Abstract
Several single incretin receptor agonists that are approved for the treatment of type 2 diabetes mellitus (T2DM) have been shown to be neuroprotective in cell and animal models of neurodegeneration. Recently, a synthetic dual incretin receptor agonist, nicknamed “twincretin,” was shown to improve upon the metabolic benefits of single receptor agonists in mouse and monkey models of T2DM. In the current study, the neuroprotective effects of twincretin are probed in cell and mouse models of mild traumatic brain injury (mTBI), a prevalent cause of neurodegeneration in toddlers, teenagers and the elderly. Twincretin is herein shown to have activity at two different receptors, dose-dependently increase levels of intermediates in the neurotrophic CREB pathway and enhance viability of human neuroblastoma cells exposed to toxic concentrations of glutamate and hydrogen peroxide, insults mimicking the inflammatory conditions in the brain post-mTBI. Additionally, twincretin is shown to improve upon the neurotrophic effects of single incretin receptor agonists in these same cells. Finally, a clinically translatable dose of twincretin, when administered post-mTBI, is shown to fully restore the visual and spatial memory deficits induced by mTBI, as evaluated in a mouse model of weight drop close head injury. These results establish twincretin as a novel neuroprotective agent and suggest that it may improve upon the effects of the single incretin receptor agonists via dual agonism.
Keywords: Traumatic brain injury, Neurodegeneration, Incretin mimetic, Incretin, Glucagon-like peptide-1, Glucose-dependent insulinotropic peptide
1. Introduction
The CDC estimates that 1.7 million Americans suffer a traumatic brain injury (TBI) each year (Faul et al., 2010), while 3.2 (Zaloshnja et al., 2008) to 5.3 (Thurman et al., 1999) million Americans are estimated to be living with TBI-related disabilities. These injuries, which primarily affect toddlers (0–4), young adults (15–19) and the elderly (older than 65) (Faul et al., 2010), are particularly alarming as they may cause a host of long-term cognitive, behavioral and physical impairments. There is a growing body of evidence suggesting that TBIs initiate various biochemical cascades and processes in the brain that potentiate neuropsychiatric disorders (Chen et al., 2014), as well as neurodegenerative diseases such as early-onset dementia (Barnes et al., 2014; Gardner et al., 2014), Parkinson's disease (Gardner et al., 2015), and Alzheimer's disease (Tweedie et al., 2013a; Tweedie et al. 2013b; Tweedie et al. 2016).
The type of TBI may be categorized as either “open,” if the skull and dura mater are perforated, or “closed,” when they are not, and the severity of the of injury may be denoted as either “mild,” “moderate,” or “severe.” These three classifications of TBI severity differ in regards to the structural imaging in the brain, the duration of lost consciousness and amnesia, and the patient's scores on the Glasgow Coma and Abbreviated Injury Scales - broadly accepted scoring evaluations to reproducibly classify the severity of TBI based on clinical/anatomical observations (Orman et al., 2011). Mild TBIs (mTBIs), which are primarily closed-head, and which are most readily characterized by normal brain imaging, <30 min of lost consciousness, and less than one day of posttraumatic amnesia, account for approximately 70–90% of all reported cases (Cassidy et al., 2004).
At the cellular level, TBI damages accumulate in a two-phase process. During the acute primary phase, which occurs at the moment of injury, brain cells undergo immediate necrotic cell death due to contusions and lacerations of brain tissue, as well as intracranial hemorrhage and diffuse axonal injury (LaPlaca et al., 2007). In the extended secondary phase, neurodegenerative processes initiated in the primary phase, such as neuroinflammation, oxidative stress and glutamate excitotoxicity, lead to progressive neuronal loss via apoptosis in mild and moderate TBI (Morganti-Kossmann et al., 2002; Schmidt et al., 2005; Greve and Zink, 2009; Bales et al., 2010; Barkhoudarian et al., 2011; Mehta et al., 2013; Rachmany et al., 2013a).
Despite the high incidence of TBI throughout the world, the capacity of these injuries to initiate debilitating neurodegenerative disorders, and the fairly sophisticated understanding of the cellular processes that underpin the extensive brain damages that occur during a TBI, no effective treatments have been developed to mitigate the deleterious effects of these injuries. To this end, incretins and incretin mimetics have been investigated in regard to their anti-apoptotic, neuroprotective and neurotrophic effects in neurons expressing incretin receptors: namely the glucagon-like peptide 1 (GLP-1) receptor (GLP-1R) and the glucose-dependent insulinotropic peptide (GIP) receptor (GIPR) (Rachmany et al., 2013b; Tweedie et al., 2013a; Li et al., 2015; Tweedie et al., 2016; Yu et al., 2016).
These peptide hormones, which were originally identified in the gut, where they are released by intestinal enteroendocrine cells in response to elevated levels of dietary glucose in the intestinal lumen, were first investigated for their use in the treatment of type 2 diabetes mellitus (T2DM). Via their receptors on pancreatic β- and α-cells, GLP-1 and GIP stimulate insulin secretion and inhibit that of glucagon in order to induce glucose metabolism (Campbell and Drucker, 2013; Wu et al., 2016). As such, GLP-1 mimetics such as exendin-4 (Ex-4) and liraglutide have been developed and approved for the treatment of T2DM.
Importantly, GLP-1 and GIP have trophic and anti-apoptotic properties (Salcedo et al., 2012) mediated through the cAMP-dependent CREB pathway (Perry and Greig, 2003; Kimet al., 2008; Shao et al., 2013). The discovery of the anti-apoptotic activity of GLP-1 and GIP, in addition to the realizations that incretin mimetics pass the blood-brain barrier (Kastin et al., 2002), and that GLP-1Rs and GIPRs are expressed on central nervous system (CNS) neurons (Alvarez et al., 2005; Nyberg et al., 2007), led to the investigation of GLP-1, GIP and their mimetics in the treatment of neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease and now TBI (Salcedo et al., 2012; Greig et al., 2014; Bassil et al., 2014; Holscher, 2014; Athauda and Foltynie, 2016).
Recently, a novel, synthetic incretin mimetic was shown to maximize the metabolic benefits of these peptides in rodent and monkey models of T2DM, as well as in humans via clinical trials (Finan et al., 2013). This peptide, nicknamed “twincretin”, which features sections of both the Ex-4 and GIP sequences, was shown to bind both the GLP-1R and GIPR, and was reported to improve upon the performance of the single receptor agonists via the combined effect of its dual receptor agonism (Finan et al., 2013).
In the current study, we investigate the neurotrophic and neuroprotective effects of this promising new peptide in cell and rodent models of mTBI. Using human neuroblastoma cells, we show that twincretin has activity at both the GLP-1R and GIPR, and that it significantly reduces cell death in response to toxic doses of glutamate and hydrogen peroxide, mimicking in part the neuroinflammatory conditions present in the secondary phase of mTBI (Morganti-Kossmann et al., 2002; Barkhoudarian et al., 2011; Baratz et al., 2011; Walker and Tesco, 2013; Greig et al., 2014; Barkhoudarian et al., 2016). In light of the vulnerability of dopaminergic neurons to TBI (Shahaduzzaman et al., 2013; Acosta et al., 2015; Impellizzeri et al., 2016), this neuroprotective effect is recapitulated in a dopaminergic neuronal cell group; specifically, primary cultures of rat ventral mesencephalon (VM) neurons. Twincretin is also shown to improve upon the effects of the single-receptor agonists, Ex-4 and GIP. Finally, we show that twincretin protects mice against mTBI-induced deficits in spatial and visual memory.
2. Methods
2.1. Materials
Twincretin was obtained from the Richard DiMarchi Research Group at Indiana University Bloomington. Ex-4 and GIP were purchased from AnaSpec Inc. (Fremont, CA, USA). Human Pro3GIP was purchased from Abgent (San Diego, CA, USA). Exendin Fragment 9–39 (Ex 9–39), L-Glutamic acid monosodium salt hydrate (glutamate), and hydrogen peroxide solution 30% (w/w) in H2O (H2O2) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Hydrochloric acid was purchased from Phoenix Pharmaceuticals Inc. (Burlingame, CA, USA) and Triton X-100 was purchased from Fisher Scientific (Waltham, MA, USA).
