Significance
Posttranslational modifications (PTMs) on histone lysines regulate gene expression and physiological functions. Succinylation is a newly discovered PTM with distinctive features. However, rarely studies have shown the function of succinylation on histone lysines. Our biochemical and structural studies demonstrate that GAS41, an oncogene-coded protein, can act as the reader of succinylation on histone H3K122. The functional significance of the pH-dependent histidine of GAS41 recognizing succinyl lysine (Ksuc) could have implications in local pH-dysregulated circumstances. The mechanism illustrated by the structures also provides an important insight into the development of specific regulators targeting the GAS41 YEATS domain in the future.
Keywords: succinyl-lysine, reader, GAS41
Abstract
Lysine succinylation is a newly discovered posttranslational modification with distinctive physical properties. However, to date rarely have studies reported effectors capable of interpreting this modification on histones. Following our previous study of SIRT5 as an eraser of succinyl-lysine (Ksuc), here we identified the GAS41 YEATS domain as a reader of Ksuc on histones. Biochemical studies showed that the GAS41 YEATS domain presents significant binding affinity toward H3K122suc upon a protonated histidine residue. Furthermore, cellular studies showed that GAS41 had prominent interaction with H3K122suc on histones and also demonstrated the coenrichment of GAS41 and H3K122suc on the p21 promoter. To investigate the binding mechanism, we solved the crystal structure of the YEATS domain of Yaf9, the GAS41 homolog, in complex with an H3K122suc peptide that demonstrated the presence of a salt bridge formed when a protonated histidine residue (His39) recognizes the carboxyl terminal of the succinyl group. We also solved the apo structure of GAS41 YEATS domain, in which the conserved His43 residue superimposes well with His39 in the Yaf9 structure. Our findings identified a reader of succinyl-lysine, and the binding mechanism will provide insight into the development of specific regulators targeting GAS41.
Posttranslational modifications (PTMs) discovered on histones provide another layer of gene transcription regulation which complement the cis-regulatory elements in our genome. Emerging evidence shows that these PTMs, also collectively termed “histone codes,” function individually or in combinations to regulate nuclear processes such as transcription, DNA damage repair, replication, and chromatin condensation (1). Lysine succinylation (Ksuc) was initially identified as a novel protein PTM on Escherichia coli isocitrate dehydrogenase, serine hydroxymethyltransferase, and glyceraldehyde-3-phosphate dehydrogenase A (GAPDH) (2). Subsequently, Xie et al. (3) confirmed lysine succinylation as part of the histone code conserved in yeast, fruit flies, mouse, and human. Succinyl-lysine carries a negative charge, which distinguishes it from acetyl- and other aliphatic forms of modified lysines, and provides the basis for more characteristic chemical interactions (Fig. 1A). The dramatic change of side-chain charge on histone lysines contributed by succinylation may imply its unique role in nucleosomal functioning distinct from competing modifications (2). The dynamic system of histone codes falls under the control of “writer” and “eraser” enzymes dedicated to specific modification sites and types (1, 4). Meanwhile, the “readers” translate the histone code and disseminate the message into myriad cell phenotypes and behaviors (4, 5). Succinyl-CoA is believed to be a cofactor for succinyl-transferase activity on various substrate proteins, analogous to the addition of acetyl groups, or alternatively, it may directly react with freely exposed lysines for succinylation to take place (2, 6, 7). Recently, acetyltransferase KAT2A was found to couple with nuclear-translocated α-Ketoglutarate Dehydrogenase complex to write succinylation from the coenzyme onto H3K79 (8). Our previous study identified SIRT5, one of the NAD-dependent deacylases, as a potent desuccinylase and demalonylase on lysines (9, 10). However, little is known about the readers for the distinct Ksuc, which motivates the studies that discover potential candidates and advance our knowledge of its biological functions.
Fig. 1.
