Abstract
Both Alzheimer’s disease (AD) and Type 2 diabetes mellitus (T2DM) share the presence of systemic and neuro-inflammation, enhanced production and accumulation of β-amyloid peptide and abnormal levels of the enzymes acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). Altered levels of AChE and BuChE both in AD as well as in T2DM imply that those two enzymes may be playing a pivotal role in the pathogenesis of the two disorders. AD and T2DM are both characterized by elevated levels of AChE and BuChE in the plasma. On the other hand, in AD the brain levels of AChE go down while those of BuChE go up, resulting in deregulation in balance between AChE and BuChE. This imbalance and change in the AChE/BuChE ratio causes cholinergic deficit in the brain, i.e. deficiency in the brain neurotransmitter acetylcholine. With better understanding of the inter-relationship of AChE and BuChE levels in normality as well as abnormality, AD and T2DM can be effectively treated. Thus, general cholinesterase inhibitors that inhibit both AChE and BuChE as well as highly selective BuChE inhibitors may have potential therapeutic benefits in the treatment of AD and other related dementias.
Keywords: Acetylcholinesterase, Alzheimer’s disease, butyrylcholinesterase, cholinergic deficit, cholinesterase inhibitors, type 2 diabetes mellitus
INTRODUCTION
The enzyme acetylcholinesterase (AChE) (EC 3.1.1.7) is a specific esterase that belongs to the carboxylesterase family of enzymes. It predominantly hydrolyzes the neurotransmitter acetylcholine (ACh). AChE is found in high concentrations mainly in the red blood cells as well as in the brain at neuromuscular junctions and cholinergic brain synapses. On the other hand, the enzyme butyrylcholin-esterase (BuChE) (also known as “pseudo” cholinesterase; EC 3.1.1.8) is a non-specific type of cholinesterase enzyme that hydrolyzes different types of choline esters and it ubiquitously exists throughout the body, most importantly, in the human liver, blood serum, pancreas and the central nervous system. In the brain, BuChE is primarily associated with glial cells and endothelial cells [1, 2].
These two enzymes share 65% of structural homology. In normality, BuChE plays a supportive role in the brain and accounts for about 10% cholinesterase activity in the temporal cortex. Just like AChE, the enzyme BuChE is involved in the hydrolysis of the neurotransmitter ACh and, hence, it has been proposed as a viable therapeutic target in AD, a disorder which is characterized by cholinergic deficit as it will be discussed later. AChE and BuChE have different Km (Michaelis Menten Constant) values and, hence, different kinetic responses to the concentrations of ACh. At low concentrations of ACh, AChE is highly efficient but BuChE is much less efficient. However, at higher ACh concentrations, AChE becomes substrate inhibited while BuChE becomes highly efficient in the hydrolysis of Ach [3].
Owing to its low expression in the normal mammalian brain as compared with AChE, the importance of BuChE in human brain was underestimated at first [4]. However, later studies confirmed the importance of BuChE within the nervous system, particularly regarding its pivotal role in coregulating the levels of ACh in the brain in neurodegenerative disorders such as AD [5]. In AD, while the levels of AChE go down by as much as 85% in certain brain areas, BuChE levels (specifically the G1 form) go up as the disease progresses [6, 7]. Consequently, the ratio of BuChE to AChE changes from 0.2 up to as much as 11 in cortical areas of the brain suffering from AD [8]. When the ratio of AChE to BuChE alters with AD progression, this changes the auxiliary role that BuChE plays in normality and, instead, makes BuChE an enzyme of increasing functional importance [9].
Low-grade systemic inflammation has been shown to be a pathological link between AD and T2DM. Various studies have demonstrated that inflammatory pathways play an important role linking T2DM and neurodegenerative diseases such as AD [10–13]. At the same time, it has been noted that altered plasma and tissue levels of AChE and BuChE may also serve as markers for the existence of low-grade systemic inflammation in disorders such as T2DM and AD [14]. In fact, it has been proposed that activities of AChE and BuChE are elevated in diseases such as T2DM and AD involving low-grade systemic inflammation even when plasma, cerebrospinal fluid and tissue concentrations of C-reactive protein, interleukin-6, tumor necrosis factor (TNF-α) and other markers of inflammation are not noticeably higher [15]. In vitro studies have demonstrated that selective BuChE inhibition results in lowering the production of pro-inflammatory cytokines such as interleukin-1b and TNF-α in peripheral blood mononuclear cell cultures [16]. Acetylcholine is anti-inflammatory in nature. The acetylcholine receptor modulates interactions between the immune system and the nervous system. Abnormally elevated levels of AChE and BuChE result in reduced level of ACh, thereby causing reduction or absence of anti-inflammatory actions exerted by ACh on the body. Hence, higher levels of AChE and BuChE in systemic circulation result in inflammation.
ROLE OF AChE AND BuChE IN THE PATHOGENESIS OF AD
Anatomical findings from the brains of AD patients have revealed damage to the nuclei containing cholinergic neurons suggesting the role of cholinergic system in AD. Earlier studies have demonstrated that nucleus basalis and hippocampus are affected in AD patients with severe deficits occurring especially in the nucleus basalis [17]. Loss of cholinergic activity in areas of the brain responsible for higher mental functions contributes to the pathogenesis of AD. Extracellular senile plaques, intracellular neurofibrillary tangles and localized Aβ formation and accumulation are the hallmarks of AD. Senile plaques found deposited in the brains of AD sufferers contain extracellular deposits of human beta-amyloid (Aβ) peptide [18, 19]. It has been reported that a region near the C-terminus of human AChE is weakly homologous to the N-terminus of Alzheimer’s disease Aβ peptide [20]. This further suggests that AChE may be involved in the formation of amyloid fibrils in senile plaques and the toxicity associated with such deposits.
