Abstract
Background
Alzheimer's disease (AD) is the most common and well-studied neurodegenerative disease (ND). Biological pathways, pathophysiology and genetics of AD show commonalities with other NDs viz. Parkinson’s disease (PD), Amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), Prion disease and Dentatorubral-pallidoluysian atrophy (DRPLA). Many of the NDs, sharing the common features and molecular mechanisms suggest that pathology may be directly comparable and be implicated in disease prevention and development of highly effective therapies.
Method
In this review, a brief description of pathophysiology, clinical symptoms and available treatment of various NDs have been explored with special emphasis on AD. Commonalities in these fatal NDs provide support for therapeutic advancements and enhance the understanding of disease manifestation.
Conclusion
The studies concentrating on the commonalities in biological pathways, cellular mechanisms and genetics may provide the scope to researchers to identify few novel common target(s) for disease prevention and development of effective common drugs for multi-neurodegenerative diseases.
Keywords: Neurodegenerative diseases, Alzheimer disease, Parkinson’s disease, Huntington's disease, Amyotrophic lateral sclerosis, biological pathways
1. INTRODUCTION
Neurodegenerative disorders are a heterogeneous and frequently hereditary group of disorders characterized by progressive degradation of neurons, leading to neuronal death. Neurodegenerative diseases (NDs) are complex since they involve selective degeneration of several types of neurons. Approximately 25% of global death and disability is caused by brain disorders [1], which account for >34% of the universal burden of diseases [2]. The human brain is a complex organ consisting of roughly 100 billion neurons and 100 trillion synapses. Its complexity and continuous operation render the brain vulnerable to abnormal functions and injuries [3]. Abnormalities in brain functions, such as memory, movement, and cognition, result in neurodegenerative disorders [4]. Various studies have reported that neurodegeneration is prompted rapidly by the combination of progressive neuronal loss and dysfunction [5, 6]. A common feature of many NDs is an extended time course until a sufficient amount of abnormal protein forms into aggregates, in addition to many symptoms over years with increasing disability [7]. Common NDs affect a significant number of individuals across all age groups [8].
The total number of reported NDs is not clear, but it is estimated to be a few hundred [9]. This large group includes diseases such as AD, PD, ALS, HD, Prion Disease, and DRPLA. All these diseases share a few common features such as clinical course, histopathology, and molecular mechanism of pathogenesis [9]. Although these conditions are mainly characterized based on clinical and histological analyses, significant progress in their diagnosis has been made due to advances in live imaging, genetic studies, and proteomic interrogation. Many of these diseases share common symptoms such as memory loss (AD, PD, DRPLA, Prion disease, and HD) and movement-related disorders (ALS, HD, PD, and DRPLA), whereas all of them are eventually fatal [10]. Neurodegenerative disorders, especially AD and PD, account for significant and increasing percentages of morbidity and mortality in the developed world [11–13]. Although each of these diseases exhibits its own modality, specific similarities become clear once their symptoms, disease-causing factors, and molecular changes are studied.
In this review article, we provide an overview of various NDs and their pathophysiology, clinical symptoms and available treatments. We particularly focus on genetics, mechanisms of disease manifestation, and reported medications relating to AD. Commonalities in cellular mechanisms and genetics among these NDs are also discussed briefly. Finally, we emphasize common therapeutics to enhance our understanding of these fatal multi-NDs.
2. ALZHEIMER'S DISEASE (AD)
Alzheimer’s disease, known to be the most common type of dementia, is a degenerative disorder leading to memory loss. AD is the major cause of dementia in Western countries and is generally caused by many factors including environmental factors, genetic factors and lifestyle [14, 15]. The symptoms of AD include cognitive changes, memory loss, and behavioral changes and are characterized by synaptic injury, followed by neuronal loss associated with neurodegeneration [16–19]. The most common symptom of AD is a gradual decline in the ability to memorize new information. During the initial phase of the disease, there is also interference in other cognitive domains affecting reasoning ability, mood, judgemental skills, and language usage. Eventually, AD patients are unable to perform daily life activities and they become dependent on others [20].
