Significance
Environmental enrichment (EE) is a neuroprotective strategy successfully employed in the treatment of cognitive deficits in different models of brain injury or disease, including Alzheimer’s disease. However, the models used to study the effects of EE include different aspects or components that go beyond the environmental changes, as animals submitted to EE also increase physical activity levels and social interaction. Here we show that EE neuroprotection in memory deficits related to amyloid-β neurotoxicity, rather than resulting from EE per se, may rely on increased physical activity and social interaction.
Keywords: Alzheimer’s disease, anaerobic physical exercise, object recognition, social recognition, oxidative stress
Abstract
Recently, nongenetic animal models to study the onset and development of Alzheimer’s disease (AD) have appeared, such as the intrahippocampal infusion of peptides present in Alzheimer amyloid plaques [i.e., amyloid-β (Aβ)]. Nonpharmacological approaches to AD treatment also have been advanced recently, which involve combinations of behavioral interventions whose specific effects are often difficult to determine. Here we isolate the neuroprotective effects of three of these interventions—environmental enrichment (EE), anaerobic physical exercise (AnPE), and social enrichment (SE)—on Aβ-induced oxidative stress and on impairments in learning and memory induced by Aβ. Wistar rats were submitted to 8 wk of EE, AnPE, or SE, followed by Aβ infusion in the dorsal hippocampus. Short-term memory (STM) and long-term memory (LTM) of object recognition (OR) and social recognition (SR) were evaluated. Biochemical assays determined hippocampal oxidative status: reactive oxygen species, lipid peroxidation by thiobarbituric acid reactive substance (TBARS) test, and total antioxidant capacity by ferric reducing/antioxidant power (FRAP), as well as acetylcholinesterase activity. Aβ infusion resulted in memory deficits and hippocampal oxidative damage. EE and AnPE prevented all memory deficits (STM and LTM of OR and SR) and lipid peroxidation (i.e., TBARS). SE prevented only the SR memory deficits and the decrease of total antioxidant capacity decrease (i.e., FRAP). Traditionally, findings obtained with EE protocols do not allow discrimination of the roles of the three individual factors involved. Here we demonstrate that EE and physical exercise have better neuroprotective effects than SE in memory deficits related to Aβ neurotoxicity in the AD model tested.
Alzheimer’s disease (AD) is a progressive brain disease that affects approximately 24 million people and causes irreversible neuronal losses, especially in the neocortex and hippocampus (1). Cognitive losses include impairments in attention, concentration, orientation, and particularly memory. The pathological hallmarks, besides the neuronal losses, include accumulation of extracellular senile plaques containing β-amyloid protein (Aβ) and several of its peptide oligomers, as well as neurofibrillary tangles of the protein tau (2–5).
The Aβ deposition results in neurotoxicity and oxidative damage, a central factor in the pathogenesis of AD (6–8). In postmortem AD brains, there is significant lipid, protein, and DNA oxidative damage, as well as reduced antioxidant defenses (6–8). Studies using cell culture and cell-free chemical systems have shown that Aβ induces higher production of reactive oxygen species (ROS) (1, 9). Therefore, Aβ injection into the brain is considered a valuable model to induce some characteristics of AD in the rat brain, especially the cognitive impairments (10, 11).
Environmental enrichment (EE) has been suggested as an efficient strategy to treat human AD and AD-like animal models (12, 13). EE has potential to ameliorate deficits resulting from the administration of Aβ protein oligomers (14, 15). However, EE as used in animal research usually includes other variables than perception and memorization, which make it difficult to determine the nature of its eventually favorable effects. Animals exposed to EE are maintained for long periods in large boxes with other conspecifics to promote interaction and socialization (16). The presence of conspecifics constitutes social enrichment (SE), which induces social interactions. Furthermore, activity wheels, tunnels, and toys that are made available in the boxes used to study EE induce intermittent physical exercise, which is known to promote neuroprotection (17). Social activity and exercise are offered in a permanent or rotational way (18, 19).
It is difficult to isolate the effects of exercise and SE on cognitive improvements. As a result, the traditional interpretations of EE results do not allow discrimination of the roles of the three individual factors involved (environment, exercise, and socialization). Although effects of EE in animals are not directly comparable vs. those used in humans, evidence suggests that increased socialization (i.e., SE) and exercise are major factors in reducing the symptoms of dementia in humans (20–22). For these reasons, we decided to compare the isolated effects of EE, anaerobic physical exercise (AnPE), and SE on the cognitive symptomatology resulting from a single Aβ infusion in the hippocampus of rats.
Results
Control Behavioral Results.
Measures of locomotion, exploration, and anxiety behaviors in the rats were not influenced by the procedures. No differences were observed among groups in open-field or plus-maze performance (Table 1).
Table 1.