2.2. Cell culture
Two immortal cell lines were used: SH-SY5Y cells, a human neuroblastoma cell line purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA), and SH-hGLP-1R#9 cells, which are SH-SY5Y cells that stably overexpress the human GLP-1R (Li et al., 2010b). All cultures were grown in a mixture of 44.5% Eagle's Minimum Essential Medium (EMEM), 44.5% Ham's F12-K (Kaighn's) Medium, 10% heat-inactivated fetal bovine serum, and 1% penicillin/streptomycin by volume (normal medium). Cultures were maintained in a humidified, 37 °C incubator comprising 5% CO2 and 95% air. Medium was replaced every other day, and the cells were split in a 1:3 ratio every week using a 0.25% trypsin, 0.53 mM ethylenediaminetetraacetic acid (EDTA) solution.
Primary cultures were prepared from embryonic (E14–15) VM tissue samples obtained from fetuses of timed-pregnant Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), according to published procedures with some modification (Lotharius et al., 1999). The whole brain was removed aseptically, and a small piece of tissue comprising the VM was dissected. After removal of blood vessels and the meninges, pooled VM tissues were trypsinized (0.25%; Invitrogen, Carlsbad, CA) with gentle mixing for 15 min at 37 °C. After rinsing with pre-warmed DMEM/F-12 (Invitrogen) to remove any remaining trypsin, cells were dissociated by trituration, counted and then plated into 96-well (6.0 × 104/well) cell culture plates pre-coated with poly-d-lysine (Sigma-Aldrich, St Louis, MO). The culture plating medium comprised of Dulbecco's modified Eagle medium/F12, supplemented with 10% heat-inactivated fetal bovine serum, 1 mM l-glutamine and 2% B27 (Invitrogen). Cultures were then maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, and were fed by exchanging 50% of media with feed media (Neurobasal medium, Invitrogen) with 0.5 mM l-glutamate and 2% B27 with antioxidants supplement on DIV (days in vitro) 3 and 5.
2.3. cAMP Assays
All cAMP assays were performed using 24-well plates containing 500 µL of cells in normal medium at a concentration of 280 × 104 cells/mL, as determined by hemocytometer cell count. Cells were treated with 500 µL of the appropriate peptide solutions for 0, 5, 10, 15, 30 or 60 min and then lysed using a solution of 0.5 M HCl with 0.5% Triton X-100. After allowing the cells to lyse for 20 min, the lysates were centrifuged at 1000 rpm for 10 min. The supernatants were extracted and frozen in a −18 °C freezer. The frozen supernatants were subsequently thawed and assessed using the Enzo Direct cAMP ELISA kit and protocol.
The results of the cAMP assays were normalized to the total protein content in each sample via Bicinchoninic acid (BCA) assay. Two 10 µL samples were taken from each frozen lysate supernatant used for the cAMP assay, dispensed on 96-well plates and then assessed using the Pierce BCA Protein Assay Kit and protocol (ThermoFisher Scientific, Waltham, MA).
2.4. Western blotting
SH-SY5Y cells were removed from 100 mm petri dishes with cell lifters and Dubelco's phosphate-buffered saline (DPBS) (ThermoFisher Scientific, Waltham, MA). After light centrifugation, the DPBS supernatant was removed, and the cells were lysed with a 100:1 M-PER Mammalian Protein Extraction Reagent (ThermoFisher Scientific, Waltham, MA), Halt Protease and Phosphatase Inhibitor Cocktail (ThermoFisher Scientific, Waltham, MA) mixture. After being lysed for 10 min, the samples were centrifuged at high speeds, and the protein-containing supernatants were removed and stored at −80 °C.
The total protein content in each sample was determined via BCA assay (ThermoFisher Scientific, Waltham, MA). 60 µg of each sample were resolved on a NuPage Novex 4–12% Bis-Tris protein gel (ThermoFisher Scientific, Waltham, MA) and then transferred to a Novex PVDF membrane with 0.2 µm pores (ThermoFisher Scientific, Waltham, MA). The membrane was then blocked with a 5% milk solution in tris-buffered saline and Tween 20 (TBST) for 1 h prior to overnight incubation with primary antibody in 5% milk solution in a cold room. The three primary antibodies used in this study were Phospho-CREB (Ser133) (87G3) Rabbit mAb (Cell Signaling Technology, Danvers, MA), CREB (48H2) Rabbit mAb (Cell Signaling Technology, Danvers, MA) and α-tubulin Mouse mAb (Sigma-Aldrich, St. Louis, MO). These three antibodies were diluted in 5% milk solution at 1:1000, 1:1000 and 1:5000 ratios, respectively. After several TBST washes, these membranes were exposed to solutions of secondary antibodies in 5% milk for 1 h at room temperature. For the membranes stained for pCREB and CREB, 1:5000 dilutions of HRP-conjugated goat anti-rabbit antibody (sc-2030, Santa Cruz Biotechnology, Dallas, TX) were used. For the membranes stained for α -tubulin, 1:50,000 dilutions of HRP-conjugated goat anti-mouse antibody (sc-2031, Santa Cruz Biotechnology, Dallas, TX) were used. After another round of washing with TBST, these membranes were exposed to SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific, Waltham, MA) for 10 min and then developed using HyBlot ES Autoradiography film (Denville Scientific Inc., South Plainfield, NJ) and an AFP Mini-Medical 90 Film Processor (AFP Imaging Corporation, Mt. Kisco, NY). If the proteins were not detected using the Pico Substrate, SuperSignal West Dura Extended Duration Substrate (ThermoFisher Scientific, Waltham, MA) was employed to detect the proteins. The developed films were scanned, and the protein bands were densitometrically quantified using ImageJ software.
2.5. Cell viability assays
Cell viability was assessed via the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and lactate dehydrogenase (LDH) assays.
All MTS assays were performed using 96-well plates containing 200 µL of cells in normal medium at a concentration of 100 × 104 cells/mL, as determined by hemocytometer cell count. For the neurotrophic MTS assays, cells were treated with 200 µL of either twincretin, Ex-4 or GIP dissolved in a low-serum medium formulation (49.25% EMEM, 49.25% F12 K, 1% penicillin/streptomycin, 0.5% heat-inactivated fetal bovine serum by volume) and were incubated overnight. The cells were subsequently assessed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit and protocol.
For the neuroprotective MTS assays, cells were treated with 150 µL of twincretin (1 to 1000 nM) in low-serum medium for 1 h before the addition of toxic solutions of either glutamate (100 mM) or H2O2 (200 to 600 µM), also dissolved in low-serum medium. The cells were incubated overnight and subsequently assessed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit and protocol.
All LDH assays, for both the neurotrophic and neuroprotective experiments, were performed in 96-well plates using 100 µL samples from the solutions used for the overnight incubations in the MTS assays. These samples were assessed using the Sigma-Aldrich In Vitro Toxicology Assay kit, Lactic Dehydrogenase based.
For the cell viability assays in primary cultures, cells were first fed with media containing B27 supplement without antioxidants (Invitrogen) on DIV7. Freshly made 6-hydroxydopamine (6OHDA, 100 µM) (in 20 µM ascorbic acid saline solution) or saline (with 20 µM ascorbic acid) was added to the wells on DIV 10. After incubation for 2 h, cultures were washed with (−) AO B27 3 times. Thereafter, either twincretin or vehicle was added to the well following the last wash. Cells were maintained at 37 °C incubator for 22 h, and then fixed with 4% paraformaldehyde (PFA) and probed for tyrosine hydoxylase (TH) immunoreactivity. Specifically, cells were incubated in a primary antibody solution of mouse monoclonal anti-TH diluted in 4% BSA and 0.3% Triton x-100 in 0.1 M PB, concentration 1:100 (Chemicon, Temecula, CA) for 17–19 h at 4 °C. They were then rinsed in 0.1 M phosphate buffer and incubated with a secondary antibody for 1 h, followed by incubation for 1 h with avidin-biotin-horseradish peroxidase complex. TH immunoreactivity was then examined under fluorescent microscope. For background control studies, cells were incubated without primary antibody.
