Abstract
Background
Alzheimer's disease (AD), a cognitive dysfunction/dementia state amongst the elders is characterized by irreversible neurodegeneration due to varied pathophysiology. Up till now, anti-AD drugs having different pharmacology have been developed and used in clinic. Yet, these medications are not curative and only lowering the AD associated symptoms. Improvement in treatment outcome required drug targeting across the blood-brain barrier (BBB) to the central nervous system (CNS) in optimal therapeutic concentration. Nanotechnology based diagnostic tools, drug carriers and theranostics offer highly sensitive molecular detection, effective drug targeting and their combination. Over the past decade, significant works have been done in this area and we have seen very remarkable outocome in AD therapy. Various nanoparticles from organic and inorganic nanomaterial category have successfully been investigated against AD.
Conclusion
This paper discussed the role of nanoparticles in early detection of AD, effective drug targeting to brain and theranostic (diagnosis and therapy) approaches in AD’s management.
Keywords: Alzheimer's disease, blood brain barrier, diagnosis, theranostic, nanoparticles, nanomedicines
1. INTRODUCTION
Alzheimer’s disease (AD) is the most disastrous neurodegenerative disorders, a frequent type of dementia which is characterized by learning and memory impairment among old age people. AD accounts between 60–80% of reported dementia cases. According to World Alzheimer Report 2014, 44 million people having dementia worldwide are projected to continue rising to approximately double and triple by 2030 and 2050, respectively [1, 2]. Brain of AD patients exhibits certain distinct neuropathological characteristics, such as deterioration of cholinergic neurons of the basal forebrain, extracellular amyloid beta (Aβ) containing plaques of abnormal protein and intracellular neurofibrillary tangles made up of abnormally phosphorylated tau protein [3]. Moreover, during advanced stage there is the occurrence of clear visibility of strong atrophy of cerebral cortex and reduction of cortical neurons [3]. The most commonly used hypothesis to describe the pathophysiology of AD is amyloid Cascade Hypothesis saying that the Aβ aggregation is the major contributor leading to progressive neurodegeneration in AD. However, the drugs developed targeting this factor have not gained much success in the AD therapy. Despite the substantial medical need and great multidisciplinary team research in biomedical and pharmaceutical sector, no effective curative therapy has been developed and therefore the pharmacotherapy in AD remains symptomatic. So far the anti-AD drugs in clinic and in clinical studies have majorly central nervous system (CNS) as the target site of action. However, due to their physicochemical nature and the protective barrier called as blood-brain barrier (BBB), these drugs failed to cross and or not in pharmacologically significant concentration in CNS. BBB is a gatekeeper of the brain towards exogenous materials that maintains the chemical composition of the neuronal “milieu” for appropriate performance of neuronal circuits and synaptic transmission. This barrier is the major interface between the blood and the brain and is formed at the level of the endothelial cells of the cerebral capillaries. BBB is the most significant issue for the design and development of new drugs for the CNS. Usually, pharmaceuticals comprise mainly small molecules which do not cross the BBB [4]. During the last two decades, various attempts have been made on this essential issue by designing different techniques that aid drug transits across the BBB.
Currently, numerous methodologies for bypassing the BBB are being tested, including nanotechnology that has shown promising outcome in delivering their payload to the target CNS area. Nanotechnogy based approaches have given the opportunity not only for delivery of drugs but also of imaging agents. Smartly designed nanoparticles (NPs) can themselves be used in AD diagnosis in addition to therapy (called as self theranostics). Nanotechnology based strategies and approaches have gained much attention because of good prospect of surmounting the limitations inherent to BBB passage. Nanotechnology based theranostics offer a combination of highly sensitive molecular detection and effective drug targeting which can improve AD therapy consequently delaying the memory loss and increasing the life expectancy. Nanoparticles of organic and inorganic nature have successfully been investigated against AD as theranostics [5–7]. This paper discusses the role of NPs in early detection of AD, effective drug targeting to brain and theranostic (diagnosis and therapy) approaches in AD management.
2. PATHOPHYSIOLOGY AND DRUG DELIVERY CHALLENGES IN AD
2.1. Pathophysiology of AD
The pathophysiology of AD can be linked to damage and dead neurons, originated in the hippocampus area of the brain (which controls learning and memory functions) followed by atrophy of cerebral cortex that ultimately affects the whole brain [8]. There are many theories yet the most acceptable theory describing the pathology of AD is Amyloid beta (Aβ) protein based hypothesis [8]. Aβ is a short peptide that is basically an atypical proteolytic byproduct of the transmembrane amyloid precursor protein. Though the role of Aβ is not clear it is thought to be involved in neuronal development. Aβ monomers contain short regions of beta sheet at significantly high concentration; they undergo a vivid alteration in conformation to form a beta sheet-rich tertiary structure that accumulates to develop amyloid fibrils. These fibrils get deposited on outer surface of the neurons in compact form called as senile or neuritic plaques. In less compact fibrils they accumulate as diffusive plaques, and occasionally inside the walls of blood vessels in the brain in a condition known as amyloid angiopathy or congophilic angiopathy. Moreover, unusual accumulation of the tau proteins, a microtubule related protein is also observed in neurons of AD patients. Tau protein also stabilizes the microtubules in the cell cytoskeleton. It is usually regulated by phosphprylation just like most of the other microtubule- associated proteins.
Hyperphosphorylated tau (P-tau) aggregates as paired helical filaments which in turn accumulates into masses within the nerve cell bodies as neurofibrillary tangles and as dystrophic neurites associated with Aβ plaques [9]. Additionally, alterations in cerebrospinal fluid levels of Aβ, tau, and P-tau are also observed in AD patient. The exact mechanism that facilitates the development of senile plaques and neurofibrillary tangles remains unclear. Senile plaques and neurofibrillary tangles elicit the injury and death of neurons and as a result memory loss and behavioural symptomatic alteration appear. Along with these above factors, abnormal release of neurotransmitters such as glutamate is also considered as the contributor to neuronal death and inflammation in AD [10]. This complex cascade involving neuroinflammation leads to AD symptoms and pathology. In recent time, significant pathological and clinical data has shown immunological alteration associated with AD, including improved concentrations of pro-inflammatory cytokine in the blood and CSF [11]. Whether these alterationsare a reason or consequence of AD remains unclear, however inflammation within the brain, including improved reactivity of the microglia towards Aβ deposits, has been involved in the progression of AD.
2.2. Barriers of Drug Delivery in AD
Brain is the most critical organ of the body having many protective barriers that have been widely investigated to discover different methods for therapeutic drugs and drug delivery systems to cross these barriers for delivery into the CNS intended for diagnosis and treatment of neurodegenerative diseases. The most significant issue for the effective therapeutic drug delivery to the CNS is BBB. Briefly, BBB is a dynamic interface which is composed of capillary endothelial cells which are extremely closely attached together by tight intercellular junctions showing high trans-endothelial electrical resistance in contrast to the other tissues [12]. It divides the brain from systemic circulation and is the main route for drugs to the CNS. The main role of the BBB is to supplement the brain with nutrients, make ionic homeostasis for neuronal functions and protect it from poisonous or toxic substances via selective transport systems. It restricts the paracellular diffusion of any molecule or drug [13]. The blood-CSF is an additional barrier which is situated at the choroid plexus and runs in the subarachnoid space enclosing the brain separating the blood from cerebrospinal fluid [14]. All through, few regions in the CNS lack BBB but these have microvessels similar to the periphery. These regions are all together called as circumventricular organs (CVOs) and are composed of the choroid plexus, median eminence, etc. The capillaries in circumventricular organs are perforated which permits the free movement of solutes between the blood and the surrounding interstitial fluid [15]. Furthermore, CNS shows functional barriers such as influx and efflux transporter which cause the inclusion and exclusion of xenobiotic into and out of the CNS [16]. Enzymes in the brain also work as a barrier to drug delivery by degrading them and therefore confining their transit to the CNS. This is a general consideration that for easily crossing the BBB by passive diffusion, neurotherapeutics should be lipid soluble, have a relatively small molecular weight of less than 500 Da, have partition coefficient (pKa) between 0.5 and 6.0, and should be either neutral or generally neutral (i.e. uncharged) at physiological pH [17]. However, many exceptions of this generalized rule have been seen in recent studies. For example, large molecule like CINC-1 can cross the BBB through transmembrane diffusion whereas fairly lipophilic molecules may not be able to do so at significant concentration [27]. Molecules having polar surface area of more than 80 Å, high H-bonding affinity, and molecules with highly branched chemical structure are poor candidates to cross the BBB [15]. Therefore, while designing the potential CNS drugs or drug delivery systems for the early detection and management of AD, it is very important to take these considerations into account. Further, the occurrence of irreversible neuronal injury can be prevented by early diagnosis which would produce opportunities to treat patients at risk of AD development. Concerning the pharmacotherapy using NPs in AD, some approaches have aimed to encapsulate various types of neurotherapeutics within NPs for targeted delivery to the CNS. Other approaches focused on development of NPs to resist the toxicity of amyloid clusters by encouraging their systemic clearance or by changing their aggregation kinetics in the brain and in the blood. In fact, the level of Aβ can be reduced in the brain by the “sink effect” i.e. by using peripheral treatment with neurotherapeutics that have significantly high affinity for Aβ.
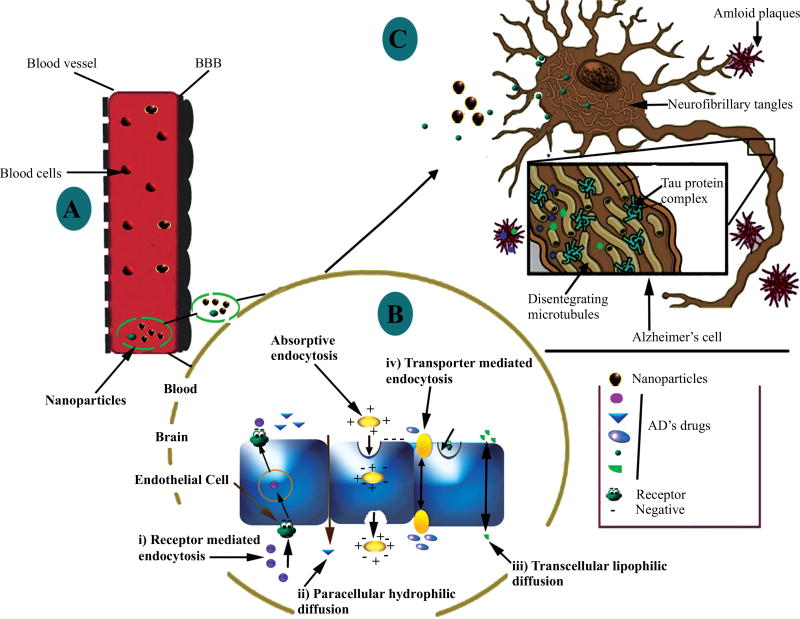
Surface engineered/targeted NPs show significantly high affinity for Aβ, where sequestered plasma Aβ is directed to hepatic and splenic macrophages for destruction [18]. This technique can significantly prevent brain amyloidosis. Fig. (1) illustrates the BBB; the obstacle in drug/biological delivery to the brain and how nanoparticles can facilitate the payload transport and targeting into the brain. Successful AD management requires early and accurate diagnosis followed by better drug targeting to CNS for effective medication. Therefore, to address these challenges the ongoing research draws attention towards theranostic nanomedicines based approaches for early detection and treatment of AD.