Control behavioral tests
| Behavioral task | Control | Aβ | ||||||
| Control | EE | AnPE | SE | Aβ | EE | AnPE | SE | |
| Exploration time in OR | ||||||||
| Total exploration time in training, s | 43.73 (8.30) | 38.91 (15.67) | 42.16 (17.09) | 32.36 (16.81) | 41.33 (22.86) | 36.05 (12.58) | 41.21 (16.62) | 39.71 (15.79) |
| Total exploration time in STM test, s | 42.16 (17.09) | 35.64 (8.05) | 40.27 (25.46) | 32.99 (12.85) | 42.09 (13.68) | 42.81 (23.84) | 41.49 (14.02) | 33.63 (11.70) |
| Total exploration time in LTM test, s | 38.91 (15.67) | 36.53 (14.24) | 42.84 (15.61) | 34.34 (11.99) | 39.62 (10.55) | 35.36 (8.82) | 36.81 (12.31) | 35.73 (5.78) |
| Exploration time in SR | ||||||||
| Total exploration time in SR test, s | 96.21 (22.21) | 93.73 (25.06) | 81.90 (16.53) | 86.94 (26.41) | 83.10 (34.64) | 85.03 (11.15) | 84.86 (31.90) | 98.93 (25.80) |
| Open field, no. | ||||||||
| Crossing | 45.00 (7.59) | 45.00 (7.59) | 35.21 (3.36) | 34.75 (3.77) | 32.88 (3.36) | 45.43 (4.49) | 36.79 (3.88) | 49.22 (5.54) |
| Rearing | 15.50 (2.51) | 15.50 (2.51) | 15.57 (1.49) | 12.33 (2.02) | 15.53 (2.71) | 17.80 (2.38) | 14.64 (1.40) | 15.78 (3.20) |
| Plus maze | ||||||||
| Total entries | 22.46 (8.10) | 23.38 (7.14) | 20.47 (9.18) | 22.84 (9.05) | 19.42 (11.12) | 22.34 (9.14) | 21.57 (8.17) | 24.84 (12.08) |
| Time in open arms, s | 25.82 (22.05) | 27.09 (18.37) | 23.64 (22.01) | 27.56 (26.35) | 27.03 (21.78) | 23.14 (19.18) | 26.93 (23.93) | 21.41 (11.31) |
Aβ, EE, AnPE, and SE did not alter the total exploration time in OR and SR training and testing sessions, locomotor and exploratory activities in the open field, and anxiety behavior evaluated by plus maze. Mean (SD); n = 8–10/group.
Object Recognition Memory.
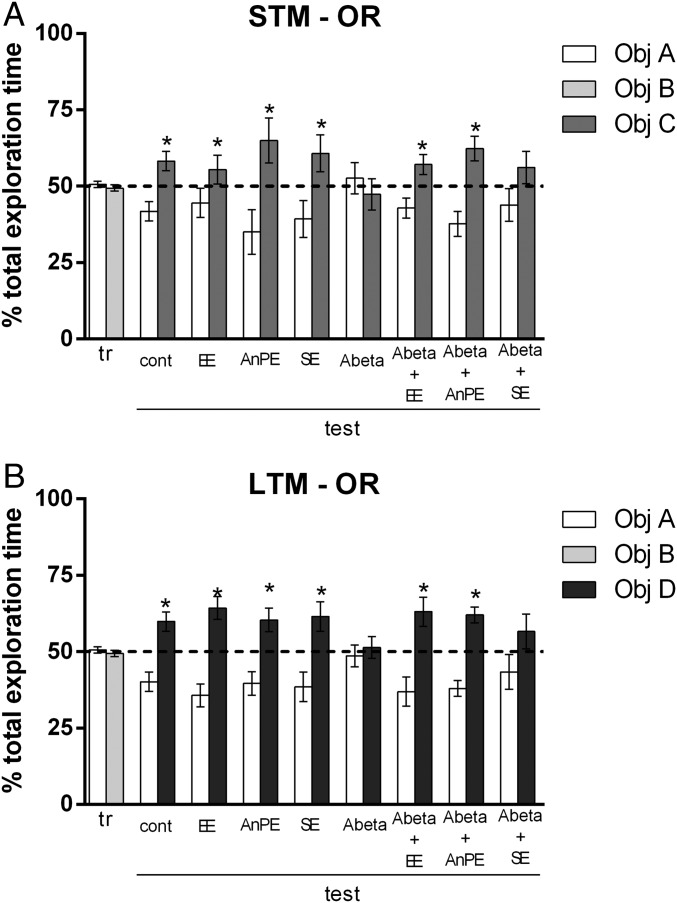
Rats explored each object (A and B) for a similar percentage of total exploration time in the training session [mean of all groups, object A = 50.60 ± 10.02%; B = 49.40 ± 10.02%; P = 0.55; t(94) = 0.58; Fig. 1, tr]. The control group did not show deficits in short-term memory (STM) in object recognition (OR) testing [P = 0.02; t(13) = 2.57; Fig. 1A, test, cont). Animals in EE, AnPE and SE groups also did not show deficits in STM OR testing (P < 0.05; Fig. 1A, test).
Fig. 1.
Aβ impairs short- (A) and long-term (B) OR memory. EE and AnPE during 8 wk prevents OR memory deficits; SE does not. Data are shown as mean and SE of the percentage of total exploration time (*P < 0.05 on one-sample t test, considering a theoretical mean of 50%; n = 8–14 per group). cont, control; tr, training.
Aβ rats presented deficits in OR STM, as they spent a similar time exploring the familiar and the novel object [P = 0.60; t(13) = 0.52; Fig. 1A, test, Abeta]. These deficits were not seen in animals exposed to EE and AnPE [P = 0.04, t(13) = 2.18 for Aβ+EE; P = 0.009, t(13) = 3.05 for Aβ+AnPE; Fig. 1A, test]. This protective effect was not seen in SE-treated rats [P = 0.28, t(8) = 1.14; Fig. 1A, test, Abeta + SE].
Control groups showed no deficits in OR testing in long-term memory (LTM) tests [P = 0.009; t(12) = 3.09; Fig. 1B, test, cont]. Animals in EE, AnPE, and SE groups did not show deficits in STM OR testing (P < 0.05; Fig. 1B, test). Aβ rats presented deficits in long-term OR memory, as they spent a similar time exploring the familiar and the novel object [P = 0.71; t(13) = 0.37; Fig. 1B, test, Abeta]. This deficit was not seen in animals subjected to EE or AnPE [P = 0.01, t(13) = 2.74 for Aβ+EE; P = 0.0005, t(13) = 4.56 for Aβ+AnPE; Fig. 1B, test]. SE did not protect animals from the deleterious effect of Aβ on OR memory [P = 0.27, t(8) = 1.16; Fig. 1B, test, Abeta + SE].
Social Recognition Memory.
