Significance
Th17 cells unlike other CD4+ T helper subsets use glutaminolysis as a source of energy and upregulate Gls1. Inhibition of Gls1 ameliorates Th17 differentiation in vitro and experimental autoimmune encephalomyelitis in mice. Mechanistically this is accomplished through the upregulation of Gls1 by the Th17-promoting transcription factor, inducible cAMP early repressor (ICER). These findings claim an essential role of glutaminolysis in the generation of Th17 cells and offer an approach to control diseases linked to their generation.
Keywords: glutaminolysis, Th17, glutaminase 1, ICER, autoimmunity
Abstract
Glutaminolysis is a well-known source of energy for effector T cells but its contribution to each T cell subset and the mechanisms which are responsible for the control of involved metabolic enzymes are not fully understood. We report that Th17 but not Th1, Th2, or Treg cell induction in vitro depends on glutaminolysis and the up-regulation of glutaminase 1 (Gls1), the first enzyme in the glutaminolysis pathway. Both pharmacological and siRNA-based selective inhibition of Gls1 reduced in vitro Th17 differentiation and reduced the CD3/TCR-mediated increase of the mammalian target of rapamycin complex 1 activity. Treatment of mice with a Gls1 inhibitor ameliorated experimental autoimmune encephalomyelitis. Furthermore, RAG1-deficient mice that received Gls1-shRNA–transfected 2D2 T cells had reduced experimental autoimmune encephalomyelitis scores compared with those that received control-shRNA–treated cells. Next we found that T cells deficient in inducible cAMP early repressor (ICER), a transcriptional factor known to promote Th17 differentiation, display reduced activity of oxidative phosphorylation rates in the presence of glutamine and reduced Gls1 expression, both of which could be restored by ICER overexpression. Finally, we demonstrate that ICER binds to the gls1 promoter directly and increases its activity. These findings demonstrate the importance of glutaminolysis in the generation of Th17 and the direct control of Gls1 activity by the IL-17–promoting transcription factor ICER. Pharmaceutical modulation of the glutaminolysis pathway should be considered to control Th17-mediated pathology.
Th17 cells are important not only in the defense against extracellular pathogens but also in the pathogenesis of autoimmune diseases, including multiple sclerosis, systemic lupus erythematosus (SLE), and psoriasis (1–4). Many studies have shown that each type of CD4+ T helper cell utilizes preferentially a source of energy production (5, 6), with naïve and regulatory T cells utilizing fatty acid oxidization (FAO) as a main source of energy production (7, 8) and effector T helper cells (Th1, Th2, and Th17) favoring glycolysis (9).
Glutaminolysis takes place in all proliferating cells, including lymphocytes, thymocytes, and tumor cells (10). Besides glycolysis, glutaminolysis is considered to be a main source of energy production in tumor cells (11). In T cells, it has been reported that glutamine (Gln) transporter-deficient T cells have decreased Th1/17 response and less TCR-mediated mammalian target of rapamycin complex 1 (mTORC1) activity (12). Gln-dependent α-ketoglutarate (α-KG) deficiency converts Th1 cells to Treg-like cells (13) and the disruption of the gene got1 converts Th17 cells to Treg-like cells by epigenetic remodeling of the Foxp3 promoter region (14). These observations suggest an essential role for glutaminolysis in the generation of Th1 and Th17 cells.
Glutaminase (Gls) is the first enzyme in the glutaminolysis pathway and converts Gln to glutamate (15). In mammals, there are two different genes encoding Gls: gls1, the kidney isoform, and gls2, the liver isoform, with Gls1 displaying more enzymatic activity than Gls2 (16). Although Gls inhibitors have been introduced as new therapeutic targets in cancer (10, 17), only a few reports have shown the efficacy of Gls inhibitors in autoimmune diseases (18). Although previous reports have indicated that glutaminolysis is essential for both Th1 and Th17 cell differentiation (12), the involved enzymes and their mechanism of induction have not been studied.
The cAMP response element modulator (CREM) controls the transcription of cAMP responsible genes. We have shown that the inducible cAMP early repressor (ICER) isoform of CREM promotes Th17 cell differentiation and ICER/CREM-deficient mice have less autoimmune disease and CD4+ T cells from the patients with SLE express more ICER/CREM than those from healthy donors (19). Because genome-wide analysis of cAMP response element (CRE) binding protein occupancy has indicated the possibility that CRE binding proteins can regulate genes involved in cell metabolism (20), we considered that ICER/CREM controls the activity of metabolic enzymes.
Here, we report that the generation of Th17 cells depends on energy produced through glutaminolysis. Mechanistically, we demonstrate that the transcription factor ICER enhances the expression of the first enzyme in the glutaminolysis pathway, Gls1. Pharmacologic inhibition of Gls1 prevented experimental autoimmune encephalomyelitis (EAE), signifying the clinical significance of our findings.
Results
Th17 Cells Depend on Glutaminolysis More than the Other T Cell Effector Subsets.