3. PATHOPHYSIOLOGY, GENETICS, AND TREATMENT
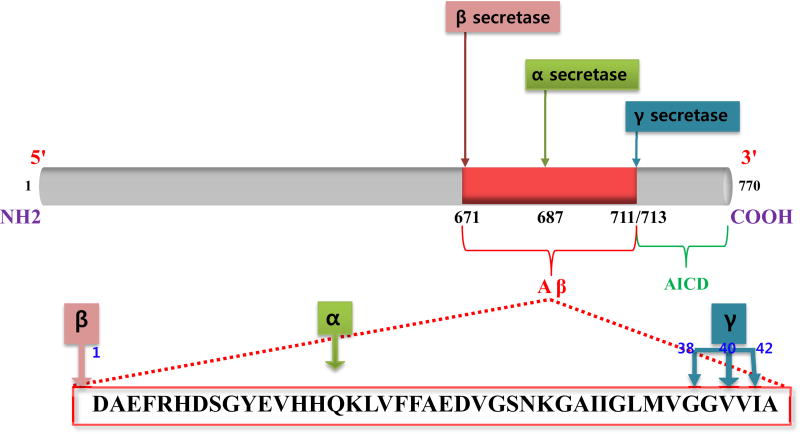
The pathophysiology of AD is complex involving many neurotransmitter systems and pathophysiological processes. Formations of amyloid plaques, neurofibrillary tangles (NFTs), and neuronal degeneration are the hallmarks of neuropathology. Amyloid-β (Aβ) is an 87 kDa transmembrane protein, amyloidogenic product of Amyloid precursor protein (APP)with evident homology to a cell-surface receptor [21]. Mutations within APP that are adjacent to the α, γ, and β-secretase cleavage sites have been identified (Fig. 1) [22–26]. Familial APP mutations increase the relative production of Aβ 42 compared to Aβ 40 [27], as Aβ 42 is more amyloidogenic than Aβ 40, and this may be an important factor in the development of AD.
Fig. (1).
APP processing by secretase enzymes.
Most AD patients suffer onset of disease during the last 7–10 years of their lives [28]. The prevalence of AD is 3% in persons between the ages of 65 and 74, 19% in people between the ages of 75 and 84, and 47% among those older than age 85 [29]. Currently used drugs do not slow the progression of AD but only alleviate the symptoms [30].
Two types of AD are distinguished as follows:
Early-Onset (Familial) AD only contributes to a small number of cases (less than 1%) with symptoms appearing before the age of 65 as well as in patients harboring gene mutations [31]. Mutations on chromosomes 21 (APP), 14 (presenilin 1), and 1 (presenilin 2) cause formation of abnormal proteins.
Late-Onset (Sporadic) (idiopathic) AD occurs most frequently, with disease onset at age 65–70. The causes of this type of AD are not completely understood, but they likely involve a combination of genetic, environmental, and lifestyle-related factors that increase the risk of disease. Specific genes related to the late-onset form of AD have not been definitively identified. However, one genetic risk factor, apolipoprotein E (APOEε4 allele) gene found on chromosome 19, does appear to increase risk of the developing the disease. Table 1 shows the list of drugs for AD that are under trial.
Table 1.
Current drugs under trial for Alzheimer's disease.
| Name | Therapy Type | Target Type | Company Name | FDA Status |
|---|---|---|---|---|
| LMTM | Small Molecule | Tau | TauRx Therapeutics Ltd | Frontotemporal Dementia (Phase 3), AD (Phase 3) |
| ALZT-OP1 | Small Molecule | Amyloid-Related, Inflammation | AZTherapies, Inc. | Phase 3 |
| Solanezumab | Immunotherapy (passive) | Amyloid-Related | Eli Lilly & Co | Phase 3 |
| Verubecestat | Small Molecule | Amyloid-Related | Merck | Phase 3 |
| Simvastatin | Small Molecule | Cholesterol | Merck | AD (Inactive), Mild Cognitive Impairment (Phase 4) |
| Resveratrol | Small Molecule | Other | AD (Phase 3), Mild Cognitive Impairment (Phase 4) | |
| AVP-923 | Small Molecule | Other Neurotransmitters | Avanir Pharmaceuticals | AD (Phase 4), ALS (Phase 3), PD (Phase 3) |
| AZD3293 | Small Molecule | Amyloid-Related | AstraZeneca, Eli Lilly & Co. | Phase 3 |
| Aducanumab | Immunotherapy (passive) | Amyloid-Related | Biogen | Phase 3 |
| Alpha- Tocopherol | Dietary Supplement | Other | Phase 3 | |
| Azeliragon | Small Molecule | Amyloid-Related, Inflammation | Pfizer, TransTech Pharma, Inc., vTv Therapeutics LLC | Phase 3 |
| Brexpiprazole | Small Molecule | Other Neurotransmitters | H. Lundbeck, Otsuka Pharmaceutical Co., Ltd. | Phase 3 |
| Crenezumab | Immunotherapy (passive) | Amyloid-Related | Genentech | Phase 3 |
| Docosahexaenoic acid (DHA) | Dietary Supplement | Other | Martek Biosciences Corporation, NeuroBioPharm, Inc. | Phase 4 |
| Gantenerumab | Immunotherapy (passive) | Amyloid-Related | Chugai Pharmaceutical Co., Ltd., Hoffmann-La Roche | Phase 3 |
| Idalopirdine | Small Molecule | Other Neurotransmitters | Eli Lilly & Co., H. Lundbeck, Otsuka Pharmaceutical Co., Ltd. | AD (Phase 3), Schizophrenia (Inactive) |
| Ketasyn | Dietary Supplement | Other | Accera, Inc. | Phase 4 |
| Masitinib | Small Molecule | Other | AB Science | Phase 3 |
| Nilvadipine | Small Molecule | Other | Archer Pharmaceuticals, Inc. | Phase 3 |
Till date, there are no drug treatments or disease-modifying therapies to cure AD. However, medicines developed and approved by FDA are used to temporarily alleviate the symptoms, or slow down disease progression. There are many failed therapeutic trials including anti-inflammatories, hormonal therapies and chelators in AD treatment [32].