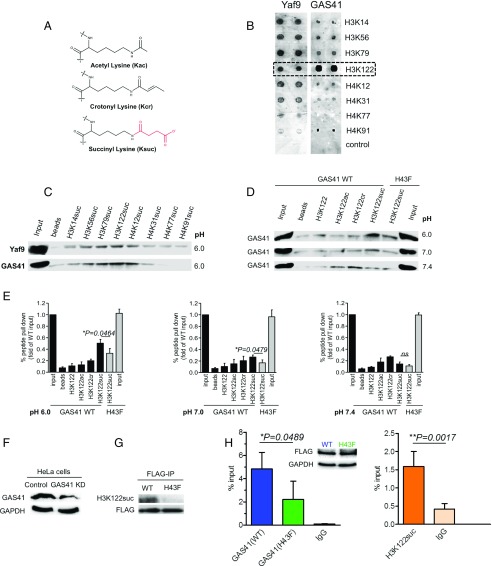
The YEATS domains of Yaf9 and GAS41 are pH-dependent succinyl readers on histones. (A) Different chemical structures of acetyl-lysine (Kac), crotonyl-lysine (Kcr), and succinyl-lysine (Ksuc). The succinyl acyl group is colored in red. (B) Microarray screening assay of Yaf9 and GAS41 YEATS domains against H3 and H4 peptides succinylated on previously identified succinyl-lysine sites. H3K122suc binds both Yaf9 and GAS41 (highlighted in dashed box). (C) Peptide pull-down assay of succinyl-lysine peptides against Yaf9 or GAS41 YEATS domain. (D) Peptide pull-down assays of GAS41 WT against different acyl groups on H3K122 peptides under pH 6.0, pH 7.0, and pH 7.4 conditions. GAS41 mutant (H43F) shows decreased binding affinity toward H3K122suc peptide. Images are representative of three independent experiments for each condition. (E) Statistical analysis of the efficiency of peptide pull-down assays in D. (F) Confirmation of GAS41 knockdown (KD) by Western blot. GAPDH was used as loading control. (G) Western blot for the identification of H3K122suc in anti-FLAG immunoprecipitates. FLAG-tagged GAS41 proteins were used as loading control. (H) The coenrichment of GAS41 and H3K122suc on p21 promoter. ChIP-qPCR analysis using anti-FLAG antibody and IgG control to probe the enrichment of FLAG-tagged WT or mutant (H43F) GAS41 on p21 promoter in cells (Left). ChIP-qPCR analysis using anti-H3K122suc antibody and IgG control to probe the enrichment of H3K122suc on p21 promoter in cells (Right). A Western blot with anti-FLAG and anti-GAPDH antibodies shows that both constructs are expressed at appropriate levels.
YEATS domain was initially characterized in Yaf9 as a crucial subunit of yeast histone acetyltransferase NuA4 (11). The YEATS domain-containing proteins, including Yaf9, Taf14, ENL, AF9, and Glioma-Amplified Sequence-41 (GAS41), are involved in acetyltransferase or methyltransferase complexes, which participate in the processes of chromatin remodeling and gene transcription (12). Shi and coworkers (13, 14) illustrated that human AF9 and ENL YEATS domains could recognize acetyl-lysine (Kac) to mediate normal physiological processes, but the readout could also underlie severe pathological conditions. Subsequently, studies have reported human AF9, YEATS2, and ENL and yeast Taf14 YEATS domains as readers of crotonyl-lysine (Kcr), whereby AF9 YEATS domain senses an enrichment of H3K18cr deposited by p300 to coordinate a mechanism for transcriptional activation of inflammation genes (15–18). YEATS domain adopts a classical immunoglobin fold structure of eight antiparallel β-strands and two α-helices (19). Currently, all solved YEATS domain structures reveal the consistent positioning of the aliphatic modified-lysine ligand sandwiched between two aromatic residues, establishing a robust π-π-π stacking as the recognition mechanism underlying crotonyl-lysine readout capability of Taf14 and AF9 (15–18).
Results and Discussion
Prediction of the Readout of GAS41 or Yaf9 Toward Ksuc.