Fig. (1).
Drawing presenting the nanoparticles facilitating drug/biologicals transport across the BBB: [A] on systemic application/absorption in blood stream, nanoparticles carrying AD’s drugs reached to the BBB, [B] get absorbed across BBB by following possible mechanisms (indicated here in figure), and [C] further reach the AD affected area and depending on their mode of action may act on the cholinergic system, amyloid plaque, tau protein and/or excessive oxidative load [Adapted and reprinted by permission from the Bentham Science for CNS Neurol Disord Drug Targets: Reference 98].
3. NANOTECHNOLOGY BASED THERANOSTICS IN AD
At present, diagnosis and drug therapy of AD are mostly carried out discretely. A personalized and efficient approach can be accomplished by simultaneous or combined approach for diagnosis and treatment in one. It will fasten the therapy with controlled monitoring on the progress of treatment of complex diseases like AD. Theranostic term was coined for agents having both therapeutic and diagnostic properties [19]. Theranostic nanocarriers can be developed from variety of organic and inorganic nanomaterials like polymeric nanoparticles, nanocapsules, nanospheres, micelles, emulsions, liposomes, quantum dots (QD), dendrimers, fullerenes and carbon nanotubes (CNT). Research over the decade in multidisciplinary nanotechnology has made the outline that can be used in designing of theranostic nanocarriers capable of crossing the BBB, and having therapeutic as well as diagnostic characteristics [18]. The nanoparticles investigated for diagnosis, therapeutics delivery and as theranostics in AD are summarized in Table 1, 2 and 3 respectively.
Table 1.
Nanoparticles investigated for effective drug delivery/targeting in Alzheimer's disease.
| Name of Bioactives /Drugs |
Nano-Carriers | In Vitro/In-Vivo Model | Remarks | Ref. |
|---|---|---|---|---|
| Curcumin | Poly(n-butyl) Cyanoacrylate (PBCA) Nanoparticles | SH-SY5Y neuroblastoma cell line | The photostability of curcumin was increased significantly in nanoparticles compared to plain curcumin. ApoE3-PBCA NPs exhibited synergetic activity against beta amyloid induced cytotoxicity along with curcumin. | [72] |
| Poly (lactide-coglycolide) (PLGA) Nanoparticles | Human SK-N-SH cell line | NPs were upto 6 months of storage and the kinetic of the release of curcumin from the nanoparticles is biphasic, PLGA nanoparticles were not toxic to neuronal cells and can protect SKN-SH cells against H2O2-induced toxicity and prevent reactive oxygen species (ROS) elevation, Glutathione (GSH) decrease, and the activation of Nrf2. | [73] | |
| PLGA nanoparticle | LAG cell line | Curcumin encapsulated-PLGA NPs destroy the amyloid aggregates and exhibit excellent anti-oxidant activity | [74] | |
| Madin–Darby canine kidney (MDCK) cell line, Tg2576 Mice | Nanoparticles exhibited significantly higher curcumin concentration in plasma (AUC) and MRT in brain than curcumin sol. Furthermore, it exhibited significant improvement in cue memory and exhibited significantly lower amyloid plaque density. | [76] | ||
| Rivastigmine (RT) | PBCA nanoparticle | Male Wistar rats | Intravenous administration of rivastigmine encapsulated polysorbate 80-coated polymeric (T-80) PBCA NPs exhibited 3.8-fold increase in rivastigmine uptake within the brain compartment compared to free rivastigmine | [78] |
| Tacrine | PBCA nanoparticle | Male Wistar rats | Tacrine encapsulated (T-80) PBCA NPs increased the tacrine brain concentration by 4-fold in comparison to the free drug. | [79] |
| Rivastigmine (RT) | PLGA and PBCA nanoparticles | Scopolamine-induced amnesia model | Sustained release of RT in brain with faster memory regain compared with RT solution | [80] |
| Chitosan nanoparticles | Wistar rats | The brain concentration achieved from intranasal (i.n) administration of rivastigmine as chitosan NPs was significantly higher than those achieved after i.v. administration and i.n. administration of RT solution. | [81] | |
| Liposome | Aluminium chloride (AlCl3)-induced Alzheimer’s model, Male albino rats | RT loaded liposome exhibited high encapsulation efficiency of RT and controlled release for a prolonged period of time, RLs formulation exhibited faster memory regain and amelioration of metabolic disturbances in AlCl3-treated rats compared with RT solution. | [83] | |
| Nanostructured lipid carriers (NLCs) | AChE inhibition assay in rat brain | NLC-based in-situ gel showed 2-fold increase in nasal permeation of the drug over plain RT solution. Furthermore, in situ gelling NLCs showed a 3-fold increase in AChE inhibition. | [84] | |
| Galanthamine hydrobromide (GH) | Liposomes | pheochromocytoma PC-12 cells, Male Sprague-Dawley rats | The efficacy of AChE inhibition of GH was greatly enhanced by intranasal administration compared with its oral administration of GH sol, bioavailability was 3.36 folds higher than those of orally administered GH. Furthermore, PC-12 cells viability tests showed that the flexible liposome carrier is not toxic to the cultured cells and the cytotoxicity of GH to cells was clearly decreased by loading in flexible liposomes. | [82] |
| Acetylcholine (ACh) | Single-wall carbon nanotubes (SWCNTs) | Male Kunming strain mice | At low dose SWCNTs effectively carried ACh into the lysosomes of the neurons and achieve excellent therapeutic effects in experimentally induced AD with a moderate safety range. | [85] |
| Estradiol | PLGA nanoparticles | Male Sprague Dawley (SD) rats | Estradiol loaded PLGA NPs exhibited zero order release, increases the Cmax and Tmax upto 1.5 and 6 folds respectively and improves the oral bioavailability up to 10 folds compared to the drug suspension. | [88] |
| Mifepristone | PLGA nanoparticles | Male Sprague-Dawley rats | Mifepristone loaded PLGA NPs exhibited up to 2 fold increase in oral bioavailability. | [90] |
| Estradiol | PLGA nanoparticles | long-term OVX animal model | NPs exhibited significantly higher brain estradiol levels after 24 h. Furthermore, these NPs prevent the expression of Aβ42 immunoreactivity in the hippocampus region of brain. | [91] |
| Epigallocatechin-3-gallate (EGCG) | Nanolipidic EGCG particles (NanoEGCG) | SweAPP N2a cells, Male Sprague Dawley rats | NanoEGCG improves 2 fold bioavailability of EGCG. Furthermore, it improves the neuronal (SweAPP N2a cells) α-secretase enhancing ability in vitro by up to 91%. | [96] |
| Selenium nanoparticles (EGCG@Se) coated with Tet-1 peptide (Tet-1-EGCG@Se) | NIH/3T3 and PC12 cells | Tet-1 peptides significantly enhance the cellular uptake of EGCG-stabilized EGCG@Se coated with Tet-1 peptide in PC12 cells and Tet-1-EGCG@Se effectively inhibit Aβ fibrillation and disaggregate preformed Aβ fibrils into nontoxic aggregates | [97] | |
| Resveratrol | Polymeric micelles | PC12 cells lines | Protect PC12 cells from Aβ- induced damage in a dose dependent manner by attenuating intracellular oxidative stress and caspase-3 activity without long-term cytotoxicity. | [103] |
| Lipid-core nanocapsules | Male Wistar rats | Nanocapsules efficiently rescuing the deleterious effects of Aβ1–42. | [104] |
Table 2.
Surface-engineered nanoparticles investigated in imaging/diagnosis of Alzheimer’s disease.
| Type of NPs | Ligands | Targets | Outcome | Ref |
|---|---|---|---|---|
| SPIONs | Ligand for Aβ | Aβ 1–40 detection | SPIONs exhibited excellent binding affinity towards Aβ1–42 and Aβ1–40 and MRI efficiently detect the Aβ plaque in transgenic mice. | [31] |
| Labeling + external magnetic field | Aβ fibril removal | SPIONs selectively mark Aβ40 fibrils that are easily detected by MRI and fluorescence microscope. | [137] | |
| Antibody | Aβ plaque | SPIONs cross the BBB and improve the detection of plaques in MRI | [33] | |
| QDs | Aβ peptide | Fibrils and AβO detection | QDs improve the detection of AβO by fluorescent microscope imaging. | [39] |
| GNPs | PEGylation + Lipoic Acid + Neutravidin lysine residues | Aβ aggregation kinetics | Significant contrast changes was observed in MRI signals during Aβ self-aggregation that correspond to the detection of Aβ protofibrillar species in the early reversible stages of aggregation. | [138] |
| SPIONs | Aβ1–42 peptide | Amyloid deposition | Targeted SPIONs selectively bind to Aβ followed by amyloid plaques detection by T2*-weighted μMRI. | [32] |
| PBCA-NPs | Fluorescent AchE inhibitor (PE154) | Aβ targeting and detection | PE154 targeted detection of hippocampal Aβ deposits with NPs by fluorescent marker in in-vivo | [139] |
| Thioflavin T | Aβ -sheet detection | Thioflavins selectively targeted fibrillar Aβ. Thioflavin T exhibited significantly stronger fluorescence. | [140] | |
| Polysorbate-80 | Aβ peptides | Crosses BBB and improved brain uptake and retention | [141] | |
| Polysorbate-80 | Aβ peptides | Crosses BBB and improved brain uptake and selectively binds to Aβ. | [142] | |
| SPIONs | 1,1-dicyano-2-[6-(dimethylamino)-naphthalene-2-yl] propene carboxyl derivative (DDNP) | Aβ plaques | Exhibited high binding affinities towards Aβ that can efficiently measured by fluorescence spectrophotometer | [143] |
Table 3.
Surface-engineered nanoparticles investigated as theranostics (simultaneous drug therapy and imaging) for Alzheimer’s disease.
| Type of NPs | Ligand | Imaging Agent | Therapeutic Agent | Outcome | Ref. |
|---|---|---|---|---|---|
| Chitosan NPs | Anti-amyloid antibody (IgG4.1) | Gd-DTPA | Curcumin/Dexamethasone | Targeting amyloid deposit/ CVA and exhibit good distribution in the brain vasculature | [144] |
| PEG-PLA NPs | TGN and QSH peptides | DiR* | Coumarin-6 | Significantly higher cellular uptake and brain distribution. Enhanced targeted delivery to amyloid plaque in the brains of mice affected by AD | [145] |
| Chitosan NPs | Putrescine modified F(ab′)2 fragment of anti-amyloid antibody, IgG4.1 (pF(ab′)24.1) | Magnevist® | Cyclophosphamide | Targeting cerebrovascular amyloid and provided contrast for imaging cerebrovascular amyloid using MRI and single photon emission computed tomography in in-vivo | [146] |
| SPIONs | anti-Aβ monoclonal antibodies (aAβmAbs) | fluorescent SPIONs | BAM10 | 5 folds greater inhibition of Aβ40 fibrillation. Selective labelling of the Aβ40 fibrils with the BAM10-conjugated SPIONs detect Aβ40 fibrils by MRI and fluorescence imaging with fair intensity | [147] |
| amyloid-derived diffusible ligands antibodies (anti-ADDLs) | SPIONs | Anti-ADDLs conjugation to SPIONs seems promising as AD theranostics. | [148] |
DiR= Coumarin-6, coumarin-7, 1,10-dioctadecyl-3,3,30,30-tetramethyl indotricarbocyanine Iodide; CVA = Cerebral amyloid angiopathy.