2.6. Mice
Male ICR mice, 6–8 weeks old, weighing 30–40 g, were housed 5-per-cage under a 12-hour light/dark cycle with food (Purina Rodent Chow) and water ad libitum. The mice were exposed to a constant temperature of 22 ± 10 °C. All experimental manipulations were conducted during the light phase of the cycle. Each mouse was used in a single experiment and for one time point only. The Ethics Committee of the Sackler Faculty of Medicine approved the experimental protocol (M-15-011) in compliance with the guidelines for animal experimentation of the National Institutes of Health (DHEW Publication 85–23, Revised, 1995). A minimal number of mice were used, and all efforts were made to minimize potential suffering.
2.7. Closed head mild traumatic brain injury
mTBIs were induced using the previously described weight drop concussive head trauma device (Zohar et al., 2003; Tweedie et al., 2007; Li et al., 2015). The device consists of a metal tube with an inner diameter of 13 mm placed vertically over the head of the mouse. Mice were lightly anesthetized with isoflurane and placed under the device. A 30 g metal weight was dropped from the top of the tube (80 cm high) in order to strike the skull at the temporal right side, between the corner of the eye and the ear. A sponge was positioned to support the head, which allowed anterior-posterior motion without any rotational head movement at the moment of impact. Immediately after injury, mice were placed in their cages for recovery.
2.8. Drug administration
50 µg/kg twincretin prepared in isotonic saline was administered to the animals once daily for 7 days by subcutaneous (s.c.) injection with the first injection administered 30 min after the injury. Mice were divided into 4 groups: a sham group (s.c. saline once daily × 7 days), a mTBI group (s.c. saline once daily × 7 days), a twincretin following sham procedure group, and a twincretin following mTBI group. The sham (saline) and sham (twincretin) groups were treated similarly to TBI challenged animals, but without being subjected to weight drop.
2.9. Core temperature
Core (rectal) body temperature was time-dependently measured with a pediatric digital thermometer in select animals prior to and post mTBI with and without twincretin administration to assess for any differences in animal core body temperature between sham and peptide treated animals. No difference was evident between these groups of mice.
2.10. Behavioral tests
Behavioral assessments were initiated 7 and 30 days after the animals received mTBIs in separate cohorts of mice. Mice cognition and emotional behavior were assessed using the novel object recognition, Y-maze and elevated plus maze paradigms. All equipment used for behavioral testing was cleaned with a 70% ethanol solution between testing sessions in order to minimize any olfactory-dependent cognitive influences on mouse behavior.
2.11. Novel object recognition paradigm
The novel object recognition task was used to evaluate recognition memory, as previously described (Edut et al., 2011). This task is based on the innate tendency of rodents to explore unfamiliar objects within their environment. This task is used in order to make assessments as to whether a mouse is able to discriminate between a familiar and a novel object.
Mice were individually habituated to an open field Plexiglas box (59 × 59 × 20 cm) for 5 min, 48 h prior to the test. 24 h later, animals were allowed to explore a set of two identical objects for a 5 min period. These objects were suitably heavy and high to guarantee that mice could neither move nor climb over them. 24 h after this acquisition phase, the animals were presented with a similar set of objects in the same environment, where one object was novel to them; they were allowed to freely explore the objects again for a 5-min period. A discrimination preference index was calculated as follows: (time near novel object − time near familiar object) / (time near novel object + time near familiar object) (Dix and Aggleton, 1999).
2.12. Y-maze paradigm
The Y-maze task was chosen to evaluate spontaneous exploration, responsiveness to novel environments and spatial memory function. The apparatus used for the Y-maze study is made of black Plexiglas and consists of three arms separated by a 120° angles (Dellu et al., 1992). Each arm is identical (8 × 30 × 15 cm), however different spatial cues were placed in each arm (i.e. a triangle, a square, or a circle). The start arm for each experiment was chosen randomly; each mouse was placed into the Y-maze environment on two occasions separated by a 2-minute interval, during which the mouse was returned to its home cage. During the first 5-minute trial, one of the two arms was randomly blocked. During the second 2-minute trial, all arms were open for exploration; the total amount of time the mouse explored in each arm was measured. A discrimination preference index was calculated as follows: (time in new arm − time in familiar arm) / (time in new arm + time in familiar arm) (Dix and Aggleton, 1999).
2.13. Elevated plus maze paradigm
The elevated plus maze apparatus is used to assess the levels of fear or anxiety-like behavior in rodents (Alcalay et al., 2004). This assessment is based upon the natural tendency of rodents to fear exploration of open, illuminated and elevated environments, and prefer exploration of enclosed, darkened environments. The maze consists of four arms in a “plus” shape formation. Two of the arms have low walls (30 × 5 × 1 cm) and the other two arms have high walls (30 × 5 × 15 cm), both with open tops. Similar arms face one another. The maze is elevated 50 cm above floor level. On the test day each mouse was placed in the center of the elevated plus maze, facing one of the open arms. During the 5-minute observation period, the numbers of entries to the open and closed arms were quantified, and the time that each mouse spent on the open arm was measured. The longer the time that mice are willing to spend on the open arms has been associated with a lower anxiety-like behavior (Belzung and Griebel, 2001).
2.14. Data analysis
All results are presented as mean ± SEM and were analyzed by either Prism or SPSS V 20 software. One-way ANOVA tests were performed for comparisons between multiple data sets, followed by either Dunnett's or Bonferroni post hoc analysis. t-tests were also used for direct comparisons in some of the cell culture data. *p < 0.05, **p < 0.01, ***p < 0.001.
3. Results
3.1. Twincretin has activity at the GLP-1R and GIPR
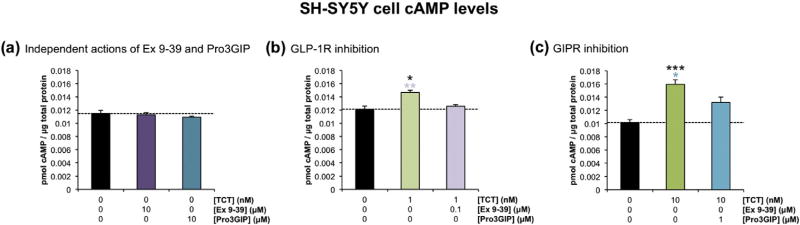
To confirm that twincretin has activity at both the GLP-1R and GIPR, cAMP production in SH-SY5Y cells was assessed after 10 min treatment with twincretin in either the absence or presence of inhibitors specific to each receptor. The two inhibitors used in this study were Exendin Fragment 9–39 (Ex 9–39), a GLP-1R antagonist, and Pro3GIP, a GIPR antagonist. A control study was first conducted in which SH-SY5Y cells were treated with either 10 µM Ex 9–39 or 10 µM Pro3GIP in order to ensure that these inhibitors do not independently reduce cAMP as compared to control. This hypothesis was confirmed, as the levels of cAMP were not altered by either 10 µM Ex 9–39 or 10 µM Pro3GIP as compared to control (Fig. 1a).
Fig. 1.
Twincretin has activity at both the GLP-1R and GIPR in human neuroblastoma cells. (a) The levels of cAMP in SH-SY5Y cells treated with 10 µM Ex 9–39 and in cells treated with 10 µM Pro3GIP are statistically equivalent to the levels of cAMP in control cells (n=6). (b) The cAMP levels in SH-SY5Y cells coincubated with 1 nM twincretin and 100 nM Ex 9–39 are significantly lower than the levels of cAMP in cells incubated with 1 nM twincretin alone. Additionally, 1 nM twincretin alone caused significant increases in cAMP levels as compared to sham control (n=4). (c) The cAMP levels in SH-SY5Y cells coincubated with 10 nM twincretin and 1 µM Pro3GIP are significantly lower than the levels of cAMP in cells incubated with 10 nM twincretin alone. Also, 10 nM twincretin alone significantly increased cAMP levels as compared to sham control (n= 3; *p < 0.05, **p < 0.01, ***p < 0.001).
Next, 1 nM twincretin significantly increased cAMP levels as compared to control. cAMP production in cells treated with 1 nM twincretin and 100 nM Ex 9–39 was significantly reduced as compared to that of cells treated with 1 nM twincretin alone (Fig. 1b). Similarly, 10 nM twincretin increased cAMP production in SH-SY5Y cells as compared to controls, while cAMP production in cells treated with 10 nM twincretin and 1 µM Pro3GIP was significantly reduced as compared to that in cells treated with 10 nM twincretin alone (Fig. 1c).
