Significance
This study defines clinical indications for using telomere length (TL) measurement as a diagnostic tool in a hospital setting. It shows that, in contrast to other methods, TL measurement by flow cytometry and FISH (flowFISH) can be standardized, and has reproducible and definable upper and lower normal boundaries. In telomerase mutation carriers and carriers of other mutant telomere maintenance genes, TL had prognostic value, correlating with the age of onset of short telomeres syndrome phenotypes, as well as the predominant complication. In a prospective study, TL results were actionable in one-fourth of cases with idiopathic bone marrow failure affecting the stem cell donor choice and/or treatment regimen. The data show that, for targeted clinical indications, and in a hospital setting, TL measurement by flowFISH informs patient care decisions.
Keywords: aplastic anemia, interstitial lung disease, primary immunodeficiency, liver disease, precision medicine
Abstract
Telomere length (TL) predicts the onset of cellular senescence in vitro but the diagnostic utility of TL measurement in clinical settings is not fully known. We tested the value of TL measurement by flow cytometry and FISH (flowFISH) in patients with mutations in telomerase and telomere maintenance genes. TL had a discrete and reproducible normal range with definable upper and lower boundaries. While TL above the 50th age-adjusted percentile had a 100% negative predictive value for clinically relevant mutations, the lower threshold in mutation carriers was age-dependent, and adult mutation carriers often overlapped with the lowest decile of controls. The extent of telomere shortening correlated with the age at diagnosis as well as the short telomere syndrome phenotype. Extremely short TL caused bone marrow failure and immunodeficiency in children and young adults, while milder defects manifested as pulmonary fibrosis-emphysema in adults. We prospectively examined whether TL altered treatment decisions for newly diagnosed idiopathic bone marrow failure patients and found abnormally short TL enriched for patients with mutations in some inherited bone marrow failure genes, such as RUNX1, in addition to telomerase and telomere maintenance genes. The result was actionable, altering the choice of treatment regimen and/or hematopoietic stem cell donor in one-fourth of the cases (9 of 38, 24%). We conclude that TL measurement by flowFISH, when used for targeted clinical indications and in limited settings, can influence treatment decisions in ways that improve outcome.
When primary human fibroblasts are grown in culture, they have a finite replicative potential; it is predictable based on the length of telomere repeat DNA (1). Telomeres define the ends of chromosomes and function to preserve genome integrity; they are comprised of TTAGGG sequences that are bound by specialized proteins (2). Telomere length (TL) shortens during DNA replication and, at a critical threshold, the shortest telomere(s) activate a DNA damage response that signals cell death or a permanent cell cycle arrest, known as cellular senescence (3, 4). The observations in cultured cells, and the fact that TL shortens with aging, have led to a hypothesized role for telomere shortening in human aging and age-related disease (1, 5); however, the short TL threshold that is clinically relevant for disease risk is not known, and whether TL measurement can influence treatment decisions in clinical settings has not been determined.
The short telomere syndromes are a group of genetic disorders that are caused by mutations in components of the telomerase enzyme and other telomere maintenance genes (6). They provide a model for understanding the causal role of short telomeres in human disease. Their manifestations include a heterogeneous spectrum of pathologies that include primary immunodeficiency and bone marrow failure (7–9). The most common short telomere syndrome pathologies are idiopathic pulmonary fibrosis (IPF) and related interstitial lung disorders (10, 11). Severe emphysema, alone or combined with fibrosis, may also manifest in short telomere patients who are smokers (12). Mutations in the essential telomerase enzyme genes, TERT, the telomerase reverse transcriptase, and TR, the telomerase RNA component (also known as TERC), are the most common cause of IPF, and the frequency of TERT mutations in severe, early-onset emphysema rivals the prevalence of α-1 antitrypsin deficiency (12). The prevalence of telomerase mutations in adult-onset lung disease makes the short telomere syndromes the most-common premature aging disorders, with an estimated 10,000 affected individuals in the United States alone (13). Identifying these patients is critical for clinical management, as they are susceptible to life-threatening toxicities from DNA damaging agents and other cytotoxic therapies (14–17). In the hematopoietic stem cell transplant setting for bone marrow failure in particular, reduced intensity regimens improve outcomes of short telomere syndrome patients (14). However, the vast majority of these patients lack recognizable clinical features at the bedside, and there is often no notable family history at the time of diagnosis (9, 18). There is therefore a clinical need for molecular diagnostic tools for the evaluation of short telomere syndromes.