Evidence from in vitro studies using electron microscopy and Congo Red binding suggests that AChE strongly stimulates Aβ peptide fibrils assembly [21]. The elongated unbranched fibrils of Aβ that bind to Congo Red are comprised of AChE and apolipoprotein E besides other things. In addition, physical, biochemical and morphological data provide evidence that AChE accelerates the assembly of Aβ peptides to form amyloid-AChE complex, strongly suggesting the role of human AChE in accelerating Aβ fibrillogenesis [22]. Those Aβ-AChE complexes were found to be more neurotoxic than Aβ aggregates alone in primary cultured chick retina neurons and PC12 cells [23]. Likewise, in an in vivo study monitoring and comparing the neurotoxicity of Aβ fibrils alone and Aβ-AChE complexes, it has been shown that when human Aβ peptide fibrils formed a complex with AChE to generate Aβ-AChE, the resulting Aβ-AChE complexes were bigger in size than those observed in matched controls injected with the same amount of Aβ peptide alone. Also, Aβ-AChE complexes proved more toxic than Aβ peptide fibrils alone. Most importantly, the hippocampal injection of Aβ-AChE complexes to rats resulted in the formation of Alzheimer-like lesions in the rat brain. In fact, Aβ-AChE injected animals showed stronger neuronal cell loss in comparison with Aβ-injected animals. This suggests that AChE is associated with some of the neurodegenerative changes seen in AD brains by promoting amyloid formation, astrocytosis and neural cell loss caused by Aβ-AChE complex formation [24].
The important role of AChE in AD pathogenesis is further supported by recent studies conducted on double-transgenic mice that overexpress both human AChE and amyloid precursor protein (APP). Those doubly-transgenic mice are produced when Tg2576 mice (which express human APP and show amyloid plaque deposits in their brains at age 6 month) are crossed with transgenic mice expressing human AChE. The resulting hybrids express both transgenes in their brains. β-amyloid plaques are formed 30–50% sooner in double-APP-AChE transgenic mice as compared to those mice that overexpress APP alone. Plaque numbers were higher and more abundant in doubly transgenic animals at 9 and 12 months and so was the total amyloid content in the brain as assessed by quantitative immunoassay via enzyme-linked immunosorbent assay. These findings provide further evidence that AChE promotes β-amyloid plaques in cerebral cortex and may play a pivotal role in the pathogenesis of AD [25].
The association of BuChE to AD has been studied in different ethnic groups. It has been shown that BuChE may promote the risk of AD either alone or in synergic association of butyrylcholinesterase-K variant and apolipoprotein epsilon 4 allele [26, 27]. It has been found that BuChE-K variant and its corresponding apolipoprotein allele act synergistically to increase the risk of late-onset AD, especially in age groups less than 75 years old [28]. Furthermore, abnormally high levels of BuChE in the brains of AD sufferers contribute to the pathogenesis of AD. Comparing the brains of AD patients versus healthy subjects, it has been observed that BuChE correlates positively with the formation of neocortical amyloid-rich neuritic plaques and neurofibrillary tangles [29].
With the progression of AD, the G1 form of BuChE increasingly accumulates in plaques and tangles. Levels of the G1 form of BuChE have been shown to correlate positively with plaque density and pathogenicity [30]. BuChE begins to be linked to amyloid plaques at around the same time when Aβ deposits start to acquire a compact beta-pleated sheet conformation. This is supported by studies on the brains of aged, demented and age-matched nondemented individuals in which the total cortical area covered by plaque-like Aβ and BuChE deposits was measured at two regions of the temporal cortex. The proportional plaque area displaying BuChE-reactivity was fivefold to six fold higher in the brains of demented subjects than in the nondemented individuals [31]. It has been suggested that BuChE could be making a noticeable contribution in the transformation of Aβ from an initially benign form to an ultimately malignant form of amyloid plaques causing brain tissue degeneration and clinical dementia in disorders such as AD [32, 33].
ROLE OF AChE AND BuChE IN THE PATHOGENESIS OF T2DM
Higher levels of AChE as well as BuChE are observed in T2DM. AChE activity has been found higher by an order of magnitude in islets of Langerhans than in the exocrine tissue from rat pancreas and this difference in activity was observed in healthy, control rats as well as rats made diabetic with streptozotocin. In addition, AChE activity in islets from diabetic rats was about 30–40% higher than in islets from control rats [34]. Likewise, when the activity of serum BuChE was investigated in diabetic versus normal controls, it was found that BuChE activity was significantly elevated in both type 1 diabetes (8.10 ±3.35 units/mL) and type 2 diabetes (7.22 ±1.95 units/mL) patients compared with the control subjects (4.23 ±1.89 units/mL) (P < 0.001). It was also found in this study that serum activity of BuChE was correlated with insulin sensitivity particularly in T2DM patients [35].
Studies have shown that BuChE may be indirectly contributing to the pathogenesis of T2DM by causing insulin resistance [36]. In fact, a locus on chromosome 3q27, which is near the chromosomal locus of BuChE (3q26), has been linked to T2DM in a French population in one study [37]. Furthermore, BuChE-K variant on chromosome 3q was found to be associated with T2DM in white Caucasian subjects. Hence, the association of the BuChE gene (3q26) with T2DM may possibly be related to an identified susceptibility on chromosome 3q27 but independent of islet function [38]. When different risk factors were studied that are independently correlated with BuChE activity for coronary artery disease, it was found that BuChE activity was positively correlated with lipoprotein synthesis, hypertension and diabetes [39].
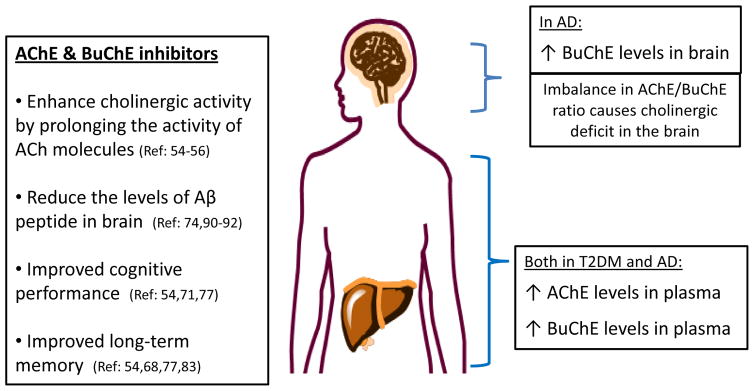
In a study conducted on 1,097 subjects from the Newfoundland adult population, evidence was found for the involvement of elevated levels of serum BuChE in the pathophysiological processes causing T2DM symptoms such as serum triglyceride levels, abdominal obesity, fasting blood glucose levels, and fasting insulin levels [40]. BuChE may be playing a role in causing T2DM through amyloid fibrils formation. It has been shown that the presence of amylin in pancreatic islet cells of T2DM patients causes apoptosis of beta cells by nitric oxide inactivation, lipid peroxidation and formation of excessive superoxide radicals (Fig. 1) [41].