3.2. Twincretin is neurotrophic
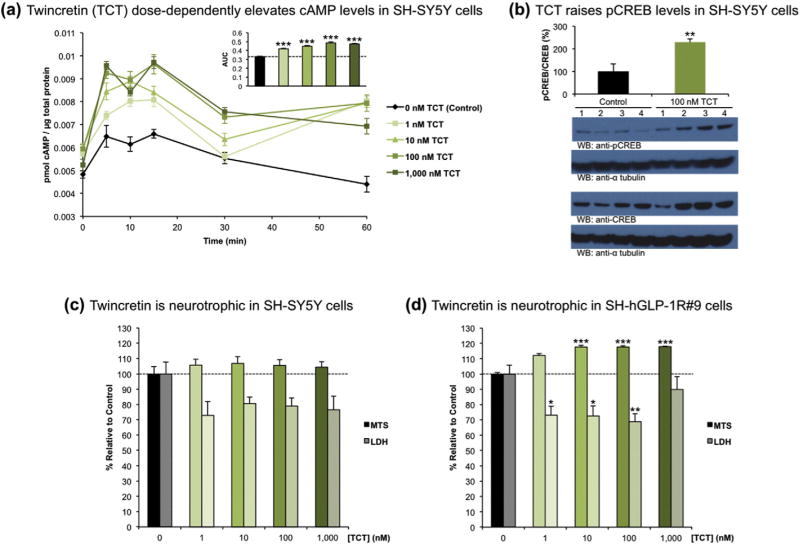
The levels of cAMP produced in SH-SY5Y cells exposed to increasing concentrations of twincretin were probed via ELISA. Cells were treated with 0 (control), 1, 10, 100 or 1000 nM twincretin and subsequently lysed at 0, 5, 10, 15, 30 or 60 min. All concentrations of twincretin significantly increased the levels of cAMP as compared to control, and dose-dependent increases in cAMP production were observed from 1 to 1000 nM twincretin (Fig. 2a). cAMP levels peaked at approximately 10 min exposure for all conditions, and maximal cAMP production was achieved at 100 nM twincretin.
Fig. 2.
Twincretin is neurotrophic in human neuroblastoma cells. (a) The levels of cAMP signicantly and dose-dependently increased in SH-SY5Y cells exposed to 1–1000 nM twincretin over the course of one hour (n=5). (b) The fraction of pCREB/CREB significantly increases in SH-SY5Y cells exposed to 100 nM TCT for 1 h as compared to control SH-SY5Y cells that were not exposed to twincretin (n= 4). (c) 1–1000 nM twincretin increased cell viability by approximately 5% as measured via MTS assay, and decreased LDH release into the extracellular space by approximately 20% as compared to controls, although neither trend was significant (n = 10). (d) In SH-hGLP-1R#9 cells, which are SH-SY5Y cells that overexpress the human GLP-1R, 1–1000 nM twincretin treatment increased cell viability by approximately 20% and reduced LDH release by approximately 20% as compared to controls (n = 10; *p < 0.05, **p < 0.01, ***p < 0.001).
Additionally, the fraction of pCREB/CREB in SH-SY5Y cells exposed to 100 nM twincretin for 1 h, as determined by Western blot quantification, was significantly greater than the pCREB/CREB fraction in SH-SY5Y cells that were not exposed to twincretin (Fig. 2b).
Having established that twincretin significantly increases the levels of two essential intermediates involved in the CREB pathway, the neurotrophic effects of twincretin were assayed via MTS and LDH assays in both SH-SY5Y and SH-hGLP-1R#9 cells. Both cell lines were treated with the same concentrations of twincretin as in the cAMP assay. In SH-SY5Y cells, 1–1000 nM twincretin consistently increased the levels of reduced MTS by approximately 5%, though these increases were not significant. Similarly, 1–1000 nM twincretin consistently reduced the levels of LDH released into the culture medium by approximately 20% as compared to control, although again, these reductions were not significant (Fig. 2c). In the GLP-1R-overexpressing SH-hGLP-1R#9 cells, however, 1–1000 nM twincretin consistently and significantly increased the levels of reduced MTS by approximately 20% and decreased the levels of extracellular LDH by approximately 20% as compared to control (Fig. 2d).
3.3. Twincretin is neuroprotective
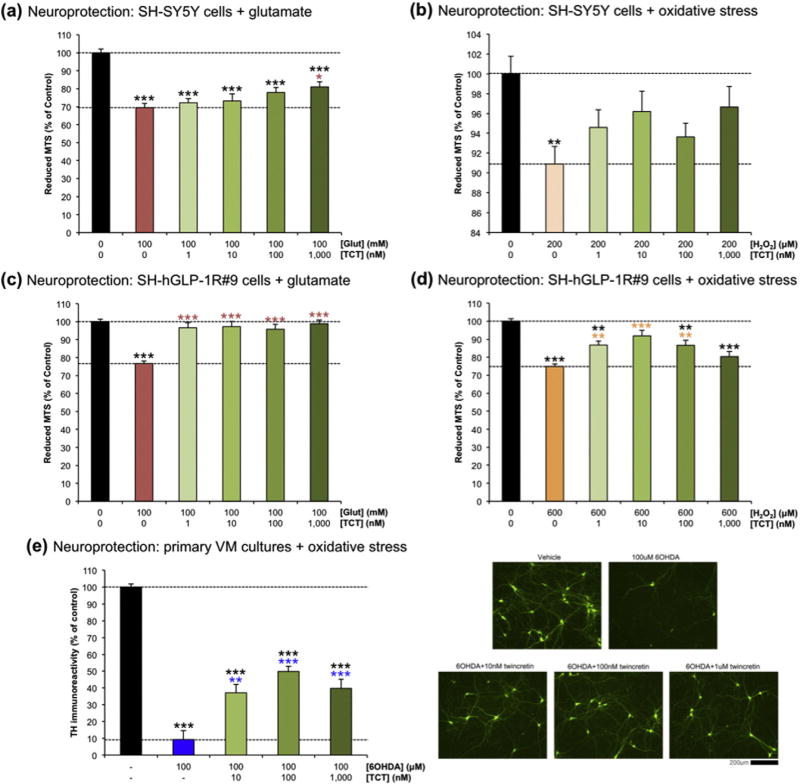
In order to assess the neuroprotective effects of twincretin in conditions similar to those experienced during the secondary phase of mTBI, 1–1000 nM twincretin was administered to SH-SY5Y and SH-hGLP-1R#9 cells that were exposed to toxic doses of either glutamate or hydrogen peroxide (H2O2). These compounds were selected in order to model glutamate excitotoxicity and deleterious reactive oxygen species (ROS) generation that are reported present within the secondary phase of TBI (Morganti-Kossmann et al., 2002; Bales et al., 2010; Barkhoudarian et al., 2011; Mehta et al., 2013; Walker and Tesco, 2013; Barkhoudarian et al., 2016). In the SH-SY5Y cells, 100 mM glutamate killed approximately 30% of cells as compared to control. 1–1000 nM twincretin dose-dependently mitigated this loss in viability, with 1000 nM twincretin significantly reducing cell death to 20% (Fig. 3a). Similarly, 200 µM H2O2 induced approximately 10% cell death that was significant compared to control, while 1–1000 nM steadily reduced the extent of cell death to approximately 3%. The cell numbers remaining after challenge with 200 µM H2O2 and treatment with 1–1000 nM twincretin were not significantly different as compared to control (Fig. 3b).
Fig. 3.