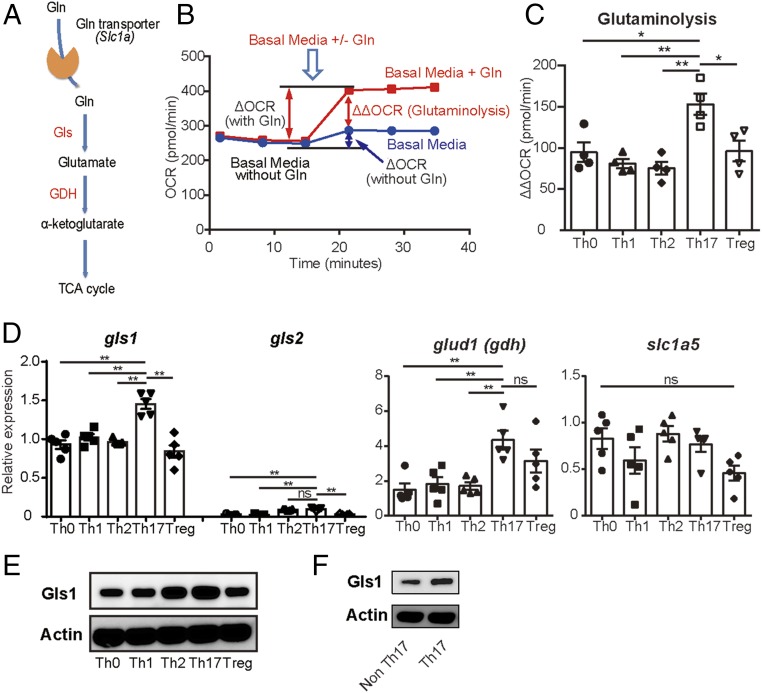
Gln enters cells using the alanine, serine, cysteine-preferring transporter 2 (ASCT2) and it is converted to glutamate by Gls1 and to α-KG by glutamate dehydrogenase (GDH) (Fig. 1A) (15). Naïve CD4+ T cells from B6 mice were cultured under Th0, Th1, Th2, Th17, or Treg conditions in vitro and oxygen consumption rate (OCR) was analyzed in the presence or absence of 2 mM Gln. Although OCR before Gln supplementation in Th17 is not higher than that recorded in Th1 or Treg cells, OCR after Gln supplementation was higher in Th17 cells compared with other T cell subsets (Fig. S1 A and B). There were no significant differences in extracellular acidification rate (ECAR) before or after Gln supplementation in any of the T cell subsets (Fig. S1 C and D). To analyze further the dependency in each subset of T cells on Gln, we calculated and compared the amount of ΔΔOCR as follows (Fig. 1B): We first determined the ΔOCR (with Gln) and ΔOCR (without Gln) which represent the change in the amount of OCR caused by supplementation of media in the presence or absence of Gln, respectively. The calculated ΔΔOCR (ΔOCR with Gln minus ΔOCR without Gln) was significantly higher in Th17 cells compared with any other T subset (Fig. 1C). These findings indicate the preferential dependence of Th17 cells on glutaminolysis as a source of energy. Then we assessed the expression levels of the involved molecules using qRT-PCR (Fig. 1A). Although the levels of the Gln transporter were comparable among various T cell effector subsets, gls1 expression was significantly increased in Th17 cells compared with other T cell subsets (Fig. 1D). gls2 was expressed at very low levels in all T cell subsets compared with the levels of gls1 but at increased levels among Th2 and Th17 cells. glud1 (GDH) was found increased in both Th17 and Tregs (Fig. 1D). Lastly, Gls1 protein was expressed at high levels in Th17 cells (Fig. 1 E and F). We conclude that Th17 cells utilize glutaminolysis as a source of energy by inducing the expression of Gls1.
Fig. 1.
Th17 cells depend on glutaminolysis more than the other T cell subsets. (A) Enzymes involved in the glutaminolysis pathway. (B) Schematic representations of the experiments performed to measure glutaminolysis [ΔΔoxygen consumption rate (OCR)] by extracellular flux analyzer. ΔOCR with Gln: change amount of oxidative consumption by supplying glutamine-containing media. ΔOCR without Gln: change amount of oxidative consumption by supplying Gln-free media. Glutaminolysis (ΔΔOCR) was determined by subtracting ΔOCR without Gln from ΔOCR with Gln. (C–E) Naïve CD4+ T cells were polarized under the indicated conditions. (C) OCR was measured by extracellular flux analyzer. Cumulative data of calculated ΔΔOCR on day 2 are shown (mean ± SEM); n = 4. (D) The relative gene expressions of the indicated molecules on day 3 were measured by qRT-PCR. Cumulative data are shown (mean ± SEM); n = 5. (E) Gls1 and actin protein expression on day 3 were assessed by Western blotting. Representative blots are shown. Data are representative of three experiments. (F) Naïve CD4+ T cells from IL-17GFP mice were polarized under Th17 conditions for 3 d. Gls1 and actin protein expression of FACS-sorted GFP+ (IL-17A–producing cells) and GFP− (IL-17A–nonproducing cells) were assessed by Western blotting. Representative blots are shown. Data are representative of three experiments. *P < 0.05; **P < 0.01. ns, not significant.
Gls1 Is Requisite for Th17 Differentiation.