Fig. 1.
Cholinesterase inhibitors in Alzheimer’s disease (AD) and Type 2 Diabetes mellitus (T2DM).
TARGETING AChE AND BuChE TO MANAGE AD AND T2DM
Clinical studies have shown that plasma levels of both AChE and BuChE are significantly higher in AD patients as compared with normal, healthy persons. Moreover, levels of β-amyloid are on average about 2-times higher in AD patients compared to normal, healthy subjects [42, 43]. Likewise, the activities of both AChE and BuChE are found to be higher in diabetic patients versus normal controls [44, 45]. This means that abnormal plasma levels of AChE and BuChE may serve as markers to predict the development of T2DM and AD in addition to serving as therapeutic targets [46]. With better understanding of the inter-relationship of AChE and BuChE levels in normality as well as abnormality, AD and T2DM can be managed.
By effectively managing the treatment of T2DM with drugs that reduce peripheral inflammation and also act as cholinesterase inhibitors, we may be able to manage and delay the onset or progression of AD as the two disorders are related [47, 48]. In this regards, one promising candidate is the Chinese herbal extract “SK0506” which is an inhibtor of BuChE. SK0506 has been used as anti-diabetic testing agent in animal studies. In one study, SK0506 treatment resulted in lowering of BuChE activity in adipose tissues and skeletal muscle of rats and also significantly lowered the levels of hepatic and serum triglycerides and inflammatory markers (interleukin-6 and TNF-α) in rats fed with high fat diet [49]. Likewise, in a separate study on rodent model of the metabolic syndrome induced by high-fat fed diet, SK0506 was found to significantly enhance glycogen synthesis and glucose uptake in adipose tissues during hyperinsulinemic clamp [50].
Similarly, α-lipoic acid and its reduced form, dihydrolipoic acid are multifunctional antioxidants and have been shown to have anti-diabetic properties [51]. α-lipoic acid is also used as a dietary supplement. Both α-lipoic acid and dihydrolipoic acid have been shown to prevent glycation reactions by binding to proteins such as albumin via hydrophobic binding [52]. Recently, a group of researchers reported a series of hybrid molecules between α-lipoic acid and the acetylated or methylated polyphenol compounds synthesized and tested for in vitro AChE and BuChE activity. The half maximal inhibitory concentration (IC50) values of all hybrid molecules for BuChE inhibition were lower than those for the parent compounds. However, one of the hybrid compounds, α-lipoic acid-acetyl protected caffeic acid, was reported to be a potent BuChE inhibitor with IC50 of 0.5 ± 0.2 μM and more than 800 fold selectivity for BuChE over AChE [53]. There is a need to conduct randomized clinical trials to confirm the potential of cholinesterase inhibitors such as SK0506, α-lipoic acid and its derivatives in treating T2DM.
As of now, cholinesterase inhibitors have been proven to be the most effective in the treatment of AD owing to their ability to affect the cognitive and functional domains of AD [54]. There is experimental evidence indicating that general cholinesterase inhibitors targeting AChE and BuChE as well as highly selective BuChE inhibitors have potential therapeutic benefits in the treatment of AD and other related dementias. Cholinesterase inhibitors enhance cholinergic activity by inhibiting enzymes (AChE and/or BuChE) that hydrolyze ACh following synaptic release and, hence, prolong the activity of ACh molecules [55, 56]. Patients treated with cholinesterase inhibitors show amelioration of AD-related behavioral disturbances [57, 58].
Physostigmine, a plant alkaloid, was the earliest drug among the AChE inhibitors to be used in the treatment of AD [59, 60]. Despite its ability to cross the blood-brain barrier, the clinical use of physostigmine was halted due to peripheral side effects, insufficient evidence of efficacy in the clinical studies and fast clearance from the body [61]. Consequently, synthetic analogues of physostigmine were synthesized such as quilostigmine, neostigmine and phenserine. Kinetic studies of human erythrocyte AChE inhibition by phenserine indicate that this compound inhibits the activity of human erythrocyte AChE in a concentration-dependent fashion with IC50 of 45.3 nM [62]. Further, investigations had led to the development of another novel experimental anti-AD therapeutic agent tolserine, a structural analog of phenserine. With an IC50 value of 8.13 nM, tolserine proved to be more potent inhibitor of human AChE compared to its structural analogues phenserine and physostigmine [63].
Currently, the most common drugs used to treat AD across the world are rivastigmine, galantamine, memantine and donepezil. With the exception of memantine, all of these drugs are AChE inhibitors [64, 65]. Rivastigmine acts as a dual reversible inhibitor of both AChE and BuChE. Clinical administration of rivastigmine has been shown to provide long-term symptomatic treatment to AD patients with dementia [66]. Donepezil, on the other hand, is a selective inhibitor of AChE. In preclinical studies on AChE-deficient mice (AChE−/− mice), rivastigmine dosing has been shown to enhance the levels of extracellular ACh by inhibiting BuChE whereas infusion of donepezil did not affect hippocampal ACh levels, although donepezil increased ACh levels by more than twofold in wild-type mice [67]. Donepezil is 1000 fold more selective for AChE over BuChE and improves working memory and long-term memory in animal studies. Clinical studies have shown that donepezil produced dose-related improvements in AD patients with statistically significant effects [68].
Galantamine is another AChE inhibitor drug commonly used to treat AD. A tertiary alkaloid extracted from several species of Amarylidacae, galantimine acts as a non-competitive inhibitor of AChE with half-life of about 10 hours and demonstrates only limited affinity for BuChE [69, 70]. Results from large randomized, double-blind, placebo-controlled trials of up to 6 months duration have demonstrated that galantamine significantly improved cognitive and global function and ability to perform every day activities in AD patients relative to placebo and baseline [71].