Twincretin is neuroprotective in human neuroblastoma cells and rat primary midbrain neurons. (a) 1–1000 nM twincretin pretreatment dose-dependently increased the viability of SH-SY5Y cells exposed to 100 mM glutamate as compared to cells that only received the toxic insult (n=10). (b) The cell viabilities of SH-SY5Y cells pretreated with 1–1000 nM twincretin before exposure to 200 µM H2O2 were statistically equivalent to control cell viability, while the cell viability of cells that received the toxic insult alone were significantly reduced (n=10). (c) 1–1000 nM twincretin pretreatment of SH-hGLP-1R#9 cells prior to 100 mM glutamate exposure fully prevented the reduction in cell viability caused by glutamate insult alone. The viabilities of cells pretreated with twincretin were statistically equivalent to control cell viability (n=10). (d) 1–100 nM twincretin pretreatment significantly increased the viability of SH-hGLP-1R#9 cells exposed to 600 µM H2O2 as compared to cells exposed to insult alone. The viability of cells pretreated with 10 nM twincretin were statistically equivalent to control viability (n = 10). (e) TH immunoreactivity significantly increased in rat primary midbrain neurons treated with 10–1000 nM twincretin after 100 µM 6OHDA insult as compared to cells that only received the insult (n = 6; *p < 0.05, **p < 0.01, ***p < 0.001).
Exposure to 100 mM glutamate killed approximately 25% of all SH-hGLP-1R#9 cells as compared to control. 96–99% of cells treated with 1–1000 nM twincretin before exposure to 100 mM glutamate survived, fully ameliorating the deleterious effects of glutamate toxicity (Fig. 3c). 600 µM H2O2 likewise killed approximately 25% of SH-hGLP-1R#9 cells as compared to control. 1–100 nM twincretin significantly increased surviving cell number by approximately 12–17%, with full amelioration at 10 nM twincretin. 1000 nM twincretin did not significantly increase cell viability as compared to 600 µM H2O2 alone (Fig. 3d).
To evaluate whether or not neuroprotective actions of twincretin evident in the described human immortal neuronal cells translate to primary cultures, tyrosine hydroxylase (TH) immunoreactivity was evaluated as a marker of dopaminergic cell viability in primary cultures of rat VM neurons. These were treated with 10–1000 nM twincretin after exposure to 100 µM of ROS-inducing 6OHDA, and were compared to unexposed neurons and neurons exposed to 6OHDA alone (Fig. 3e). Whereas 6OHDA induced a 90% reduction in TH immunoreactivity, the addition of twincretin significantly mitigated this loss.
3.4. Twincretin improves upon constituent single receptor agonists
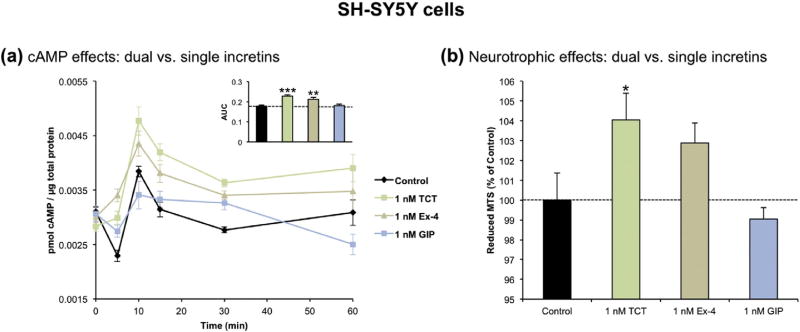
To complete the in vitro studies, the cAMP production and neurotrophic effects of twincretin were compared to those of the two single receptor agonist peptides that are incorporated in its sequence: Ex-4 and GIP. The cAMP levels in SH-SY5Y cells treated with 1 nM twincretin, Ex-4 or GIP over the course of 1 h were first assessed. 1 nM twincretin and Ex-4 significantly increased the levels of cAMP as compared to control, whereas 1 nM GIP did not. Though the difference in cAMP levels between 1 nM twincretin and 1 nM Ex-4 was not significant, those produced by twincretin were higher. Again, cAMP peaked at approximately 10 min (Fig. 4a).
Fig. 4.
Twincretin provides maximal cAMP production and neurotrophic effects in human neuroblastoma cells as compared to equimolar Ex-4 and GIP. (a) Treatment with 1 nM twincretin and 1 nM Ex-4 significantly raised the levels of cAMP in SH-SY5Y cells as compared to sham control cells over the course of one hour. 1 nM GIP treatment did not significantly alter the levels of cAMP as compared to control (n=5). (b) Only SH-SY5Y cells treated with 1 nM twincretin had significantly increased cell viability as compared to controls. The viability of cells treated with 1 nM Ex-4 and 1 nM GIP were statistically equivalent to controls (n = 15; *p < 0.05, **p < 0.01, ***p < 0.001).
Secondly, the neurotrophic effects of 1 nM twincretin, Ex-4 and GIP were assessed via MTS assay. 1 nM twincretin and Ex-4 increased the amount of surviving cells by 4 and 3% respectively, with the former being significantly different from the control group. 1 nM GIP did not significantly alter the amount of surviving cells (Fig. 4b).
3.5. Twincretin prevents visual and spatial memory deficits in mice post-mTBI
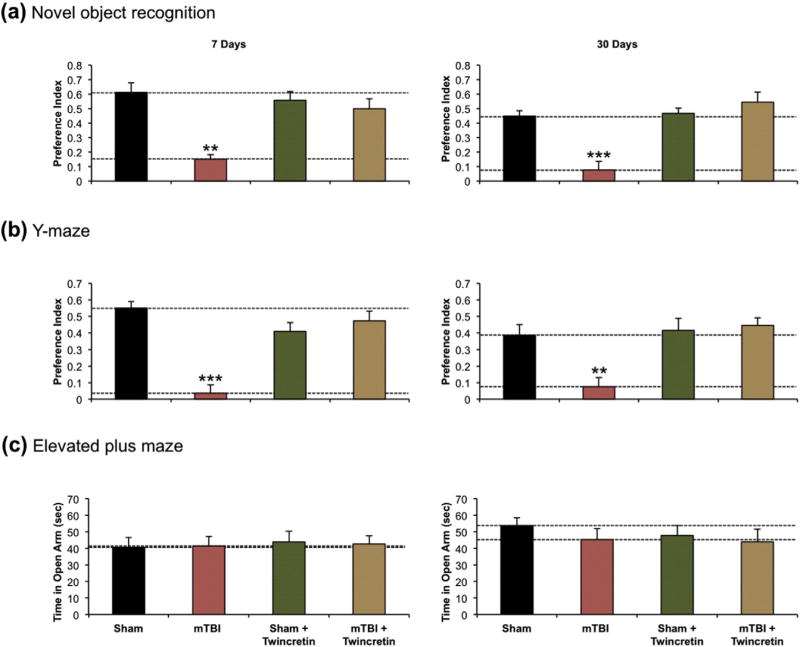
To assess the effects of twincretin administration on post-mTBI memory formation, the novel object recognition and Y-maze paradigms were performed on mice that received an mTBI with or without twincretin (50 µg/kg once daily × 7 days, s.c.) therapy afterwards, as both paradigms are consistently reported to be impaired by m-TBI (Zohar et al., 2011; Dachir et al., 2014; Doulames et al., 2015; Deselms et al., 2016). The novel object recognition test was applied in order to examine the visual recognition memory of the mice. The test was performed at 7 and 30 days after either sham procedure or actual injury. Vehicle-treated mTBI mice suffered from visual memory deficits and spent less time near the novel object, as compared to all other groups. High preference for the new object, similar to the sham group, was seen in mice that were treated with twincretin following mTBI induction (Fig. 5a).
Fig. 5.
Twincretin administration (50 µg/kg once daily × 7 days, s.c.) prevents deficits in visual and spatial memory in mice at 7 and 30 days post-mTBI. (a) The preference index of mTBI mice in the novel object recognition test was significantly reduced at both and 7 and 30 days post insult, as compared to sham control. Mice that received twincretin treatment after mTBI demonstrated equivalent preference indices to sham controls (n=9–12). (b) The preference index of mTBI mice in the Y-maze paradigm was significantly reduced as compared to sham control at 7 and 30 days post-mTBI. mTBI + twincretin mice did not demonstrate these reductions in preference indices at either 7 or 30 days post-mTBI (n=12–16). (c) All conditions of mice spent equivalent times in the open arm of the elevated plus maze at both 7 and 30 days post-mTBI (n= 9–14; *p < 0.05, **p < 0.01, ***p < 0.001).