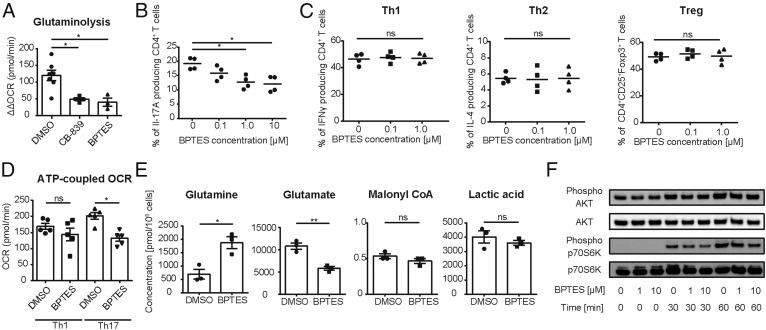
To confirm that Gls1 is crucial for Th17 differentiation, we used two selective Gls1 inhibitors [CB-839 and Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES)] in cultures of naïve CD4+ cells undergoing Th17 differentiation and assessed glutaminolysis and glycolysis by measuring OCR and associated ECAR, respectively. Both inhibitors suppressed OCR (Fig. 2A) and reduced the percentage of IL-17–producing CD4+ T cells in a dose-dependent manner (Fig. 2B and Fig. S2 A–C). Interestingly, as shown in Fig. 2C, neither of the inhibitors changed the differentiation of other T helper subsets, including Th1 cells. Indeed, when we measured the ATP-coupled OCR in in vitro Th1 and Th17 polarized cells, we found a reduction of ATP-coupled OCR in Th17 cells by BPTES but not in Th1 cells (Fig. 2D), suggesting the dependence of Th17 cells on the activity of Gls1.
Fig. 2.
Gls1 is requisite for Th17 differentiation. (A) Naïve CD4+ T cells were cultured under Th17-polarizing conditions and DMSO, CB-839, or BPTES was added on day 0. Oxygen consumption rate (OCR) was measured by extracellular flux analyzer on day 2. Cumulative data of calculated ΔΔOCR are shown (mean ± SEM); n = 3–7. (B) Naïve CD4+ T cells were cultured under Th17-polarizing conditions in the presence of increasing concentration of BPTES (0–10 μM) for 3 d. Percentage of IL-17A–positive cells was measured by flow cytometry. Cumulative data are shown (mean ± SEM); n = 4. (C) Naïve CD4+ T cells were cultured under Th1-, Th2-, and Treg-polarizing conditions for 3 d in the presence of increasing concentration of BPTES (0–1 μM) for 3 d. Percentage of IFNγ+ (Th1), IL-4+ (Th2), or CD25+Foxp3+–cells (Tregs) were measured by flow cytometry. Cumulative data are shown (mean ± SEM); n = 4. (D) Naïve CD4+ T cells were cultured under Th1- and Th17-polarizing conditions in the presence of DMSO or BPTES for 2 d. ATP-coupled OCR was assessed by extracellular flux analyzer. Cumulative data are shown (mean ± SEM); n = 5. (E) Naïve CD4+ T cells were cultured under Th17-polarizing conditions in the presence of DMSO or BPTES for 2 d. Absolute concentrations of each indicated metabolite were determined by CE-MS analysis. Cumulative data are shown (mean ± SEM); n = 3. (F) Naïve CD4+ T cells were stimulated in the presence of BPTES (0, 1, or 10 μM). Expression of phosphorylated-AKT, AKT, phosphorylated-p70S6K, and p70S6K were assessed by Western blot analysis. Representative blots of those proteins at 0, 30, and 60 min of stimulation are shown. Data are representative of three independent experiments. *P < 0.05; **P < 0.01. ns, not significant.
To assess the effect of BPTES in Th17 cell metabolism we measured the absolute amount of intracellular metabolites in Th17-polarized T cells cultured in the presence or absence of BPTES by capillary electrophoresis (CE)-MS analysis (Fig. 2E and Fig. S2D). Since the amount of Gln in BPTES-treated Th17 cells was increased, whereas that of glutamate was decreased (Fig. 2E), we were able to confirm that BPTES treatment inhibits glutaminolysis by inhibiting Gls that catalyzes Gln-to-glutamate conversion. FAO metabolism-related malonyl-CoA (Fig. 2E) and HMG-CoA (Fig. S2D) were comparable between the two groups, suggesting that BPTES treatment did not affect the FAO pathway. In reference to the glycolysis pathway, we observed a reduction in the levels of metabolites that relate to glycolysis, e.g., pyruvic acid (Fig. S2D). However, the amount of lactate that specifically reflects the glycolytic metabolism, which is favored during Th17 differentiation (9), did not change (Fig. 2E), and none of the metabolites upstream of the reduced metabolites were increased after BPTES treatment (Fig. S2D). Furthermore, as shown in Fig. S2E, we performed a glycolysis stress test by using extracellular flux analyzers and confirmed that glycolysis and glycolytic capacity did not change significantly after BPTES treatment. It appears that the recorded changes in the glycolysis pathway metabolites can be explained by compensatory reduction by consumption of glucose-related metabolites.
To confirm the effect of gls1 inhibition in Th17-polarized T cells, we transfected two different Gls1-specific siRNA, and we found the decreased generation of IL-17–producing CD4+ T cells compared with cells transfected with control siRNA (Fig. S2 F–H).
Since Gln transporter-deficient T cells have less TCR-mediated mTORC1 activity, we analyzed the phosphorylation of AKT and p70S6K by Western blotting. CD4+ T cells from B6 mice were cultured in the presence or absence of BPTES and then stimulated with CD3 and CD28 antibodies. AKT phosphorylation and p70S6K phosphorylation, which represent upstream and downstream events of mTORC1 activity, respectively, were measured by Western blotting. In agreement with previous observations in Gln transporter-deficient T cells (12), BPTES-treated CD4+ T cells displayed reduced phosphorylation of p70S6K, while the levels of AKT phosphorylation were not affected (Fig. 2F). These observations confirm the indispensable role of Gls1 and glutaminolysis in the generation of Th17 cells.