3.1. Nanotechnology in Diagnosis of AD
The promise for early diagnosis of AD is the current focus in developing NPs for imaging and molecular detection of biomarkers. Highly potent signal transduction can be achieved with nanotechnology that may help in early diagnosis of AD. This potential application of nanotechnology in imaging/detection is primarily based on the physical (magnetic, optical, or electrical), chemical and/or biological quality of smartly designed nanoparticles [20]. Conventionally, the soluble bio-markers of AD can be detected by two general approaches. The first is based on determining the entire tau protein or Aβ concentration in CSF or in plasma [21]. This approach has led to sometimes unconvincing result as it is inhibited by overlap of such biological marker in normal and abnormal subjects [22]. In the second approach, only the suspected pathogenic biomarkers are targeted for instance phosphorylated tau protein, cleaved tau protein or Aβ derived diffusible ligands (ADDL) [23]. Though this approach leads to more decisive results, such pathogenic markers cannot be measured precisely with conventional ELISA or western blotting assays as their concentrations in CSF are quite low in the early stages of the AD [24].
3.1.1. In-Vitro Diagnostics
3.1.1.1. Nanoparticle Conjugates
DNA-NPs complexes are capable of detecting the protein biomarkers at a very low molar concentration (even at the level of 10–18 moles per liter) [25]. This technique of detection (termed as Bio-barcode technique) takes advantage of Gold NPs tagged targeted antibody for specific protein [25]. This technique has shown satisfactory results in detection of ADDL in CSF of in vitro diagnosis of AD [25].
3.1.1.2. Localized Surface Plasmon Resonance Based Nanosensor (LSPR)
The surface plasmon resonance (SPR) is a state of resonant and collective oscillation of valence electrons in solid material upon irradiation by incident light. The absorption and emission of photon take place with similar frequency in all directions [26]. The unique phenomena of SPR in some inorganic NPs make them valuable for imaging and theranostic application in various conditions including AD. Based on SPR effect, recently many metallic NPs based ultra-sensitive and economical techniques have been established for detection of AD’s biomarkers such as ADDL [27]. Gold nanoparticles (GNPs) and triangular silver nanoparticles (AgNPs) have been successfully studied as LSPR in early and sensitive diagnosis of AD. GNPs, based on LSPR provide opportunity to analyse the Aβ expression level. Similarly, triangular silver nanoparticles (AgNPs) are used because of their LSPR effect in the determination of ADDL [27]. Any changes in the external environment around AgNPs can cause an alteration in the refractive index (RI) of the surrounding magnetic field that alters the absorption wavelength (λmax) of AgNPs which can be detected via UV–Visible spectroscopy. LSPR based AgNPs are ultrasensitive to detect the concentration change of biomarker of AD such as ADDL that causes the change in refractive index with their concentration change [27].
3.1.1.3. Scanning Tunneling Microscopy [STM] and Two Photon Rayleigh Spectroscopy
Recently, a molecular detection system was developed on the basis of electrical detection by STM [28]. STM involves immobilization of particular antibody fragment on GNPs. The tunneling current profile due to change in the concentration of substrate or antigen depdenpds upon the frequency of the generated pulse, which appear every time on the scanning tip of instrument when STM crosses GNPs. This technique is very sensitive and can detect the Aβ at concentrations as low as 10 fg/ml [28]. Recently, Two Photon Rayleigh spectroscopy was effectively used to analyze the tau protein by measuring the transformed signal from the scattering of GNPs [29]. This technique is fast, specific and highly sensitive that can determine the concentration as low as 1pg/ml [29].
3.1.2. Nanodiagnostics for AD in In-Vivo
3.1.2.1. Magnetic Resonance Imaging (MRI)
Iron oxide nanoparticles (IONPs) or magnetic nanoparticles (MNPs) have been widely investigated in recent decades as MRI contrast agent [30]. Two independent groups of scientists have accounted the significance of monocrystalline IONPs and ultra-small SIONPs as MRI probes. Th groups performed in vivo imaging of Aβ plaques in the brain of mouse bearing AD [31, 32]. MRI enhancement agent (NPs) infused intravenously were considered as minimal invasive [32]. Recently, Sillerud et al. synthesized antibody-superparamagnetic iron oxide nanoparticles conjugate targeted in vivo MR imaging of Aβ plaques in transgenic mice model of AD. Their results revealed that developed SPIONs exhibited excellent enhancement of MRI contrast in Aβ plaques in the brain of the mice [33]. Antibody conjugated SPIONs exhibited 2-fold higher detectability compared to control group. Nevertheless, the limitation of this technique is that it cannot be adopted well for the early detection of AD as the Aβ plaques targeted by this method are the ones whose formation appears in advanced stages of AD. Interestingly, shifting the target from Aβ plaques to Aβ oligomers (AβO) that appears at the early stage of the AD may make the procedure feasible for early detection of the disease. It has been recently demonstrated that MNPs can be designed in the way that is capable as molecular MRI probe for the early detection of Aβ in in vitro and in vivo [30]. Selective antibodies for AβO when conjugated to superpara-MNPs were specific and sensitive in detection of AβO [30]. This MNPs based probe can be readily adapted to other AD’s targets and therefore providing advantage in early detection over PET probes for AβO (AβO is the toxins considered for the neuron damage in the early stage of AD.
3.1.2.2. Optical Imaging (OI)
A recent approach being developed for in vivo imaging of molecular biomarkers in various ailments including AD is optical imaging (OI) with specific near-infrared (NIR) fluorescent dye [34]. Most common requisites for a diagnostic investigation of AD involves the ability of imaging probe to cross the BBB and specificity to target of AD associated biomarkers. For in vivo molecular detection of Aβ, Nesterov et al. proposed a fluorescent dye working in NIR range called NIAD-4. It can rapidly traverse through BBB due to its unique structure and its quite low molecular weight [34]. The chemical structure of NIAD-4 core resembles the structure of Thioflavin T (ThT). ThT is a renowned Aβ fibril detection molecule. ThT resemblance makes them an extremely specific binding agent for Aβ masses. The structural characteristics of NIAD-4 also significantly enhanced the quantum yield after binding with Aβ masses. The in vivo studies of NIAD-4 in transgenic mouse model of AD were highly auspicious [34]. ThT which is capable of recognizing β-sheet structures corresponding to Aβ aggregates both in vitro and in vivo. The encapsulation of ThT into polystyrene-block-poly (n-butyl cyanoacrylate) (PS-b-PnBCA) NPs was lately studied showing that release of developed NPs into the brain after intracerebral injection, and its interaction with Aβ and clearly indicated the Aβ aggregates [35]. Quantum dots (QDs), a nanotechnology based in-organic material are another very important member of fluorescent dyes used in imaging studies [36]. These are semiconductor materials based NPs. Commonly used QDs are cadmium selenide (CdSe), Cadmium telluride (CdTe) and Indium arsenide (InAs). QDs as an imaging and theranostic agents offer extraordinary optical properties like high stability, high signal to noise ratio, high quantum yield, negligible photo bleaching and resistance to intrinsic fluorescence emission spectra due to which NPs become extremely capable of sensing [36]. Simultaneous multiple labeling properties offer by QDs is significant for diagnosis of AD as there are numerous molecular biomarkers in the pathology of AD [37]. However, the in vivo applications of QDs are questionable due to the toxicity of the semiconductor materials used. However, attempt has been made to reduce the toxicity of QDs by entrapping these in phospholipids [38] or by pegylation [39]. Moreover, to further enhance their delivery across BBB, transferrin conjugated QDs have been developed that are able to transmigrate through BBB [40].
3.2. Nanotechnology in AD Therapy
As discussed somewhere else in this article, the currently available therapy of AD acts only to lower its symptoms [37]. In the last two decades, researches mainly focused on the development of “neuroprotective agents” i.e. development of therapeutics that prevent the disease progression by preventing several mechanisms in the pathology of AD [37]. Nevertheless, more innovative approaches are the one that could rebuild the damaged tissue, called as “regenerative agents”. These two (neuroprotective and neuroregenerative) techniques are collectively called “disease-modifying techniques” in AD. Nanoparticle based approaches focused on the neuroprotective and neuroregenerative techniques in AD can extensively benefit from NPs based research conducted with advances in AD’s cell biology, its pathology and neurophysiology, and the upcoming smart NPs design that are capable of crossing BBB and targeting to the desired site into the CNS. Over the past decade, several NPs have been investigated to enhance the bioavailability and therapeutic efficacy of AD drugs [37].
3.2.1. Neuroprotection Facilitated by Nanotechnology
The two major causes of neurotoxicity in AD are free radicals and Aβ. Neurons can be protected from Aβ toxicity by some of the NPs based techniques. These approaches can prevent Aβ oligomerization and/or accumulation of Aβ oligomeric species [58]. The other nanotechnology mediated neuroprotective techniques include agents that can inhibit oxidative stress induced by free radicals and protect neurons [37]. Numberous NPs have been explored for the delivery of therapeutic agents to the brains in AD.
3.2.1.1. Nanogels, Fullerene and Nano-Ceria
Nanogels are promising drug delivery devices as they offer better drug encapsulation, stability, and can also be targeted based several factors like pH, temperature and ionic strength [41]. Its therapeutic targeting potential was explored by Ikeda et al. who developed a novel monomeric peptide incorporated pullulan (CHP) nanogels bearing cholesterol. The developed colloidal NPs significantly inhibited the aggregation of Aβ thereby reducing its toxicity against PC12 cells [41]. Further, the same group of scientists investigated the capability of these colloidal NPs to interact with the AβO. These revealed that the developed NPs significantly decrease toxicity on primary cortical and microglial cells. CHPs exhibited the knockdown effect on AβO (e.g. Aβ1–42) toxicity and significantly decreased the accumulation in lysosomes [42].