4. PARKINSON'S DISEASE
Parkinson's disease (PD), the second most common ND, is a severe progressive movement disorder involving degeneration of nigrostriatal dopaminergic neurons [33–35]. Parkinson’s disease affects approximately 1.5 million people in U.S., with about 60,000 additionally diagnosed patients every year. In the population above the age of 65, nearly 2% of individuals have PD [34]. PD is a disease caused by a multitude of factors, including genetic and environmental factors. Environmental factors consist of exposure to household pesticides containing rotenone as well as to toxins such as the neurotoxin MPTP (1-methyl-4-phenyl-1, 2, 3,6-tetrahydropyridine). The use of certain drugs and consumption of a specific diet may also be contributing factors [35]. Interestingly, it has been shown that smoking and high intake of coffee and caffeine contribute to a lower risk of developing PD [36].
5. CLINICAL SYMPTOMS AND TREATMENT
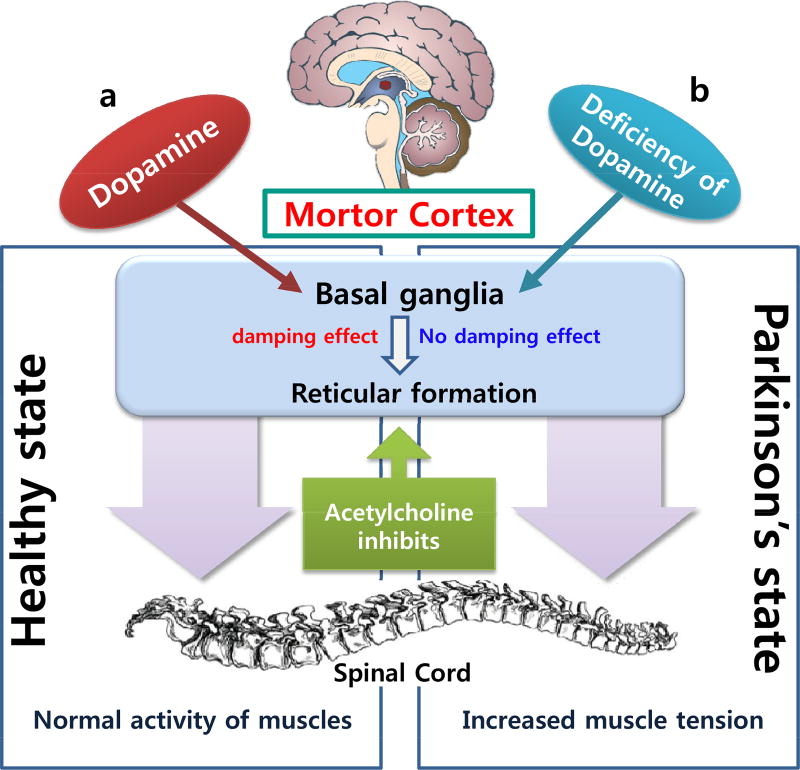
The onset of PD symptoms is triggered by the loss of 50–70% of nigrostriatal dopaminergic neurons. Symptoms of PD include the presence of Lewy bodies, resting tremors, and cytoplasmic accumulation of fibrous proteins in brain cells [33, 37]. Hand tremors are more common than resting foot tremors, whereas difficulty with fine motor tasks is one of the first signs of bradykinesia. Postural instability or impaired balance leads to increased risk of falls, and rigidity of movement increases during performance of mental tasks. Dementia is reported in 80% of end-stage PD patients [37]. It is a key component of Lewy bodies and neurites [32], and mutation of the α-synuclein gene may promote aggregation of α-synuclein protein or inhibition of its degradation pathway (Fig. 2) [36]. This ultimately leads to aggregation and fibrillization of α-synuclein in Lewy bodies and Lewy neurites, resulting in neurodegeneration [34]. The genetic studies of PD explore that mutations in at least five genes namely α-synuclein, parkin, DJ-1, PTEN-induced putative kinase I (PINK1) and leucine-rich repeat kinase 2 or dardarin are responsible for familial early-onset of the disease [34].
Fig. (2).
Schematic comparison between normal and Parkinson’s disease.
Current treatment regimens for PD focus on symptomatic treatment. Understanding of the apoptotic process is essential in developing treatments that could alter the course of the disease [33]. All potential medicines available to date are either under clinical trials or awaiting approval by the U.S. Food and Drug Administration (FDA). Around 40 medicines have been developed for the treatment of PD and related conditions [38]. Levodopa, dopamine agonists, COMT inhibitors, and anti-cholinergics are the classes/types of drugs most effectively used for the treatment of PD.