Gls1 Inhibition Ameliorates EAE.
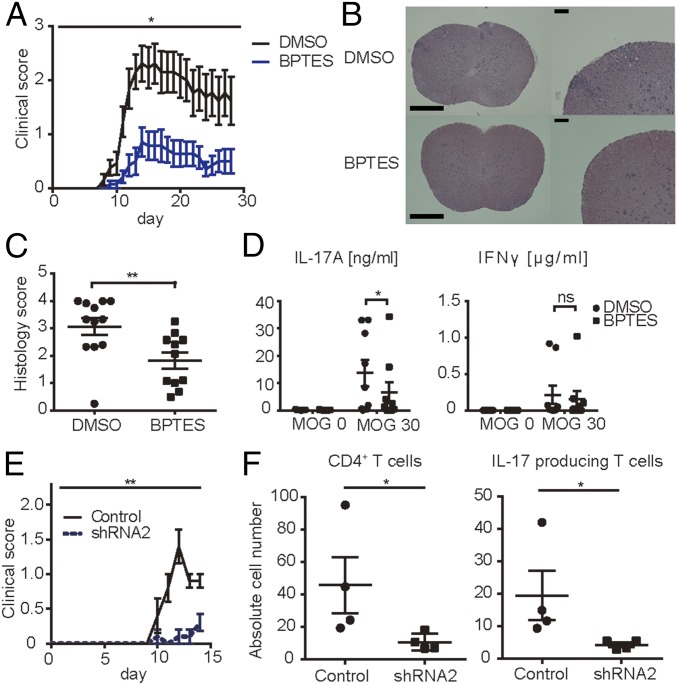
Our in vitro data suggest that Gls1 inhibitors represent therapeutics for Th17-related autoimmune diseases. We subjected B6 mice to EAE while treated with BPTES or dimethyl sulfoxide (DMSO). As shown in Fig. 3A and Fig. S3A, treatment of mice with BPTES significantly reduced both the clinical score and body weight loss compared with treatment with DMSO. Histology scores of spinal cords from diseased animals were significantly decreased in the BPTES-treated group (Fig. 3 B and C). This observation was further confirmed by assessing the absolute numbers of spinal cord-infiltrating cells by flow cytometry. Treatment with BPTES reduced the numbers of CD4+ T cells, IL-17A, and IFNγ-producing CD4+ T cells in the spinal cord compared with those of animals treated with DMSO (Fig. S3B).
Fig. 3.
Gls1 inhibition ameliorates EAE. (A–D) EAE was induced in B6 mice by immunization with MOG35–55 emulsified in complete Freund’s adjuvant. Mice were treated with DMSO or BPTES twice a week intraperitoneally. (A) Clinical scores. Cumulative results of three independent experiments with three to five mice per group are shown (mean ± SEM). (B) Spinal cords were harvested at day 14 and stained with H&E to assess inflammation. [Scale bars, 500 μm or 100 μm (magnified panels).] (C) Quantitative cumulative data are shown (mean ± SEM); n = 11–12. (D) Mononuclear cells harvested from inguinal lymph nodes of DMSO- or BPTES-treated mice on day 8 were activated in vitro with MOG35–55 for 3 d. IL-17A and IFNγ concentrations were measured by ELISA. Cumulative data are shown (mean ± SEM); n = 8–9. (E and F) Naïve CD4+ T cells from 2D2 mice were cultured under Th17-polarizing conditions. Gls1 shRNA- or control shRNA-containing lentiviral particles were infected on day 1. On day 4 of culture, those harvested cells were transferred to recipient Rag1-deficient mice intravenously. (E) Clinical scores of recipient mice. Cumulative results of five mice per group are shown (mean ± SEM). (F) Absolute cell numbers of spinal cord-infiltrated CD4+ T cells, IL-17A–producing CD4+ T cells, and IFNγ-producing CD4+ T cells from DMSO- or BPTES-treated mice evaluated by flow cytometry on day 14. Cumulative data are shown (mean ± SEM); n = 4. *P < 0.05; **P < 0.01. ns, not significant.
Next we evaluated the in vitro response of T cells from animals immunized in vivo to develop EAE to MOG35–55. We harvested draining lymph nodes from B6 mice subjected to EAE and treated with DMSO or BPTES on day 8 and cultured T cells with MOG35–55 for 3 d in vitro. IL-17A production was significantly decreased in BPTES-treated mice, whereas IFNγ production was not affected (Fig. 3D). Furthermore, when we treated cells isolated from DMSO-treated mice with MOG35–55 in the presence or absence of BPTES in vitro for 3 d, we noticed a significant reduction in the production of IL-17A but not of IFNγ (Fig. S3C). These data document the ability of the Gls1 inhibitor BPTES to suppress the generation of Th17 cells in vivo.
To investigate further the physiological importance of Gls1 in vivo in CD4+ T cells, we performed an adoptive transfer EAE experiment. To this end, we generated two shRNAs specific for Gls1. After confirming that shRNAs suppressed Th17 polarization in vitro (Fig. S3 D–F), we prepared in vitro Th17-polarized cells and transfected them with Gls1 shRNA or control shRNA using naïve CD4+ T cells from 2D2 mice and transferred them into Rag1-deficient mice. As shown in Fig. 3E, mice that received Gls1-shRNA–transfected 2D2 T cells had reduced EAE disease compared with those that received control-shRNA. We confirmed the effect of Gls1 silencing by assessing the absolute numbers of spinal cord-infiltrating cells by flow cytometry. Rag1-deficient mice, which received gls1 shRNA-treated 2D2 T cells, had reduced numbers of CD4+ T cells, and IL-17A–producing CD4+ T cells in the spinal cord compared with those that received control-shRNA (Fig. 3F).