Another important NPs coming from carbon family is Fullerene (C60) which has been directly studied as antioxidant and free radical scavenger intended for AD therapy as neuroprotective [43]. Carboxyfullerene, a malonic acid derivative has been studied for its protective effect against Aβ42 induced oxidative stress and neurotoxicity [44, 45]. Later, it has also been revealed that C60 hydrated fullerene can prevent amyloid oligomerization [44]. Dugan et al. has demonstrated complete neuroprotective effect of Fullerene against NMDA receptor mediated neurotoxicity [44]. The functionalized fullerene; a water- soluble hydroxyl (Fullerenol) has been found to be neuroprotective against Aβ [45]. Anti-oxidant reactions and inhibition of Ca2+ neurotoxicity induced by Aβ are assumed as the reason of neuroprotective effect of fullerenols [45]. Inhibition of Ca2+ mediated neurotoxicity by fullerenol-1 was validated by Huang et al [45]. Another water soluble C60 NP (WS[60]FD) has been studied by Kotelnikova et al. demonstrating the neuroprotective effect [46]. The developed C60 NPs significantly inhibited the in vitro catalytic activity of monoaminooxidase B (MAO-B), and decreased the free radicals level. Moreover, C60 NPs exhibited the cognitive stimulation in vivo [46]. In another report, Zhou et al. demonstrated the binding process and the interaction between fullerene derivative 1, 2-(dimethoxymethano) fullerene (DMF) which is water-soluble and fibrillar Aβ42 hexamer (a protofibril model) simultaneously [47]. Moreover, the D23–K28 salt bridge in this site can be destabilized by DMF, which is thought to be significant in the Aβ fibrillation. Additionally, Xie et al. studied the molecular mechanism of inhibition of Alzheimer's Aβ peptide aggregation by fullerene [48]. Their results showed that significantly higher inhibition of β-sheet formation through fullerene is due to extreme lipophilic and aromatic π -stacking interactions of the C60 hexagonal rings. These strong interactions between the C60 NPs and Aβ peptides extensively lessens the peptide-peptide interaction that is necessary for β-sheet formation, hence hindering Aβ fibrillation. In general, it can be said that novel NPs of functionalized fullerene derivatives have promising potential applications in the discovery of new drugs for AD. Likewise, Cerium oxide (CeO2) nanoparticle (called as nano-ceria) have been found to have neuroprotective effect in in vitro models of AD primarily due to anti-oxidant activity [49]. Two redox states of cerium; Ce2+ and Ce4+ and the developed oxygen vacancies make them good antioxidant [49]. Of late, it was found that nano-ceria also helps in shielding the neurons from cytotoxic effects of Aβ [49]. Cimini et al. developed PEGylated anti-Aβ antibody- conjugated cerium oxide NPs (Ab-CNPs-PEG) to examine the effects of (Ab-CNPs-PEG) on neuronal survival and brain-derived neurotrophic factor (BDNF) signaling pathways [50]. They reported that Aβ-CNPs-PEG targets the Aβ aggregates specifically, and simultaneous rescue of neuronal survival was better than Aβ-CNPs, due to alteration in BDNF signaling pathway. Later, Li et al. studied a novel double delivery proposal based on hydrogen peroxide responsive system [51]. They merged the anti-aggregation property of CeO2 caged metal chelators and antioxidant property of CeO2-NPs in one system as a formulation. It was found that this two-in-one system NPs can efficiently inhibit Aβ aggregation, reduce cellular reactive oxygen species (ROS) and shield the cells from AβO related toxicity [51].
3.2.1.2. Dendrimers
Dendrimers are monodispersed macromolecules at the nanoscale comprising of a regular and extremely branched 3D architecture exhibiting a distinct number of spatially arranged peripheral functional groups [52]. Generally, they are prepared by an iterative sequence of reaction steps. In recent years, such NPs have gained attention of pharmaceutical and biomedical researchers. Particularly, the opportunity to functionalize the peripheral functional groups with desirable ligands is a smart strategy to target the Aβ peptide [52]. Some investigations have been undertaken on the Aβ aggregation procedure involving some critical peptidic sequences concerned with the Aβ aggregation. For example, the KLVFF sequence i.e., the 16–20 residues of Aβ hydrophobic core, is critical for the development of β-sheet of Aβ peptide [53]. This region of peptides binds to its corresponding sequence in Aβ and inhibits Aβ peptides aggregation into Aβ fibrils [53]. This particular sequence has been utilized for the preparation of drugs as inhibitors for blocking Aβ peptide aggregation into Aβ fibrils in vivo [53]. Chafekar et al. synthesized KLVFF-functionalized Dendrimer NPs and demonstrated their inhibitory effect on Aβ aggregation and their capability to dismantle preexisting amyloid aggregates [54]. Some independent researchers have also suggested that Aβ are capable of binding to cells by interacting with glycolipids or glycoproteins at the peripheral surface of the cellular membrane. Moreover, the affinity for interaction increases with increased concentration of sialic acid on the exterior surface of cell membrane [55]. Patel et al. fabricated sialic acid-functionalized polyamidoamine (PAMAM) dendrimers to develop Aβ binding competing agents. Their results showed that the affinity constant between sialic acidconjugated PAMAM dendrimers and Aβ and dendrimers significantly decreases the Aβ-induced toxicity in comparison with the cells treated with free sialic acid [56]. Furthermore, it was also found that the position of the covalent bond between the dendrimers and the sialic acid is critical for modulation of biological activity of the developed sialic acid-functionalized PAMAM dendrimers [57]. Glycosaminoglycans entities, like heparin, which contain linear arrangements of polysaccharides, have been found to be extremely significant in development of Aβ aggregation [58]. Klajnert et al. reported that the heparin induced Aβ aggregation could be altered by the presence of third generation PAMAM dendrimers. It has been suggested that lower concentrations of dendrimers can decrease the Aβ peptide aggregation, while higher concentrations increase the effect [59]. Ciepluch et al. developed viologen-phosphorus dendrimers and demonstrated its effect on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities [60]. Their results showed that it can inhibit the activities of both AChE & BChE, thus demonstrating their potential as new drugs for treating AD. Moreover, Wasiak et al. designed cationic phosphorus containing dendrimers (CPDs) and illustrated interactions of phosphorus incorporated dendrimers with the fragment of Aβ1–28 peptide and MAP-Tau protein [61]. They found that CPDs can modulate the gathering of both Aβ1–28 and MAP-Tau protein. In addition, CPDs also decreased toxicity of aggregated forms of Aβ1–28 [61].
3.2.1.3. Gold Nanoparticles (GNPs)
GNPs have been extensively studied for their drug delivery and theranostics features in various biomedical applications including AD therapy. Kogan et al. employed GNPs in weak microwave fields to dissolve amyloid aggregates [62]. GNPs dissolved Aβ peptide aggregates and inhibited further Aβ peptide aggregations by developing local thermal energy. GNPs provided a dissipating thermal energy of 10–14 J/s making them capable of smashing a Aβ fibril bond (10–20 J binding energy per non-covalent bond) per microsecond without smashing of covalent bonds, which are two times stronger [62]. Kogan et al. developed a method which appeared to be beneficial for noninvasive study and exploitation of Aβ aggregates in AD. Negatively charged GNPs were developed by Liao et al. and examined their effects on amyloids by taking Aβ40 as a model system [63]. Their results showed that GNPs inhibited Aβ fibrillization and redirected Aβ forming fragmented fibrils and spherical oligomers. When these GNPs were added to preformed fibrils, GNPs bound preferentially to Aβ fibrils and resulted in formational of smaller and ragged Aβ species. Similar results were observed when carboxyl conjugated GNPs but not with amine-conjugated AuNPs. Thus, the negative surface potential of GNPs is extremely critical for this phenomenon. Prades et al. has developed GNPs-CLPFFD conjugate and introduced the peptide sequence THRPPMWSPVWP [64]. The results of in vitro and in vivo experiments showed that the there was interaction between peptide sequence and transferrin receptor present in the endothelial cells of the BBB which caused enhanced permeability in to the brain.
3.2.1.4. Diamondoid and its Derivatives
Diamondoids and their derivatives are the sources of various antibacterial and antiviral drugs which are already being marketed or which are at different developmental stages [65]. Memantine is an FDA approved commercially used diamondoid derivative for moderate to severe AD. Memantine is a neuroprotective agent having activity against excitotoxicity, excitatory neurotransmitter glutamate, or over activation of its membrane receptors, that causes neuronal cell death. Neuronal cell death due to excitotoxicity is mediated, partly, through over activation of N-methyl-d-aspartate (NMDA)-type glutamate receptors. Memantine primarily inhibits excessive NMDA receptor activity without affecting normal activity [66]. NMDA receptor activity too is vital for normal neuronal function. This suggests that potential neuroprotective agents that practically block/inhibit all NMDA receptor activity would possibly have undesirable adverse effects. Clinical investigations are currently in progress for the development of other diamondoid derivatives intended to be more potent neuroprotective agents and possibly having regenerative potential. Such agensts can also be used for the management of diseases associated to glutamatergic dysfunction. Sozio et al. developed memantine-sulfur bearing antioxidant glutathione (GSH) (i) and (R)-α-lipoic acid (LA) (ii) conjugates as prodrugs for improved treatment of AD [67]. Prodrugs (i) and (ii) exhibited free radical scavenging activity for both H2O2 and superoxide anion radical. They exhibited no interference with the proliferative ability of the GL15 astroglial cell line, supporting their use “in vivo”. It was found that the prodrug (ii) was able to cross the BBB and significantly inhibit Aβ1–42 aggregation [67]. Gauthier and Molinuevo investigated the advantages of combined therapy of memantine and donepezil (cholinesterase inhibitor) in moderate to severe AD [68]. Their results illustrated that combination therapy can yield significant efficacy in cognition behavior function and global outcome in comparison to donepezil alone.
3.2.2. Nanocarriers in Brain Delivery of Neuroprotectives
The main obstruction in the successful development of both large and small neurotherapeutic molecules (such as Aβ fragments, recombinant peptides, antisense oligonucleotides) is BBB [6]. The BBB affects the therapeutic efficacy and tolerance, as large doses of neurotherapeutic molecules are required to achieve levels above the minimum effective concentration in the brain. Nanotechnology based approaches provide an opportunity to prevail over such challenges and can be employed as “Trojan systems” for transport of neurotherapeutic molecules as payload across the BBB, and consequently decrease the toxicity and enhance therapeutic efficacy of drugs [7, 37].
3.2.2.1. Curcumin Loaded Nanoparticles
Curcumin has been extensively investigated in recent decade for various biological activities including neuroprotective effect [69]. Many investigators have accounted that curcumin can cause considerable reduction in Aβ aggregate related toxicity on neurons [70]. However, it exhibits poor aqueous solublity and stability. Furthermore, it is prone to oxidation and photodegradation [71]. Nanocapsules made up of poly n-butylcyanoacrylate (PBCA) have been used to transport curcumin across the BBB. Apolipoprotein E3 (APoE-3), a protein ligand, has been used to coat these nanocapsules so that LDL receptors onto the BBB could be targeted. Curcumin n nanoparticles was also reported to have enhanced photostability compared to free curcumin. Cytotoxicity in SH-SY5Y neuroblastoma cells showed that ApoE3-C-PBCA exhibited significantly improved efficacy against Aβ induced cytotoxicity compared to free curcumin. Additionally, ApoE3 with curcumin showed excellent therapeutic efficacy against Aβ induced cytotoxicity [72]. Doggui et al. developed curcumin encapsulated PLGA-NPs and evaluated neuronal uptake and neuroprotective effect in the human SK-N-SH cell lines [73]. It was found that these NPs not only protected SK-N-SH cells against H2O2 but also prevented increase in ROS level and GSH consumption. Mathew et al. successfully developed curcumin encapsulated PLGA-NPs and coupled the NPs with the Tet-1 peptide, which has affinity for neurons [74]. It was observed that curcumin incorporated PLGA-NPs were able to dismantle Aβ aggregates. The PLGA-NPs also showed excellent antioxidative properties and were non-toxic. Recently, Lazar et al. designed curcumin conjugated nanoliposomes [75]. The developed liposomes were non-toxic in vitro, down regulated the Aβ peptide secretion and partially inhibited Aβ induced toxicity. Intracerebral injection of developed liposomes showed that curcumin encapsulated nanoliposomes exhibited significantly higher accumulation in the Aβ aggregates in vivo [75]. Of late, other studies showed improved bioavailability and long circulation time for curcumin based NPs [76].