6. HUNTINGTON'S DISEASE (HD)
Huntington’s disease is a well-known progressive and devastating ND characterized by motor dysfunction and early death, and it affects 4 to 8 persons per 100,000 with an average disease onset between the ages 35 and 45 [39, 40]. The pathophysiology of HD is linked to an 'expanded trinucleotide repeat' (CAG) in the IT-15 gene on chromosome 4 [41]. Current treatments for HD are unable to delay the progression and onset of the disease [42].
7. PATHOPHYSIOLOGY, CLINICAL SYMPTOMS, AND TREATMENT
Although there is currently no cure for HD, there are a few drugs that help manage some of the disease symptoms. In 2008, the U.S. FDA approved tetrabenazine to treat Huntington's chorea, making it the first drug approved in the U.S. to treat the disease. Available medicines for HD reduce involuntary movements, mood stabilizers and antipsychotic drugs can help with some emotional disturbances, and antidepressant medicines may alleviate depression.
8. AMYOTROPHIC LATERAL SCLEROSIS (ALS)
Amyotrophic Lateral Sclerosis (also known as Lou Gehrig's disease) is an ND that develops during adulthood and is characterized by the selective loss of motor neurons in the spinal cord, brainstem, and cerebral cortex, which results in paralysis and death within 5 years [43]. The frequency of ALS is about 6 per 100,000 individuals [44, 45], and about 10% of ALS cases are genetically related [46].
9. PATHOPHYSIOLOGY AND TREATMENT
Evidence for autosomal dominant inheritance has been found in the etiology of ALS, and 10–20% of this type of inheritance is due to mutation of the Cu/Zn superoxide dismutase 1 (SOD1) gene on chromosome 21 [47]. SOD1 is a protein that is ubiquitously expressed in the cytosol and implicated in the conversion of superoxide radical to hydrogen peroxide (although hydrogen peroxide can be inactivated by catalase, thus preventing oxidative damage) [46]. Risk factors for ALS include old age, family history of dementia, pseudobulbar palsy, male gender, low forced vital capacity, bulbar site of onset, and environmental factors [47, 48].
Complete physical examination, medical history, as well as neurological examination are required for diagnosis of ALS. An electromyogram is part of the neurologic exam during diagnosis of ALS. There is no cure for ALS available to date. In 1998, riluzole (trade name Rilutek) was approved for treatment of ALS patients. The drug reduces loss of muscle strength to some extent. No other drug has been found to halt progression of ALS. Recently, Neuralstem, Inc. (NYSE MKT: CUR) announced NSI-566 spinal cord-derived neural stem cells under development, which are in Phase II trials and hold great prospects for the treatment of ALS.
10. DENTATORUBRAL-PALLIDOLUYSIAN ATROPHY (DRPLA)
DRPLA, also known as Naito-Oyanagi disease and Haw River Syndrome, is an autosomal dominant spinocerebellar degenerative disease caused by an expansion in the CAG repeat, which encodes a polyglutamine tract in atrophin-1 protein [49]. Dentatorubral-pallidoluysian atrophy is found in three forms: (i) juvenile-onset (< 20 years), with symptoms involving ataxia and consistent with progressive myoclonus epilepsy; (ii) early adult-onset (20–40 years), including seizures and myoclonus; and (iii) late adult-onset (>40 years), characterized by ataxia, choreoathetosis and dementia.
11. PATHOPHYSIOLOGY AND TREATMENT
Patients with DRPLA show worsening disease symptoms over time. ATN1 is the only gene known to be coupled with DRPLA. Discovery of pathogenic mutations in ATN1 has led to the identification of neuronal intra-nuclear inclusions in the brains of individuals with DRPLA [44, 45]. Accumulation of mutant ATN1 in neuronal nuclei is the most prominent neuropathological finding and is detected as diffused nuclear staining by antibody specifically detecting expanded polyglutamine stretches. There is currently no cure for DRPLA, and the treatment is supportive only. Epilepsy is treated with anti-seizure medications, i.e. antiepileptic drugs.
12. PRION DISEASE
Prion diseases are a group of fatal NDs caused by abnormally conformed infectious proteins called prions [50]. Prion diseases can be genetic or acquired as well as impair brain function and cause memory changes, dementia, personality changes, and problems with movement that worsen over time. The symptoms of these conditions typically commence in adulthood, and these disorders lead to death within a few months to several years. One type of prion disease in humans, known as variant Creutzfeldt-Jakob disease, is assimilated by consumption of cattle with prion disease. In cows, this form of disease is known as bovine spongiform encephalopathy, or more commonly, "mad cow disease".