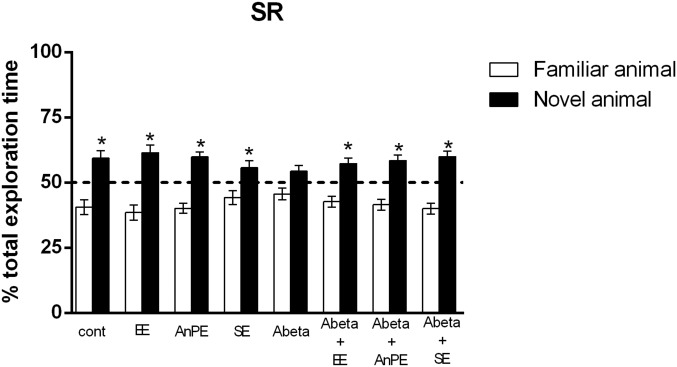
Control rats explored the new rat for a longer time than the familiar one [P < 0.001; t(16) = 10.23; Fig. 2, sham]. Animals in EE, AnPE, and SE groups did not differ significantly from controls (P < 0.05; Fig. 2). Aβ impaired social recognition memory by reducing the difference in the time spent exploring the familiar and the new rat [P = 0.07, t(11) = 1.93; Fig. 2, Aβ]. EE, AnPE, and SE protected rats against this deleterious effect of Aβ [P < 0.01, t(12) = 3.52 for Aβ+EE; P = 0.003, t(8) = 4.00 for Aβ+AnPE; and P < 0.01, t(7) = 4.83 for Aβ+SE; Fig. 2].
Fig. 2.
Aβ impairs social recognition memory. EE, AnPE, and SE for 8 wk prevent SR memory deficits. Data are presented as mean and SE of the percentage of the total exploration time (*P < 0.05 on one-sample t test, considering a theoretical mean of 50%; n = 8–13 per group). cont, control.
Hippocampal Oxidative Status.
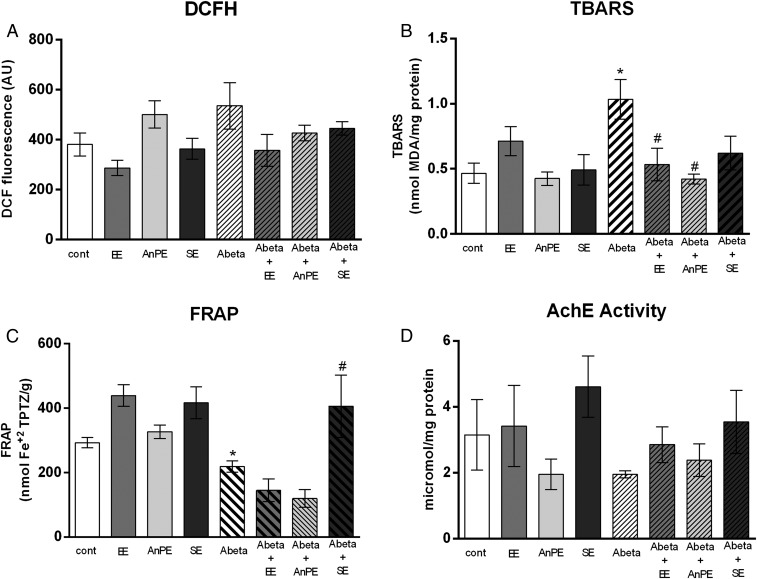
No significant differences in hippocampal ROS levels were observed among groups [P = 0.11, H(8) = 11.64; Fig. 3A]. An effect of the treatment was observed in hippocampal lipid peroxidation [i.e., thiobarbituric acid reactive substance (TBARS) test; P = 0.03, H(8) = 15.20; Fig. 3B]. Aβ rats showed greater lipid peroxidation than control rats (P = 0.004; Fig. 3B). Aβ rats subjected to EE and AnPE presented lower lipid peroxidation than Aβ-treated animals not subjected to any intervention [P = 0.02 for EE; P = 0.01 for AnE; Fig. 3B].
Fig. 3.
Effects of Aβ, EE, AnPE, and SE on the hippocampal oxidative status and AChE activity. (A) ROS determined by DCFH. (B) Lipid peroxidation determined by TBARS test. (C) Total antioxidant capacity determined by FRAP. (D) AChE activity. Data are presented as mean and SE (*P < 0.05, control vs. Aβ; #P < 0.05, Aβ vs. Aβ + specific intervention; Kruskal–Wallis test followed by t tests; n = 5–8 per group). cont, control.
Effect of treatment was observed in hippocampal total antioxidant capacity [i.e., ferric reducing/antioxidant power (FRAP); P < 0.01, H(8) = 36.42; Fig. 3C]. The Aβ group showed lower total antioxidant capacity than the control group (P = 0.02; Fig. 3C). Aβ rats subjected to SE presented higher antioxidant capacity than Aβ rats not subjected to any intervention (P = 0.02; Fig. 3C). Acetylcholinesterase (AChE) activity did not differ between the groups [P = 0.22; H(8) = 9.43; Fig. 3D].
Discussion
We investigated the isolated neuroprotective effects of EE, AnPE, and SE on the impairments of OR and SR memory and on those of hippocampus oxidative stress related to Aβ neurotoxicity. We demonstrated that EE and AnPE prevent impairments in object recognition memory (i.e., STM and LTM), whereas social recognition memory deficits were prevented by all interventions, including SE. Therefore, EE and AnPE interventions seem to be more effective in memory protection compared with SE. This result has important implication for basic research and further implication in behavioral interventions in conditions of degenerative brain diseases.
A single intrahippocampal injection of amyloid does not reproduce all of the pathological characteristics of AD and cannot be expected to do so. In fact, there is no single animal model of AD that reproduces all of the characteristics of the disease, and there are advantages of using genetic mouse models and injection of amyloid proteins in the rat brain. Although transgenic models more closely reflect the pathologic processes elicited in familiar AD (both are genetically driven), models with a minimal genetic invasiveness, like the one used here, offer the closest available analogy to the human sporadic AD pathology (23). Therefore, Aβ injection is an alternative AD animal model to the use of transgenic animals (10). The injection of Aβ into rat brain serves as a stimulus to induce proinflammatory reactivity, oxidative stress, and neurotoxicity (10). Here we opted for a model that we and other authors have used to describe neurotoxicity related to amyloid after Aβ 25–35 brain injection (24–26). In fact, evidence shows that the relative ratio of Aβ peptides is more crucial than the absolute amounts of peptides for the induction of neurotoxic conformations (27).