Berberine is a natural isoquinoline alkaloid that holds promise as a potential anti-AD candidate. It acts as a dual AChE/BuChE inhibitor [72]. Berberine can inhibit AChE with an IC50 of 0.44 μM and it can inhibit BuChE with an IC50 of 4.44 μM [73]. Berberine also causes reduction in Aβ levels. Asai et al. has reported that berberine can alter APP processing and, hence, cause reduction of Aβ levels in human neuroglioma H4 cells that stably express Swedish-type of APP at the range of berberine concentration without cellular toxicity [74].
In disorders such as AD, AChE activity goes down to only 33–45% of normal values as the disease progresses while the activity of BuChE goes up by as much as 40–90% in certain brain areas [75, 76]. This drastic alteration of AChE to BuChE ratio causes a change in the normally supportive role of BuChE in hydrolyzing excess ACh only. This implies that BuChE inhibition may serve as an appropriate therapeutic target to treat AD. Furthermore, when transgenic mice with overexpressed human mutant APP were treated with selective and potent BuChE inhibitors, it resulted in lowering of Aβ peptide brain levels than in control mice and reduced intra- and extracellular β-amyloid precursor protein. Selective BuChE inhibition in rat brain slices resulted in improved long-term potentiation. Furthermore, those potent and brain-targeted BuChE inhibitors also resulted in improved cognitive performance in animal studies [77].
Various selective BuChE inhibitors have been tested as potential therapeutic anti-AD candidates. Notable among those compounds are the two structural analogs of cymserine, namely, bisnorcymserine and phenethylcym-serine, which have proven to be 15, 110 and 5000-fold more selective for BuChE than AChE respectively [78–82]. It has been demonstrated in animal studies that these two cymserine-derivatives, i.e. phenethylcymserine and bisnorcymserine, are nontoxic and they improve learning in elderly rats in a 14-unit T maze compared with untreated controls [83]. Preclinical studies conducted on animals with cholinergic forebrain lesions have demonstrated that phenethylcymserine and bisnorcymserine cause in vivo reduction in the levels of APP found in cerebrospinal fluid [84]. Dosing of both of these BuChE inhibitors results in reduced intracellular and extracellular levels of APP in a dose and time dependent manner [85–87]. Likewise, MF-8622 is a BuChE-specific carbamate inhibitor that holds promise in the treatment of AD. Animal studies have reported a 15-fold increase in the extracellular concentration of ACh in rat brains injected intracortically with MF-8622 [88, 89].
Ethopropazine is another drug that inhibits BuChE selectively and it has been shown clinically to cause reduction in the formation of β-amyloid in patients suffering from AD [90, 91]. Two other noteworthy anti-AD compounds that show weak BuChE inhibition are HLA20 and M30D, even though they also exhibit strong AChE inhibition in vitro. HLA20 and M30D were designed by merging three FDA-approved drugs rasagline, rivastigmine and donepezil or tacrine. Both of these compounds have demonstrated the ability to modulate APP regulation and β-amyloid reduction (Table 1) [92].
Table 1.
Summary of natural and synthesized cholinesterase inhibitors.
| Category | Compound/Drug Name | Type (Natural or Synthesized) | Mechanism of Action | Reference(s) |
|---|---|---|---|---|
| AChE inhibitors | Phenserine | Synthetic analogue of a physostigmine (plant alkaloid) | Inhibits the activity of human erythrocyte AChE in a concentration-dependent fashion with nanomolar IC(50) | [62] |
| Tolserine | Synthetic analogue of a physostigmine | More potent inhibitor of human AChE than its structural analogue phenserine | [63] | |
| Berberine | Plant alkaloid | Dual inhibitor of AChE and BuChE; it can alter APP processing and cause reduction of β-amyloid levels in human neuroglioma H4 cells that stably express Swedish-type of APP | [72–74] | |
| Rivastigmine | Miotine derivative of physostigmine | Dual inhibitor of AChE and BuChE; Clinical administration shown to provide long-term symptomatic treatment to AD patients with dementia | [66] | |
| Donepezil | Synthetic novel drug | 1000 fold more selective for AChE over BuChE; improves long-term memory in animal studies; produced dose-related improvements in AD patients with statistically significant effects | [68] | |
| Galantamine | Tertiary alkaloid extracted from botanical sources | Non-competitive, selective inhibitor of AChE; significantly improved cognitive and global function and ability to perform every day activities in AD patients | [71] | |
| BuChE inhibitors | Bisnorcymserine & Phenethylcymserine | Cymserine analogs | Dosing resulted in reduced intracellular and extracellular levels of APP; nontoxic; improved cognitive performance (maze navigation) in aged rats | [78–87] |
| MF-8622 | Synthetic BuChE-specific carbamate | Animal studies reported a 15-fold increase in the extracellular concentration of ACh in rat brains injected intracortically with MF-8622 | [88, 89] | |
| Ethopropazine | Synthetic novel drug | Shown clinically to cause reduction in the formation of β-amyloid in patients suffering from AD | [90, 91] | |
| HLA20 & M30D | Designed by merging three FDA-approved drugs rasagline, rivastagmine and donepezil or tacrine | Shown weak BuChE inhibition; demonstrated the ability to modulate APP regulation and β-amyloid reduction | [92] | |
| 3f | Synthetic derivative of thienothiazine | Highly active against the human BuChE (in vitro) with an IC(50) of 0.51 microM | [93] | |
| Metaproterenol, Isoproterenol and their racemic Bisdimethylcarbamate derivatives | Synthetic novel drugs used as bronchodilators | This class of compounds works by selectively inhibiting BuChE five times stronger than AChE | [94] |
List of Abbreviations: Acetylcholine (ACh); Acetylcholinesterase (AChE); Alzheimer’s Disease (AD); Amyloid Precursor Protein (APP); Butyrylcholinesterase (BuChE); Half Maximal Inhibitory Concentration (IC(50)); Type 2 Diabetes Mellitus (T2DM).
Recently, various thienothiazines have also been explored as anti-AD agents due to their potent BuChE inhibitory activities and also due to the fact that this class of compounds share common structural features with phenothiazines, which are well-known potent BuChE inhibitors. After screening 45 thienothiazines for their BuChE inhibitory activity, Karlsson et al. have reported a compound, 3f, which is so far the most active against human BuChE with an IC50 of 0.51 μM [93]. The bronchodilating compounds metaproterenol and isoproterenol as well as their racemic bisdimethylcarbamate derivatives are another class of compounds that work by selectively inhibiting BuChE five times stronger than AChE [94].