13. PATHOPHYSIOLOGY AND TREATMENT
Familial forms of prion disease are caused by inherited mutations within the PRNP gene, which ultimately encodes a protein called prion protein (PrP). Normally, this protein plays a role in copper transport in cells. Mutations in the PRNP gene cause cells to produce an abnormal form of the prion protein known as PrPSC, which can convert the normal prion protein PrPC into additional PrPSC. Accumulation of this abnormal protein in the brain results in the formation of clumps that damage or destroy nerve cells, creating microscopic sponge-like holes in the brain and eventually the signs and symptoms of prion disease. To date, no drug, supplement, or lifestyle measurement have been shown to delay the onset of genetic prion diseases. Drugs from various molecular families have been found to impede prion replication, but none are of practical use due to efficacy, pharmacology, or toxicity issues [51].
14. COMMONALITIES IN NEURODEGENERATIVE DISEASES
Many NDs share common features and molecular mechanisms, suggesting that their pathologies may be directly comparable and implicated in disease prevention and development of effective therapies. The mechanisms of pathogenesis of different NDs such as AD, PD, and Prion diseases are all characterized by abnormal accumulations of proteins and selective neuronal degeneration. The accumulated proteins usually form intracellular inclusions or extracellular aggregates in specific brain areas. An intracellular or extracellular aggregate is known as ‘amyloid’ [52–54]. The proteins that accumulate in NDs are usually misfolded and yield a β-sheet structure that promotes aggregation and fibril formation.
NDs share many other common features as most of them manifest late in life, and their pathophysiologies are characterized by neuronal loss and synaptic abnormalities [55]. Another shared feature of NDs is the formation of toxic protein aggregates [56–58]. Mitochondrial dysfunction, oxidative stress, neuronal apoptosis, and impaired protein homeostasis are also common in many NDs. Further, in NDs such as HD and DRPLA, a family history of the disease can be detected in almost every case [59], whereas in AD [60], PD [61], and ALS [62], about 1–10% of all cases are inherited.
A common conception related to the genetic makeup of neurodegenerative diseases is the “common disease/common variant” (CD/CV) hypothesis. This hypothesis explains that common diseases are also governed by common DNA variants (e.g. SNPs). These variants definitely elevate disease risk but are not causative factor of any specific disease [42]. Previously it has been reported that in many NDs, oxidative damage to protein-coding or non-coding RNA potentially causes dysregulation of gene expression and oxidative RNA damage described in many NDs including AD and PD [63]. The apolipoprotein E (APOE) gene, encodes a 299 amino acids long glycoprotein and located on chromosome 19 is estimated to be the major genetic factor in AD (up to 50%) and also reported in case of PD [64]. The similarities in the pathogenesis of NDs suggest that certain genes can be targeted for intervention of multiple NDs [65]. There are many proteins that play an important role in the regulation of multiple NDs. Although research on each ND is very active and advanced, only limited studies have focused on common regulatory proteins and protein–protein interaction networks across multiple NDs [66, 67].
15. COMPUTATIONAL ANALYSIS OF DISEASE PATHWAYS AND PROTEIN-PROTEIN INTERACTION NETWORKS
Rapid developments have established protein-protein interactions (PPIs) as a key part in the topological study of biological networks. Analysis of molecular pathways has been performed on differentially expressed genes identified in DNA array experiments. These identified genes along with their respective PPI information provide better insight into the pathways involved in NDs as well as other diseases.
Analysis of the molecular networks of these genes on the basis of their PPI data can provide insights into the mechanisms upon which the intricacy and compliance of a living cell is found [68]. Such information provided by the molecular heterogeneity of cancer will help us decode the processes involved in various disorders as well as diseases [69]. However, in accordance to the multifactorial mode of disease progression, it is difficult to identify genes that exhibit direct genotype-phenotype correlations [70].
The modeling of a gene pathway network and its topological analysis at different scales may provide us with detailed descriptions of the precise functions of genes and proteins involved in diseases of multifactorial nature in which pathogenesis is not dependent on the malfunction of a single gene or protein [71]. Pathway analysis of different ND provides better understanding of functional and metabolic processes. Pathway analysis is meant to analyze a predetermined aggregation of a set of genes contained in a functional unit as defined by prior biological knowledge. Currently, there are many pathway-based approaches, which correspond to different research designs and data types. A few common pathway databases are listed in Table 2 [72].
Table 2.
Pathway databases.