The Aβ 25–35 fragment used here shares with the Aβ 1–42 peptide the ability to self-aggregate and to induce neurotoxicity (11). The early soluble forms of Aβ peptides may interfere with neuronal network function related to AD learning and memory deficits (28–30). Furthermore, the presence of soluble forms of Aβ peptides could be more related to memory deficits of AD compared with fibrillar forms of Aβ, as well as those observed in transgenic AD animal models (31–33). Although Aβ 1–42 or 1–40 are more abundant than the 25–35 fragment in the extracellular aggregations of the AD brain, it is important to consider that they include the latter and that 25–35 is the most toxic region of Aβ (30, 34–36) and recently has been found to play a relevant role in AD because of its peculiar aggregation properties (36). Additionally, Aβ 25–35 seems to retain the toxicity of the full-length Aβ (1−40/42) peptides (37). Several other peptides, including numerous fragments, inversions, and variants of Aβ 25–35 or Aβ 1–42, have been used over the years as controls or otherwise studied for their effects since their introduction by Cotman and coworkers and others and found to be ineffective (11, 34–37). We injected Aβ directly into the dorsal hippocampus considering the role of hippocampus in memory and our previous results (26), but other hippocampal subregions or cortical or subcortical regions could also be considered.
Evidence suggests that OR (38, 39) and SR (40) involve, to a large extent, the hippocampus. In previous research studying hippocampal damage, these memory tests were sensitive to detect memory deficits, including those resulting from amyloid injection (26). OR and SR involve elements of exploration. In addition, EE generally alters exploratory behavior (41, 42), which could influence memory tests involving locomotion and exploration. To ensure that exploration would have no effect on our results, we performed a number of control behavioral tests to show that EE, AnPE, and SE do not influence locomotion and exploration in the animals (Table 1). Additionally, we compared the total exploration time on OR and SR tests and found no differences between the groups. These control measurements support the idea that the results represent cognitive/memory performance.
In addition to the behavioral results, we show that Aβ hippocampal infusion promotes oxidative damage (i.e., TBARS) and impairs the total antioxidant capacity (i.e., FRAP). In the AD brain, imbalances in homeostasis lead to the increase of ROS and lipid peroxidation, resulting in oxidative stress and damage (43, 44). The oxidative damage observed in the hippocampus after Aβ injection supports the concept that oxidative stress in the AD brain is associated with Aβ deposition and cognitive deficits observed (43, 45–47). Therefore, interventions avoiding or reducing oxidative stress or increasing antioxidant defenses are of interest in conditions of AD/Aβ neurotoxicity. Here we show that EE and AnPE (EE was performed without increases in physical exercise or socialization levels) prevent the effects of Aβ on lipid peroxidation in the hippocampus. ROS was not altered in the other group of animals, possibly because of the short t1/2 of the ROS produced. The same interventions that avoid TBARS increases (EE and AnPE) protected all memory parameters studied. SE was not sufficient to avoid TBARS increases, but did prevent the decrease of FRAP. We can link the TBARS results to the OR memory results (i.e., no oxidative damage indicates no memory deficit). FRAP results, on the contrary, could be linked to SR results. It is important to highlight that, even though lipid peroxidation is a common effect of oxidative stress, the antioxidant protection could come from different sources. Here, we investigated only the total antioxidant capacity. It does not ensure that other antioxidant enzymes are not involved and cannot be modulated by the interventions. Additionally, it is important to consider that the oxidative status is not static, but is modified continuously. Antioxidant defenses exhibit several adaptations to combat the insults generated all of the time. We verified the hippocampus antioxidant potential only at the end of the observation period.
We also measured AChE activity considering that the cholinergic system is the neurotransmitter system usually investigated in AD model conditions. We found no differences among groups regarding AChE. Further research is needed to determine if there is a change in the role of other neurotransmitter systems. It is also possible that the brain memory protection found might rely on other protective factors, as EE and AnPE have been shown to increase brain neuroplasticity (45–51). EE has been shown to increase neurogenesis, neurotrophin levels, neuronal survival, synaptogenesis, cellular proliferation, and dendritic arborization in different brain regions (52–55). Additionally, a reduction in oxidative stress in brain tissue was described for a genetic model of AD rat subjected to EE protocol (56). However, how many of these effects observed in response to EE protocols rely on EE per se and how many result from physical exercise and increase of social interaction (i.e., SE) was not determined. When the effects of physical exercise and social interaction components of an EE protocol were investigated after brain ischemia/reperfusion, physical exercise and SE reduced the brain lesion (i.e., infarct volume) and increased astrocyte proliferation and brain-derived neurotrophic factor expression (57). It suggests that the physical activity component of EE might be the most important factor concerning positive results on functional, biochemical, and histological outcomes. On the contrary, EE carried out in the absence of physical exercise can increase synaptogenesis and improves memory in normal rats (48), which suggests a time-dependent cognitive-enhancing effect of EE regardless of physical activity. Our results permit important interpretations in the discussion of each component of behavioral intervention that might have effects on specific parameters of behavioral and biochemical manifestations of AD.
Aerobic balanced training of at least moderate intensity is widely regarded as protective of cognition in AD (58, 59). On the contrary, “strength” training and AnPE seem to be particularly more effective to improve postural and motor functions (60–62). Here we considered the exercise as anaerobic because the EE protocols used in animal models include more anaerobic exercise performed in an intermittent configuration (e.g., animals climbing ramps and stairs, passing by tunnels). Indeed, AD is more common in elderly patients, in whom strength training is recommended to promote better muscular function, avoid falls, and improve balance and posture (63, 64).
