of transfected cells were resolved by SDS/PAGE, the SNARE complex migrated as smear bands above 40 kDa that were detected by the anti-syntaxin1 antibody (Fig. 6D, Left, lane 1), in agreement with previous reports (44). Boiling of the samples disrupted the complex, and the SNARE proteins migrated as individual bands to the predicted molecular weights (Fig. 6D, Right, lane 1). Intriguingly, coexpression of Nrg3 reduced the complex formation in a dose-dependent manner, without changing levels of individual SNARE proteins (Fig. 6D, lanes 2–4, and Fig. 6E; Fig. S8 A and B). Together, these results indicate that increasing Nrg3 inhibits the assembly of the SNARE complex, revealing the pathophysiological mechanism of a high Nrg3 level in inhibiting glutamate release (Fig. 6F).
Discussion
The major findings of this study are as follows. First, loss of Nrg3 in GFAP-Nrg3f/f mice specifically increased sEPSC frequency but had no effect on sIPSCs. Second, the same phenotypes were observed in Nex-Nrg3f/f mice, where Nrg3 was specifically knocked out in pyramidal neurons, indicating that Nrg3 regulates glutamatergic transmission by a cell-autonomous mechanism. Consequently, Nex-Nrg3f/f mice displayed various behavioral deficits related to schizophrenia. Third, the Nrg3 mutation decreased the PPR, increased decay of NMDAR currents when treated with MK801, and increased minimal stimulation-elicited response, providing evidence that the Nrg3 mutation increases glutamate release probability. Fourth, Nrg3 is a presynaptic protein and regulates the assembly of the SNARE complex. Finally, we showed that increasing Nrg3 levels in neurons impaired glutamate release. Together, these observations indicate that Nrg3 is critical in controlling glutamatergic transmission (Fig. 6F).
Nrg3 is thought to be a ligand that specifically binds ErbB4. However, the following evidence suggests that Nrg3 regulation of glutamate transmission is independent of ErbB4. First, the Nrg3 mutation had no effect on GABA transmission (Figs. 1 and 2), in contrast to the ErbB4 mutation, which impairs GABA transmission (37–39). The Nrg3 mutation also reduces PPR in pyramidal neurons whereas ErbB4 deletion from interneurons has no effect (45). Second, sEPSCs were recorded in the presence of BMI, a GABA antagonist, excluding a possible secondary effect (if any). Third, the ErbB4 level or phosphorylation was not altered in Nex-Nrg3f/f mutant mice (Fig. S9 A and B). Finally, pharmacological inhibition of ErbB4 had no effect on the increase in glutamate release in Nex-Nrg3f/f hippocampal slices, excluding the involvement of ErbB4 (Fig. S9 C and D). Rather, our study suggests that Nrg3 regulation of glutamate release is cell-autonomous. First, Nex-Cre–mediated ablation reduced the protein level of Nrg3 by 87%, indicating that Nrg3 is expressed mainly in pyramidal neurons. Second, subcellular Nrg3 is localized in the presynaptic fraction. Third, we show that Nrg3 overexpression in cultured neurons reduced glutamate release. At the molecular level, Nrg3 can interact with syntaxin1 (via the SNARE domain) and thus inhibit SNARE-complex formation and reduce glutamate release. Thus, Nrg3 joins a group of proteins that control SNARE-complex formation, including Munc18-1 that binds syntaxin (41), complexin that inserts to the four-helical bundle of the SNARE complex (46), and Synip that binds t-SNARE proteins (47). Together, a parsimonious explanation of these observations is that Nrg3 is a presynaptic protein that controls SNARE-complex assembly.
Hypofunction of the glutamatergic pathway is considered an important pathological mechanism of severe mental illnesses including schizophrenia (48–50). Phencyclidine (PCP), an NMDAR uncompetitive antagonist, produces psychotic symptoms, thought disorder, blunted affect, and cognitive impairments that resemble those in schizophrenia patients (51). In healthy individuals, ketamine, a PCP derivative, produces transient schizophrenia-like (positive and negative) symptoms and impairs prefrontal cortex-dependent cognitive function (52). In schizophrenic patients, on the other hand, ketamine increases signs and symptoms of the disorder (53). The mechanisms that cause hypoglutamatergic function are beginning to be understood. Both protein and mRNA levels of GluN1, a subunit of the NMDA receptor, are reduced in the dorsolateral prefrontal cortex in postmortem subjects with schizophrenia (54, 55). In agreement, pharmacological or genetic manipulations of the NMDA receptor in rodents cause behaviors relevant to schizophrenia (56, 57). Our study reveals an unexpected pathophysiological mechanism for hypofunction of the glutamatergic pathway. An increasing Nrg3 level in the medial prefrontal cortex enhances impulsivity (15). Developmental overexposure of mice to Nrg3 causes an anxiogenic-like phenotype and deficits in social behavior in adulthood (17). We demonstrate that an increased level of Nrg3 reduces glutamate release likely by disrupting the assembly of the SNARE complex.
Materials and Methods
Reagents, antibodies, plasmids, animals, cell culture and transfection, lentivirus production, Western blotting, immunostaining, subcellular fractionation, coimmunoprecipitation (co-IP), SNARE-complex analysis, electrophysiological analysis, behavior tests, and statistical analysis are described in SI Materials and Methods. Experimental procedures were approved by the Institutional Animal Care and Use Committee of Augusta University.