| Category | Description | Examples |
|---|---|---|
| Metabolic pathways | Primarily contain a series of biochemical reactions, especially the chemical modifications of the small molecule substrates of enzymes | KEGG (http://www.genome.ad.jp/kegg) BioCyc (http://www.biocyc.org) BIOPATH (http://www.mol-et.de/databases/biopath.html) EMP (http://emp.mcs.anl.gov) |
| Signal transduction pathways | Describe the information spread from one part or subprocess of the cell to another, generally through a series of covalent modifications of protein | CSNDB (http://geo.nihs.go.jp/csndb) SPAD (http://www.grt.kyushu-u.ac.jp/spad) TransPath (http://transpath.gbf.de/) BBID (http://bbid.grc.nia.nih.gov/) |
| Protein–protein interaction pathways | Focus on interactions between proteins, most of which are derived from various large-scale experimental methods | CYGD (http://mips.gsf.de/genre/proj/yeast/index.jsp) DIP (http://dip.doe-mbi.ucla.edu) GRID (http://biodata.mshri.on.ca/grid/servlet/Index) HPRD (http://www.hprd.org/) |
| Transcriptional regulation pathway | Mainly concerned with the relationships between transcription factors and the corresponding genes they regulate | STKE (http://www.stke.org/) BTITE (http://www.genome.ad.jp/brite/) TRANSFAC (http://transfac.gbf.de/) CST (http://www.cellsignal.com/) |
Determination of protein-protein interactions is becoming most important objectives of systems biology. The major target of bioinformatics progression has now been more inclined from understanding networks encoded by model species to understanding the networks underlying human diseases due to an increase in human protein-protein interaction data [73]. Protein-protein interactions involve a broad range of biological processes, including cellular interactions, metabolic and developmental control [74]. The many functional affiliations and connections between proteins are at the core of cellular processing, and their systematic characterization will provide context in molecular systems biology. However, experimental and virtually predicted interactions are available over many resources, and the available data represent quite differences in terms of quality and completeness.
CONCLUSION
Alzheimer disease pathophysiology and genetics show many commonalities with other NDs, which provide unprecedented opportunities to explore common therapeutic possibilities. Biological pathways of multi-NDs provide common interacting genes/proteins, which may be targeted for common treatment and rational therapeutic intervention. Understanding of multi-NDs may become more clear and advanced by tackling all common features. It is expected that these molecular genetic signatures will guide clinicians in clinical decision-making and establishing accurate clinical diagnosis of NDs, thus allowing classification of NDs based on molecular deficits.
Acknowledgments
This study was supported by Research Grant of Yeungnam University, Republic of Korea (2016). This work was supported by the grant K16281 awarded to the Korea Institute of Oriental Medicine (KIOM) from Ministry of Education, Science and Technology (MEST), Republic of Korea.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- Aβ
Amyloid-β
- PD
Parkinson’s disease
- ALS
Amyotrophic lateral sclerosis
- HD
Huntington's disease
- DRPLA
Dentatorubral-pallidoluysian atrophy
- NDs
Neurodegenerative diseases
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Silberberg D. The high impact of neurologic disorders in developing countries: the struggle for global recognition. Neurology. 2011;77(3):307–8. doi: 10.1212/WNL.0b013e3182285da9. [DOI] [PubMed] [Google Scholar]
- 2.Pedro AV. Coping with Brain Disorders using Neurotechnology. Malays J Med Sci. 2012;19(1):1–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Teplow DB. Molecular biology of neurodegenerative diseases. Preface Prog Mol Biol Transl Sci. 2012;107:xiii–xiv. doi: 10.1016/B978-0-12-385883-2.00014-X. [DOI] [PubMed] [Google Scholar]
- 4.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–65. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruttgen A, Saxena S, Evangelopoulos ME, Weis J. Neurotrophins and neurodegenerative diseases: receptors stuck in traffic? J Neuropathol Exp Neurol. 2003;62(4):340–50. doi: 10.1093/jnen/62.4.340. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad K, Balaramnavar VM, Baig MH, Srivastava AK, Khan S, Kamal MA. Identification of potent caspase-3 inhibitors for treatment of multi- neurodegenerative diseases using pharmacophore modeling and docking approaches. CNS Neurol Disord Drug Targets. 2014;13(8):1346–53. doi: 10.2174/1871527313666141023120843. [DOI] [PubMed] [Google Scholar]
- 7.Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol. 2009;256(3):270–9. doi: 10.1007/s00415-009-5243-y. [DOI] [PubMed] [Google Scholar]
- 8.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3(3):186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 9.Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111(1):3–10. doi: 10.1172/JCI17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340(25):1970–80. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 11.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15(4):169–73. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 13.Russell A, Drozdova A, Wang W, Thomas M. The impact of dementia development concurrent with Parkinson's disease: a new perspective. CNS Neurol Disord Drug Targets. 2014;13(7):1160–8. doi: 10.2174/1871527313666140917122739. [DOI] [PubMed] [Google Scholar]