Unlike EE and AnPE, in our experimental conditions, SE was able to prevent only social recognition memory deficits, and not object recognition deficits. In humans, an increase in social engagement with the surrounding environment has been positively correlated with angiogenesis, synaptogenesis, and neurogenesis, which are important factors for delaying the development of AD and cognitive dysfunctions (65). However, a recent study in rats has shown that SE may have only minor effects on neuroplasticity and cognition (17). There is little research on the effects of social engagement on AD prognosis related to AD animal models. Our results indicate that, in the rat model of Aβ injection, memory protection by SE was less effective than that by EE and AnPE. On the contrary, SE increases total antioxidant capacity, which might be viewed as a desirable effect. SE resulting in improvement of social recognition memory has been reported before (17). This result might come from the sheer practice of this behavior, given the presence of conspecifics in the training box that the rodents were accustomed to interacting with in their home cage environments.
In summary, we show that intrahippocampal Aβ infusion causes oxidative and memory damages that mimic those described or suggested to occur in AD. EE and AnPE cause a reversion of memory deficits (in OR and SR tests) and the effect of Aβ on lipid peroxidation (i.e., TBARS) induced by Aβ. SE prevents only the SR memory deficits and the decrease of antioxidant capacity (i.e., FRAP) caused by Aβ. Although further research is needed to elucidate the effects/mechanisms by which each one of the manipulations investigated act in the brain, we conclude that environmental and physical training components of EE protocols seem to be more important than SE in terms of neuroprotective effects. Our results are important because most of the studies that investigate the effects of EE do not consider that EE protocols usually involve an increment of physical activity and social interaction.
Materials and Methods
Animals and Experimental Design.
Male Wistar rats (age 3 mo; 350–380 g) were purchased from the Central Vivarium of the Federal University of Santa Maria (Brazil). During all of the experimental period, they were housed four per cage (except in SE group) and maintained under controlled light and environmental conditions (12 h light/12 h dark cycle; temperature of 23 ± 2 °C; humidity of 50 ± 10%) with food and water ad libitum.
All experiments were conducted in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication 80–23, revised 1996) and in agreement with the guidelines established by the local institutional animal care and use committee to ensure that the number of rats and their suffering were kept to a minimum (protocol 032/2016).
At the age of 3 mo, the animals were randomly assigned to one of the four experimental groups:
-
i)
Control (cont; n = 20): rats from this group were maintained in standard laboratory conditions (temperature 23 °C).
-
ii)
EE (n = 20): Rats subjected to EE for 8 wk;
-
iii)
AnPE (n = 20): Rats subjected to AnPE for 8 wk;
-
iv)
SE (n = 20): Rats subjected to SE for 8 wk.
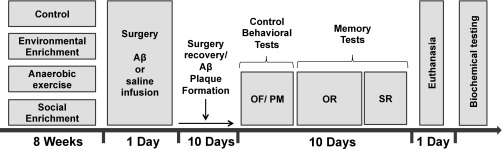
After 8 wk of intervention, half of the animals from each group underwent stereotaxic surgery to induce Aβ toxicity, an important characteristic of AD; the half was subjected to sham surgery without Aβ injection. After recovery from surgery, all rats were subjected to behavioral tests. The experimenter who was scoring task performance in OR and SR was blinded to the treatment status of the rats. When behavioral tests were finished, rats were euthanized for posterior brain tissue preparation and biochemical assays (Fig. 4).
Fig. 4.
Experimental design. Rats were maintained in standard housing conditions (control) or subjected to EE, anaerobic exercise, or SE during 8 wk. Afterward, they were subjected to stereotaxic surgery (66) with injection of Aβ or saline solution (sham surgery). Behavioral testing started 10 d after surgery to ensure surgery recovery and Aβ plaque formation. Euthanasia occurred 20 d after surgery; biochemical testing was the last step of the study. OF, open field; OR, OR memory test; PM, plus maze; SR, SR memory test.
Interventions.
EE.
Rats subjected to EE were housed in large polycarbonate cages (80 × 60 × 60 cm), four rats per cage, and provided with various objects of different shapes, sizes, colors, textures, and material (wood, plastic, and metal) such as plastic tubes, small balls, sound objects, and wooden houses (19, 67). During the 8 wk of intervention, the objects and their locations were renewed once per week to ensure novelty.
SE.
Rats subjected to SE were housed in large polycarbonate cages (80 × 60 × 60 cm), 10 rats per cage [adapted from Pascual et al. (68)].
AnPE.
The AnPE was resistance training that used a 1-m ladder with steps separated by 2 cm from one another, as described by Cassilhas et al. (69). Rats were familiarized with the exercise (i.e., climbing the ladder) for 3 d. In the first week after familiarization, the exercise was performed by using only the rat’s body mass as the resistance. To increase the workload, from the second week onward, additional mass was attached to the base of the rats’ tails. The initial workload was of 50% of the rat’s body weight and gradually increased throughout the subsequent 8-wk training period. The resistance training consisted of one set of eight repetitions with a 1-min rest interval between repetitions. The training was conducted 3 d per week for 8 wk. When the rats reached the top of the ladder, they were allowed to recover in the resting area.
Preparation of Aβ 25–35.
Aβ peptide 25–35 (A4559; Sigma Aldrich) was dissolved in saline solution (i.e., vehicle) at a concentration of 100 μM. Before intrahippocampal injection, Aβ was incubated at 37 °C for 4 d to induce Aβ 25–35 aggregation (70).
Stereotaxic Surgery.