To identify an ideal method for TL measurement in clinical settings, we considered such a method must be reproducible and amenable to standardizing. One method that was developed for epidemiologic studies measures TL by quantitative PCR; however, this method has shown a 20% variability rate across laboratories (19, 20). The variability lies in part due to its sensitivity to DNA quality and extraction methods, while also being prone to error propagation with PCR amplification (21, 22). Circulating leukocytes additionally have variable TL depending on their replicative histories (23), thus making the analysis of total leukocyte TL, by quantitative PCR as well as by the Southern blotting method, confounded by fluctuations in leukocyte ratios. Importantly, in contrast to other genotypes, TL is a continuous variable, and the absence of standardized normal ranges and abnormal TL thresholds presents an impediment to translating research findings to clinical settings. Here, we report a hospital-based experience of TL testing by automated flow cytometry and FISH (flowFISH) (24). This method was developed to measure single-cell TL using a fluorescently labeled probe that hybridizes to telomere DNA (24). We show that peripheral blood TL measurement by flowFISH is a gold standard for clinical use; it informs diagnostic and patient care decisions in clinical settings.
Results
TL Has a Discrete and Reproducible Normal Range in the Human Population.
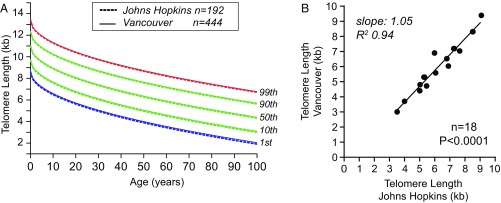
To test the relevance of TL measurement for patient care decisions, we first sought to establish a normal range based on values obtained from 192 controls we recruited across the age spectrum at The Johns Hopkins Hospital (Table S1). TL shortened with age in lymphocytes and showed a normal distribution at every age, similar to prior reports (11, 23) (Fig. 1A, Fig. S1A, and Table S2). The reproducibility for TL measured by flowFISH was superior to quantitative PCR with an intra- and interassay coefficient of variation (CV) of 2.2% and 2.5% for lymphocytes, compared with 8.0% and 25.0%, respectively (Fig. S1 B–D). We compared the interlaboratory reproducibility with a Vancouver laboratory that uses flowFISH and found an outstanding concordance (R2 0.94, slope 1.04, P < 0.0001, linear regression) (Fig. 1B and Fig. S1 B and C). To test the generalizability of this normal range, we compared the nomogram from the Baltimore population with data published from an ethnically different population of 444 controls recruited in Vancouver (23), and found they were nearly identical in both range and percentile distributions (Fig. 1A). The concordance was tighter for lymphocytes than granulocytes, likely in part because of the known effects of freezing on granulocyte TL (23) (Fig. S1 E and F). These data indicated that TL, across different populations and as measured by flowFISH, has reproducible upper and lower boundaries.
Fig. 1.
TL by flowFISH shows a reproducible normal range. (A) Nomogram of lymphocyte lengths from Johns Hopkins controls (n = 192) and controls from Vancouver (n = 444) with percentile lines as annotated. (B) Interlaboratory reproducibility of lymphocyte TL measurements from 18 samples, processed independently, shows outstanding concordance by linear regression.
There Is an Age-Dependent Short TL Threshold Associated with Disease Risk.