In favor of hydrophobic interactions, the extremely low binding affinity between AF9 and Ksuc was attributed to a key hydrophobic aromatic residue (Phe28) on loop 1 located at the end of the acyl-binding pocket of its YEATS domain (17). The residue at this position may have a crucial role in the recognition of different acyl groups, which has displayed a major discrepancy across human YEATS domains with a phenylalanine (Phe) in AF9 and ENL, a serine (Ser) in YEATS2, and a histidine (His) in GAS41 (SI Appendix, Fig. S1) (15). As a comparative case, YEATS2 can favorably bind 2-hydroxyisobutyryl-lysine (Khib) while AF9 cannot, probably due to the contribution of a hydrogen bond by the Ser254 hydroxyl group in YEATS2 (15, 17). Intriguingly, GAS41 has a histidine residue at this position. GAS41, otherwise known as YEATS4 (YEATS Domain-containing Protein 4), is a newly discovered oncogene regulating the p53-p21 pathway (20). However, despite the availability of crystal structures of its homolog Yaf9, the role of GAS41 YEATS domain has been left unexplored (19). In concordance with GAS41, Yaf9 has a histidine residue that surrogates the key Phe residue recognizing Kcr in human AF9 when superimposed (SI Appendix, Fig. S2 A and B). As histidine could gain a positive charge when protonated (21), therefore, we proposed that human GAS41, as well as yeast Yaf9, may have the preference for an acyl group with a negative charge, such as the succinyl group.
Screening of the Potential Ksuc Sites Binding to GAS41 or Yaf9.
To test our hypothesis, we first applied peptide microarray assay to profile the binding affinities of yeast Yaf9 or human GAS41 YEATS domain against N-terminal biotinylated peptides bearing a subset of succinyl-lysine sites on histone H3 or H4 (Ksuc-H3/H4) (19). Different experimental methods have been used to measure pH of resting yeast at 5.8 and 6.35, and that of fermenting yeast at 6.1 and 7.1 (22, 23); the intracellular pH of yeast could be acidic, thus prompting us to choose pH 6.0 for testing. We also chose the succinyl-lysine sites that are physiologically detectable in HeLa cells (3). The screening results showed that both Yaf9 and GAS41 YEATS domains could interact with Ksuc on H3 and H4 peptides with binding preferences for H3. Both Yaf9 and GAS41 displayed strong binding affinity toward the H3K14suc, H3K56suc, H3K79suc, and H3K122suc peptides while Yaf9 could also bind the H4K12suc and H4K31suc peptides (Fig. 1B). GAS41 showed a stronger binding affinity toward the H3K122suc peptide than the others. Moreover, we coupled these peptides onto streptavidin agarose to perform a peptide pull-down assay (24). The results confirmed that both Yaf9 and GAS41 YEATS domains were able to bind Ksuc on histone H3 and H4 peptides upon a protonated histidine under pH 6.0, but their affinities with H3K122suc were relatively stronger than the others (Fig. 1C). Therefore, H3K122suc stood out for further studies because of its similarly strong binding toward both Yaf9 and GAS41.
Measurement of GAS41 or Yaf9 Binding Toward Acetyl-, Crotonyl-, Succinyl H3K122 Peptides.