ICER Promotes Gls1 Expression.
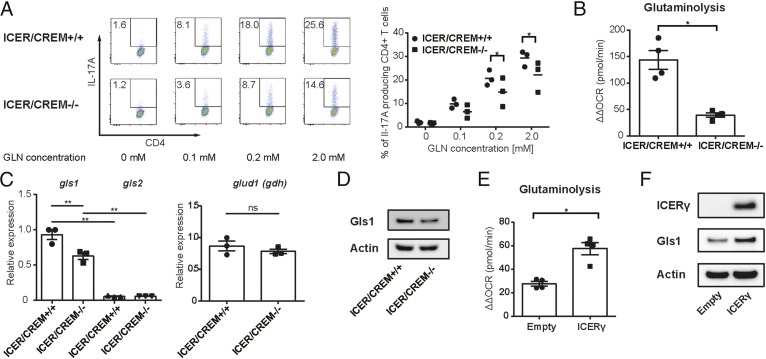
We previously reported that the transcriptional factor ICER/CREM is predominantly induced during Th17 cell differentiation and promotes Th17 differentiation both in vivo and in vitro. Because we found putative CRE sites, conserved in both humans and mice, to reside within the promoter region of Gls1 gene, we considered that ICER/CREM controls gls1 expression in Th17 cells. First, we determined whether Gln is required for Th17 differentiation in ICER/CREM-sufficient and -deficient mice. ICER/CREM-sufficient CD4+ cells polarized to Th17 in the presence of Gln at significantly higher levels compared with ICER/CREM-deficient CD4+ cells (Fig. 4A). The use of glutaminolysis as estimated by measuring the levels of ΔΔOCR was significantly reduced in ICER/CREM-deficient mice compared with those in ICER/CREM-sufficient mice (Fig. 4B). Next we determined the levels of gls1 gene expression in ICER/CREM-sufficient and -deficient cells. Both gls1 gene (Fig. 4C) and protein expression (Fig. 4D) were significantly decreased in ICER/CREM-deficient cells compared with those in ICER/CREM-sufficient counterparts, whereas the other glutaminolysis-related genes were not significantly different (Fig. 4C and Fig. S4A).
Fig. 4.
ICER/CREM-deficient mice display decreased Gls1 expression, glutaminolysis, and Th17 polarization. (A) ICER/CREM-deficient or -sufficient naïve CD4+ T cells as cultured under Th17-polarizing condition in media containing the indicated doses of glutamine (Gln) (0–2.0 mM) for 3 d. Representative flow plots (Left) and cumulative data (Right) are shown (mean ± SEM); n = 3. (B–D) ICER/CREM-deficient or -sufficient naïve CD4+ T cells as cultured under Th17-polarizing conditions. (B) Calculated ΔΔOCRs on day 2. Cumulative data are shown (mean ± SEM); n = 4. (C) Relative gene expressions of the gls and glud1 (gdh) on day 3 as assessed by qRT-PCR. Cumulative data are shown (mean ± SEM); n = 3. (D) Gls1 and actin protein expression on day 3 as assessed by Western blotting. Representative blots are shown. Data are representative of three experiments. (E and F) ICER/CREM-deficient naïve CD4+ T cells as cultured under Th17-polarizing conditions. Empty vector (empty) or ICERγ expressing (ICERγ) plasmids were transfected to cultured T cells on day 1. (E) Calculated ΔΔOCRs on day 2. Cumulative data are shown (mean ± SEM); n = 4. (F) ICERγ, Gls1, and actin protein expression on day 3 as assessed by Western blotting. Representative blots are shown. Data are representative of three experiments. *P < 0.05; **P < 0.01. ns, not significant.
To confirm that ICER regulates glutaminolysis in in vitro Th17-polarized cells we overexpressed ICERγ in ICER/CREM-deficient Th17 cells and measured ΔΔOCR and Gls1 expression. Indeed, ICER overexpression restored ΔΔOCR and Gls1 expression levels (Fig. 4 E and F and Fig. S4B).
ICER Is a Transcriptional Enhancer for Gls1.