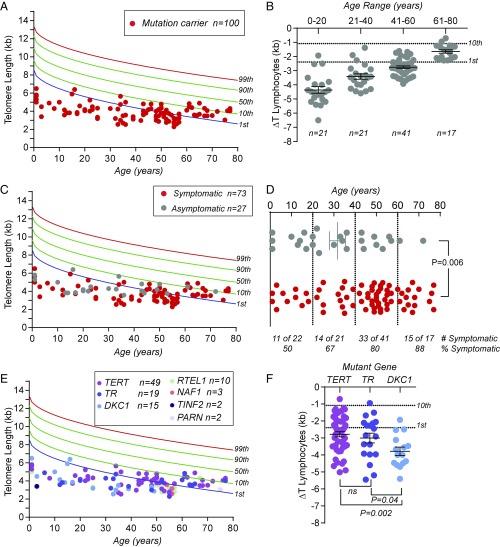
We next examined whether TL can be used to identify patients with germline defects in telomere maintenance. We recruited 100 individuals from 60 families who carried known pathogenic mutations in telomerase and other telomere maintenance genes (Table S3). These mutations, which were deemed pathogenic based on segregation in families and functional molecular evidence, fell in seven genes: TERT, TR, DKC1, RTEL1, PARN, TINF2, and NAF1 (Table S4). TERT mutations were most common, seen in 49 of the cases. The mean age of mutation carriers was 41 y (range, 8 mo to 77 y) and 54% were male. None of these patients had TL above the age-adjusted 50th percentile, indicating a 100% negative predictive value for identifying a clinically meaningful mutation in telomerase/telomere maintenance genes (Fig. 2A). We examined whether there is a TL threshold, relative to controls, that helps to identify telomerase and telomere maintenance gene-mutation carriers, and found an age-dependent effect. Because the trends we will report hereafter also hold for granulocytes, we summarized the analyses for lymphocytes, and the respective granulocyte data are shown in Fig. S2. There was no single short TL threshold that encompassed all mutation carriers across the entire age spectrum. This finding is in contrast to conclusions from a study that was limited to a predominantly pediatric population (25). We found that, whereas in children under the age of 20 y TL deviated significantly from the age-adjusted median control length, adults over the age of 60 y usually overlapped with the lowest decile of healthy controls [mean deviation from age-adjusted median (ΔT) −4.4 vs. −1.6 kb, respectively, P < 0.0001, Mann–Whitney U test] (Fig. 2 A and B). In a similar analysis, 39 of 42 (93%) of patients who were identified before the age of 40 y had TL below the first percentile, while only 9 of 58 (16%) diagnosed after 40 y fell in that range (Fig. 2B). There was also a notable dropout of mutation carriers who had TL below the first percentile after age of 60 y, possibly reflecting attrition related to more severe telomere-mediated pathology (Fig. 2 A and B). These data indicated that TL testing has an outstanding negative predictive value for excluding individuals with clinically relevant mutations across the age spectrum; however, its highest sensitivity is in patients who are younger than 40 y.
Fig. 2.
TL has age-dependent diagnostic thresholds. (A and B) Lymphocyte TL measurements from 100 telomerase and telomere maintenance gene mutation carriers relative to the nomogram with the age-adjusted deviation from the median (ΔT) shown by two-decade intervals. In B, 1st and 10th percentile thresholds are annotated to the right. (C) Data from A separated by mutation carriers who had symptoms, defined as primary immunodeficiency, bone marrow failure, liver disease, or pulmonary fibrosis-emphysema, and those who had no symptoms. (D) The proportion of symptomatic patients increases with age, as indicated by the red circles and quantified in the proportions listed below each age group. (E) TL in lymphocytes annotated by mutant gene. (F) Shorter TL in DKC1 mutation carriers (males) relative to TERT and TR. Graphs in B, D, and E indicate means ± SEM, Mann–Whitney U test.
The Degree of Telomere Shortening Correlates with Disease Onset and Type in Mutation Carriers.
To assess if TL correlates with clinical outcomes, we queried the phenotypes of these 100 mutation carriers. We found a significant subset was asymptomatic (27%); these patients had been identified because of a diagnosis in a symptomatic relative. The extent of telomere shortening in these asymptomatic individuals was similar to symptomatic patients (mean ΔT −3.1 vs. −3.0, P = 0.7, Mann–Whitney U test), suggesting the short telomere defect itself is not pathognomonic for a disease state. However, in this cross-sectional analysis, we noted that the proportion of symptomatic patients increased with age: 10 of 21 (50%) who were younger than 20 y showed symptoms compared with 15 of 17 (88%) above 60 y [P = 0.015, Fisher’s exact test (Fig. 2D); odds ratio 8.3 (95% confidence interval, 1.5–45.4)]. On average, symptomatic individuals were 13 y older than asymptomatic mutation carriers (mean 44.6 vs. 31.7 y, P = 0.006, Mann–Whitney U test) (Fig. 2D). There was no significant correlation between TL and the mutant gene (Fig. 2E), except for DKC1 mutation carriers (all male) who had shorter TL relative to TERT and TR mutation carriers (Fig. 2E and Fig. S2B). Notably, among these 100 mutation carriers, only 7, all who were male, had any of the classic mucocutaneous features of dyskeratosis congenita. These data underscore the importance of molecular diagnostic tools for identifying this population.