Spatial memory was evaluated using the Y-maze paradigm at 7 and 30 days following injury. mTBI-challenged mice demonstrated a significant impairment in spatial memory, as compared to sham animals. This mTBI-induced spatial memory deficit was ameliorated by twincretin treatment when appraised at 7 and 30 days post injury (Fig. 5b).
Finally, as a control, anxiety-like behavior following mTBI was evaluated by the use of the elevated plus maze paradigm. Time spent in the open arm of the maze and the number of entries to each arm was measured. Mice were examined at either 7 or 30 days post-mTBI or post-sham injury, with or without twincretin treatment. All groups spent approximately equal time in the open arm of the maze and could not be differentiated from one another at either 7 or 30 days post-injury in regards to their anxiety-like behavior (Fig. 5c), indicating that anxiety-like behavior was not a confound in the prior novel object recognition and Y-maze paradigms.
4. Discussion
This study is the first to show that twincretin has activity at both the GLP-1R and GIPR in neural cells. The inhibitor studies (Fig. 1) demonstrate that twincretin induces cAMP production via agonism at both the GLP-1R and GIPR. As cAMP is the first intermediate in the neurotrophic CREB pathway, it may be inferred that the neurotrophic and neuroprotective effects of twincretin are accordingly mediated via both receptors. Notably, although the GIPR antagonist Pro3GIP has been consistently shown to be specific for the GIPR, the GLP-1R antagonist Ex 9–39 has been reported to possibly bind both the GLP-1R and GIPR (Gault et al., 2003). Recent studies suggest that Ex 9–39 concentrations of 1 µM and greater (that are higher than those used in our study) result in a loss of incretin receptor subtype selectivity (Al-Sabah et al., 2014). Nevertheless, a possibility exists that the reduction in cAMP production induced by the addition of 100 nM Ex 9–39 to 1 nM twincretin solution may not be due solely to the inhibition of GLP-1R binding. This leaves the prospect that twincretin does not bind the GLP-1R in neural cells, but rather induces its neurotrophic effects solely through the GIPR. Such a scenario is highly unlikely, however, as our data shows that the GLP-1R-overexpressing SH-hGLP-1R#9 cell line dramatically enhances the neurotrophic and neuroprotective effects of twincretin. Additionally, although one recent publication suggests that human (Pro3)GIP may actually be a full agonist of the human GIPR, rather than an antagonist (Sparre-Ulrich et al., 2016), several results obtained in the current study (data not shown), in addition to the results displayed in Fig. 1c, suggest that (Pro3)GIP is in fact an antagonist of the human GIPR. Our findings coincide with a large body of research demonstrating the antagonistic effects of (Pro3)GIP at the GIPR (Gault et al., 2002; Gault et al., 2003; De Toro-Martin et al., 2014). The summation of these results, as well as previous data demonstrating the efficacy of twincretin at both receptors in vivo (Finan et al., 2013) suggests that twincretin almost certainly induces significant cAMP production via both receptors in neural cells.
In this study, twincretin is also shown to be neurotrophic in cultured human neuroblastoma cells. SH-SY5Y cells have been widely used to evaluate the neurotrophic and neuroprotective actions of candidate therapeutics in neurological drug development (Melo et al., 2011), as they possess multiple biochemical and functional properties of primary neurons (Ciccarone et al., 1989); albeit they are less vulnerable to toxic insults. Twincretin dose-dependently increased levels of cAMP and pCREB, two pivotal intermediates in the neurotrophic CREB pathway (Sakamoto et al., 2011), and reduced markers of cell death, such as extracellular LDH and oxidized MTS, in SH-SY5Y cells. Whereas the results of the MTS and LDH assays performed on SH-SY5Y cells showed neurotrophic trends, they did not reach significance when compared to control conditions. For this reason the GLP-1R-overexpressing SH-hGLP-1R#9 cells were used. In these cells, the neurotrophic trends observed in the SH-SY5Y cells were amplified and became statistically significant. It must be noted that these cells overexpress the GLP-1R, but not the GIPR, meaning that their GLP-1R:GIPR ratio does not exactly mirror that of SH-SY5Y cells. If both receptors were overexpressed in proportion, however, the neurotrophic trends may likely have been yet further exaggerated.
Twincretin is also shown to have significant neuroprotective effects in both SH-SY5Y and SH-hGLP-1R#9 cells exposed to toxic concentrations of glutamate and hydrogen peroxide (as the insults of gluatamate excitotoxicity and oxidative stress are reported involved in the secondary phase of mTBI (Morganti-Kossmann et al., 2002; Barkhoudarian et al., 2011; Walker and Tesco, 2013; Greig et al., 2014 Barkhoudarian et al., 2016)). Again, 1–1000 nM twincretin displayed neuroprotective trends in SH-SY5Y cells that were amplified when experiments were repeated in SH-hGLP-1R#9 cells. Remarkably, full amelioration of the toxic effects of glutamate and hydrogen peroxide were achieved at 1 and 10 nM, respectively, in SH-hGLP-1R#9 cells. In light of the reported dopaminergic neuronal cell loss following mild to moderate TBI in rodent models (Hutson et al., 2011; Bales et al., 2010; Acosta et al., 2015; Impellizzeri et al., 2016), up regulation of PD associated pathways instigated by mTBI in rodents (Tweedie et al., 2013b), elevated risk of PD following TBI in humans (Gardner et al., 2015; Crane et al., 2016), and progression of PD in humans by TBI (Schiehser et al., 2016; Crane et al., 2016), we evaluated the ability of Twincretin to protect cultured dopaminergic primary VM neurons from cellular demise. Twincretin (10 to 1000 nM) significantly increased TH immunoreactivity in these neurons exposed to toxic doses of 6OHDA, as compared to neurons exposed to the toxic insult alone. Taken together, these cellular studies in primary and immortal neuronal cells indicate that twincretin is a potent neuroprotective compound.
The comparison among equimolar concentrations of twincretin, Ex-4 and GIP in regards to their cAMP production, CREB phosphorylation and neurotrophism in SH-SY5Y cells suggests that twincretin improves upon the effects of its constituent single receptor agonists. These improvements are in line with prior results of the dual agonism of twincretin and demonstration of EC50 (effective concentration that stimulates half-maximal receptor number) values that are similar to both Ex-4 and GIP at their respective target receptors (Finan et al., 2013).
Finally, our behavioral results in mice demonstrate that daily twincretin (50 µg/kg) administration for seven days beginning 30 min post-mTBI fully ameliorated the deficits in visual and spatial memory that were evident in vehicle-treated mTBI mice. These effects were observed at both 7 and 30 days post-mTBI, indicating that the beneficial effects of a clinically translatable dose of twincretin are sustained long after injury and, importantly, long after the cessation of twincretin (7 day) dosing. The results of the elevated plus maze bolster these findings, as they show that the results of the novel object recognition and Y-maze paradigms are not confounded by different levels of anxiety among experimental groups, which is important in light of recent reports that GLP-1 has potential anxiogenic and antidepressant actions (Isacson et al., 2011; Anderberg et al., 2016). Notably, persistent spatial and visual learning deficits have been detected after both mild and moderate TBI (Beers, 1992; Konrad et al., 2010; Skelton et al., 2000; Chuah et al., 2004). In summary, the results of this study show that twincretin is neurotrophic and neuroprotective in human neuroblastoma cells and primary cultures of rat neurons, has activity at both the GLP-1R and GIPR in human neuroblastoma cells, improves upon the effects of single incretin receptor agonists in vitro, and, likely due to these neuroprotective effects, fully prevents the impairments in visual and spatial memory exhibited in mice post-mTBI.