The stereotaxic surgeries for intrahippocampal injection of 2 μL Aβ 25–35 or vehicle (saline solution) were performed after the intervention period. Rats were anesthetized with ketamine and xylazine (i.p. 75 mg/kg and 10 mg/kg, respectively). When the anesthetic plan had been confirmed, the rats were mounted into stereotaxic frames, and the CA1 region of the dorsal hippocampus was located based in the Paxinos and Watson brain atlas (anterior–posterior, −4.2; lateral–lateral, ±3.0; ventral–medial, −2.0 mm) (66). Bilateral infusions were performed by using a Hamilton syringe and an infusion bomb. After surgery, rats were returned to their cages and monitored for a 10-d period required for surgery recovery and to induct the aggregation of Aβ protein in rats’ hippocampi (25).
Control Behavioral Tasks.
To analyze exploratory and locomotor activities and ensure that any procedure impaired such behaviors, altering the memory tests results, 10 d after surgery, rats were placed on the left quadrant of a 50 × 50 × 50-cm open-field made with white-painted wood. Black lines were drawn on the floor to divide it into 12 equal quadrants. Crossing the quadrant lines and rearing were measured over a period of 5 min as measures for locomotor activity and exploration, respectively (40). To evaluate anxiety state, rats were exposed to an elevated plus maze, and the time spent in the open arms and the number of entries into them were recorded over a 5-min session (71).
Memory Behavioral Testing.
OR memory test.
Training and testing in the OR task were carried out in an open-field arena (50 × 50 × 50 cm) built with white-painted wood (72). Rats were first habituated individually in the apparatus and left to freely explore it for 20 min during four consecutive days before the training session. In the training session, two different objects (A and B) were placed in the apparatus, and rats were allowed to freely explore for 5 min. The objects were made of metal, glass, or glazed ceramic. Exploration was defined as sniffing or touching the objects with the nose and/or forepaws. Sitting on or turning around the objects were not considered as exploratory behaviors. After 3 h and 24 h, in the STM and LTM test sessions, one of the objects was randomly exchanged for a novel object (C and D, respectively), and the rats were reintroduced into the apparatus for 5 min. The time spent exploring the familiar and the novel object was recorded. To avoid confounding effects of lingering olfactory stimuli and preferences, the object and the arena were cleaned with 70% ethanol after testing each animal.
SR memory test.
The SR memory task is an adaptation of the social interaction test proposed by Kaidanovich et al. (73) as recently used by Garrido Zinn et al. (40). The task was completed in 3 d. First, the rats were placed in an arena for habituation (the same size and characteristics previously described for OR) with two small cages during 20 min for free exploration. The following day, training was performed with inclusion of one unfamiliar rat in the cages for 1 h of free exploration. After 24 h, testing was performed when the same rat from the training (i.e., familiar rat) and a new rat were placed for exploration for 5 min. The time spent exploring the new and the familiar rat were recorded. Exploration of the conspecific animal was defined as sniffing or touching the small cages with the nose and/or forepaws.
Biochemical Testing.
Tissue preparation.
Rats were euthanized 24 h after the behavioral experiments. The brain was removed, and bilateral hippocampi were quickly dissected out and homogenized in 50 mM Tris⋅HCl, pH 7.4. Then, samples were centrifuged at 2,400 × g for 20 min, and supernatants (i.e., S1) were used for the assay.
ROS levels.
ROS content was assessed by a spectrofluorometric method using 20,70-dichlorofluorescein diacetate (DCFH-DA) as a probe (74). The sample (S1) was incubated in darkness with 5 μL DCFH-DA (1 mM). The oxidation of DCHF-DA to fluorescent dichlorofluorescein (DCF) was measured for the detection of intracellular ROS. The formation of the oxidized fluorescent derivative (i.e., DCF), measured by DCF fluorescence intensity, was recorded at 520 nm (480-nm excitation) 30 min after the addition of DCFH-DA to the medium. Results were expressed as arbitrary units.
Detection of lipid peroxidation via TBARS test.
Lipoperoxidation was evaluated by the TBARS test (75). One aliquot of S1 was incubated with a 0.8% thiobarbituric acid solution, acetic acid buffer (pH 3.2), and SDS solution (8%) at 95 °C for 2 h, and the color reaction was measured at 532 nm. Results were expressed as nanomoles of malondialdehyde per milligram protein.
FRAP assay.
The working FRAP reagent was prepared by mixing 25 mL acetate buffer, 2.5 mL TPTZ solution, and 2.5 mL FeCl3⋅6H2O solution. Homogenate (10 μL) was added to the 300-μL working FRAP reagent in microplate (76). Additionally, a standard curve with 10 μL Trolox concentrations (15, 30, 60, 120, and 240 mM) and 300-μL working FRAP reagent was used. The microplate was incubated at 37°C for 15 min before reading in a SpectraMax M5 Microplate Reader at 593 nm.
AChE activity.
AChE is a marker of the loss of cholinergic neurons in the forebrain. The AChE activity was assessed by the Ellman method (77). The reaction mixture was composed of 100 mM phosphate buffer, pH 7.4, and 1 mM 5,5′-dithio-bis-2-nitrobenzoic acid. The method is based on the formation of a yellow anion, 4,4′-dithio-bis nitrobenzoic acid, after adding 0.8 mM acetylthiocholine iodide. The change in absorbance was measured for 2 min at 30-s intervals at 412 nm (SpectraMax M5; Molecular Devices). Results were expressed as micromoles of acetylthiocholine iodide hydrolyzed per minute per milligram of protein. Proteins were measured according to Bradford (78) using BSA as a standard.
Statistical Analysis.
Normality of data distribution was checked by using the Shapiro–Wilk test. Object exploration time in OR and conspecific animal exploration time in SR were converted to percentage of total exploration time, and a one-sample t test was used to compare the percentage of total time of exploration spent on each object or rat considering a theoretical mean of 50%. In open-field and plus-maze tests, data of all groups were compared by one-way ANOVA. Biochemical results of all groups were compared by using a Kruskal–Wallis test followed by specific t test. Significance level was set at 0.05.