CONCLUSION
To sum up, numerous in vitro and in vivo animal and human studies have demonstrated that activities and abnormal levels of AChE and BuChE appear to be closely associated with the pathogenesis of T2DM and AD. Both AChE and BuChE seem to play a crucial role in the pathogenesis of AD and T2DM and this class of esterase enzymes could possibly be an underlying connection responsible for the coexistence of these two disorders [95]. Currently, cholinesterase inhibitors are the treatment of choice for AD albeit the fact that they only provide symptomatic relief. Of course, cholinesterase inhibitors, especially AChE inhibitors, have potential unwanted side effects on the brain as well as the skeletal muscles and digestive system. In terms of the future of targeting AChE and BuChE inhibitors for the treatment of AD and T2DM, research should be geared towards designing such selective cholinesterase inhibitors that can potentially treat both AD and T2DM at the same time. Furthermore, there exists a need to design safe and efficacious cholinesterase inhibitors with a favorable pharmacological profile that would rapidly cross the blood-brain barrier and disappear quickly from the body with minimal or no side-effects on the body.
Acknowledgments
The authors would like to thank the facilities provided by Department of Biochemistry, College of Science and KFMRC, King Abdulaziz University, Jeddah, Saudi Arabia as well as Drug Design & Development Section, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, National Institutes of Health, Baltimore, USA.
LIST OF ABBREVIATIONS
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- Aβ
Beta-amyloid
- BuChE
Butyrylcholinesterase
- IC50
Half maximal inhibitory concentration
- T2DM
Type 2 diabetes mellitus
- TNF-α
Tumor necrosis factor
Footnotes
Send Orders for Reprints to reprints@benthamscience.net
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Kaplay SS. Acetylcholinesterase and butyrylcholinesterase of developing human brain. Biol Neonate. 1976;28:65–73. doi: 10.1159/000240805. [DOI] [PubMed] [Google Scholar]
- 2.Jope RS, Walter-Ryan WG, Alarcon RD, Lally KM. Cholinergic processes in blood samples from patients with major psychiatric disorders. Biol Psychiatry. 1985;20:1258–66. doi: 10.1016/0006-3223(85)90110-6. [DOI] [PubMed] [Google Scholar]
- 3.Silver A. The biology of cholinesterases. Elsevier/Agricultural Research Council Institute; New York: 1974. pp. 426–47. [Google Scholar]
- 4.Li B, Stribley JA, Ticu A, et al. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J Neurochem. 2000;75(3):1320–31. doi: 10.1046/j.1471-4159.2000.751320.x. [DOI] [PubMed] [Google Scholar]
- 5.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4(2):131–8. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 6.Perry EK. The cholinergic system in old age and Alzheimer’s disease. Age Ageing. 1980;9(1):1–8. doi: 10.1093/ageing/9.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Atack JR, Perry EK, Bonham JR, Candy JM, Perry RH. Molecular forms of acetylcholinesterase and butyrylcholinesterase in the aged human central nervous system. J Neurochem. 1986;47(1):263–77. doi: 10.1111/j.1471-4159.1986.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 8.Giacobini E. Cholinergic function and Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18(Suppl):S1–5. doi: 10.1002/gps.935. [DOI] [PubMed] [Google Scholar]
- 9.Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int Psychogeriatr. 2002;14(Suppl 1):77–91. doi: 10.1017/s1041610203008676. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg PB. Clinical aspects of inflammation in Alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–14. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 11.Sheng JG, Bora SH, Xu G, et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–45. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 12.Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–41. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturchler-Pierrat C, Abramowski D, Duke M, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–92. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampon N, Hermida-Cadahia EF, Riveiro A, Tutor JC. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann Hepatol. 2012;11(3):356–63. [PubMed] [Google Scholar]
- 15.Das UN. Acetylcholinesterase and butyrylcholinesterase as markers of low-grade systemic inflammation. Ann Hepatol. 2012;11(3):409–11. [PubMed] [Google Scholar]
- 16.Kamal MA, Bellante V, Greig NH. Butyrylcholinesterase inhibitors modulate cytokines production in peripheral blood mononuclear cells. Alzheimer’s & Dementia: J Alzh Assoc. 2011;7(4 Suppl):671–2. [Google Scholar]
- 17.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 18.Glenner GG. The pathobiology of Alzheimer’s disease. Annu Rev Med. 1989;40:45–51. doi: 10.1146/annurev.me.40.020189.000401. [DOI] [PubMed] [Google Scholar]
- 19.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–82. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 20.Cottingham MG, Hollinshead MS, Vaux DJ. Amyloid fibril formation by a synthetic peptide from a region of human acetylcholinesterase that is homologous to the Alzheimer’s amyloid-beta peptide. Biochemistry. 2002;41(46):13539–47. doi: 10.1021/bi0260334. [DOI] [PubMed] [Google Scholar]