These results establish twincretin as a further incretin mimetic that may prove effective in the prevention of cognitive and memory deficits resulting from mTBI, as well as other forms of neurodegeneration. This subset of incretin mimetics includes Ex-4 and liraglutide (Salcedo et al., 2012; Greig et al., 2014; Holscher, 2014; Li et al., 2015), GLP-1R agonists that were approved for the treatment of T2DM in 2005 and 2010, respectively, and more recently lixisenatide (Holscher, 2014). The neurotrophic and neuroprotective properties of Ex-4 have been researched extensively, and cross-validated across independent laboratories (Duarte et al., 2013; Bassil et al., 2014; Holscher, 2014; Liu et al., 2015b). Ex-4 has been shown to be neuroprotective in multiple neuronal cell lines and to have beneficial effects in cell and rodent models of multiple diseases and injuries, such as peripheral neuropathy (Perry et al., 2007), stroke (Li et al., 2009; Briyal et al., 2012), Parkinson's disease (Bertilsson et al., 2008, Li et al., 2009, Harkavyi and Whitton, 2010; Athuada & Foltynie, 2016), Huntington's disease (Martin et al., 2009), Alzheimer's disease (Perry et al., 2003; Li et al., 2010a), Amyotrophic lateral sclerosis (Li et al., 2012) and TBI (Eakin et al., 2013; Rachmany et al., 2013b; Tweedie et al., 2013a; Tweedie et al., 2016). Currently, multiple clinical trials are ongoing investigating the use of Ex-4 in the treatment of Alzheimer's disease (NCT01255163), Parkinson's disease (NCT01174810 and NCT01971242), diabetic neuropathy (NCT00855439) and TBI (NCT02058940). Liraglutide has just recently been investigated in preclinical models of neurodegeneration. It has been shown to be neuroprotective in multiple cell and mouse models of Alzheimer's disease (McClean et al., 2010; Hansen et al., 2015), Parkinson's disease (Liu et al., 2015a) and mTBI (Li et al., 2015). Research into the neuroprotective effects of liraglutide has, likewise, made its way into ongoing clinical trials in Alzheimer's disease (NCT01469351 and NCT01843075) and diabetic neuropathy (NCT02138045).
Whereas it is far too early to make any definitive claims, the benefits of twincretin therapy over Ex-4 and liraglutide therapy in mouse and monkey models of T2DM suggest that twincretin may turn out to be as or more promising a neuroprotective agent than Ex-4 and liraglutide. Studies in T2DM show that twincretin reduces blood glucose, fat mass and body weight more than equimolar concentrations of Ex-4 and liraglutide do in mice. Similarly, twincretin improves upon the insulinotropic response of liraglutide in monkeys and has promising actions in preliminary human T2DM studies (Finan et al., 2013). As these effects are mediated through the GLP-1R and GIPR, it is possible that the neurotrophic and neuroprotective effects of twincretin, which are mediated via the same receptors on neurons, will improve upon those of Ex-4 and liraglutide in a similar manner. Side-by-side dose-dependent studies in animal models of TBI as well as other neurological disorders would be required to test this hypothesis.
In synopsis, the results of the current study, as well as those of several recent studies demonstrating the neuroprotective effects of twincretin treatment in rat and mouse models of transient focal cerebral ischemia and Parkinson's disease (Cao et al., 2016; Han et al., 2016; Ji et al., 2016), provide strong impetus for further investigation of twincretin therapy for neurodegeneration. Future studies should assess the neuroprotective effects of twincretin in additional neuronal cell lines, particularly primary cultures, and across multiple animal models of neurodegenerative disease, with a particular emphasis on the specific mechanism and benefits of dual agonism as compared to those of the other viable single receptor incretin mimetics.
5. Conclusion
Our results in cellular and animal models of mTBI establish twincretin as an effective neurotrophic and neuroprotective agent and suggest that it may improve upon the effects of the single incretin receptor agonists via dual agonism. Twincretin mediated mitigation of mTBI-induced impairments in visual and spatial memory at a clinically translatable dose highlight the agent for evaluation both across additional models of TBI as well as other neurodegenerative conditions.
Acknowledgments
R.D.D. is a cofounder of Marcadia Biotech and is currently a research consultant to Roche that supports ongoing scientific collaborations. R.D.D. is a co-inventor on patent applications (US2011/0166062 A1; US 12/999,285; “GIP-based mixed agonists for treatment of metabolic disorders and obesity”) owned by Indiana University that are licensed to Roche Pharmaceuticals (32993-214815).
This research was supported in part by (i) the Intramural Research Program of the National Institute on Aging, National Institutes of Health, grant number AG000333 (2016), (ii) the Ari and Regine Aprijaskis Fund at Tel-Aviv University, and (iii) a grant from the Israel Science Foundation, grant number 108/09.
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations
- BCA
bicinchoninic acid
- cAMP
cyclic AMP
- CDC
Centers for Disease Control and Prevention
- CREB
cyclic AMP response element-binding protein
- Ex-4
exendin-4
- Ex 9–39
exendin fragment 9–39
- GIP
glucose-dependent insulinotropic peptide
- GIPR
glucose-dependent insulinotropic peptide receptor
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- H2O2
hydrogen peroxide
- LDH
lactate dehydrogenase
- mTBI
mild traumatic brain injury
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- pCREB
phosphorylated cyclic AMP response element-binding protein
- ROS
reactive oxygen species
- T2DM
type 2 diabetes mellitus
- TBI
traumatic brain injury
- TBST
tris-buffered saline and Tween 20
- TCT
twincretin
- TH
tyrosine hydroxylase
- VM
ventral mesencephalon.
Footnotes
Competing interests
The other authors declare no competing interests.
References
- Acosta SA, et al. Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson's disease. J. Cell. Physiol. 2015;230:1024–1032. doi: 10.1002/jcp.24830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalay RN, et al. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci. Lett. 2004;361:12–131. doi: 10.1016/j.neulet.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Al-Sabah S, Al-Fulaij M, Ahmed HA. Selectivity of peptide ligands for the human incretin receptors expressed in HEK-293 cells. Eur. J. Pharmacol. 2014;741:311–315. doi: 10.1016/j.ejphar.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Alvarez E, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 2005;92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- Anderberg RH, et al. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology. 2016;65:54–66. doi: 10.1016/j.psyneuen.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action. Drug Discov. Today. 2016;21:802–818. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Baratz R, et al. Tumor necrosis factor-α synthesis inhibitor, 3,6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J. Neurochem. 2011;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 2011;30(33–48):vii–viii. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys. Med. Rehabil. Clin. N. Am. 2016;27:373–393. doi: 10.1016/j.pmr.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Barnes DE, et al. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83:312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil F, et al. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog. Neurobiol. 2014;118:1–18. doi: 10.1016/j.pneurobio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Beers SR. Cognitive effects of mild head injury in children and adolescents. Neuropsychol. Rev. 1992;3:281–320. doi: 10.1007/BF01108414. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J. Neurosci. Res. 2008;86:326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- Briyal S, Gulati K, Gulati A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res. 2012;1427:23–34. doi: 10.1016/j.brainres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Cao L, et al. A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson's disease by reducing chronic inflammation in the brain. Neuroreport. 2016 doi: 10.1097/WNR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, et al. Injury, W.H.O.C.C.T.F.O.M.T.B. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. The involvement of secondary neuronal damage in the development of neuropsychiatric disorders following brain insults. Front. Neurol. 2014;5:22. doi: 10.3389/fneur.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah YM, Maybery MT, Fox AM. The long term effects of mild head injury on short term memory for visual form, spatial location and their conjunction in a well-functioning university students. Brain Cogn. 2004;56:304–312. doi: 10.1016/j.bandc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Ciccarone V, et al. Phenotypic diversification in human neuroblastoma cells: Expression of distinct neural crest lineages. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- Crane PK, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73:1062–1069. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachir S, et al. Inosine improves functional recovery after experimental traumatic brain injury. Brain Res. 2014;1555:78–88. doi: 10.1016/j.brainres.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- De Toro-Martin J, et al. Predominant role of GIP in the development of a metabolic syndrome-like phenotype in female Wistar rats submitted to forced catch-up growth. Endocrinology. 2014;155:3769–3780. doi: 10.1210/en.2013-2043. [DOI] [PubMed] [Google Scholar]
- Deselms H, et al. Novel pharmaceutical treatments for minimal traumatic brain injury and evaluation of animal models and methodologies supporting their development. J. Neurosci. Methods. 2016 doi: 10.1016/j.jneumeth.2016.02.002. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav. Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- DHEW Publication (NIH) 85-23, Revised. 1995 https://www.ncbi.nlm.nih.gov/books/NBK54050/