3.2.2.2. Cholinesterase Inhibitors Loaded Nanocarriers
Defective cholinergic neurotransmission is thought to be one of the main reasons for the learning and memory impairment of AD patients [5]. To date, enhancement of cholinergic neurotransmission remains the most effective therapeutic strategy for AD treatment. In 2000, FDA approved rivastigmine (RT) for AD treatment. Nevertheless, clinical efficacy of rivastigmine is still limited largely because of poor brain translocation which leads to undesirable cholinergic effects on peripheral organs and frequent administration [77]. Nanotechnology based approach for transportation of rivastigmine to the brain is a promising strategy to overcome above limitations. Wilson et al. developed rivastigmine (RT) loaded polysorbate 80 coated Poly n-butylcyanoacrylate nanoparticles (PnBCA-NPs) to increase the brain delivery of RT and to decrease the side effects [78]. Their The developed NPs exhibited 3.8 fold improved uptake of RT in the brain in comparison with free RT after i.v. administration in rats. Similar approach was used to enhance the brain uptake of tacrine (another AChE inhibitor) by means of PnBCA-NPs [79]. The delivery mediated by NPs enhanced the concentration of tacrine in brain by 4-folds as compared to the free drug. Joshi et al. further reported that rivastigmine (RT) entrapped PLGA and PnBCA NPs [80]. exhibit quicker memory regain in scopolamine-induced amnesic mice compared to RT solution. Fazil et al. developed rivastigmine (RT) encapsulated chitosan nanoparticles (CS-RT NPs) to enhance the uptake and bioavailability of RT in the brain after intranasal (i.n.) administration [81]. Their results showed that CS-RT NPs exhibited ~3 fold improved RT concentration in the brain after i.n. administration compared to i.v. administration. CS-RT NPs exhibited significantly higher RT transport efficiency. Furthermore, Li et al. formulated galanthamine hydrobromide (GH) loaded flexible liposomes and investigated their acetylcholinesterase inhibition efficiency in the brain of male Sprague-Dawley rat after i.n. administration [82]. The results showed that the developed liposomes exhibited significantly enhanced acetylcholinesterase inhibition efficacy of GH after i.n. administration compared to oral administration. Similarly, Ismail et al. developed rivastigmine (RT) loaded liposomes and investigated the efficacy in aluminium chloride induced AD model [83]. Their results showed that the developed RT loaded liposomes exhibited faster memory regain and amelioration of metabolic disturbances in aluminium chloride treated rats compared to free RT. Wavikar et al. also developed RT loaded nanostructured lipid carriers gel (RT-NLC gel) for brain delivery after i.n. administration [84]. The developed RT-NLC gel exhibited 2-fold improved i.n. permeation of the RT compared to plain RT solution. RT-NLC gel also exhibited 3-fold enhanced enzyme inhibition efficiency.
3.2.2.3. Acetylcholine (ACh) Loaded Nanocarrier
It is well established that ACh concentration has direct co-relation with the memory loss, the main symptom of AD. In fact, the presently available major drug therapy for AD is based on the strategy to sustain the ACh level by inhibiting its hydrolysis by the enzyme. However, drugs working as enzyme inhibitor have many side effects. Direct use of neurotransmitter ACh as a drug offers a good approach in safer management of AD. However, its delivery to brain through BBB is challenging. Recently, a nanotechnology based approach has been proposed for brain delivery of ACh using single-wall carbon nanotubes (SWCNTs) [85]. Though, SWCNTs are associated with the concern of toxicity which was addressed by careful controlling of the dosages and by doing surface PEGylation [85].
3.2.2.4. Hormone loaded NPs
It has been discovered through recent investigations that sex hormones, particularly andorgens and estrogen have neuroprotective activity against some pathogenic mechanisms of AD, such as Aβ aggregation, neurotoxicity and cytotoxicity [86]. It has been found that estradiol can elevate the development of cholinergic neurons and extensively decrease cerebral Aβ peptide deposition [87]. Recently, for the effective delivery of such hormone, Mittal et al. developed estradiol loaded PLGA NPs [88]. They effectively increased 10-fold oral bioavailability of estradiol in comparison with free estradiol by manipulating the copolymer molecular weight and composition. Similarly, mifepristone (antiprogesterone molecule) was found to inhibit the progression of cognitive decline in AD patients by altering P-gp mediated efflux of Aβ peptide [89]. He et al. has reported an increase in oral bioavailability of mifepristone by its encapsulated PLGA NPs [90]. Later, Mittal et al. developed oral estradiol loaded polysorbate 80 coated PLGA NPs for brain delivery [91]. Oral administration of polysorbate 80 coated PLGA NPs exhibited significantly enhanced levels of estradiol within the brain in comparison with the uncoated ones. Moreover, PLGA NPs successfully prevented the expression of Aβ42 immunoreactivity in the brains of treated animals.
3.2.2.5. Polyphenol drugs loaded NPs
Recently, epigallocatechin-3-gallate (EGCG), a polyphenol from green tea, was found to have therapeutic efficacy against AD [92]. Apart from antioxidant effects, EGCG also reduced Aβ peptide production. Aβ peptides are produced after proteolysis of the APP by a sequence of enzymatic actions of β- and γ-secretase. Alternatively, the nonamyloidogenic molecular pathway includes successive APP cleavages by α-secretase (thereby preventing Aβ formation) and γ- secretase, leading to the development of nonamyloidogenic fragments. Interruption in these two important pathways and the aggregative property of Aβ peptides might be the reason which triggers AD [93]. Therefore, α-, β-, and γ-secretases enzymes can be considered as potential therapeutic targets. However, β- secretase could be the most appropriate and attractive target as α- and γ-secretases have multiple biological functions [94]. Encouraging results have been obtained in several studies, except the ones involving intracranial delivery of the inhibitors by invasive mode [95]. Smith et al. proposed that the incorporation of EGCG into lipidic NPs might enhance its oral bioavailability [96]. Zhang et al. developed Tet-1 peptide modified EGCG conjugated selenium nanoparticles to decrease cytotoxicity and Aβ peptide aggregation [97]. It significantly inhibited Aβ fibrillation and disaggregated preformed Aβ fibrils into nontoxic aggregates.
Another polyphenolic compound commonly used in precilical and clinical studies for the treatment of AD is Resveratrol [98]. Resveratrol is a non-flavonoid, mainly found in red wine and grape skin. Its antioxidant, anti-inflammatory, anti-carcinogenic and anti-mutagenic activities have been proven by many researchers [99]. Its exerts anti-oxidant activity by the prevention of β-amyloid induced nuclear translocation of NF-κB and inhibition of the release of nitric oxide (NO) and prostaglandin E2 (PGE2) in AD [100]. Resveratrol can induce protein kinase C to protect cells from toxic effects of Aβ [100]. The neuroprotective effect of resveratrol against toxic effects of Aβ may also be mediated by advancing the intracellular degradation of Aβ by the ubiquitin proteasome system [101]. It was found that resveratrol can reduce nitric oxide (NO) toxicity in rat hippocampal mixed neuronal/glial cultures [102]. Lu et al. developed resveratrol loaded polymeric micelles and assessed their protective effects on cells against Aβ-induced oxidative stress for treatment of AD [103]. They investigated the efficacy of resveratrol encapsulated polymeric micelles for Aβ protection using PC12 cell lines. The 12-h pre-incubation of resveratrol encapsulated NPs guarded PC12 cells from Aβ-induced injuries in a dose dependent manner by reducing intracellular oxidative stress and caspase-3 activity. In another study, Frozza et al. developed resveratrol lipid-core nanocapsules and assessed their neuroprotective effects against intracerebroventricular injection of Aβ1–42 in male adult Wistar rats for management of AD [104]. Resveratrol lipid- core nanocapsules efficiently rescued the toxic effects of Aβ1–42. Furthermore, da Rocha Lindner et al. developed resveratrol (RVT) loaded poly(lactic acid) (PLA) and PLA-PEG NPs and assessed its antioxidant activity for management of AD [105]. They found that RVT-loaded nanoparticles, particularly PLA-PEG nanoparticles, were very effective as a hunter for the ABTS radical, signifying that the polymeric nanoparticles can be used as RVT carriers for prophylactic or therapeutic effects.
3.2.3. NPs in Metal Chelation
Brain contains certain trace metal ions such as Zn2+, Cu2+ and Fe2+, and participates in variety of physiologically important roles [106]. These trace metal ions are kept under strict homeostatic condition and compartmentalization. Any disturbance in the homeostasis of these trace elements resulting in higher concentrations of these ions which affects several proteins and causes membrane lipid damage and ultimately cause production of reactive oxygen species (ROS) [106]. Aβ possesses few selective metal binding sites, therefore, it is considered as a metalloprotein. It is known that Zn2+ and Cu2+ released in traces from synaptic terminals of brain cortical neurons might induce Aβ aggregation by interacting with Aβ histidine [106]. This precipitation can be reversed by metal chelation prior to fibrillization that can reduce the progress of AD. Furthermore, the interaction of Fe3+ and Cu2+ with Aβ may cause generation of H2O2 due to double electron transfer to O2 [106]. The production of H2O2 is partially accountable for oxidative injury as observed in AD. Additionally, the interaction of Aβ with cell membrane is increased by Zn2+ as well as Cu2+ and could be responsible for neurotoxicity of Aβ [107].
Therefore, clearance of body tissues from excessive metal ions by metal chelation could be considered as one of the approach to control disease progression in AD [106].
An effective metal chelation approach for AD may significantly reduce both the extracellular oxidative stress as well as Aβ aggregation that ultimately retard the progression of AD. Conventional mode faces obstacle in an efficient therapeutic response for most current metal chelators. These comprise threat of non-specific metal chelation from other tissues, difficulty to cross the BBB and pooling of metal ions into amyloid plaques [106]. Nanotechnology has also been exploited to design metal chelator systems that would surmount these limitations [108]. The significance of iron and copper chelators are very well reported in AD modification.
3.2.3.1. Iron Chelators
Liu et al. conjugated the Desferrioxamine (FDA approved metal chelator) with NPs to design a robust chelator nanoparticulate system (CNPS) [109]. It was found that designed CNPS was able to retain the chelating efficacy of metal chelator. The iron chelating CNPS was found to cross the BBB in the reverse direction by preferential adsorption of Apo A-I and was ultimately removed through LDL transport system. Furthermore, it was found that the inherent toxicity of metal chelators gets reduced after conjugation with NPs [109]. Later, another study has reported that nanoparticle-chelator conjugate (Nano-N2PY) can inhibit the cytotoxic effects of Aβ on human cortical neurons [110]. Carboxyl functionalized polystyrene NPs were used in this study. Anticytotoxic mechanism of the Nano-N2PY was probably related to its preventive effect on the Aβ aggregation. Apart from improved efficacy of metal chelating strategy, these nanoparticle conjugates were also found to reduce the toxicity by decreasing the lipophilicity of the chelators [110].