- 21.Inestrosa NC, Alvarez A, Perez CA, et al. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16:881–91. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 22.De Ferrari GV, Canales MA, Shin I, et al. A structural motif of acetylcholinesterase that promotes amyloid β-peptide fibril formation. Biochemistry. 2001;40:10447–57. doi: 10.1021/bi0101392. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez A, Alarcon R, Opazo C, et al. Stable complexes involving acetylcholinesterase and amyloid-β peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J Neurosci. 1998;18:3213–23. doi: 10.1523/JNEUROSCI.18-09-03213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes AE, Chacon MA, Dinamarca MC, et al. Acetylcholinesterase-Abeta complexes are more toxic than Abeta fibrils in rat hippocampus: effect on rat beta-amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am J Pathol. 2004;164(6):2163–74. doi: 10.1016/s0002-9440(10)63774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees T, Hammond PI, Soreq H, Younkin S, Brimijoin S. Acetylcholinesterase promotes β-amyloid plaques in cerebral cortex. Neurobiol Aging. 2003;24:777–87. doi: 10.1016/s0197-4580(02)00230-0. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann DJ, Johnston C, Smith AD. Synergy between the genes for butyrylcholinesterase K variant and apolipoprotein E4 in late-onset confirmed Alzheimer’s disease. Hum Mol Genet. 1997;6(11):1933–6. doi: 10.1093/hmg/6.11.1933. [DOI] [PubMed] [Google Scholar]
- 27.Mattila KM, Rinne JO, Roytta M, et al. Dipeptidyl carboxypeptidase 1 (Dcp1) and butyrylcholinesterase (BuChe) gene interactions with the apolipoprotein E epsison4 allele as risk factors in Alzheimer’s disease and in Parkinson’s disease with coexisting Alzheimer pathology. J Med Genet. 2000;37(10):766–70. doi: 10.1136/jmg.37.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raygani AV, Zahrai M, Soltanzadeh A, et al. Analysis of association between butyrylcholinesterase K variant and apolipoprotein E genotypes in Alzheimer’s disease. Neurosci Lett. 2004;371(2–3):142–6. doi: 10.1016/j.neulet.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 29.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978;4:273–7. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 30.Arendt T, Bruckner MK, Lange M, Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic development - a study of molecular forms. Neurochem Int. 1992;21(3):381–96. doi: 10.1016/0197-0186(92)90189-x. [DOI] [PubMed] [Google Scholar]
- 31.Mesulam M, Geula C. Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann Neurol. 1994;36:722–7. doi: 10.1002/ana.410360506. [DOI] [PubMed] [Google Scholar]
- 32.Geula C, Mesulam MM. Cholinesterases and the pathology of Alzheimer disease. Alzheimer Dis Assoc Disord. 1995;9(Suppl 2):23–8. doi: 10.1097/00002093-199501002-00005. [DOI] [PubMed] [Google Scholar]
- 33.Guillozet A, Smiley J, Mash D, Mesulam M. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann Neurol. 1997;42(6):909–18. doi: 10.1002/ana.410420613. [DOI] [PubMed] [Google Scholar]
- 34.Godfrey DA, Matschinsky FM. Enzymes of the cholinergic system in islets of Langerhans. J Histochem Cytochem. 1975;23(9):645–51. doi: 10.1177/23.9.126256. [DOI] [PubMed] [Google Scholar]
- 35.Abbott CA, Mackness MI, Kumar S, et al. Relationship between serum butyrylcholinesterase activity, hypertriglyceridaemia and insulin sensitivity in diabetes mellitus. Clin Sci. 1993;85(1):77–81. doi: 10.1042/cs0850077. [DOI] [PubMed] [Google Scholar]
- 36.Sridhar GR, Nirmala G, Allam AR, et al. Serum butyrylcholinesterase in type 2 diabetes mellitus: a biochemical and bioinformatics approach. Lipids Health Dis. 2005;4(1):18. doi: 10.1186/1476-511X-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vionnet N, Hani EH, Dupont S, et al. Genomewise search for type 2 diabetes susceptibility genes in French Whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2 diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 2000;67:1470–80. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashim Y, Shepherd D, Wiltshire S, et al. Butyrylcholinesterase K variant on chromosome 3q is associated with Type II diabetes in white Caucasian subjects. Diabetologia. 2001;44:2227–30. doi: 10.1007/s001250100033. [DOI] [PubMed] [Google Scholar]
- 39.Alcantara VM, Chautard-Freire-Maia EA, Scartezini M, et al. Butyrylcholinesterase activity and risk factors for coronary artery disease. Scand J Clin Lab Invest. 2002;62:399–404. doi: 10.1080/00365510260296564. [DOI] [PubMed] [Google Scholar]
- 40.Randell EW, Mathews MS, Zhang H, Seraj JS, Sun G. Relationship between serum butyrylcholinesterase and the metabolic syndrome. Clin Biochem. 2005;38(9):799–805. doi: 10.1016/j.clinbiochem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Johansen AE-M, Nielsen D, Andersen G, et al. Large-scale studies of the functional K variant of the butyrylcholinesterase gene in relation to Type 2 diabetes and insulin secretion. Diabetologia. 2004;47(8):1437–41. doi: 10.1007/s00125-004-1459-7. [DOI] [PubMed] [Google Scholar]
- 42.Reale MA, Di Nicola M, Velluto L, et al. Selective acetyl- and butyrylcholinesterase inhibitors reduce amyloid-b ex vivo activation of peripheral chemo-cytokines from Alzheimer’s disease subjects: exploring the cholinergic anti-inflammatory pathway. Curr Alzheimer Res. 2014;11(6):608–22. doi: 10.2174/1567205010666131212113218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mushtaq G, Khan JA, Kamal MA. Biological Mechanisms linking Alzheimer’s disease and Type 2 Diabetes Mellitus. CNS Neurol Disord Drug Targets. 2014;13(7):1192–1201. doi: 10.2174/1871527313666140917114537. [DOI] [PubMed] [Google Scholar]
- 44.Sato KK, Hayashi T, Maeda I, et al. Serum butyrylcholinesterase and the risk of future type 2 diabetes: the Kansai Healthcare Study. Clin Endocrinol. 2014;80(3):362–7. doi: 10.1111/cen.12171. [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki T, Yoneda M, Nakajima A, Terauchi Y. Serum butyrylcholinesterase is strongly associated with adiposity, the serum lipid profile and insulin resistance. Intern Med. 2007;46(19):1633–9. doi: 10.2169/internalmedicine.46.0049. [DOI] [PubMed] [Google Scholar]