Next, we compared the binding capabilities of different acyl groups on H3K122 against GAS41 YEATS domain under different pH conditions. As reported, the intracellular pH value varies in different cellular organelles (from ∼pH 4.7 to ∼pH 7.4), owing to the activities of various metabolic pathways such as ATP production in the cytoplasm (25). The regulation of nuclear pH is hypothesized to have a mechanism similar to that in the cytoplasm, but this requires experimental validation (25). Therefore, we chose pH 6.0, pH 7.0, or pH 7.4 for the following experiments. The results of peptide pull-down revealed that the H3K122suc peptide, rather than the others, showed prominent binding ability toward GAS41 under pH 6.0 or pH 7.0 (Fig. 1D). Mutation of the proposed key residue from His to Phe (H43F) significantly decreased the binding affinity. When the pH reached 7.4, H3K122suc demonstrated dramatically decreased binding toward GAS41, while H3K122cr and H3K122ac showed increased affinity to GAS41 (Fig. 1D). Quantification by statistical analysis supported the scenario of the above peptide pull-down experiment (Fig. 1E). Together, the GAS41 YEATS domain can interact with H3K122suc presumably upon a protonated histidine. Furthermore, we measured the binding affinities of both GAS41 and Yaf9 YEATS domains against different acylations on H3K122 peptide with surface plasmon resonance spectroscopy (SPR). In line with the peptide pull-down assay, the SPR experiment was also performed at pH 6.0, 7.0, and 7.4 (Table 1). Each measurement was repeated three times. At pH 7.4, affinity of H3K122suc binding was not determined, but at pH 7.0, it was calculated to be 48.34 ± 3.8 μM. This suggests that the proportion of protonated His43 increases from pH 7.4 to 7.0. Affirmatively, a stronger binding affinity between GAS41 and the H3K122suc peptide was determined to be 2.937 ± 0.21 μM at pH 6.0. The mutation H43F in GAS41 sharply decreased its affinity toward the H3K122suc peptide (Table 1). Together, upon pH variation from pH 7.4 to pH 6.0, we observed an increase in binding affinity of GAS41 toward H3K122suc, in contrast to the corresponding decrease in affinity toward H3K122cr (Table 1). The affinities did not vary as much toward H3K122ac from pH 7.4 to 7.0. Additionally, Yaf9 was able to bind H3K122suc with an affinity of 27.44 ± 3.4 μM at pH 6.0 while it has no detectable binding toward H3K122cr and H3K122ac. Altogether, the results suggest that GAS41 and Yaf9 YEATS domains can interact with H3K122suc peptide in a pH-dependent manner, probably through the protonation of His43, which facilitates recognition of the Ksuc carboxyl terminal.
Table 1.
The binding affinities of GAS41 and Yaf9 YEATS domains against different acylations on H3K122 peptides measured by surface plasmon resonance spectroscopy
| Peptides | pH | Kd, μM | |
| GAS41 | Yaf9 | ||
| H3K122suc | 6.0 | 2.937 ± 0.21* (WT) | 27.44 ± 3.4 |
| 103.7 ± 16 (H43F) | |||
| 7.0 | 48.34 ± 3.8* (WT) | ND | |
| 102.0 ± 1.5 (H43F) | |||
| 7.4 | ND (WT) | ND | |
| ND (H43F) | |||
| H3K122cr | 6.0 | 79.82 ± 1.0 | ND |
| 7.0 | 31.27 ± 2.6 | ||
| 7.4 | 18.23 ± 4.3 | ||
| H3K122ac | 6.0 | ND | ND |
| 7.0 | 51.94 ± 6.1 | ||
| 7.4 | 47.68 ± 6.7 | ||
Kd values are calculated with an equilibrium model, except when marked with an *, the values are calculated with kinetic rate constants. ND, not detectable. Choice of analysis and the original rate constants are available in the SI Appendix.
Cellular Studies of GAS41 Binding H3K122suc on Histones.