3.2.3.2. Copper Chelators
Cui et al. examined d-penicillamine, an FDA approved drug for chelation of copper in Wilson’s disease, as a metal chelator in AD [107]. The conjugation of d-penicillamine with the NPs containing 1,2-Dioleoyl-snglycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl) butyramide] and pyridyldithio-propionylphosphoethanolamine known as MPB-PE and PDP-PE respectively, facilitated the transport of d-penicillamine across the BBB in spite of its high hydrophilicity showing the ability of d-penicillamine for chelating copper and desolubilizing Aβ42. Cui et al. also showed that the BBB integrity and permeability remained unaffected and no changes in the cerebral perfusion flow were evident. Further, it was suggested that the transport mechanism for these NPs was either endocytosis, transcytosis or passive diffusion [107].
3.2.3.3. Zinc Chelators
Clioquinol (Iodochlorohydroxyquin), a USP drug which possesses chelation properties and ability to penetrate BBB, has shown potential therapeutic advantage in AD [111]. Clioquinol, is a quinoline derivative and an antibiotic drug having strong chelation affinity for copper and zinc which dissolves the Aβ plaques in vitro and impedes Aβ accretion in mice bearing AD [112]. Clioquinol showed advanced Aβ disaggregation which leads to a significant reduction in Aβ deposition in the APP transgenic mice brain compared to non-treated animals after oral administration [112]. Clinical studies showed that clioquinol exhibits strong deacceleration of cognition rate in AD patients compared to control group [111]. Mufamadi et al. developed surface decorated nanoliposomes (NLPs) using different chelating agents viz. ethylenediaminetetraacetic acid (EDTA), histidine residues, copper acetate (CuAc) and zinc acetate (ZnAc) to modulate neurotoxicity associated with Aβ aggregates of AD [113]. The result of their in vitro studies in PC12 neuronal cell line confirmed that the incubation of Cu(II) or Zn(II) with Aβ1–42 peptide stimulated Aβ aggregation. Modified NLPs (with EDTA, histidine and ZnAc) induced disaggregation of CuAβ1–42 and ZnAβ1–42 aggregates in vitro. Further, modified NLPs exhibited significantly higher survival of PC12 neuronal cells; thereby protecting PC12 neuronal cells from toxicity associated with Aβ1–42 aggregate. Thus, the surface modified NLPs showed strong protection of PC12 neuronal cells from insoluble Aβ peptides associated with neurotoxicity in AD.
4. TOXICITY CONCERNS WITH NANOTECHNOLOGY IN AD
Advancement nanotechnology based drug delivery, along with positive implications, has also raised the concerns related to toxicity. The particle size and surface morphology of nanoparticulate systems are reported to modify the pharmacokinetics and tissue distribution profile of the loaded drugs/bioactive [114–116]. Such investigations particularly in reference to brain delivery are still limited and benefit-to-risk ratio is yet to be assessed for therapeutic considerations. The constituents of nanoparticulate system are found to modulate the P-gp pumps expressed over the luminal side of capillary endothelial cells in brain and potentially mediate transportation of hemostatic mediators [117]. The different polycationic nanoparticulate systems are reported to stimulate the necrotic or apoptotic cell death through various pathways upon internalization [114, 118]. Besides this, some other polymeric delivery systems such as polyplexes, lipoplexes and lipopolyplexes are also found to modify gene expression in case of nucleic acid delivery to the capillary endothelial cells in brain. Such an issue has been addressed with low expression of ATP-binding cassette genes after polymer treatment in some cells. Polymers and partially degraded component of nanoparticulate system may bind to endogenous nucleic acids and cause hindrance with normal processes of cell as well as triggering off-target effects. This is found to be one of the reasons related to polycationinduced “gene-signature” issue [119]. Furthermore, it has also been reported that nanoparticulate delivery system, through a complex intercellular signaling processes, may mediate DNA and chromosomal damage in the tissues present across the cellular barriers [120]. More recently, clinically approved nanomedicines were found to exhibit the hypersensitivity reactions known as complement activation-related pseudoallergy (CARPA) due to activation of the complement system in some patients [121]. In addition, accelerated blood clearance (ABC) phenomenon also resulted after the administration of first injection of PEGylated nanoparticulate delivery system leading to failure of drug therapy only because of low plasma drug concentration. Polymeric and lipid-based nanomedicines have been reported to exhibit neuropsychosomatic and vegetative responses in animals studes [121]. Various PEGylated or non-PEGylated nano delivery systems like certain liposomes, polymeric nanospheres, carbon nanotubes, and metallic nanoparticulate system have been found to trigger complement activation through one or more established initiation pathways such as classical, alternative, and lectin pathways [121, 122]. These pathways exploit different recognition molecules to perceive foreign particle and are activated by similar mechanism to secrete convertase enzyme that responsible to cleave central complement protein C3. The nanoparticulate system-mediated complement activation results into surface opsonization by the opsonic fragments (like C3b and iC3b) of C3 cleavage [116, 121]. Overall, this promotes recognition and fast clearance through macrophages of the reticuloendothelial system decorated with complement receptors. Consequently, it may be advantageous for intravenous nanomedicines that are considered to stimulate sink effect in AD. Furthermore, complement cascade activation results into production of potent anaphylatoxins such as C4a, C3a, and C5a. These are found to elicit the secretion of secondary mediators from various immune cells and consequently commence anaphylaxis in susceptible individuals to further complicate AD [116]. Additionally, the lytic membrane attacks complex (C5b-9 or MAC) assembled from the terminal complement components (C5, C6, C7, C8, and C9) on cleavage of C5. This complex has ability to provoke nonlytic stimulatory responses from vascular endothelial cells and results into modification in hemostasis and inflammatory cell recruitment [116]. The complement system is sturdily activated in AD brain (specifically at senile-plaque site) and functions in association with activated microglia having high expression of complement receptors [123]. Complement proteins as well as activation products have also been reported to be associated with cerebrovascular Aβ [124]. Therefore, activation of complement system results intro exacerbation of pathologic changes in AD. More recently, it was confirmed that the classical components of pathway like C1q might be extremely upregulated specifically in the cortex region in AD [125]. The binding interaction between Aβ and C1q is mainly ionic and such interactions also result in increase in amyloid aggregation [126]. Hence, it seems rational that design of nanomedicine for brain delivery should not induce further activation of complement system (specifically activation of the terminal pathway) principally via C1q-dependent triggering mechanisms. Future directions may focus on the development of brain-specific nanomedicine that promote release of complement inhibitors specifically that block binding to the collagen tail of C1q [127]. Therefore, the complement system plays an important role in performance of nanomedicine specifically designed for brain delivery and thorough understanding of material properties involved in complement activation remains critical factor for rational design of nanoparticulate system in management as well as treatment of AD.
5. FUTURE DIRECTION
In the perspective of AD therapy, remarkable studies have been carried showing the worth of nanotechnology application in diagnosis, therapeutics delivery and theranostics in AD. However, the majority of research is at the preclinical level, the outcome promises that in near future significant transition is expected from the clinical studies and the nanotechnology-based therapy in AD could lead to improved therapeutic outcomes. Many upcoming novelties together with nanotechnology based strategies have shown potential in drug/biomolecules and/or imaging agent’s delivery across the BBB intended for AD therapy. Among them, few which are comprehensively used in research are briefly dicussed here. These techniques can help in improving the performance of NPs mediated CNS delivery without any complication.
5.1. Ultrasound Mediated BBB Distruption
The permeability of drug and drug carriers can be increased across blood-brain barrier (BBB) by focused ultrasound (FUS) and can be used in drug and or NPs targeting in brain [128]. In the past few years, biomedical research in drug delivery and disgnosis concerning brain diseases particularly AD and brain cancer focused on developing MRI guided FUS to selectively and focally disrupt the BBB to increase its permeability to therapeutics and NPs. MRI guided FUS technique serves the unique purpose of theranostics along with smartly designed payload carrying NPs. FUS induced BBB disruption is mainly affected by the ultrasound setting that includes amplitude of applied pressure, frequency, duty cycle, number of cycles per pulse, dose and the size of microbubble [129]. Control of such parameter is very critical as in vivo studies have showed that endothelial damage may happen after sonication [130]. Moreover, increase in vascular permeability across the tissue near to the sonication area often occurs due to the interaction of the sonication and vasculature. This is linked to the destruction of microbubbles, presence of radiation forces as well as microstreaming [131]. The disruption needs to be continued for longer period in order to attain therapeutic drug concentration as the co-administered drug extravasation remains dependent on diffusion. Opening of the BBB is reported to lasts for several minutes to hours with sonication [132]. Therefore, ultrasound mediated BBB distruption technology can be used to increase the permeability of drug for brain delivery for better treatment of AD. Though, the FUS induced BBB disruption showed improvement in brain cancer therapy, its application in AD is critical in many aspects. BBB is important in maintaining homeostasis of CNS. Therefore, disruption in BBB can be responsible for various problems such as inward flow of circulating substances into the CNS that might be neurotoxic, transporter dysfunction, altered protein expression, inflammatory activation, oxidative stress as well as neuronal damage. These effects have been seen in AD and there is conflicting evidence that BBB disruption can be considered as a feature of progressive AD.
5.2. Direct Convection Therapy in AD
Convection-enhanced delivery (CED) is a technique to deliver the different diagnostic/ therapeutic agents directly into the brain that by pass the blood-brain barrier [133]. Traditional local delivery system like parenteral administration of various therapeutic agents into the brain has been based on diffusion principles and depends on concentration gradient to overcome hindrance due to biological barriers. It results into limited distribution of delivered agents and drug infiltrates remain confined to few millimeters from the site of administration. In human studies, administration of therapeutics into non-malignant brain is associated with reflux as well as leakage by the injection site. However, CED utilizes a fluid pressure gradient at the infusion catheter tip and bulk flow to disseminate the substances within extracellular fluid space [133]. It also permits the extracellular infused material to spread further by means of the perivascular spaces as well as rhythmic contractions of blood vessels which act as an efficient motive force for the infusate [134]. Consequently, high drug concentration can be more uniformly distributed over the larger area of targeted tissue in comparison to simple injection mode of administration. Presently, CED is being clinically tested in the areas of neurodegenerative diseases [135] and neuro-oncology [136].
CONCLUSION
Considering the role of nanotechology in AD’s therapy ‘engineered tuneable surface and size of NPs in nanoscale which can go down upto 1nm makes them potential drug/diagnostic agent/ carrier as a theranostic agent for the management of AD. Nanoparticles offer the opportunity to design smart therapeutics carriers that can simultaneously cross the BBB and deliver the payload to the specific targets. Moreover, significant work has been done in nanoparticles enabled imaging sofar for making detection of early biomarkers in AD with high sensitivity possible. Though, research carried in this area is promising but yet not good enough to bring such technology from bench to bed side in AD therapy. Many important aspects such as pharmacokinetics, metabolism and toxicity concerns with many nanomaterials particularly inorganic nanoparticles (significantly used in imaging studies) are still to be properly addressed. Lastly, other form of nanoparticles investigated in AD therapy have shown their performace majorly in preclinical studies only.
Acknowledgments
Declared none.