- 46.Rao AA, Sridhar GR, Das UN. Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer’s disease. Med Hyp. 2007;69(6):1272–6. doi: 10.1016/j.mehy.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Mushtaq G, Khan JA, Kumosani TA, Kamal MA. Alzheimer’s disease and Type 2 Diabetes via Chronic Inflammatory Mechanisms. Saudi J Biol Sci. 2014 doi: 10.1016/j.sjbs.2014.05.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamal MA, Greig NH, Reale M. Anti-Inflammatory Properties of Acetylcholinesterase Inhibitors Administered in Alzheimer’s disease. Anti Inflamm Anti Allergy Agents Med Chem. 2009;8(1):85–100. [Google Scholar]
- 49.Kamal MA, Tan Y, Seale JP, Qu X. Targeting BuChE-Inflammatory Pathway by SK0506 to Manage Type 2 Diabetes and Alzheimer Disease. Neurochem Res. 2009;34(12):2163–9. doi: 10.1007/s11064-009-0011-z. [DOI] [PubMed] [Google Scholar]
- 50.Tan Y, Kamal MA, Wang ZZ, et al. Chinese herbal extracts (SK0506) as a potential candidate for the therapy of the metabolic syndrome. Clin Sci. 2011;120(7):297–305. doi: 10.1042/CS20100441. [DOI] [PubMed] [Google Scholar]
- 51.Borcea V, Nourooz-Zadeh J, Wolff SP, et al. alpha-Lipoic acid decreases oxidative stress even in diabetic patients with poor glycemic control and albuminuria. Free Rad Biol Med. 1999;26:1495–500. doi: 10.1016/s0891-5849(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 52.Packer L, Witt EH, Tritschler HJ. Alpha-Lipoic acid as a biological antioxidant. Free Rad Biol Med. 1995;19:227–50. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 53.Woo YJ, Lee BH, Yeun GH, et al. Development of Selective Butyrylcholinesterase Inhibitors Using (R)-Lipoic Acid-Polyphenol Hybrid Molecules. Bull Korean Chem Soc. 2011;32(8):2997–3002. [Google Scholar]
- 54.Cummings JL. The role of cholinergic agents in the management of behavioural disturbances in Alzheimer’s disease. Int J Neuropsychopharmacol. 2000;3(7):21–9. doi: 10.1017/S1461145700001917. [DOI] [PubMed] [Google Scholar]
- 55.Verma A, Siddiqui S, Ahmad SS, et al. Current acetylcholinesterase-inhibitors: A Neuroinformatics perspective. CNS Neurol Disord Drug Targets. 2014;13(3):391–401. doi: 10.2174/18715273113126660166. [DOI] [PubMed] [Google Scholar]
- 56.Kamal MA, Al-Jafari AA, Greig NH. Interaction of new anti-Alzheimer’s disease agents with cholinesterase. J Neurochem. 2005;94(s2):168. [Google Scholar]
- 57.Corey-Bloom J. The ABC of Alzheimer’s disease: cognitive changes and their management in Alzheimer’s disease and related dementias. Int Psychogeriatr. 2002;14(Suppl 1):51–75. doi: 10.1017/s1041610203008664. [DOI] [PubMed] [Google Scholar]
- 58.Sheikh IA, Ali R, Dar TA, Kamal MA. An overview on potential neuroprotective compounds for management of Alzheimer’s Disease. CNS Neurol Disord Drug Targets. 2012;11(8):1006–11. doi: 10.2174/1871527311211080010. [DOI] [PubMed] [Google Scholar]
- 59.Davis KL, Mohs RC. Enhancement of memory processes in Alzheimer’s disease with multiple-dose intravenous physostigmine. Am J Psychiatry. 1982;139:1421–4. doi: 10.1176/ajp.139.11.1421. [DOI] [PubMed] [Google Scholar]
- 60.Thal LJ, Fuld PA, Masur DM, Sharpless NS. Oral physostigmine and lecithin improve memory in Alzheimer disease. Ann Neurol. 1983;13:491–6. doi: 10.1002/ana.410130504. [DOI] [PubMed] [Google Scholar]
- 61.Perola E, Cellai L, Lamba D, Filocamo L, Brufani M. Long chain analogs of physostigmine as potential drugs for Alzheimer’s disease: New insights into the mechanism of action in the inhibition of acetylcholinesterase. Biochim Biophys Acta. 1977;1343:41–50. doi: 10.1016/s0167-4838(97)00133-7. [DOI] [PubMed] [Google Scholar]
- 62.Al-Jafari AA, Kamal MA, Greig NH, Alhomida AS, Perry ER. Kinetics of human erythrocyte acetylcholinesterase inhibition by a novel derivative of physostigmine: Phenserine. Biochem Biophys Res Commun. 1998;248:180–5. doi: 10.1006/bbrc.1998.8931. [DOI] [PubMed] [Google Scholar]
- 63.Kamal MA, Greig NH, Alhomida AS, Al-Jafari AA. Kinetics of human acetylcholinesterase inhibition by the novel experimental Alzheimer therapeutic agent, tolserine. Biochem Pharmacol. 2000;60:561–70. doi: 10.1016/s0006-2952(00)00330-0. [DOI] [PubMed] [Google Scholar]
- 64.Ali R, Sheikh IA, Jabir NR, Kamal MA. Comparative Review of Decade’s Research on Cholinesterase Inhibition. Am J Neuroprot Neuroregen. 2012;4:1–8. [Google Scholar]
- 65.Pohanka M. Alzheimer’s disease and related neurodegenerative disorders: Implication and counteracting of melatonin. J Appl Biomed. 2011;9:185–96. [Google Scholar]
- 66.Muller T. Rivastigmine in the treatment of patients with Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:211–8. doi: 10.2147/nedt.2007.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naik RS, Hartmann J, Kiewert C, et al. Effects of rivastigmine and donepezil on brain acetylcholine levels in acetylcholinesterase-deficient mice. J Pharm Pharm Sci. 2009;12:79–85. doi: 10.18433/j3mk59. [DOI] [PubMed] [Google Scholar]
- 68.Heydorn WE. Donepezil (E2020): A new acetylcholinesterase inhibitor. Review of its pharmacology, pharmacokinetics, and utility in the treatment of Alzheimer’s disease. Expert Opin Investig Drugs. 1997;6:1527–35. doi: 10.1517/13543784.6.10.1527. [DOI] [PubMed] [Google Scholar]
- 69.Huang F, Fu Y. A review of clinical pharmacokinetics and pharmacodynamics of galantamine, a reversible acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease, in healthy subjects and patients. Curr Clin Pharmacol. 2010;5:115–24. doi: 10.2174/157488410791110805. [DOI] [PubMed] [Google Scholar]