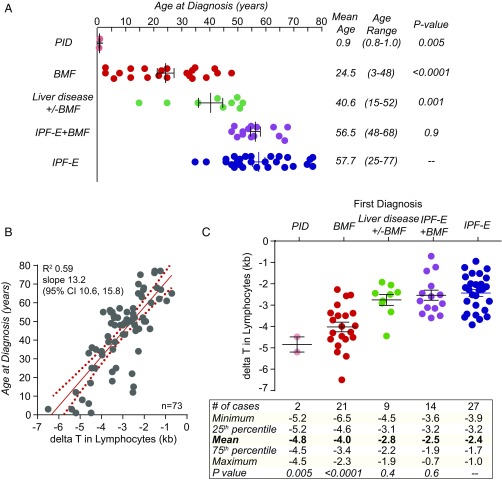
We next tested whether in the 73 symptomatic mutation carriers, TL had predictive value. We focused on four common degenerative, fatal complications, and noted several significant associations. There was strong evidence for an age-dependent predilection as to the predominant short telomere syndrome phenotype, as has been hypothesized (18). Patients who developed bone marrow failure first were on average three decades younger than pulmonary fibrosis-emphysema patients with normal blood counts (mean 24.5 vs. 57.3 y, P < 0.001, Mann–Whitney U test) (Fig. 3A). There was also a continuous correlation between the severity of the telomere defect and the age at diagnosis as quantified by the ΔT in lymphocytes (Fig. 3B and Fig. S2 C and D). Patients with more severe telomere shortening manifested earlier in life, while patients with milder defects developed disease in adulthood (R2 0.59, slope 13.2, 95% confidence interval 10.6–15.8). The correlation between TL severity and disease onset resembled the causal relationship seen between short telomeres and disease onset in mice with short telomeres (26, 27). There was also a correlation between TL and disease complication. Patients with bone marrow failure had significantly shorter age-adjusted TL than patients with lung disease (mean ΔT −4.0 kb vs. −2.0 kb, P < 0.001, Mann–Whitney U test) (Fig. 3C). In contrast, patients with liver disease presented at an intermediate age (mean 40.6 y, range 15–52), and had intermediate TL (mean ΔT −2.8 kb) relative to bone marrow failure and pulmonary fibrosis patients (Fig. 3 A and C). Notably, there was a demarcation of lung disease to adulthood, and we found no cases of de novo pulmonary fibrosis (i.e., outside of the posthematopoietic stem cell transplant setting) before the age of 35 y (41 of 41 cases) (Fig. 3A). These data have implications for counseling and surveillance of patients with mutations in telomerase and telomere maintenance genes.
Fig. 3.
TL correlates with disease onset and disease type. (A) Dot plot shows age-dependent manifestations of the four common short telomere syndrome features. IPF-E refers to idiopathic pulmonary fibrosis with or without emphysema. BMF refers to bone marrow failure. PID refers to severe immunodeficiency, presenting usually in the setting of enterocolitis in infants. The P values to the right indicate difference in age relative to IPF-emphysema (Mann–Whitney U test). (B) Linear regression shows a correlation between deviation of lymphocyte TL from the age-adjusted median (ΔT) and the age at diagnosis of one of four short telomere syndrome features. The linear regression line and 95% confidence intervals are shown for the 73 symptomatic individuals from A. (C) The disease type correlates with ΔT with the percentile ranges, as shown below in the tabulated data. P values in C reflect ΔT comparisons relative to idiopathic pulmonary fibrosis-emphysema patients (Mann–Whitney U test).
TL Results Are Actionable for Bone Marrow Failure Patients.