- 14.Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, et al. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51(3):347–72. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 15.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer's and vascular types. Biomed Res Int. 2014;2014:908915. doi: 10.1155/2014/908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10(2):184–92. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- 17.Katzman R. Alzheimer's disease. N Engl J Med. 1986;314(15):964–73. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- 18.Masliah E, Mallory M, Alford M, DeTeresa R, Iwai A, Saitoh T. Molecular Mechanisms of Synaptic Disconnection in Alzheimer’s Disease. In: Hayman BT, Duyckaerts C, Christen Y, editors. Connections, Cognition and Alzheimer’s Disease. Berlin, Heidelberg: Springer Berlin Heidelberg; 1997. pp. 121–40. [Google Scholar]
- 19.Budson AE, Price BH. Memory dysfunction. N Engl J Med. 2005;352(7):692–9. doi: 10.1056/NEJMra041071. [DOI] [PubMed] [Google Scholar]
- 20.LaFerla FM, Oddo S. Alzheimer's disease: Abeta, tau and synaptic dysfunction. Trends Mol Med. 2005;11(4):170–6. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–6. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 22.Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, et al. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353(6347):844–6. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 23.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349(6311):704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 24.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992;360(6405):672–4. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 25.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–7. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 26.Cole SL, Vassar R. The Alzheimer's disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264(5163):1336–40. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 28.Adlard PA, Cummings BJ. Alzheimer's disease--a sum greater than its parts? Neurobiol Aging. 2004;25(6):725–33. doi: 10.1016/j.neurobiolaging.2003.12.016. discussion 43-6. [DOI] [PubMed] [Google Scholar]
- 29.Holscher C. Possible causes of Alzheimer's disease: amyloid fragments, free radicals, and calcium homeostasis. Neurobiol Dis. 1998;5(3):129–41. doi: 10.1006/nbdi.1998.0193. [DOI] [PubMed] [Google Scholar]
- 30.Bolognesi ML, Banzi R, Bartolini M, Cavalli A, Tarozzi A, Andrisano V, et al. Novel class of quinone-bearing polyamines as multi-target-directed ligands to combat Alzheimer's disease. J Med Chem. 2007;50(20):4882–97. doi: 10.1021/jm070559a. [DOI] [PubMed] [Google Scholar]
- 31.Gotz J, Schild A, Hoerndli F, Pennanen L. Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models. Int J Dev Neurosci. 2004;22(7):453–65. doi: 10.1016/j.ijdevneu.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Waite LM. Treatment for Alzheimer's disease: has anything changed? Aust Prescr. 2015;38(2):60–3. doi: 10.18773/austprescr.2015.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lev N, Melamed E, Offen D. Apoptosis and Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):245–50. doi: 10.1016/S0278-5846(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 34.Corti O, Hampe C, Darios F, Ibanez P, Ruberg M, Brice A. Parkinson's disease: from causes to mechanisms. CR Biol. 2005;328(2):131–42. doi: 10.1016/j.crvi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Booth TC, Nathan M, Waldman AD, Quigley AM, Schapira AH, Buscombe J. The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 1. AJNR Am J Neuroradiol. 2015;36(2):229–35. doi: 10.3174/ajnr.A3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shastry BS. Parkinson disease: etiology, pathogenesis and future of gene therapy. Neurosci Res. 2001;41(1):5–12. doi: 10.1016/s0168-0102(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 37.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363(9423):1783–93. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 38.Payami H, Factor SA. Promise of pharmacogenomics for drug discovery, treatment and prevention of Parkinson's disease. A perspective. Neurotherapeutics. 2014;11(1):111–6. doi: 10.1007/s13311-013-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman AO, Murgatroyd PR, Medina-Gomez G, Wood NI, Finer N, Vidal-Puig AJ, et al. The metabolic profile of early Huntington's disease--a combined human and transgenic mouse study. Exp Neurol. 2008;210(2):691–8. doi: 10.1016/j.expneurol.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Kim HS, Lyoo CH, Lee PH, Kim SJ, Park MY, Ma HI, et al. Current Status of Huntington's Disease in Korea: A Nationwide Survey and National Registry Analysis. J Mov Disord. 2015;8(1):14–20. doi: 10.14802/jmd.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Block RC, Dorsey ER, Beck CA, Brenna JT, Shoulson I. Altered cholesterol and fatty acid metabolism in Huntington disease. J Clin Lipidol. 2010;4(1):17–23. doi: 10.1016/j.jacl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fink KD, Deng P, Torrest A, Stewart H, Pollock K, Gruenloh W, et al. Developing stem cell therapies for juvenile and adult-onset Huntington's disease. Regen Med. 2015;10(5):623–46. doi: 10.2217/rme.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patzke H, Tsai LH. Cdk5 sinks into ALS. Trends Neurosci. 2002;25(1):8–10. doi: 10.1016/s0166-2236(00)02000-2. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi Y, Kakita A, Yamada M, Egawa S, Oyanagi S, Naito H, et al. Hereditary dentatorubral-pallidoluysian atrophy: ubiquitinated filamentous inclusions in the cerebellar dentate nucleus neurons. Acta Neuropathol. 1998;95(5):479–82. doi: 10.1007/s004010050828. [DOI] [PubMed] [Google Scholar]