- 70.Bartolucci C, Perola E, Pilger C, Fels G, Lamba D. Three dimensional structure of a complex of galanthamine (Nivalin) with acetylcholinesterase from Torpedo californica: Implications for the design of new anti-Alzheimer drugs. Proteins. 2001;42:182–91. doi: 10.1002/1097-0134(20010201)42:2<182::aid-prot50>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 71.Lilienfeld S. Galantamine - a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev. 2002;8(2):159–76. doi: 10.1111/j.1527-3458.2002.tb00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji HF, Shen L. Berberine: A Potential Multipotent Natural Product to Combat Alzheimer’s Disease. Molecules. 2011;16:6732–40. doi: 10.3390/molecules16086732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung HA, Min BS, Yokozawa T, et al. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol Pharm Bull. 2009;32:1433–8. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 74.Asai M, Iwata N, Yoshikawa A, et al. Berberine alters the processing of Alzheimer’s amyloid precursor protein to decrease Abeta secretion. Biochem Biophys Res Commun. 2007;352:498–502. doi: 10.1016/j.bbrc.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 75.Perry EK, Tomlinson BE, Blessed G, et al. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978;2(6150):1457–9. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chatonnet A, Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem J. 1989;260(3):625–34. doi: 10.1042/bj2600625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greig NH, Utsuki T, Ingram DK, et al. Selective butyrylcholinesterase inhibition elevated brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc Natl Acad Sci USA. 2005;102:17213–8. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu Q, Holloway HW, Flippen-Anderson JL, et al. Methyl analogues of the experimental Alzheimer drug phenserine: Synthesis and structure/activity relationships for acetyl- and butyrylcholinesterase inhibitory action. J Med Chem. 2001;44:4062–71. doi: 10.1021/jm010080x. [DOI] [PubMed] [Google Scholar]
- 79.Kamal MA, Yu Q-S, Holloway HW, et al. Kinetics of human serum butyrylcholinesterase and its inhibition by a novel experimental Alzheimer therapeutic, bisnorcymserine. J Alz Disease. 2006;10(1):43–51. doi: 10.3233/jad-2006-10108. [DOI] [PubMed] [Google Scholar]
- 80.Giacobini E. Cholinesterases: new roles in brain function and in Alzheimer’s disease. Neurochem Res. 2003;28(3–4):515–22. doi: 10.1023/a:1022869222652. [DOI] [PubMed] [Google Scholar]
- 81.Yu Q, Holloway HW, Utsuki T, Brossi A, Greig NH. Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer’s disease. J Med Chem. 1999;42:1855–61. doi: 10.1021/jm980459s. [DOI] [PubMed] [Google Scholar]
- 82.Kamal MA, Al-Jafari AA, Yu Q, Greig NH. Kinetic analysis of the inhibition of human butyrylcholinesterase with cymserine. Biochem Biophys Acta. 2006;1760:200–6. doi: 10.1016/j.bbagen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Greig NH, Utsuki T, Yu Q, et al. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr Med Res Opin. 2001;17:159–65. doi: 10.1185/0300799039117057. [DOI] [PubMed] [Google Scholar]
- 84.Haroutunian V, Ahlers SA, Greig NH, Wallace WC. Induction, secretion and pharmacological regulation of β-APP in animal model systems. In: Fisher A, Hanin I, Yoshinda M, editors. Progress in Alzheimer’s and Parkinson’s Disease. Plenum; New York: 1997. pp. 695–9. [Google Scholar]
- 85.Lahiri DK, Farlow MR, Hintz N, Utsuki T, Greig NH. Cholinesterase inhibitors, beta-amyloid precursor protein and amyloid beta-peptides in Alzheimer’s disease. Acta Neurol Scand Suppl. 2000;176:60–7. doi: 10.1034/j.1600-0404.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 86.Shaw KT, Utsuki T, Rogers J, et al. Phenserine regulates translation of beta-amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development. Proc Natl Acad Sci USA. 2001;98:7605–10. doi: 10.1073/pnas.131152998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greig NH, Utsuki T, Yu QS, et al. Dissociation between the potent β-amyloid protein pathway inhibition and cholinergic actions of the Alzheimer drug candidates phenserine and cymserine. In: Fisher A, Memo M, Stocchi F, Hanin I, editors. Advances in Alzheimer’s and Parkinson’s Disease: Insights, Progress, and Perspectives. Springer Science + Business Media; USA: 2008. pp. 445–62. [Google Scholar]
- 88.Giacobini E, Griffini P, Maggi T, Mascellani G, Mandelli R. Butyrylcholinesterase: is it important for cortical acetylcholine regulation? Soc Neurosci. 1996;22:203. [Google Scholar]
- 89.Giacobini E. Selective inhibitors of butyrylcholinesterase: a valid alternative for therapy of Alzheimer’s disease? Drugs Aging. 2001;18:891–8. doi: 10.2165/00002512-200118120-00001. [DOI] [PubMed] [Google Scholar]
- 90.Inestrosa NC, Alvarez A, Godoy J, Reyes A, De Ferrari GV. Acetylcholinesterase-amyloid-beta-peptide interaction and Wnt signaling involvement in Abeta neurotoxicity. Acta Neurol Scand Suppl. 2000;176:53–9. doi: 10.1034/j.1600-0404.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- 91.Darvesh S, MacKnight C, Rockwood K. Butyrylcholinesterase and cognitive function. Int Psychogeriatr. 2001;13(4):461–4. doi: 10.1017/s1041610201007876. [DOI] [PubMed] [Google Scholar]
- 92.Zheng H, Fridkin M, Youdim MBH. Site-activated chelators derived from anti-Parkinson drug rasagiline as a potential safer and more effective approach to the treatment of Alzheimer’s disease. Neurochem Res. 2010;35(12):2117–23. doi: 10.1007/s11064-010-0293-1. [DOI] [PubMed] [Google Scholar]
- 93.Karlsson D, Fallarero A, Brunhofer G, et al. The exploration of thienothiazines as selective butyrylcholinesterase inhibitors. Eur J Pharm Sci. 2012;47:190–205. doi: 10.1016/j.ejps.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Bosak A, Gazic Smilovic I, Sinko G, Vinkovic V, Kovarik Z. Metaproterenol, isoproterenol and their bisdimethylcarbamate derivatives as human cholinesterase inhibitors. J Med Chem. 2012;55(15):6716–23. doi: 10.1021/jm300289k. [DOI] [PubMed] [Google Scholar]
- 95.Allam AR, Sridhar GR, Thota H, et al. Alzheimer’s disease and Type 2 diabetes mellitus: the cholinesterase connection? Lipids Health Dis. 2006;5(1):28. doi: 10.1186/1476-511X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]