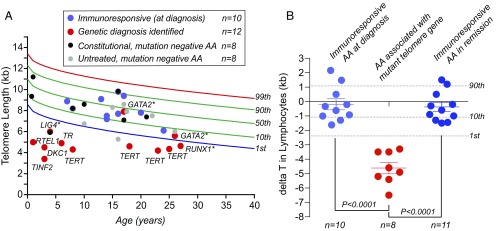
We next examined whether TL can distinguish the etiology of aplastic anemia, given the risk of morbidity with standard regimens in patients with short telomere–mediated bone marrow failure. We recruited 38 patients younger than the age of 40 y as this is the age range when telomere-mediated aplastic anemia first manifests (Table S5). The study was observational, with results disclosed to the treating clinician in real time. Its endpoints were either a genetic diagnosis or response to immunosuppression. In parallel, we screened for the common inherited bone marrow failure genes (details in Materials and Methods), and correlated the genetic data with TL. Among 38 recruited patients, 22 reached an informative endpoint with a documented response to immunosuppression (n = 10) or an identifiable genetic cause (n = 12) (Fig. 4A). All 10 patients who were treated with immunosuppression and responded had lymphocyte TL longer than the age-adjusted first percentile (Fig. 4 and Table S4). All of them had documented improvements in blood counts (partial response n = 5, complete response n = 5) (Fig. 4). In contrast, all of the patients with mutations in telomerase or telomere maintenance genes had short TL below the first percentile (8 of 8), with mutations identified in TERT, TR, DKC1, TINF2, and RTEL1 (Fig. 4). None of these eight patients had a family history or any stigmata of a short telomere syndrome at the time of diagnosis, underscoring the importance of TL as a diagnostic tool in this setting. In addition, two other patients, with LIG4 and RUNX1 mutations, also had TL shorter than the first percentile, suggesting that this range may enrich for other inherited bone marrow failure syndromes. The two patients we identified with GATA2 mutations had lymphocyte TL in the range of immunoresponders. To test if prior immunosuppression may affect TL in the diagnostic setting, we studied aplastic anemia patients who were in remission after treatment initiation (median 67 mo, range 14–180) (Table S6). Similar to the de novo aplastic anemia patients who went on to respond to immunosuppression, these retrospectively studied patients (11 of 11, 100%) had TL above the first percentile (Fig. 4B). These data suggested that longer lymphocyte TL enriches for immune-mediated forms of aplastic anemia and distinguishes them from short telomere–mediated bone marrow failure. We finally tracked whether TL results altered the management for the patients we recruited. In 9 of the 38 patients we prospectively followed (24%); the short TL result was actionable. It altered the treatment program by the primary clinician, including in changing the choice of hematopoietic stem cell donor through an evaluation of TL or genetic causes in relatives, reducing the transplant preparative regimen intensity, or in avoiding the use of empiric trials of immunosuppression therapy.
Fig. 4.
Utility of TL in the diagnosis of idiopathic bone marrow failure. (A) Lymphocyte TL in 38 prospectively recruited patients with idiopathic aplastic anemia (AA) relative to controls. The red circles denote patients for whom a genetic diagnosis was identified with documentation of a mutation in a telomere maintenance gene (TERT n = 4, TR n = 1, RTEL1 n = 1, DKC1 n = 1, TINF2 n = 1) or nontelomere gene (GATA2 n = 2, RUNX1 n = 1, LIG4 n = 1). The latter group is denoted by an asterisk (*). The blue circles denote patients treated with immunosuppression who responded (all the treated patients responded). The remaining cases, denoted by black and gray circles, denote cases of constitutional aplastic anemia and untreated cases, respectively. Larger circles indicate the 22 patients who reached an informative endpoint (either a genetic diagnosis made or treatment with immunosuppression). (B) The degree of deviation from the age-adjusted median (ΔT) from three groups is shown: prospectively recruited patients who had a response to immunosuppression at 1 y (n = 10, 5 complete response, 5 partial response), patients with telomerase and telomere gene mutations identified in A (TERT, TR, RTEL1, DKC1, TINF2, n = 8), as well as 11 patients successfully treated with immunosuppression who were in remission for 2 y or more. Means ± SEM are shown, Mann–Whitney U test. The 1st, 10th, and 90th percentiles are annotated to the right in B.
Discussion
Although telomere shortening has been associated in epidemiologic studies with numerous conditions, its causal role in mediating disease is most strongly linked to genetic disorders caused by mutations in telomerase and other telomere maintenance genes. In this hospital-based study, we found TL by flowFISH had outstanding interlaboratory reproducibility, superior to any other TL measurement method heretofore tested. Importantly, we found the flowFISH-derived normal TL range is definable and can be standardized. Given the narrow normal range of TL in the human population (Fig. 1A), our data indicate that flowFISH is a gold standard for TL testing in patient care settings.
Our study focused on four degenerative disease presentations that are most commonly associated with fatal complications, but patients with mutations in telomerase and telomere maintenance genes may also manifest with enteropathy, infertility, and cancer (28–30). short telomere syndrome patients have an increased risk of myelodysplastic syndrome and acute myeloid leukemia, as well as squamous cell cancers (30); recognizing these patients has similar implications and vigilance with cytotoxic treatments. TL testing provides distinct information from DNA sequencing as it can clarify the functional significance of DNA sequence variants. The degree of TL deviation from the median is also predictive and holds prognostic information as to the approximate timing of disease onset and the predominant phenotype likely to develop. Such findings make TL measurement critical for genetic counseling and clinical symptom assessment in patients with pathogenic mutations. The genetic basis of short telomere phenotypes is still uncharacterized in approximately one-third to one-half of cases, and TL testing may also be useful in recognizing some of these patients.