Subsequently, we investigated whether the interaction between GAS41 YEATS domain and H3K122suc is physiologically relevant in cells. It is previously described that GAS41 could act as a transcription factor involved in cancer development, while H3K122suc was detected in HeLa cells (3, 20). Peptide dot-blot assays showed that the anti-H3K122suc antibody specifically recognizes H3K122suc, but not other modification types or sites including unmodified histone H3K122, H3K122ac, H3K122cr, and other identified Ksuc sites on H3 (SI Appendix, Fig. S3). We then generated GAS41 knockdown (KD) HeLa cells (Fig. 1F), in which FLAG-tagged constructs of full-length GAS41 WT or mutant (H43F) were overexpressed. FLAG immunoprecipitation (FLAG-IP) was then carried out to analyze the content of H3K122suc in FLAG immunoprecipitates. Western blot analysis showed that GAS41 WT, rather than the mutant (H43F), had prominent interaction with H3K122suc in cells (Fig. 1G), suggesting the essential role of this key histidine residue during the interaction. Furthermore, we also performed ChIP-qPCR analysis to investigate cellular coenrichment of GAS41 and H3K122suc. As reported, GAS41 was located on p14ARF and the p21 promoter in proliferating cells (26). Our data underscored the fact that GAS41 WT enriches on the p21 promoter and showed the low occupancy of the mutant (H43F) accordingly (Fig. 1H, Left histogram). H3K122ac was also detected on the p21 promoter (26–29). Thus, H3K122suc is proposed to competitively occupy the p21 promoter region. ChIP-qPCR results confirmed the enrichment of H3K122suc on the p21 promoter in cells (Fig. 1H, Right histogram). Therefore, GAS41, but not the mutant, could be coenriched with H3K122suc on the p21 promoter, implicating a relevant physiological interaction in cells. Collectively, the cellular data supported that GAS41 could act as a reader of histone succinylation in cells.
Molecular Mechanism of H3K122suc Readout.
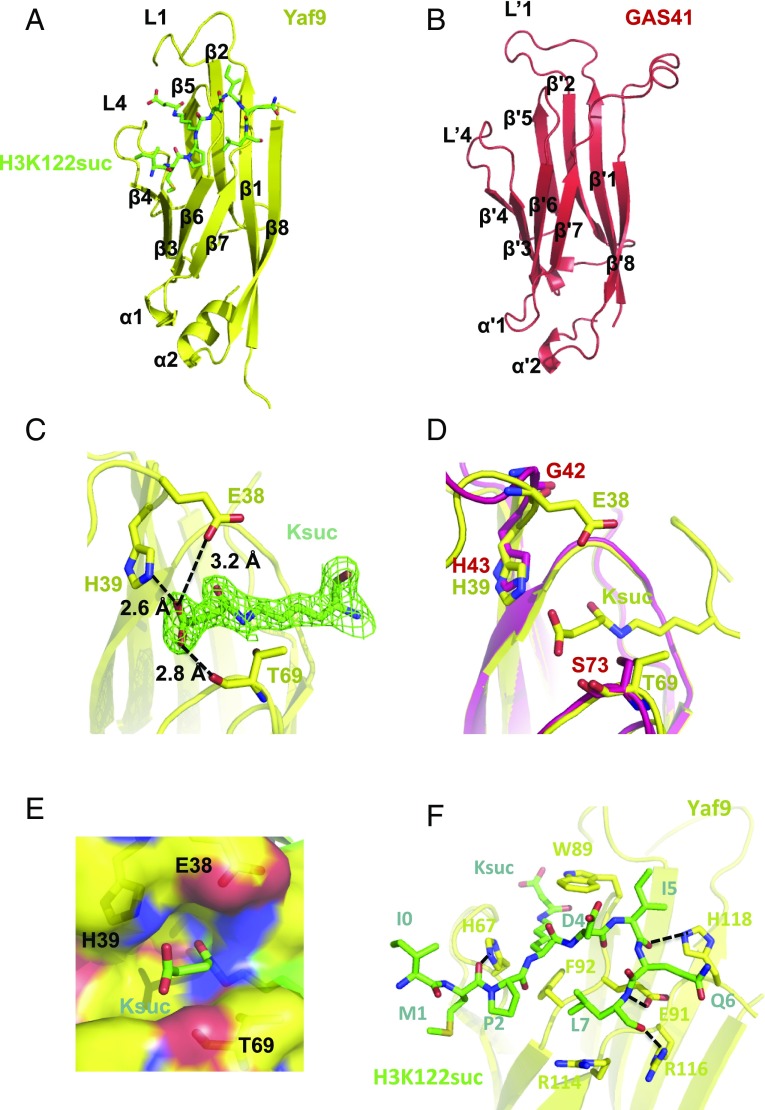
To gain insight into the molecular basis underlying Ksuc readout by GAS41, we performed crystallographic studies of GAS41 and Yaf9 YEATS domains in complex with the H3K122suc peptide. Crystals of the Yaf9/H3K122suc complex could diffract to 2.0-Å resolution after optimization. Statistics of the crystallographic data collection and refinement are summarized in SI Appendix, Table S1. The overall structure of Yaf9 shows a typical YEATS domain with eight antiparallel β-strands capped by two short α-helices (Fig. 2A). Despite our efforts to cocrystallize GAS41 with H3K122suc peptide, only the apo structure of GAS41 was obtained at 2.6-Å resolution (SI Appendix, Table S1). The structure of GAS41 again demonstrates a classical YEATS domain that superimposes well with Yaf9 (Fig. 2B and SI Appendix, Fig. S4). In the complex, the succinyl-lysine is anchored in the groove of Yaf9 formed between loops 1 and 4 (Fig. 2A). 