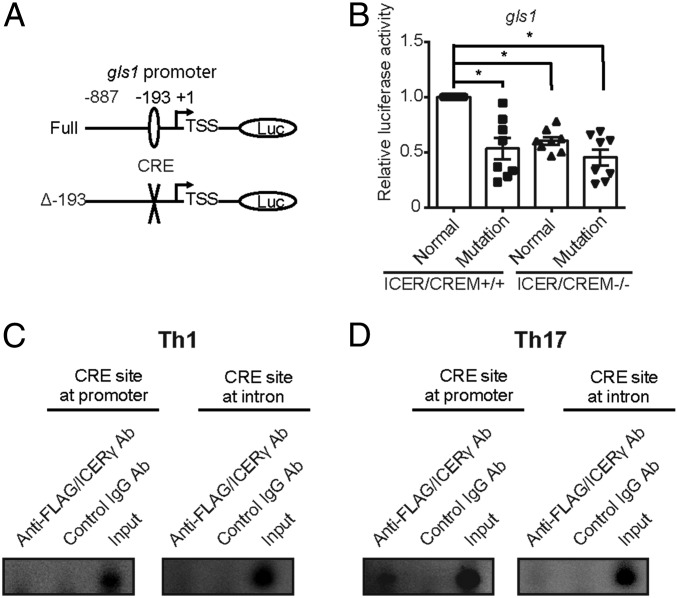
Finally, we assessed whether the transcriptional factor ICER can regulate Gls1 expression directly by binding to the gls1 promoter. To this end, we generated a luciferase reporter vector driven by the full-length gls1 promoter or the gls1 promoter in which the CRE (Δ-193) site had been mutated (Fig. 5A). We found that the gls1 promoter reporter activity was decreased in the Th17-polarized cells that had been transfected with the mutated vector compared with cells transfected with the reporter vector driven by the full gls1 promoter (Fig. 5B). To demonstrate that ICERγ accessed the gls1 promoter at the CRE site, we transfected a Flag-tagged ICERγ overexpression vector into Th17-polarized ICER/CREM-deficient T cells and measured the recruitment of ICERγ to the gls1 promoter using ChIP assays. As we show in Fig. 5C, ICERγ accumulated at the promoter region of gls1, which contains the CRE but not at the intron 1 region of gls1, which also contains putative CRE, suggesting that ICERγ accumulates at the promoter region of gls1 in Th17-polarized T cells. In contrast, we did not detect any accumulation of ICERγ in any region of Gls1 when we performed the same experiment using Th1-polarized cells (Fig. 5D), suggesting that ICERγ induces gls1 in Th17 cells in a specific manner. These data demonstrate that the transcription factor ICER promotes glutaminolysis by inducing Gls1 expression by binding directly to the gls1 promoter in Th17 cells.
Fig. 5.
ICERγ binds to the gls1 promoter directly and increases its activity. (A) Schematic representations of the reporter constructs. Numbers represent the position from transcription start site (TSS) of the murine gls1 gene. (B–D) ICER/CREM-deficient or -sufficient naïve CD4+ T cells as cultured under Th17- (B and C) and Th1- (D) polarizing conditions. (B) The full-length gls1 promoter region (full) or a version containing a mutated CRE binding site (Δ-193) transfected to Th17-polarized T cells on day 1. Cells were harvested and lysed on day 2. Cumulative results of eight independent experiments are shown (mean ± SEM). (C and D) FLAG-tagged ICERγ overexpression vector transfected to ICER/CREM-deficient CD4+ T cells on day 1. Cells were harvested and lysed on day 3 and binding of FLAG/ICERγ to the CRE was assessed by chromatin immunoprecipitation (ChIP) assay. CRE at the first intron of the gls1 gene was used as a negative control for ChIP enrichment. Representative blots from three experiments are shown. *P < 0.05.
Discussion
In this study we demonstrate glutaminolysis as the major source of energy for the generation of Th17 cells both in vitro and in vivo. Gls1, the first enzyme in the glutaminolysis pathway responsible for the conversion of Gln to glutamate, is up-regulated in a specific manner in Th17 cells and its transcription is regulated by ICER. At the translational level we report that pharmaceutical inhibition of Gls1 suppresses EAE in mice.
It is now well established that cell metabolism processes serve as main regulators of T cell differentiation and function (6, 21). Although Th17 cells depend on glycolysis and glutaminolysis as sources of energy (9, 22), Treg cells depend mainly on FAO (7, 8). Previous reports have shown that blocking glycolysis with 2-deoxy-d-glucose (2DG, a glycolysis inhibitor) can inhibit Th17 cell differentiation (9) and treatment of lupus-prone mice with a combination of metformin, a mitochondrial metabolism inhibitor, and 2DG normalized T cell metabolism and reversed disease manifestations (23), suggesting that modulation of metabolic pathways may have therapeutic value for autoimmune diseases (24).
Previous reports have shown that Gln transporter deficiency or depletion of Gln in culture media reduces Th17 differentiation (12). We demonstrate here that inhibition of the conversion of Gln to glutamate, the first step in the glutaminolysis pathway, can preferentially suppress Th17 over other T cell subsets. Gls1, the enzyme involved in this step is up-regulated only in Th17 cells. Recently it was claimed that inhibition of glutamate oxaloacetate transaminase 1 (GOT1) reduced Th17 differentiation (14). GOT1 represents one of the three metabolic pathways involved in the conversion of glutamate to α-KG (GDH pathway, GOT pathway, and glutamic pyruvic transaminase pathway). In addition, since GOT also catalyzes the conversion of oxaloacetate to aspartate, it was not made clear whether the suppression of Th17 generation following the inhibition of GOT was due to limited production of α-KG or aspartate. Our experiments demonstrate the exclusivity of the glutaminolysis pathway in the generation of Th17 with evidence generated both in vitro and in vivo. Interestingly though, and as claimed before, Gln deprivation can inhibit both Th1 and Th17 differentiation (12), and pharmacologic inhibition or silencing of Gls1 suppressed significantly the generation of Th17, while it had a minor effect on the differentiation of other T cell subsets, including Th1. Treatment of mice with a Gls1 inhibitor limited significantly the development of EAE after immunization with the MOG peptide.
Inhibition of Gls1 reduced the phosphorylation of p70S6K, which is downstream of mTORC1. Previous reports have shown that p70S6K1-deficient mice display reduced Th17 differentiation and developed less severe EAE, while the development of Th1 was not affected (25). Since the acetylation of histone 3 at the promoters of il17a and il17f was reduced by the absence of p70S6K1 (25), we propose that Gls inhibition alters the epigenetic status of the il17a and il17f promoters by preventing the phosphorylation of p70S6K.