- 45.Igarashi S, Koide R, Shimohata T, Yamada M, Hayashi Y, Takano H, et al. Suppression of aggregate formation and apoptosis by transglutaminase inhibitors in cells expressing truncated DRPLA protein with an expanded polyglutamine stretch. Nat Genet. 1998;18(2):111–7. doi: 10.1038/ng0298-111. [DOI] [PubMed] [Google Scholar]
- 46.Simpson CL, Al-Chalabi A. Amyotrophic lateral sclerosis as a complex genetic disease. Biochim Biophys Acta. 2006;1762(11–12):973–85. doi: 10.1016/j.bbadis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–41. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 48.Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- 49.Mohan RD, Abmayr SM, Workman JL. The expanding role for chromatin and transcription in polyglutamine disease. Curr Opin Genet Dev. 2014;26:96–104. doi: 10.1016/j.gde.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takada LT, Geschwind MD. Prion diseases. Semin Neurol. 2013;33(4):348–56. doi: 10.1055/s-0033-1359314. [DOI] [PubMed] [Google Scholar]
- 51.Karapetyan YE, Sferrazza GF, Zhou M, Ottenberg G, Spicer T, Chase P, et al. Unique drug screening approach for prion diseases identifies tacrolimus and astemizole as antiprion agents. Proc Natl Acad Sci U S A. 2013;110(17):7044–9. doi: 10.1073/pnas.1303510110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 53.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10:S2–9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 54.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10–7. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 55.Kamal MA, Mushtaq G, Greig NH. Current Update on Synopsis of miRNA Dysregulation in Neurological Disorders. CNS Neurol Disord Drug Targets. 2015;14(4):492–501. doi: 10.2174/1871527314666150225143637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carrell RW, Lomas DA. Conformational disease. Lancet. 1997;350(9071):134–8. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 57.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24(9):329–32. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 58.Soto C. Protein misfolding and disease; protein refolding and therapy. FEBS Lett. 2001;498(2–3):204–7. doi: 10.1016/s0014-5793(01)02486-3. [DOI] [PubMed] [Google Scholar]
- 59.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 60.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 61.Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16(Spec No. 2):R183–94. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 62.Ince PG, Highley JR, Kirby J, Wharton SB, Takahashi H, Strong MJ, et al. Molecular pathology and genetic advances in amyotrophic lateral sclerosis: an emerging molecular pathway and the significance of glial pathology. Acta Neuropathol. 2011;122(6):657–71. doi: 10.1007/s00401-011-0913-0. [DOI] [PubMed] [Google Scholar]
- 63.Grunblatt E. Commonalities in the genetics of Alzheimer's disease and Parkinson's disease. Expert Rev Neurother. 2008;8(12):1865–77. doi: 10.1586/14737175.8.12.1865. [DOI] [PubMed] [Google Scholar]
- 64.Giau VV, Bagyinszky E, An SS, Kim SY. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr Dis Treat. 2015;11:1723–37. doi: 10.2147/NDT.S84266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathisen PM. Gene discovery and validation for neurodegenerative diseases. Drug Discov Today. 2003;8(1):39–46. doi: 10.1016/s1359644602025400. [DOI] [PubMed] [Google Scholar]
- 66.Goehler H, Lalowski M, Stelzl U, Waelter S, Stroedicke M, Worm U, et al. A protein interaction network links GIT1, an enhancer of huntingtin aggregation, to Huntington's disease. Mol Cell. 2004;15(6):853–65. doi: 10.1016/j.molcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Giorgini F, Muchowski PJ. Connecting the dots in Huntington's disease with protein interaction networks. Genome Biol. 2005;6(3):210. doi: 10.1186/gb-2005-6-3-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hiesinger PR, Hassan BA. Genetics in the age of systems biology. Cell. 2005;123(7):1173–4. doi: 10.1016/j.cell.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Rhodes DR, Chinnaiyan AM. Integrative analysis of the cancer transcriptome. Nat Genet. 2005;37:S31–7. doi: 10.1038/ng1570. [DOI] [PubMed] [Google Scholar]
- 70.Gunsalus KC, Ge H, Schetter AJ, Goldberg DS, Han JD, Hao T, et al. Predictive models of molecular machines involved in Caenorhabditis elegans early embryogenesis. Nature. 2005;436(7052):861–5. doi: 10.1038/nature03876. [DOI] [PubMed] [Google Scholar]
- 71.Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabasi AL. The human disease network. Proc Natl Acad Sci U S A. 2007;104(21):8685–90. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin L, Zuo XY, Su WY, Zhao XL, Yuan MQ, Han LZ, et al. Pathway-based analysis tools for complex diseases: a review. Genom Proteom Bioinform. 2014;12(5):210–20. doi: 10.1016/j.gpb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kann MG. Protein interactions and disease: computational approaches to uncover the etiology of diseases. Brief Bioinform. 2007;8(5):333–46. doi: 10.1093/bib/bbm031. [DOI] [PubMed] [Google Scholar]
- 74.Rao VS, Srinivas K, Sujini GN, Kumar GN. Protein-protein interaction detection: methods and analysis. Int J Proteomics. 2014;2014:147648. doi: 10.1155/2014/147648. [DOI] [PMC free article] [PubMed] [Google Scholar]