2Fo-Fc omit map (1σ) shows the complete density of Ksuc anchored inside the pocket (Fig. 2C). Remarkably, the carboxyl terminal of Ksuc could be recognized by His39 via a salt bridge as well as by Glu38 and Thr69 through hydrogen bonds (Fig. 2C). The pattern of H3K122suc bound to Yaf9 YEATS domain is similar to that of H3K9cr being recognized by human AF9 (PDB ID code 5HJB) (SI Appendix, Fig. S5). The succinyl-lysine superimposes well with crotonyl-lysine in the pockets of both yeast Yaf9 and human AF9 YEATS domains (SI Appendix, Fig. S5). The His39 of Yaf9 aligns best with Phe28 of human AF9, which distinguishes Yaf9 as a reader of Ksuc (SI Appendix, Fig. S5). Superimposition of the residues Trp89 and Tyr70 of Yaf9 to Tyr78 and Phe59 of AF9, respectively, demonstrates the typical binding pocket constituents adopted by the YEATS domains for the readouts of different acyl-lysines (SI Appendix, Fig. S5). The distance between the imidazole ring of His39 and the carboxyl group is 2.6 Å, which is comparable to that between Arg105 and H3K9suc (2.7 Å) in the SIRT5 complex structure (10), indicating a strong electrostatic interaction.
Fig. 2.
Molecular basis of H3K122suc readout by Yaf9 and GAS41 YEATS domains. (A) The overall structure of Yaf9 YEATS domain (yellow) in complex with H3K122suc (green). (B) The apo structure of GAS41 YEATS domain (red). (C) The Ksuc readout by Yaf9 via the hydrophilic interactions between H39, E38, and T69 residues and carboxyl terminal of succinyl group. 2Fo-Fc omit map (1σ) shows that the succinyl-lysine is well-defined in the pocket. (D) Superimposition of the pockets of Yaf9/H3K122suc (yellow) and GAS41 (Red) YEATS domains. (E) The electrostatic potential map for the interactions between residues and succinyl group. The negative potential is colored in red while the positive potential is colored in blue. (F) Interactions between H3 peptide (green) and Yaf9 YEATS domain (yellow).
Notably, His43 of GAS41 is conserved and superimposes well with His39 of Yaf9 in the pocket, demonstrating a similar layout of the H3K122suc-binding pocket residues (Fig. 2D). As aforementioned, mutation of this His to Phe (H43F) significantly decreased the binding affinity of GAS41 to the H3K122suc peptide in both pull-down and SPR assays (Fig. 1 D and E and Table 1), which again emphasizes the key role of this histidine for acyl recognition. Additionally, the electrostatic potential was calculated to illustrate charge distribution in the interaction environment within the binding pocket (Fig. 2E). Meanwhile, the structure revealed an aromatic sandwiching cage, which specifically accommodates the flat carbonyl group (C = O) on the ligand by snugly clamping it via π stacking into a well-oriented position and permits other associated intermolecular interactions (SI Appendix, Fig. S6). Moreover, 2Fo-Fc omit map (1σ) shows that the flanking residues of the H3K122suc peptide could be well-defined in the structure (SI Appendix, Fig. S7). They form intimate contacts with the surface residues of Yaf9 YEATS domain (Fig. 2F). The hydrophobic interaction between the residues of H3K122suc peptide, including M1, P2, I4, Q6, and L7, and the residues H67, W89, F92, and H118 of Yaf9 stabilizes the binding (Fig. 2F). It may explain why H3K122suc could standout to bind both Yaf9 and GAS41, probably due to the additional interactions available for the specific peptide sequence. Moreover, hydrogen bonds formed between the residues H67, E91, R116, and H118 of Yaf9 and the main chain of H3K122suc peptide also contribute to the interaction (Fig. 2F). Therefore, GAS41 is able to recognize Ksuc with a mechanism similar to Yaf9, utilizing the characteristic salt bridge established with a protonated histidine residue.