LIST OF ABBREVIATION
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- ADDL
Aβ -derived diffusible ligands
- AgNPs
Silver nanoparticles
- AUC
Area under the curve
- Aβ
Amyloid beta
- AβO
Aβ oligomers
- BBB
Blood-brain barrier
- BChE
Butyrylcholinesterase
- BDNF
Brain-derived neurotrophic factor
- CHP
Cholesterol-bearing pullulan
- CnLs
Curcumin-conjugated nanoliposomes
- CNPS
Chelator nanoparticle system
- CNS
Central nervous system
- CNTs
Carbon nanotubes
- CSF
Cerebrospinal fluid
- DMF
1,2-(dimethoxymethano)fullerene
- EGCG
Epigallocatechin-3-gallate
- GH
Galanthamine hydrobromide
- GNPs
Gold nanoparticles
- LSPR
Localized surface plasmon resonance
- MNPs
Magnetic nanoparticles
- MRI
Magnetic Resonance Imaging
- MRT
Mean residence time
- NLCs
Nanostructured lipid carriers
- NMDA
N-methyl-d-aspartate
- NPs
Nanoparticles
- PBCA
Poly n-butylcyanoacrylate
- P-tau
Hyperphosphorylated tau
- QDs
Quantum dots
- RIVA
Rivastigmine
- ROS
Reactive oxygen species
- RT
Rivastigmine
- SPR
Surface plasmon resonance
- ThT
Thioflavin T
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Prince M, Albanese E, Guerchet M, Prina M. World alzheimer report 2014: Dementia and risk reduction. 2014 www.alz.co.uk/research/WorldAlzheimerReport.
- 2.Nabeshima T, Nitta A. Memory impairment and neuronal dysfunction induced by beta-amyloid protein in rats. Tohoku J Exp Med. 1994;174(3):241–49. doi: 10.1620/tjem.174.241. [DOI] [PubMed] [Google Scholar]
- 3.Popovic N, Brundin P. Therapeutic potential of controlled drug delivery systems in neurodegenerative diseases. Int J Pharm. 2006;314(2):120–26. doi: 10.1016/j.ijpharm.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Potschka H. Targeting the brain-Surmounting or bypassing the blood-brain barrier. Handb Exp Pharmacol. 2010:411–31. doi: 10.1007/978-3-642-00477-3_14. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad MZ, Akhter S, Rahman Z, Ahmad J, Ahmad I, Ahmad FJ. Nanomedicine based drug targeting in Alzheimer’s disease: High impact of small carter. In: Atta-ur-Rahman, Choudhary MI, editors. Front Drug Design Discov. Vol. 6. 2014. pp. 716–39. [Google Scholar]
- 6.Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64(7):686–700. doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Brambilla D, Le Droumaguet B, Nicolas J, Hashemi SH, Wu LP, Moghimi SM, et al. Nanotechnologies for Alzheimer's disease: diagnosis, therapy, and safety issues. Nanomedicine. 2011;7(5):521–40. doi: 10.1016/j.nano.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Shaffer JL, Petrella JR, Sheldon FC, Choudhury KR, Calhoun VD, Coleman RE, et al. Azheimer’s disease neuroimaging initiative. Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology. 2013;266(2):583–91. doi: 10.1148/radiol.12120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goedert M, Klug A, Crowther RA. Tau protein, the paired helical filament and Alzheimer disease. J Alzheimers Dis. 2006;9(3):195–207. doi: 10.3233/jad-2006-9s323. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer disease begin? Curr Opin Neurol. 2012;25(6):708–14. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- 11.Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid β peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38(1):6–23. doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasha S, Gupta K. Various drug delivery approaches to the central nervous system. Exp Opin Drug Deliv. 2010;7(1):113–35. doi: 10.1517/17425240903405581. [DOI] [PubMed] [Google Scholar]
- 13.Mayeux R, Stern Y. Epidemiology of Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;2:a006239. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma HS, Sharma A. Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology. Prog Brain Res. 2007;162:245–73. doi: 10.1016/S0079-6123(06)62013-X. [DOI] [PubMed] [Google Scholar]
- 15.Pavan B, Dalpiaz A, Ciliberti N, Biondi C, Manfredini S, Vertuani S. Progress in drug delivery to the central nervous system by the prodrug approach. Molecules. 2008;13(5):1035–65. doi: 10.3390/molecules13051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53(4):569–96. [PubMed] [Google Scholar]
- 17.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 18.Aulic S, Bolognesi ML, Legname G. Small-molecule theranostic probes: a promising future in neurodegenerative diseases. Int J Cell Biol. 2013;2013:150952. doi: 10.1155/2013/150952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhter S, Ahmad MZ, Ahmad FJ, Storm G, Kok RJ. Gold nanoparticles in theranostic oncology: current state-of-the-art. Exp opin drug deliv. 2012;9(10):1225–43. doi: 10.1517/17425247.2012.716824. [DOI] [PubMed] [Google Scholar]
- 20.Nazem A, Mansoori GA. Nanotechnology for Alzheimer's disease detection and treatment. Insciences J. 2011;1(4):169–93. [Google Scholar]
- 21.Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, et al. Evaluation of CSF-tau and CSFAbeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58(3):373–79. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- 22.Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, et al. Improved discrimination of AD patients using beta-amyloid (1–42) and tau levels in CSF. Neurology. 1999;52(8):1555–62. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 23.Maddalena A, Papassotiropoulos A, Muller-Tillmanns B, Jung HH, Hegi T, Nitsch RM, et al. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch Neurol. 2003;60(9):1202–6. doi: 10.1001/archneur.60.9.1202. [DOI] [PubMed] [Google Scholar]
- 24.Fluri F. Clinical nanomedicine: nanomedical approaches in Alzheimer's disease. Eur J Nanomed. 2010;3(1):7–12. [Google Scholar]
- 25.Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, et al. Nanoparticle based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc Natl Acad Sci USA. 2005;102(7):2273–76. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svenson S. Theranostics: are we there yet? Mol Pharm. 2013;10(3):848–56. doi: 10.1021/mp300644n. [DOI] [PubMed] [Google Scholar]
- 27.Moghimi SM. Bionanotechnologies for treatment and diagnosis of Alzheimer's disease. Nanomedicine. 2011;7(5):515–18. doi: 10.1016/j.nano.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Kang DY, Lee JH, Oh BK, Choi JW. Ultra-sensitive immunosensor for amyloid-beta (1–42) using scanning tunneling microscopy-based electrical detection. Biosens Bioelectron. 2009;24(5):1431–36. doi: 10.1016/j.bios.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Neely A, Perry C, Varisli B, Singh AK, Arbneshi T, Senapati D, et al. Ultrasensitive and highly selective detection of Alzheimer's disease biomarker using two-photon Rayleigh scattering properties of gold nanoparticle. ACS Nano. 2009;3(9):2834–40. doi: 10.1021/nn900813b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viola KL, Sbarboro J, Sureka R, De M, Bicca MA, Wang J, et al. Towards non-invasive diagnostic imaging of early-stage Alzheimer's disease. Nat Nanotechnol. 2015;10(1):91–98. doi: 10.1038/nnano.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadghiri YZ, Sigurdsson EM, Sadowski M, Elliott JI, Li Y, Scholtzova H, et al. Detection of Alzheimer's amyloid in transgenic mice using magnetic resonance microimaging. Magn Reson Med. 2003;50(2):293–302. doi: 10.1002/mrm.10529. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Wadghiri YZ, Hoang DM, Tsui W, Sun Y, Chung E, et al. Detection of amyloid plaques targeted by USPIO-A-beta 1–42 in Alzheimer's disease transgenic mice using magnetic resonance microimaging. Neuro Image. 2011;55(4):1600–9. doi: 10.1016/j.neuroimage.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sillerud LO, Solberg NO, Chamberlain R, Orlando RA, Heidrich JE, Brown DC, et al. SPION-enhanced magnetic resonance imaging of Alzheimer’s disease plaques in AβPP/PS-1 transgenic mouse brain. J Alzheimer’s Dis. 2013;34(2):349–65. doi: 10.3233/JAD-121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nesterov EE, Skoch J, Hyman BT, Klunk WE, Bacskai BJ, Swager TM. In vivo optical imaging of amyloid aggregates in brain: design of fluorescent markers. Angew Chem Int Ed Engl. 2005;44(34):5452–56. doi: 10.1002/anie.200500845. [DOI] [PubMed] [Google Scholar]
- 35.Henley DB, May PC, Dean RA, Siemers ER. Development of semagacestat (LY450139), a functional gamma-secretase inhibitor, for the treatment of Alzheimer’s disease. Exp Opin Pharmacother. 2009;10:1657–64. doi: 10.1517/14656560903044982. [DOI] [PubMed] [Google Scholar]
- 36.Akhter S, Ahmad Z, Singh A, Ahmad I, Rahman M, Anwar M, Jain GK, et al. Cancer targeted metallic nanoparticle: targeting overview, recent advancement and toxicity concern. Curr Pharm Des. 2011;17(18):1834–50. doi: 10.2174/138161211796391001. [DOI] [PubMed] [Google Scholar]
- 37.Alam Q, Alam MZ, Karim S, Gan SH, Kamal MA, Jiman Fatani A, et al. A nanotechnological approach to the management of Alzheimer disease and type 2 diabetes. CNS Neurol Disord Drug Tar. 2014;13(3):478–86. doi: 10.2174/18715273113126660159. [DOI] [PubMed] [Google Scholar]
- 38.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298(5599):1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 39.Tokuraku K, Marquardt M, Ikezu T. Real-time imaging and quantification of amyloid-beta peptide aggregates by novel quantum-dot nanoprobes. PLoS One. 2009;4(12):e8492. doi: 10.1371/journal.pone.0008492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu G, Yong KT, Roy I, Mahajan SD, Ding H, Schwartz SA, et al. Bioconjugated quantum rods as targeted probes for efficient transmigration across an in vitro blood-brain barrier. Bioconjug Chem. 2008;19(6):1179–85. doi: 10.1021/bc700477u. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda K, Okada T, Sawada S, Akiyoshi K, Matsuzaki K. Inhibition of the formation of amyloid beta-protein fibrils using biocompatible nanogels as artificial chaperones. FEBS Lett. 2006;580(28–29):6587–95. doi: 10.1016/j.febslet.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Boridy S, Takahashi H, Akiyoshi K, Maysinger D. The binding of pullulan modified cholesteryl nanogels to Aβ oligomers and their suppression of cytotoxicity. Biomaterials. 2009;30(29):5583–91. doi: 10.1016/j.biomaterials.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O'Malley KL. Fullerene-based Antioxidants and neurodegenerative disorders. Parkinsonism Relat Disord. 2001;7(3):243–46. doi: 10.1016/s1353-8020(00)00064-x. [DOI] [PubMed] [Google Scholar]
- 44.Dugan LL, Gabrielsen JK, Yu SP, Lin TS, Choi DW. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol Dis. 1996;3(2):129–35. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 45.Huang H, Ou H, Hsieh S, Chiang L. Blockage of amyloid beta peptide-induced cytosolic free calcium by fullerenol-1, carboxylate C60 in PC12 cells. Life Sci. 2000;66(16):1525–33. doi: 10.1016/s0024-3205(00)00470-7. [DOI] [PubMed] [Google Scholar]
- 46.Kotelnikova RA, Smolina AV, Grigoryev VV, Faingold II, Mischenko DV, Rybkin AY, et al. Influence of water-soluble derivatives of [60]fullerene on therapeutically important targets related to neurodegenerative diseases. Med Chem Commun. 2014;5(11):1664–68. [Google Scholar]