An aspect of our work is the identification of ICER/CREM as the transcriptional controller of the expression of the first enzyme of the glutaminolysis pathway, Gls1. CREM belongs to the CREM/ATF family that can bind to the CRE site directly. The CREM gene is expressed in many alternatively spliced transcript variants that are tightly regulated at the epigenetic and posttranscriptional levels. The alternative splice variants of the primary CREM gene are known to generate isoforms that exert opposing effects on target gene expression (26). One of the splice variants, ICER, is unique because it has an alternative transcription initiation site and is induced by a private alternative promoter (27). Recently we demonstrated that ICER is induced predominantly in Th17 cells and binds to the il17a promoter site (19) and ICER/CREM-deficient mice displayed limited ability to generate Th17 cells both in vivo and in vitro (19). In the present study we demonstrate that ICER positively regulates the expression of Gls1 and the utilization of Gln in the generation of Th17 cells. Indeed, ICER/CREM-deficient mice have less glutaminolysis and less Gls1 expression without affecting the expression of other glutaminolysis-related genes. More specifically, we show that forced ICERγ expression in ICER/CREM-deficient Th17 cells can restore Gln utilization and Gls1 expression and ICER binds to the gls1 promoter directly and increases its activity. These data provide a molecular link between a transcription factor and the expression of a specific metabolic enzyme in the induction of Th17 cells.
At the translational level, Gls1 inhibitors are being considered for the treatment of various cancers in mice (28, 29), and some of them have entered clinical trials (17, 30). Although inhibition of glutaminolysis is linked to side effects, including neurotoxicity, gastrointestinal toxicity, and myelosuppression (10), we have succeeded in suppressing EAE in mice by using only 10–20% of the dose of BPTES used in the treatment of cancer. If the low dose proves efficacious in the treatment of other autoimmune diseases in mice, then it should be justifiable to use Gls1 inhibitors to treat human autoimmune diseases.
In summary, we have shown that Th17 cells depend on the induction of Gls1 and glutaminolysis more than Th1, Th2, or Tregs. Inhibition of Gls1 reduces in vitro Th17 differentiation and ameliorates EAE in mice. More importantly, we demonstrate that the transcriptional factor ICER favors Th17 differentiation by promoting the transcription of gls1, which encodes the first enzyme involved in the glutaminolysis pathway.
Materials and Methods
More detailed information, including single cell isolation, in vitro T cell differentiation, CE-MS analysis, Western blotting, flow cytometry, ELISA, RNA isolation, and quantitative PCR, is provided in SI Materials and Methods.
Mice.
SV129/Bl6.ICER/CREM−/−mice were originally generated by Günther Schuetz (Das Deutsche Krebsforschungszentrum, Heidelberg, Germany) (31). Animals were crossed to C57BL/6J mice for over nine generations to transfer the ICER/CREM−/− locus to the B6 background. C57BL6J mice, C57BL/6-Il17atm1Bcgen/J (IL-17GFP) mice, C57BL/6-Tg(Tcra2D2, Tcrb2D2)1Kuch/J (2D2) mice, and B6.129S7-Rag1tm1Mom/J (Rag KO) mice were purchased from The Jackson Laboratory. B6.ICER/CREM−/−.IL-17GFP mice were made by crossing B6.ICER/CREM−/− mice with IL-17GFP mice. Animals were killed at 8–12 wk of age for in vitro experiments and indicated number of weeks for in vivo experiments. All mice were maintained in a specific pathogen-free animal facility [Beth Israel Deaconess Medical Center (BIDMC)]. Experiments were approved by the Institutional Animal Care and Use Committee of BIDMC.
Metabolism Assays.
ECAR and OCR were measured using a 96-well XFp Extracellular Flux Analyzer. Assay buffer was made of XF base medium (without Gln) with 10 mM glucose and 1.0 mM sodium pyruvate. Cell-Tak Cell and Tissue Adhesive was used for coating plates and 0.15 × 106 T cells per well were seeded. OCR and ECAR were measured before and after exposure of the assay buffer with or without 2.0 mM Gln. All other procedures were performed according to the manufacturer’s instructions.
In Vitro Gene-Overexpressing Culture, siRNA Treatment, and Luciferase Assay.
Vectors and siRNAs are described in SI Materials and Methods. We used the Amaxa nucleofector following established protocols (19).
Chromatin Immunoprecipitation Assays.
Freshly isolated naïve CD4+ T cells from B6.ICER/CREM−/− mice were cultured in Th17-polarizing condition for 3 d. N′-FLAG-tagged ICERγ overexpressing vectors (19) were transfected as described above on day 1. Harvested cells were lysed and ChIP assay was performed using the MAGnify Chromatin Immunoprecipitation System (Invitrogen). Anti-FLAG antibody produced in rabbit (Sigma-Aldrich) was used for immunoprecipitation. Primers used for this study are shown in SI Materials and Methods. All procedures were performed according to the manufacturer’s instructions.
Generation of Lentiviral Particles Contains shRNAs.