Conclusion
With a rapidly expanding library of PTM marks on histones, it is exciting to understand their interplay and the emerging important roles that lysine acylations play in epigenetic regulation on nucleosomes. Succinylation is a newly discovered PTM with distinct structural and chemical properties. YEATS domain-containing proteins have been reported as readers of Kac or Kcr on histones. Our cellular and structural studies revealed that GAS41 is a lysine succinylation reader owing to a protonated histidine residue located inside the pocket. As reported, intracellular pH could be regulated by global histone acetylation at a genomic level by the relevant physiological functions in normal or cancer cells (30). The wax and wane of the binding affinities of GAS41 toward H3K122suc and H3K122cr indicate the switching role of GAS41 upon pH variation. The functional significance of the pH-dependent histidine switch of GAS41 for Ksuc recognition could have implications in local pH-dysregulated circumstances (31). This cross-talk for GAS41 recognizing Kac, Kcr, or Ksuc upon intracellular pH variation would be an interesting area for further investigation. Currently, there are more than 20 Ksuc sites identified on various histones in cancer cells (3), which urges further investigation on profiling GAS41 readouts of other potential histone Ksuc sites and other types of acyl-lysines with a terminal carboxyl group including malonyl- and glutaryl-lysine, to comprehensively elucidate GAS41-mediated epigenetic regulation.
Materials and Methods
Full details are provided in SI Materials and Methods. The in vitro screening of the potential Ksuc sites binding to GAS41 or Yaf9 was achieved by microarray assay using proteins against N-terminal biotinylated peptides that bear a subset of succinyl-lysine sites on histone H3 or H4. The measurement of GAS41 or Yaf9 binding toward acetyl-, crotonyl-, succinyl H3K122 was performed by peptide pull-down and SPR assay. For the cellular study, the coenrichment of GAS41 and H3K122suc on the p21 promoter was detected by ChIP-qPCR. Then, the crystallizations of GAS41 and Yaf9 with H3K122suc were carried out and diffraction data were collected at the Shanghai and Taiwan synchrotron facilities.
Supplementary Material
Acknowledgments
We thank Dr. Michael S. Kobor from the Centre for Molecular Medicine and Therapeutics, Canada, for providing the Yaf9 construct. The crystallographic data were collected on BL17U of the Shanghai Synchrotron Radiation Facility and beamline BL13C1 at the Taiwan National Synchrotron Radiation Research Center. This work was supported by Hong Kong Research Grants Council Grants C7037-14G, AoE/P-705/16, and HKU2/CRF/13G and National Natural Science Foundation of China Grant 21572190.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 5WYI and 5Y8V).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717664115/-/DCSupplemental.