- Doulames VM, et al. Social interaction attenuates the extent of secondary neuronal damage following closed head injury in mice. Front. Behav. Neurosci. 2015;9:275. doi: 10.3389/fnbeh.2015.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte AI, et al. Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim. Biophys. Acta. 2013;1832:527–541. doi: 10.1016/j.bbadis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Eakin K, et al. Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PLoS One. 2013;8:e82016. doi: 10.1371/journal.pone.0082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edut S, Rubovitch V, Schreiber S, Pick CG. The intriguing effects of ecstasy (MDMA) on cognitive function in mice subjected to a minimal traumatic brain injury (mTBI) Psychopharmacology (Berl) 2011;214:877–889. doi: 10.1007/s00213-010-2098-y. [DOI] [PubMed] [Google Scholar]
- Faul M, et al. Ctr. Dis. Control Prev. National Center for Injury Prevention and Control; 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- Finan B, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- Gardner RC, et al. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, et al. Traumatic brain injury in later life increases risk for Parkinson disease. Ann. Neurol. 2015;77:987–995. doi: 10.1002/ana.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault VA, et al. Characterization of the cellular and metabolic effects of a novel enzyme-resistant antagonist of glucose-dependent insulinotropic polypeptide. Biochem. Biophys. Res. Commun. 2002;290:1420–1426. doi: 10.1006/bbrc.2002.6364. [DOI] [PubMed] [Google Scholar]
- Gault VA, et al. Effects of the novel (Pro3)GIP antagonist and exendin(9–39)amide on GIP- and GLP-1-induced cyclic AMP generation, insulin secretion and postprandial insulin release in obese diabetic (ob/ob) mice: evidence that GIP is the major physiological incretin. Diabetologia. 2003;46:222–230. doi: 10.1007/s00125-002-1028-x. [DOI] [PubMed] [Google Scholar]
- Greig NH, et al. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014;10:S62–S75. doi: 10.1016/j.jalz.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J. Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- Han L, et al. A novel dual-glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide receptor agonist is neuroprotective in transient focal cerebral ischemia in the rat. Neuroreport. 2016;27:23–32. doi: 10.1097/WNR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- Hansen HH, et al. The GLP-1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence-accelerated mouse model of Alzheimer's disease. J. Alzheimers Dis. 2015;46:877–888. doi: 10.3233/JAD-143090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkavyi A, Whitton PS. Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br. J. Pharmacol. 2010;159:495–501. doi: 10.1111/j.1476-5381.2009.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J. Endocrinol. 2014;221:T31–T41. doi: 10.1530/JOE-13-0221. [DOI] [PubMed] [Google Scholar]
- Hutson CB, et al. Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. J Neurotrauma. 2011;28:1783–1801. doi: 10.1089/neu.2010.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impellizzeri D, et al. Traumatic brain injury leads to development of Parkinson's disease related pathology in mice. Front. Neurosci. 2016;10:458. doi: 10.3389/fnins.2016.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacson R, et al. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur. J. Pharmacol. 2011;650:249–255. doi: 10.1016/j.ejphar.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Ji C, et al. A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson's disease by increasing expression of BNDF. Brain Res. 2016;1634:1–11. doi: 10.1016/j.brainres.2015.09.035. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- Kim SJ, et al. Glucose-dependent insulinotropic polypeptide-mediated up-regulation of beta-cell antiapoptotic Bcl-2 gene expression is coordinated by cyclic AMP (cAMP) response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol. Cell. Biol. 2008;28:1644–1656. doi: 10.1128/MCB.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, et al. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2010;2010:1–15. doi: 10.1017/S0033291710001728. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, et al. CNS injury biomechanics and experimental models. Prog. Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and parkinsonism. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J. Alzheimers Dis. 2010a;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J. Neurochem. 2010b;113:1621–1631. doi: 10.1111/j.1471-4159.2010.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7:e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice. J. Neurochem. 2015;135:1203–1217. doi: 10.1111/jnc.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, et al. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience. 2015a;303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Liu W, et al. Neuroprotective effects of geniposide on Alzheimer's disease pathology. Rev. Neurosci. 2015b;26:371–383. doi: 10.1515/revneuro-2015-0005. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Dugan LL, O'Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J. Neurosci. 1999;19:1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean PL, et al. The novel GLP-1 analogue liraglutide has neuroprotective properties in a mouse model of Alzheimer's disease. Regul. Pept. 2010;164:40. [Google Scholar]
- Mehta A, et al. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Melo A, et al. Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxidative Med. Cell. Longev. 2011;2011:467180. doi: 10.1155/2011/467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, et al. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Nyberg J, et al. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J. Neurosci. Res. 2007;85:2099–2119. doi: 10.1002/jnr.21349. [DOI] [PubMed] [Google Scholar]
- Orman JAL, Kraus JF, Zaloshnja E, M SJ, W MT, C YS. Textbook of Traumatic Brain Injury. American Psychiatric Pub; Washington, DC: 2011. Epidemiology; pp. 3–22. [Google Scholar]
- Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol. Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Perry T, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J. Neurosci. Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- Perry T, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp. Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmany L, et al. Cognitive impairments accompanying rodent mild traumatic brain injury involve p53-dependent neuronal cell death and are ameliorated by the tetrahydrobenzothiazole PFT-α. PLoS One. 2013a;8:e79837. doi: 10.1371/journal.pone.0079837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmany L, et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr.) 2013b;35:1621–1636. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo I, et al. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012;166:1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiehser DM, et al. Cognitive functioning in individuals with Parkinson's disease and traumatic brain injury: a longitudinal study. Parkinsonism Relat. Disord. 2016;30:58–61. doi: 10.1016/j.parkreldis.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Schmidt OI, et al. Closed head injury - an inflammatory disease? Brain Res. Brain Res. Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Shahaduzzaman M, Acosta S, Bickford PC, Borlongan CV. α-Synuclein is a pathological link and therapeutic target for Parkinson's disease and traumatic brain injury. Med. Hypotheses. 2013;81:675–680. doi: 10.1016/j.mehy.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Shao W, et al. GLP-1(28–36) improves beta-cell mass and glucose disposal in streptozotocin-induced diabetic mice and activates cAMP/PKA/beta-catenin signaling in beta-cells in vitro. Am. J. Physiol. Endocrinol. Metab. 2013;304:E1263–E1272. doi: 10.1152/ajpendo.00600.2012. [DOI] [PubMed] [Google Scholar]
- Skelton RW, et al. Humans with traumatic brain injuries show place-learning deficits in computer-generated virtual space. J. Clin. Exp. Neuropsychol. 2000;22:157–175. doi: 10.1076/1380-3395(200004)22:2;1-1;FT157. [DOI] [PubMed] [Google Scholar]
- Sparre-Ulrich AH, et al. Species-specific action of (Pro3)GIP - a full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors. Br. J. Pharmacol. 2016;173:27–38. doi: 10.1111/bph.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, et al. Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Tweedie D. Apoptotic and behavioral sequelae of mild brain trauma in mice. J. Neurosci. Res. 2007;85:805–815. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- Tweedie D, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp. Neurol. 2013a;239:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, et al. Changes in mouse cognition and hippocampal gene expression observed in a mild physical- and blast-traumatic brain injury. Neurobiol. Dis. 2013b;54:1–11. doi: 10.1016/j.nbd.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, et al. Blast traumatic brain injury-induced cognitive deficits are attenuated by preinjury or postinjury treatment with the glucagon-like peptide-1 receptor agonist, exendin-4. Alzheimers Dement. 2016;12:34–48. doi: 10.1016/j.jalz.2015.07.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 2013;5:29. doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Rayner CK, Horowitz M. Incretins. Handb. Exp. Pharmacol. 2016;233:137–171. doi: 10.1007/164_2015_9. [DOI] [PubMed] [Google Scholar]
- Yu YW, et al. Glucose-dependent insulinotropic polypeptide ameliorates mild traumatic brain injury-induced cognitive and sensorimotor deficits and neuroinflammation in rats. J Neurotrauma. 2016 doi: 10.1089/neu.2015.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E, et al. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- Zohar O, et al. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Zohar O, et al. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol. Exp. (Wars) 2011;71:36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]
- Bales JW, et al. Targeting dopamine in acute traumatic brain injury. Open Drug Delivery J. 2010;2:119–128. doi: 10.2174/1877381801002010119. [DOI] [PMC free article] [PubMed] [Google Scholar]