MISSION pLKO.1-puro empty vector control plasmid DNA (Sigma-Aldrich) was used for this cloning. We designed two gls1-shRNAs as listed in SI Materials and Methods and cloned them into the empty vector following the manufacturer’s protocols. MISSION pLKO.1-puro nonmammalian shRNA control plasmid DNA control-shRNA (Sigma-Aldrich) was used for control shRNA. Those vectors were transfected to 40% confluent HEK-293T cells by polyethyleneimine “Max” (Polysciences, Inc.) according to the manufacturer’s protocol. Culture media with shRNA contained-lentiviral particles were collected on day 4.
EAE.
Methodology for EAE and for the adaptive transfer EAE was described in SI Materials and Methods. A total of 60 μg/body BPTES or DMSO in PBS was treated twice a week intraperitoneally. The following clinical scores were used: 1, limp tail; 2, hind-limb paresis; 3, hind-limb paralysis; 4, tetraplegia; and 5, moribund (32). For the priming assay of MOG35–55 immunizations, cells were purified from inguinal lymph nodes on day 8 of the experiment, then cultured ex vivo with the same lot of MOG35–55 peptide for 5 d.
Histological Staining and Analysis.
Sections from 10% formalin-fixed spinal cords were stained with H&E. Spinal cord sections were scored by an investigator blinded to experimental group as follows: 0, no infiltration (<50 cells); 1, mild infiltration of nerve or nerve sheath (50–100 cells); 2, moderate infiltration (100–150 cells); 3, severe infiltration (150–200 cells); and 4, massive infiltration (>200 cells).
Statistics.
Statistical analyses were performed in GraphPad Prism version 6.0 software. Statistical significance was determined by t tests (two tailed) for two groups or one-way ANOVA with Bonferroni’s multiple comparisons tests for three or more groups. For the EAE model, clinical scores and body weight changes of each treatment group were compared using two-way ANOVA. P values of <0.05 were considered statistically significant (**P < 0.01, *P < 0.05).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R37 AI49954, a SENSHIN Medical Research Foundation grant (to M.K.), and Japan Society for the Promotion of Science postdoctoral fellowships for research abroad (to N.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714717115/-/DCSupplemental.
References
- 1.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 3.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 4.Ooi JD, Kitching AR, Holdsworth SR. Review: T helper 17 cells: Their role in glomerulonephritis. Nephrology (Carlton) 2010;15:513–521. doi: 10.1111/j.1440-1797.2010.01343.x. [DOI] [PubMed] [Google Scholar]
- 5.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton R, Priyadharshini B, Turka LA. Immunometabolism of regulatory T cells. Nat Immunol. 2016;17:618–625. doi: 10.1038/ni.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beier UH, et al. Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerriets VA, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–3625. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Venneti S, Nagrath D. Glutaminolysis: A hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017;19:163–194. doi: 10.1146/annurev-bioeng-071516-044546. [DOI] [PubMed] [Google Scholar]
- 12.Nakaya M, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klysz D, et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci Signal. 2015;8:ra97. doi: 10.1126/scisignal.aab2610. [DOI] [PubMed] [Google Scholar]
- 14.Xu T, et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature. 2017;548:228–233. doi: 10.1038/nature23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30:671–678. doi: 10.1038/nbt.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botman D, Tigchelaar W, Van Noorden CJ. Determination of phosphate-activated glutaminase activity and its kinetics in mouse tissues using metabolic mapping (quantitative enzyme histochemistry) J Histochem Cytochem. 2014;62:813–826. doi: 10.1369/0022155414551177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLaBarre B, Hurov J, Cianchetta G, Murray S, Dang L. Action at a distance: Allostery and the development of drugs to target cancer cell metabolism. Chem Biol. 2014;21:1143–1161. doi: 10.1016/j.chembiol.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S, et al. Glutaminase 1 plays a key role in the cell growth of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2017;19:76. doi: 10.1186/s13075-017-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida N, et al. ICER is requisite for Th17 differentiation. Nat Commun. 2016;7:12993. doi: 10.1038/ncomms12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Y, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel L. Immunometabolism in systemic lupus erythematosus. Nat Rev Rheumatol. 2017;13:280–290. doi: 10.1038/nrrheum.2017.43. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki CY, et al. p(70S6K1) in the TORC1 pathway is essential for the differentiation of Th17 Cells, but not Th1, Th2, or Treg cells in mice. Eur J Immunol. 2016;46:212–222. doi: 10.1002/eji.201445422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foulkes NS, Sassone-Corsi P. More is better: Activators and repressors from the same gene. Cell. 1992;68:411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- 27.Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: An alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki A, et al. Glutaminase and poly(ADP-ribose) polymerase inhibitors suppress pyrimidine synthesis and VHL-deficient renal cancers. J Clin Invest. 2017;127:1631–1645. doi: 10.1172/JCI87800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elgogary A, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci USA. 2016;113:E5328–E5336. doi: 10.1073/pnas.1611406113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla K, et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55:10551–10563. doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schütz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- 32.Koga T, et al. CaMK4-dependent activation of AKT/mTOR and CREM-α underlies autoimmunity-associated Th17 imbalance. J Clin Invest. 2014;124:2234–2245. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen HX, Beck KD, Anderson AJ. Quantitative assessment of immune cells in the injured spinal cord tissue by flow cytometry: A novel use for a cell purification method. J Vis Exp. 2011:2698. doi: 10.3791/2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Rodríguez N, et al. Pro-inflammatory self-reactive T cells are found within murine TCR-αβ(+) CD4(-) CD8(-) PD-1(+) cells. Eur J Immunol. 2016;46:1383–1391. doi: 10.1002/eji.201546056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.