References
- 1.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, et al. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Z, et al. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics. 2012;11:100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seet BT, Dikic I, Zhou MM, Pawson T. Reading protein modifications with interaction domains. Nat Rev Mol Cell Biol. 2006;7:473–483. doi: 10.1038/nrm1960. [DOI] [PubMed] [Google Scholar]
- 5.Collignon J, Perea-Gomez A. Epigenetic regulation of pluripotency in the early mouse embryo. Med Sci (Paris) 2007;23:679–681. doi: 10.1051/medsci/20072389679. [DOI] [PubMed] [Google Scholar]
- 6.Weinert BT, et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4:842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Peng C, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10:M111.012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyltransferase. Nature. 2017;552:273–277. doi: 10.1038/nature25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, et al. The bicyclic intermediate structure provides insights into the desuccinylation mechanism of human sirtuin 5 (SIRT5) J Biol Chem. 2012;287:28307–28314. doi: 10.1074/jbc.M112.384511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Masson I, et al. Yaf9, a novel NuA4 histone acetyltransferase subunit, is required for the cellular response to spindle stress in yeast. Mol Cell Biol. 2003;23:6086–6102. doi: 10.1128/MCB.23.17.6086-6102.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze JM, Wang AY, Kobor MS. YEATS domain proteins: a diverse family with many links to chromatin modification and transcription. Biochem Cell Biol. 2009;87:65–75. doi: 10.1139/O08-111. [DOI] [PubMed] [Google Scholar]
- 13.Wan L, et al. ENL links histone acetylation to oncogenic gene expression in acute myeloid leukaemia. Nature. 2017;543:265–269. doi: 10.1038/nature21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, et al. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell. 2014;159:558–571. doi: 10.1016/j.cell.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, et al. Molecular coupling of histone crotonylation and active transcription by AF9 YEATS domain. Mol Cell. 2016;62:181–193. doi: 10.1016/j.molcel.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao D, et al. YEATS2 is a selective histone crotonylation reader. Cell Res. 2016;26:629–632. doi: 10.1038/cr.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, et al. Structural insights into histone crotonyl-lysine recognition by the AF9 YEATS domain. Structure. 2016;24:1606–1612. doi: 10.1016/j.str.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews FH, et al. The Taf14 YEATS domain is a reader of histone crotonylation. Nat Chem Biol. 2016;12:396–398. doi: 10.1038/nchembio.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang AY, et al. Asf1-like structure of the conserved Yaf9 YEATS domain and role in H2A.Z deposition and acetylation. Proc Natl Acad Sci USA. 2009;106:21573–21578. doi: 10.1073/pnas.0906539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pikor LA, et al. YEATS4 is a novel oncogene amplified in non-small cell lung cancer that regulates the p53 pathway. Cancer Res. 2013;73:7301–7312. doi: 10.1158/0008-5472.CAN-13-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava J, Barber DL, Jacobson MP. Intracellular pH sensors: Design principles and functional significance. Physiology (Bethesda) 2007;22:30–39. doi: 10.1152/physiol.00035.2006. [DOI] [PubMed] [Google Scholar]
- 22.Conway EJ, Downey M. pH values of the yeast cell. Biochem J. 1950;47:355–360. doi: 10.1042/bj0470355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slavík J. Intracellular pH of yeast cells measured with fluorescent probes. FEBS Lett. 1982;140:22–26. doi: 10.1016/0014-5793(82)80512-7. [DOI] [PubMed] [Google Scholar]
- 24.Wysocka J. Identifying novel proteins recognizing histone modifications using peptide pull-down assay. Methods. 2006;40:339–343. doi: 10.1016/j.ymeth.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Roeder RG. GAS41 is required for repression of the p53 tumor suppressor pathway during normal cellular proliferation. Mol Cell Biol. 2006;26:4006–4016. doi: 10.1128/MCB.02185-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tropberger P, et al. Regulation of transcription through acetylation of H3K122 on the lateral surface of the histone octamer. Cell. 2013;152:859–872. doi: 10.1016/j.cell.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Gévry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L. p21 transcription is regulated by differential localization of histone H2A.Z. Genes Dev. 2007;21:1869–1881. doi: 10.1101/gad.1545707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradeepa MM, et al. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet. 2016;48:681–686. doi: 10.1038/ng.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBrian MA, et al. Histone acetylation regulates intracellular pH. Mol Cell. 2013;49:310–321. doi: 10.1016/j.molcel.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: A perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.