Our data highlight the importance of integrating clinical and genetic findings with TL in diagnostic settings because we did not find a threshold that encompassed all mutation carriers. In our study, which included patients across entire age spectrum, we found that an arbitrary threshold (such as the first percentile) would miss a subset of children with short telomere syndromes and nearly all adult mutation carriers. While TL in this older age group has less diagnostic specificity than in patients younger than 40 y, the negative predictive value remains outstanding and nearly all of these patients fall below the 10th age-adjusted percentile. Moreover, the severity of the short telomere defect within this group of patients with predominantly pulmonary fibrosis may predict certain complications. We also found that the first percentile threshold is not specific to short telomere syndromes, and other inherited disorders that cause bone marrow failure may be associated with very short TL in leukocytes. The mechanisms are unknown, but the short TL in these cases may reflect an acquired attrition related to increased cell turnover, such as with the mutant transcription factor RUNX1, or to the fact that mutant genes may possibly affect TL maintenance, such as with LIG4. Regardless, the biology and natural history of these disorders are distinct from short telomere syndromes even though the leukocyte TL may at times be short. The diagnostic criteria for short telomeres syndromes thus require integrating TL results with clinical findings, and where possible, genetic information. It is important to note that we focused our analyses on lymphocyte and granulocyte TL measurements. We note that while TL measurement in lymphocyte subsets is technically feasible (23), it has failed to show superiority over total lymphocyte TL in distinguishing dyskeratosis congenita from other inherited bone marrow failure syndromes (31).
There has been a recent emerging trend of direct to consumer advertising of TL measurement claiming it may be used to predict biological age and fitness. These methods generally rely on PCR-based quantification, which has shown low reproducibility in the literature (19) and in our study. This hospital-based experience provides an opportunity to caution clinicians and patients. TL has a normal distribution at every age and small deviations from the median within this distribution still fall in the normal range, and their significance should not be overinterpreted or equated with aging or youthfulness. Rigor in TL measurement is also critical, as the predicted effect size of TL changes with some environmental exposures, as reported in the literature, is often within the error rate of the measurement for the quantitative PCR method (32). Data from recently published large Mendelian randomization studies have identified links between polymorphisms associated with longer TL and the risk of common cancers, including lung adenocarcinoma, melanoma, and glioma (33). The upper threshold that increases the risk of these cancers is not known, but these recent findings add significant warning to the oversimplified interpretation of short TL being linked to aging and long telomeres to youth. Overall, the evidence we present suggests that TL testing should be targeted, and that it is most informative in high-yield diagnostic settings, such as suspected cases of short telomere syndromes, the interpretation of genetic variation in telomerase and telomere maintenance genes, and in the evaluation of bone marrow failure and related disorders. When a robust and validated method is utilized in these definable settings, the results have the potential to avert significant morbidity and alter treatment decisions in a way that advances patient care outcomes.
Materials and Methods
Study Review and Approval.
The clinical studies included here were reviewed and approved by the Johns Hopkins Medicine Institutional Review Board, and all of the research subjects gave written, informed consent.
Healthy Controls.
To establish a normal range for TL, we recruited 183 volunteers from the Baltimore area across the age spectrum from 2010 to 2011. Volunteers were interviewed and excluded if they reported having a history of HIV, cancer, hemophilia, or pulmonary fibrosis, or if they answered “yes” when asked about a history of a major illness. Race was self-reported as African American, Asian, or Caucasian. Peripheral blood was collected in EDTA-containing vacutainer tubes (20 cc; Becton-Dickinson). Cord blood was harvested from 25 discarded placentae. Donor demographics are summarized in Table S1. Mononuclear cells and residual granulocytes were separated by density gradient and frozen at −80 °C until analysis. TL measurements were completed by June 2012. Among 208 samples analyzed by flowFISH, 192 (92%) passed preset quality control measures: that is, less than 10% intraassay CV among triplicate values, and more than 1,000 cell events for each of lymphocyte and granulocyte populations. These values were then used to construct a nomogram, as described below. The raw lymphocyte and granulocyte TL values are listed in Table S2.
Telomerase and Telomere Maintenance Gene Mutation Carriers.
Subjects with mutations in telomerase and telomerase maintenance genes were recruited from 2003 to 2016 as part of the Johns Hopkins Telomere Syndrome Registry (28, 34, 35). Entry criteria for this substudy to assess the value of TL testing included familial and sporadic cases with a pathogenic mutation in a telomerase or telomere maintenance gene. Rare variants in the known telomere or telomerase genes were deemed pathogenic if they segregated with short telomere phenotypes or had functional molecular evidence of pathogenicity (e.g., telomerase activity assay), as previously described (11, 29, 35–37). The mutations are listed in Table S4. The age at diagnosis and telomere phenotype were extracted from the medical record, as previously described (11, 12, 18). Patients with liver disease had hepatopulmonary syndrome or biopsy-proven cirrhosis. Primary immunodeficiency in this study referred to severe combined immunodeficiency.