Acknowledgments
This work was supported by research grants and fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (to I.I. and P.B.M.-C.); Conselho Nacional de Desenvolvimento Científico e Tecnológico (to I.I., P.B.M.-C., and F.P.C.); Federal University of Pampa (to F.P.C. and P.B.M.-C.); and For Women in Science Program - L’Oréal Foundation and UN Educational, Scientific, and Cultural Organization (to P.B.M.-C.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Chakrabarti S, Sinha M, Thakurta IG, Banerjee P, Chattopadhyay M. Oxidative stress and amyloid beta toxicity in Alzheimer’s disease: Intervention in a complex relationship by antioxidants. Curr Med Chem. 2013;20:4648–4664. doi: 10.2174/09298673113209990152. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Clark CM, Ewbank D, Lee VM-Y, Trojanowski JQ. Molecular pathology of Alzheimer’s disease: Neuronal cytoskeletal abnormalities. In: Growdon JH, Rossor MN, editors. The Dementias. Butterworth–Heinemann; Boston: 1998. pp. 285–304. [Google Scholar]
- 4.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 5.Dong S, Duan Y, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer’s disease: A re-evaluation of amyloid cascade hypothesis. Transl Neurodegener. 2012;1:18. doi: 10.1186/2047-9158-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield DA, Swomley AM, Sultana R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid Redox Signal. 2013;19:823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polidori MC, Nelles G. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease–challenges and perspectives. Curr Pharm Des. 2014;20:3083–3092. doi: 10.2174/13816128113196660706. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki H, et al. Amyloid β induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2005;12:19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- 10.McLarnon JG. Correlated inflammatory responses and neurodegeneration in peptide-injected animal models of Alzheimer’s disease. BioMed Res Int. 2014;2014:923670. doi: 10.1155/2014/923670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delobette S, Privat A, Maurice T. In vitro aggregation facilities beta-amyloid peptide-(25-35)-induced amnesia in the rat. Eur J Pharmacol. 1997;319:1–4. doi: 10.1016/s0014-2999(96)00922-3. [DOI] [PubMed] [Google Scholar]
- 12.Quattromani MJ, Cordeau P, Ruscher K, Kriz J, Wieloch T. Enriched housing down-regulates the Toll-like receptor 2 response in the mouse brain after experimental stroke. Neurobiol Dis. 2014;66:66–73. doi: 10.1016/j.nbd.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Hase Y, et al. Effects of environmental enrichment on white matter glial responses in a mouse model of chronic cerebral hypoperfusion. J Neuroinflammation. 2017;14:81. doi: 10.1186/s12974-017-0850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, et al. Environmental enrichment potently prevents microglia-mediated neuroinflammation by human amyloid β-protein oligomers. J Neurosci. 2016;36:9041–9056. doi: 10.1523/JNEUROSCI.1023-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart KE, et al. Mid-life environmental enrichment increases synaptic density in CA1 in a mouse model of Aβ-associated pathology and positively influences synaptic and cognitive health in healthy ageing. J Comp Neurol. 2017;525:1797–1810. doi: 10.1002/cne.24156. [DOI] [PubMed] [Google Scholar]
- 16.Vivinetto AL, Suárez MM, Rivarola MA. Neurobiological effects of neonatal maternal separation and post-weaning environmental enrichment. Behav Brain Res. 2013;240:110–118. doi: 10.1016/j.bbr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Brenes JC, et al. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J Comp Neurol. 2016;524:1586–1607. doi: 10.1002/cne.23842. [DOI] [PubMed] [Google Scholar]
- 18.Fiala B, Snow FM, Greenough WT. “Impoverished” rats weigh more than “enriched” rats because they eat more. Dev Psychobiol. 1977;10:537–541. doi: 10.1002/dev.420100607. [DOI] [PubMed] [Google Scholar]
- 19.Newberry RC. Environmental enrichment: Increasing the biological relevance of captive environments. Appl Anim Behav Sci. 1995;44:229–243. [Google Scholar]
- 20.Seo TB, et al. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J Exerc Rehabil. 2013;9:220–229. doi: 10.12965/jer.130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garland T, Jr, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: Human and rodent perspectives. J Exp Biol. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Do Carmo S, Cuello AC. Modeling Alzheimer’s disease in transgenic rats. Mol Neurodegener. 2013;8:37. doi: 10.1186/1750-1326-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vedagiri A, Thangarajan S. Mitigating effect of chrysin loaded solid lipid nanoparticles against amyloid β25-35 induced oxidative stress in rat hippocampal region: An efficient formulation approach for Alzheimer’s disease. Neuropeptides. 2016;58:111–125. doi: 10.1016/j.npep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Zussy C, et al. Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid β fragment in rats. Am J Pathol. 2011;179:315–334. doi: 10.1016/j.ajpath.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schimidt HL, Garcia A, Martins A, Mello-Carpes PB, Carpes FP. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer-like rat model. Food Res Int. 2017;100:442–448. doi: 10.1016/j.foodres.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Kuperstein I, et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lue LF, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Näslund J, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 30.Peña F, et al. Beta-amyloid protein (25-35) disrupts hippocampal network activity: Role of Fyn-kinase. Hippocampus. 2010;20:78–96. doi: 10.1002/hipo.20592. [DOI] [PubMed] [Google Scholar]
- 31.Moechars D, et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 32.Giacchino J, Criado JR, Games D, Henriksen S. In vivo synaptic transmission in young and aged amyloid precursor protein transgenic mice. Brain Res. 2000;876:185–190. doi: 10.1016/s0006-8993(00)02615-9. [DOI] [PubMed] [Google Scholar]
- 33.Stéphan A, Laroche S, Davis S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pike CJ, et al. Structure-activity analyses of beta-amyloid peptides: Contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- 35.Gruden MA, et al. Differential neuroimmune markers to the onset of Alzheimer’s disease neurodegeneration and dementia: Autoantibodies to Abeta((25-35)) oligomers, S100b and neurotransmitters. J Neuroimmunol. 2007;186:181–192. doi: 10.1016/j.jneuroim.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Millucci L, Ghezzi L, Bernardini G, Santucci A. Conformations and biological activities of amyloid beta peptide 25-35. Curr Protein Pept Sci. 2010;11:54–67. doi: 10.2174/138920310790274626. [DOI] [PubMed] [Google Scholar]