Supplementary Material
Acknowledgments
We thank members of the L.M. and W.-C.X. laboratories for helpful discussion; Dr. Hongjun Song for lentivirus vectors; and Dr. Cary Lai for the anti-ErbB4 antibody. This work is supported in part by National Institutes of Health Grants MH083317, MH109280, NS082007, and NS090083 (to L.M.) and AG051773 and AG045781 (to W.-C.X.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716322115/-/DCSupplemental.
References
- 1.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 2.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 3.Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–167. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson SB, Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rico B, Marín O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev. 2011;21:262–270. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Buonanno A. The neuregulin signaling pathway and schizophrenia: From genes to synapses and neural circuits. Brain Res Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, et al. Neuregulin-3 (NRG3): A novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci USA. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallin MD, et al. Bipolar I disorder and schizophrenia: A 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YC, et al. Neuregulin 3 genetic variations and susceptibility to schizophrenia in a Chinese population. Biol Psychiatry. 2008;64:1093–1096. doi: 10.1016/j.biopsych.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Chen PL, et al. Fine mapping on chromosome 10q22-q23 implicates Neuregulin 3 in schizophrenia. Am J Hum Genet. 2009;84:21–34. doi: 10.1016/j.ajhg.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao WT, et al. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc Natl Acad Sci USA. 2010;107:15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterson C, et al. Temporal, diagnostic, and tissue-specific regulation of NRG3 isoform expression in human brain development and affective disorders. Am J Psychiatry. 2017;174:256–265. doi: 10.1176/appi.ajp.2016.16060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loos M, et al. Neuro-BSIK Mouse Phenomics Consortium Neuregulin-3 in the mouse medial prefrontal cortex regulates impulsive action. Biol Psychiatry. 2014;76:648–655. doi: 10.1016/j.biopsych.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Hayes LN, et al. Neuregulin 3 knockout mice exhibit behaviors consistent with psychotic disorders. Mol Neuropsychiatry. 2016;2:79–87. doi: 10.1159/000445836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson C, Law AJ. Transient overexposure of neuregulin 3 during early postnatal development impacts selective behaviors in adulthood. PLoS One. 2014;9:e104172. doi: 10.1371/journal.pone.0104172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartolini G, et al. Neuregulin 3 mediates cortical plate invasion and laminar allocation of GABAergic interneurons. Cell Rep. 2017;18:1157–1170. doi: 10.1016/j.celrep.2016.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plani-Lam JH, et al. PTPN21 exerts pro-neuronal survival and neuritic elongation via ErbB4/NRG3 signaling. Int J Biochem Cell Biol. 2015;61:53–62. doi: 10.1016/j.biocel.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Carteron C, Ferrer-Montiel A, Cabedo H. Characterization of a neural-specific splicing form of the human neuregulin 3 gene involved in oligodendrocyte survival. J Cell Sci. 2006;119:898–909. doi: 10.1242/jcs.02799. [DOI] [PubMed] [Google Scholar]
- 21.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 23.Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 24.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 25.Goebbels S, et al. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- 26.Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- 28.Keefe RS, Harvey PD. Novel Antischizophrenia Treatments. Springer; Berlin: 2012. Cognitive impairment in schizophrenia; pp. 11–37. [DOI] [PubMed] [Google Scholar]
- 29.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: Investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- 31.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 32.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 33.Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 34.Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- 35.Vullhorst D, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bean JC, et al. Genetic labeling reveals novel cellular targets of schizophrenia susceptibility gene: Distribution of GABA and non-GABA ErbB4-positive cells in adult mouse brain. J Neurosci. 2014;34:13549–13566. doi: 10.1523/JNEUROSCI.2021-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo RS, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Chen YJ, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci USA. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen L, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vullhorst D, Ahmad T, Karavanova I, Keating C, Buonanno A. Structural similarities between neuregulin 1-3 isoforms determine their subcellular distribution and signaling mode in central neurons. J Neurosci. 2017;37:5232–5249. doi: 10.1523/JNEUROSCI.2630-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dulubova I, et al. A conformational switch in syntaxin during exocytosis: Role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acuna C, et al. Microsecond dissection of neurotransmitter release: SNARE-complex assembly dictates speed and Ca2+ sensitivity. Neuron. 2014;82:1088–1100. doi: 10.1016/j.neuron.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi T, et al. Synaptic vesicle membrane fusion complex: Action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burré J, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin DM, et al. Regulation of spine formation by ErbB4 in PV-positive interneurons. J Neurosci. 2013;33:19295–19303. doi: 10.1523/JNEUROSCI.2090-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Rathore SS, Shen J. Synip arrests soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent membrane fusion as a selective target membrane SNARE-binding inhibitor. J Biol Chem. 2013;288:18885–18893. doi: 10.1074/jbc.M113.465450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder MA, Adelman AE, Gao W-J. Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning. Neuropsychopharmacology. 2013;38:328–340. doi: 10.1038/npp.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kantrowitz J, Javitt DC. Glutamatergic transmission in schizophrenia: From basic research to clinical practice. Curr Opin Psychiatry. 2012;25:96–102. doi: 10.1097/YCO.0b013e32835035b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 51.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 52.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 53.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 54.Clinton SM, Haroutunian V, Davis KL, Meador-Woodruff JH. Altered transcript expression of NMDA receptor-associated postsynaptic proteins in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2003;160:1100–1109. doi: 10.1176/appi.ajp.160.6.1100. [DOI] [PubMed] [Google Scholar]
- 55.Weickert CS, et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 2013;18:1185–1192. doi: 10.1038/mp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 57.Rung JP, Carlsson A, Rydén Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats: A model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.