Bone Marrow Failure Study.
Newly diagnosed, treatment-naïve children and adults with idiopathic bone marrow failure from birth and up to the age of 40 y were prospectively recruited from patients who were evaluated at or referred to the Johns Hopkins Hospital from 2012 to 2014. Exclusion criteria included a positive chromosome breakage study, indicating Fanconi anemia, a history of a known inherited bone marrow failure syndrome, personal history of classic mucocutaneous features of dyskeratosis congenita, or a family history of bone marrow failure, pulmonary fibrosis, liver cirrhosis, or hematologic malignancy. Patients were classified as having constitutional aplastic anemia if they had a history of developmental delay, intellectual disability, or other congenital anomaly. Clinical data were extracted from medical records and study endpoints were assessed at the 2-y time point. Table S5 summarizes the clinical characteristics of these 38 patients. Subjects who were retrospectively studied were treated for severe aplastic anemia at the Johns Hopkins Hematology Clinics and were recruited from 2011 and 2012 based on a history of durable remission (>1 y) after immunosuppressive therapy. Their characteristics are summarized in Table S6.
TL Measurement.
TL was measured by flowFISH following the detailed protocol in Baerlocher et al. (24), except that peripheral blood mononuclear cell were extracted by density gradient separation before preserving in freezing media. Red cells were lysed at the time of thawing (RBC Lysis Buffer; eBioscience). Cow thymus was obtained from a local butcher and fixed thymocytes were mixed with each sample (24). A peptide nucleic acid (PNA) labeled probe containing the sequence (CCCTAA)3 was used for the hybridization (Alexa 488; PNA Bio). Each sample was run in triplicate in addition to a no probe to account for background (24). The average lymphocyte and granulocyte TL were then calculated. A reference sample with known TL, obtained from a large leukapheresis product, was used to normalize fluorescence values on each plate. For this sample, the leukocyte TL was measured by Southern blot (1), as well as by flowFISH at a company (Repeat Diagnostics). Intra- and interassay CV was calculated by dividing the SD (σ) by the mean (µ).
Nomogram Generation.
Lymphocyte and granulocyte TL from healthy volunteers were read into R/Bioconductor (38). A linear model (39) with the TL as response and square root of age as predictor was fitted based on a strong correlation and supported by evidence of the same relationship in long-lived marine birds (40) (Fig. S1A). Using the fitted model, we predicted the TL for individuals 0–100 y with the 1-y step. For every predicted value, the 1st, 10th, 50th, 90th, 95th, and 99th percentile predictions were generated. The same analyses were then performed for the raw control data from Aubert et al. (23) (termed Vancouver). Comparisons between the Johns Hopkins and Vancouver laboratories were limited to similarly prepared samples (i.e., frozen), given the known effect of freezing on TL (23).
DNA Sequencing and Analysis.
DNA was extracted from peripheral blood (PureGene Blood Core Kit; Qiagen). Mutations were detected by either PCR amplification and Sanger sequencing, as described previously (11, 41), whole-exome sequencing (29, 37), whole-genome sequencing (35), or custom amplicon sequencing followed by confirmation with Sanger sequencing (12). For targeted panel sequencing, we designed a TruSeq Custom Amplicon probe set (Illumina) that included the coding and flanking intronic sequences of telomere genes (TERT, TR, DKC1, RTEL1, NAF1, TINF2, CTC1, PARN, NOP10, NHP2, TCAB1) and GATA2 (including the intron 4 enhancer). Libraries were generated from 500 ng of DNA according to manufacturer recommendations and analyzed on a MiSeq sequencer (Illumina) (12). The mean coverage of target sequence was 238× and 88% of the target sequences were covered at or greater than 8×.
Statistics.
We used GraphPad Prism to graph the data and analyze the statistics, except for the nomogram generation and comparison of distributions, which were performed as detailed above. All P values are two-sided.