- 37.Naldi M, et al. Amyloid β-peptide 25-35 self-assembly and its inhibition: A model undecapeptide system to gain atomistic and secondary structure details of the Alzheimer’s disease process and treatment. ACS Chem Neurosci. 2012;3:952–962. doi: 10.1021/cn3000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2009;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Silveira CK, Furini CRG, Benetti F, Monteiro SC, Izquierdo I. The role of histamine receptors in the consolidation of object recognition memory. Neurobiol Learn Mem. 2013;103:64–71. doi: 10.1016/j.nlm.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Garrido Zinn C, et al. Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc Natl Acad Sci USA. 2016;113:E4914–E4919. doi: 10.1073/pnas.1609883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: Effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- 42.Van Waas M, Soffié M. Differential environmental modulations on locomotor activity, exploration and spatial behaviour in young and old rats. Physiol Behav. 1996;59:265–271. doi: 10.1016/0031-9384(95)02151-5. [DOI] [PubMed] [Google Scholar]
- 43.Chauhan V, Chauhan A. Oxidative stress in Alzheimer’s disease. Pathophysiology. 2006;13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: An update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 46.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Migliore L, et al. Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer’s disease and in other neurodegenerative diseases. Neurobiol Aging. 2005;26:587–595. doi: 10.1016/j.neurobiolaging.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Birch AM, McGarry NB, Kelly AM. Short-term environmental enrichment, in the absence of exercise, improves memory, and increases NGF concentration, early neuronal survival, and synaptogenesis in the dentate gyrus in a time-dependent manner. Hippocampus. 2013;23:437–450. doi: 10.1002/hipo.22103. [DOI] [PubMed] [Google Scholar]
- 49.Neidi R, et al. Late-life environmental enrichment induces acetylation events and nuclear factor κ-dependent regulations in the hippocampus of aged rats showing improved plasticity and learning. J Neurosci. 2016;36:4351–4361. doi: 10.1523/JNEUROSCI.3239-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- 51.Sakalem ME, et al. Environmental enrichment and physical exercise revert behavioral and electrophysiological impairments caused by reduced adult neurogenesis. Hippocampus. 2017;27:36–51. doi: 10.1002/hipo.22669. [DOI] [PubMed] [Google Scholar]
- 52.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 53.Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 54.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 55.Mohammed AH, et al. Environmental enrichment and the brain. Prog Brain Res. 2002;138:109–133. doi: 10.1016/S0079-6123(02)38074-9. [DOI] [PubMed] [Google Scholar]
- 56.Herring A, et al. Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathol. 2010;20:166–175. doi: 10.1111/j.1750-3639.2008.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Zhang X, Liao W, Wan Q. Effect of physical and social components of enriched environment on astrocytes proliferation in rats after cerebral ischemia/reperfusion injury. Neurochem Res. 2017;42:1308–1316. doi: 10.1007/s11064-016-2172-x. [DOI] [PubMed] [Google Scholar]
- 58.Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26:381–388. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winchester J, et al. Walking stabilizes cognitive functioning in Alzheimer’s disease (AD) across one year. Arch Gerontol Geriatr. 2013;56:96–103. doi: 10.1016/j.archger.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011;41:289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Granacher U, Gollhofer A, Strass D. Training induced adaptations in characteristics of postural reflexes in elderly men. Gait Posture. 2006;24:459–466. doi: 10.1016/j.gaitpost.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Malling AS, Jensen BR. Motor intensive anti-gravity training improves performance in dynamic balance related tasks in persons with Parkinson’s disease. Gait Posture. 2016;43:141–147. doi: 10.1016/j.gaitpost.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Hurley BF, Hagberg JM. Optimizing health in older persons: Aerobic or strength training? Exerc Sport Sci Rev. 1998;26:61–89. [PubMed] [Google Scholar]
- 64.Rubenstein LZ, et al. Effect of a group exercise program on strength, mobility, and falls among fall-prone elderly men. J Gerontol Med Sci. 2000;55:317–321. doi: 10.1093/gerona/55.6.m317. [DOI] [PubMed] [Google Scholar]
- 65.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 66.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Ed Academic; New York: 1986. [Google Scholar]
- 67.Leger M, et al. Environmental enrichment improves recent but not remote memory in association with a modified brain metabolic activation profile in adult mice. Behav Brain Res. 2012;228:22–29. doi: 10.1016/j.bbr.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 68.Pascual R, Zamora-León SP, Valero-Cabré A. Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta Neurobiol Exp (Warsz) 2006;66:7–14. doi: 10.55782/ane-2006-1582. [DOI] [PubMed] [Google Scholar]
- 69.Cassilhas RC, et al. Resistance exercise improves hippocampus-dependent memory. Braz J Med Biol Res. 2012;45:1215–1220. doi: 10.1590/S0100-879X2012007500138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghasemi R, Zarifkar A, Rastegar K, Maghsoudi N, Moosavi M. Repeated intra-hippocampal injection of beta-amyloid 25–35 induces a reproducible impairment of learning and memory: Considering caspase-3 and MAPKs activity. Eur J Pharmacol. 2014;726:33–40. doi: 10.1016/j.ejphar.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 71.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 72.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 73.Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J Vis Exp. 2011;48:2473. doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loetchutinat C, et al. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiat Phys Chem. 2005;72:323–331. [Google Scholar]
- 75.Ohkawa K. Promotion of renewal canes in greenhouse roses by 6-benzylaminopurine without cutback. HortScience. 1979;5:612–613. [Google Scholar]
- 76.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 77.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 78.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]