Supplementary Material
Acknowledgments
We thank the patients and study volunteers and all their referring clinicians; the Genetic Resources Core Facility staff for their support; Michael Ochs, Alan Scott, and Christopher Gocke for helpful discussions; and Laura Kasch-Semenza and M.A. laboratory members for technical help. This work was supported by NIH Grants K99-R00 HL113105 (to J.K.A.), K23 HL123601 (to A.E.D.), R37 AG009383 (to C.W.G.), and R01 CA160433 and R01 HL119476 (to M.A.); the Maryland Stem Cell Research and the Commonwealth Foundation; the Johns Hopkins inHealth initiative; the Flight Attendants Medical Research Institute; and the Gary Williams Foundation (M.A.); a gift from Dr. Sachiko Kuno to the Telomere Center at Johns Hopkins. S.E.S. received support from NIH T32 GM007309.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720427115/-/DCSupplemental.
References
- 1.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 2.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 3.d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 4.Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 5.Allsopp RC, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley SE, Armanios M. The short and long telomere syndromes: Paired paradigms for molecular medicine. Curr Opin Genet Dev. 2015;33:1–9. doi: 10.1016/j.gde.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokal I, Vulliamy T. Dyskeratosis congenita: Its link to telomerase and aplastic anaemia. Blood Rev. 2003;17:217–225. doi: 10.1016/s0268-960x(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 8.Jyonouchi S, Forbes L, Ruchelli E, Sullivan KE. Dyskeratosis congenita: A combined immunodeficiency with broad clinical spectrum—A single-center pediatric experience. Pediatr Allergy Immunol. 2011;22:313–319. doi: 10.1111/j.1399-3038.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- 9.Fogarty PF, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 10.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armanios MY, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 12.Stanley SE, et al. Telomerase mutations in smokers with severe emphysema. J Clin Invest. 2015;125:563–570. doi: 10.1172/JCI78554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz AC, et al. Disease-specific hematopoietic cell transplantation: Nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2010;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silhan LL, et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J. 2014;44:178–187. doi: 10.1183/09031936.00060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley SE, Rao AD, Gable DL, McGrath-Morrow S, Armanios M. Radiation sensitivity and radiation necrosis in the short telomere syndromes. Int J Radiat Oncol Biol Phys. 2015;93:1115–1117. doi: 10.1016/j.ijrobp.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabe M, et al. Fatal interstitial pulmonary disease in a patient with dyskeratosis congenita after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19:389–392. doi: 10.1038/sj.bmt.1700674. [DOI] [PubMed] [Google Scholar]
- 18.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Ruiz CM, et al. Reproducibility of telomere length assessment: An international collaborative study. Int J Epidemiol. 2015;44:1673–1683. doi: 10.1093/ije/dyu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagnall CL, et al. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLoS One. 2017;12:e0184098. doi: 10.1371/journal.pone.0184098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham JM, et al. Telomere length varies by DNA extraction method: Implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2013;22:2047–2054. doi: 10.1158/1055-9965.EPI-13-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8:e1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 25.Alter BP, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armanios M, et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 28.Jonassaint NL, Guo N, Califano JA, Montgomery EA, Armanios M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell. 2013;12:319–323. doi: 10.1111/acel.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alder JK, et al. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. 2015;147:1361–1368. doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alter BP, et al. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97:353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: Roles in cellular aging. Mutat Res. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Haycock PC, et al. Telomeres Mendelian Randomization Collaboration Association between telomere length and risk of cancer and non-neoplastic diseases: A mendelian randomization study. JAMA Oncol. 2017;3:636–651. doi: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorgy AI, et al. Hepatopulmonary syndrome is a frequent cause of dyspnea in the short telomere disorders. Chest. 2015;148:1019–1026. doi: 10.1378/chest.15-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanley SE, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8:351ra107. doi: 10.1126/scitranslmed.aaf7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alder JK, et al. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS Genet. 2011;7:e1001352. doi: 10.1371/journal.pgen.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alder JK, et al. Telomere phenotypes in females with heterozygous mutations in the dyskeratosis congenita 1 (DKC1) gene. Hum Mutat. 2013;34:1481–1485. doi: 10.1002/humu.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers JM, Hastie T. 1992. Statistical Models in S (Wadsworth & Brooks/Cole Advanced Books & Software, Pacific Grove, CA) p xv, 608 p.
- 40.Juola FA, Haussmann MF, Dearborn DC, Vleck CM. Telomere shortening in a long-lived marine bird: Cross-sectional analysis and test of an aging tool. Auk. 2006;123:775–783. [Google Scholar]
- 41.Parry EM, et al. Decreased dyskerin levels as a mechanism of telomere shortening in X-linked dyskeratosis congenita. J Med Genet. 2011;48:327–333. doi: 10.1136/jmg.2010.085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.