- 47.Zhou X, Xi W, Luo Y, Cao S, Wei G. Interactions of a water-soluble fullerene derivative with amyloid-β protofibrils: dynamics, binding mechanism, and the resulting salt-bridge disruption. J Phys Chem B. 2014;118(24):6733–41. doi: 10.1021/jp503458w. [DOI] [PubMed] [Google Scholar]
- 48.Xie L, Luo Y, Lin D, Xi W, Yang X, Wei G. The molecular mechanism of fullerene-inhibited aggregation of Alzheimer's b-amyloid peptide fragment. Nanoscale. 2014;6(16):9752–62. doi: 10.1039/c4nr01005a. [DOI] [PubMed] [Google Scholar]
- 49.D'Angelo B, Santucci S, Benedetti E, Di Loreto S, Phani RA, Falone S, et al. Cerium oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathway. Curr Nanosci. 2009;5(2):167–76. [Google Scholar]
- 50.Cimini A, D’Angelo B, Das S, Gentile R, Benedetti E, Singh V, et al. Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Aβ aggregates modulate neuronal survival pathways. Acta Biomater. 2012;8(6):2056–67. doi: 10.1016/j.actbio.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Shi P, Xu C, Ren J, Qu X. Cerium oxide caged metal chelator: anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer's disease treatment. Chem Sci. 2013;4(6):2536–42. [Google Scholar]
- 52.Stiriba SE, Frey H, Haag R. Dendritic polymers in biomedical applications: from potential to clinical use in diagnostics and therapy. Angew Chem Int Ed Engl. 2002;41(8):1329–34. doi: 10.1002/1521-3773(20020415)41:8<1329::aid-anie1329>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 53.Lowe TL, Strzelec A, Kiessling LL, Murphy RM. Structure-function relationships for inhibitors of β-amyloid toxicity containing the recognition sequence KLVFF. Biochemistry. 2001;40(26):7882–89. doi: 10.1021/bi002734u. [DOI] [PubMed] [Google Scholar]
- 54.Chafekar SM, Malda H, Merkx M, Meijer EW, Viertl D, Lashuel HA, et al. Branched KLVFF tetramers strongly potentiate inhibition of β-amyloid aggregation. Chembiochem. 2007;8(15):1857–64. doi: 10.1002/cbic.200700338. [DOI] [PubMed] [Google Scholar]
- 55.Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem. 2001;276(27):24985–90. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- 56.Patel DA, Henry JE, Good TA. Attenuation of β-amyloid induced toxicity by sialic acid-conjugated dendrimeric polymers. Biochim Biophys Acta. 2006;1760(12):1802–9. doi: 10.1016/j.bbagen.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Patel DA, Henry JE, Good TA. Attenuation of β-amyloid-induced toxicity by sialic acid-conjugated dendrimers: role of sialic acid attachment. Brain Res. 2007;1161:95–105. doi: 10.1016/j.brainres.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLaurin J, Franklin T, Zhang X, Deng J, Fraser PE. Interactions of Alzheimer amyloid-β peptides with glycosaminoglycans effects on fibril nucleation and growth. Eur J Biochem. 1999;266(3):1101–10. doi: 10.1046/j.1432-1327.1999.00957.x. [DOI] [PubMed] [Google Scholar]
- 59.Klajnert B, Cladera J, Bryszewska M. Molecular interactions of dendrimers with amyloid peptides: pH dependence. Biomacromolecules. 2006;7(7):2186–91. doi: 10.1021/bm060229s. [DOI] [PubMed] [Google Scholar]
- 60.Ciepluch K, Weber M, Katir N, Caminade AM, El Kadib A, Klajnert B, et al. Effect of viologen-phosphorus dendrimers on acetylcholinesterase and butyrylcholinesterase activities. Int J Biol Macromol. 2013;54:119–24. doi: 10.1016/j.ijbiomac.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Wasiak T, Ionov M, Nieznanski K, Nieznanska H, Klementieva O, Granell M, et al. Phosphorus dendrimers affect Alzheimer’s (Aβ1–28) peptide and MAP-tau protein aggregation. Mol Pharm. 2011;9(3):458–69. doi: 10.1021/mp2005627. [DOI] [PubMed] [Google Scholar]
- 62.Kogan MJ, Bastus NG, Amigo R, Grillo-Bosch D, Araya E, Turiel A, et al. Nanoparticle mediated local and remote manipulation of protein aggregation. Nano Lett. 2006;6(1):110–15. doi: 10.1021/nl0516862. [DOI] [PubMed] [Google Scholar]
- 63.Liao YH, Chang YJ, Yoshiike Y, Chang YC, Chen YR. Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-β fibrillization, induce fibril dissociation and mitigate neurotoxicity. Small. 2012;8(23):3631–39. doi: 10.1002/smll.201201068. [DOI] [PubMed] [Google Scholar]
- 64.Prades R, Guerrero S, Araya E, Molina C, Salas E, Zurita E, et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33(29):7194–205. doi: 10.1016/j.biomaterials.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 65.Mansoori GA. Diamondoid Molecules. Advances in Chemical Physics. 2007:207–58. [Google Scholar]
- 66.Lipton SA. Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer's disease and other neurologic disorders. J Alzheimers Dis. 2004;6:61–74. doi: 10.3233/jad-2004-6s610. [DOI] [PubMed] [Google Scholar]
- 67.Sozio P, Cerasa LS, Laserra S, Cacciatore I, Cornacchia C, Di Filippo ES, et al. Memantine-sulfur containing antioxidant conjugates as potential prodrugs to improve the treatment of Alzheimer’s disease. Eur J Pharm Sci. 2013;49(2):187–98. doi: 10.1016/j.ejps.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Gauthier S, Molinuevo JL. Benefits of combined cholinesterase inhibitor and memantine treatment in moderate-severe Alzheimer’s disease. Alzheimers Dement. 2013;9(3):326–31. doi: 10.1016/j.jalz.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Ahmad MZ, Akhter S, Mohsin N, Abdel-Wahab BA, Ahmad J, Warsi MH, et al. Transformation of curcumin from food additive to multifunctional medicine: nanotechnology bridging the gap. Curr Drug Discov Technol. 2014;11(3):197–213. doi: 10.2174/1570163811666140616153436. [DOI] [PubMed] [Google Scholar]
- 70.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res. 2005;2(2):131–36. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 72.Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR. ApoE3 mediated poly(butyl) cyanoacrylate nanoparticles containing curcumin: study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol Pharm. 2010;7(3):815–25. doi: 10.1021/mp900306x. [DOI] [PubMed] [Google Scholar]
- 73.Doggui S, Sahni JK, Arseneault M, Dao L, Ramassamy C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J Alzheimers Dis. 2012;30(2):377–92. doi: 10.3233/JAD-2012-112141. [DOI] [PubMed] [Google Scholar]
- 74.Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS One. 2012;7(3):e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lazar AN, Mourtas S, Youssef I, Parizot C, Dauphin A, Delatour B, et al. Curcumin-conjugated nanoliposomes with high affinity for Aβ deposits: possible applications to Alzheimer disease. Nanomedicine. 2013;9(5):712–21. doi: 10.1016/j.nano.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AHL, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 Mice. AAPS J. 2013;15(2):324–36. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gauthier S, Juby A, Dalziel W, Rehel B, Schecter R. Effects of rivastigmine on common symptomatology of Alzheimer's disease. Curr Med Res Opin. 2010;26(5):1149–60. doi: 10.1185/03007991003688888. [DOI] [PubMed] [Google Scholar]
- 78.Wilson B, Samanta MK, Santhi K, Kumar KP, Paramakrishnan N, Suresh B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res. 2008;1200:159–68. doi: 10.1016/j.brainres.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 79.Wilson B, Samanta MK, Santhi K, Kumar KP, Paramakrishnan N, Suresh B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur J Pharm Biopharm. 2008;70(1):75–84. doi: 10.1016/j.ejpb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 80.Joshi SA, Chavhan SS, Sawant KK. Rivastigmine-loaded PLGA and PBCA nanoparticles: preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur J Pharm Biopharm. 2010;76(2):189–99. doi: 10.1016/j.ejpb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 81.Fazil M, Md S, Haque S, Kumar M, Baboota S, Sahni JK, et al. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci. 2012;47(1):6–15. doi: 10.1016/j.ejps.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 82.Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol. 2012;34(2):272–79. doi: 10.1016/j.etap.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Ismail MF, Elmeshad AN, Salem NA. Potential therapeutic effect of nanobased formulation of rivastigmine on rat model of Alzheimer's disease. Int J Nanomedicine. 2013;8:393–406. doi: 10.2147/IJN.S39232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wavikar PR, Vavia PR. Rivastigmine-loaded in situ gelling nanostructured lipid carriers for nose to brain delivery. J Liposome Res. 25(2):141–9. doi: 10.3109/08982104.2014.954129. (215) [DOI] [PubMed] [Google Scholar]
- 85.Yang Z, Zhang Y, Yang Y, Sun L, Han D, Li H, et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine. 2010;6(3):427–41. doi: 10.1016/j.nano.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30(2):239–58. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amtul Z, Wang L, Westaway D, Rozmahel RF. Neuroprotective mechanism conferred by 17β-estradiol on the biochemical basis of Alzheimer's disease. Neuroscience. 2010;169(2):781–86. doi: 10.1016/j.neuroscience.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 88.Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MN. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119(1):77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 89.Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, et al. β-Amyloid efflux mediated by P-glycoprotein. J Neurochem. 2001;76(4):1121–28. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- 90.He W, Horn SW, Hussain MD. Improved bioavailability of orally administered mifepristone from PLGA nanoparticles. Int J Pharm. 2007;334(1–2):173–78. doi: 10.1016/j.ijpharm.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 91.Mittal G, Carswell H, Brett R, Currie S, Kumar MNVR. Development and evaluation of polymer nanoparticles for oral delivery of estradiol to rat brain in a model of Alzheimer's pathology. J Control Release. 2011;150(2):220–28. doi: 10.1016/j.jconrel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 92.Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–87. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 93.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 94.Vassar R. β-secretase (BACE) as a drug target for Alzheimer's disease. Adv Drug Deliv Rev. 2002;54(12):1589–602. doi: 10.1016/s0169-409x(02)00157-6. [DOI] [PubMed] [Google Scholar]
- 95.Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, et al. Efficient inhibition of the Alzheimer's disease β-secretase by membrane targeting. Science. 2008;320(5875):520–23. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- 96.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and α-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer's disease. Int J Pharm. 2010;389(1–2):207–12. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J, Zhou X, Yu Q, Yang L, Sun D, Zhou Y, et al. Epigallocatechin-3-gallate (EGCG)-stabilized selenium nanoparticles coated with Tet-1 peptide to reduce amyloid-β aggregation and cytotoxicity. ACS Appl Mater Interfaces. 2014;6(11):8475–87. doi: 10.1021/am501341u. [DOI] [PubMed] [Google Scholar]
- 98.Ahmad MZ, Ahmad J, Amin S, Rahman M, Anwar M, Mallick N, et al. Role of nanomedicines in delivery of anti-Acetylcholinesterase compounds to the brain in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2014;13(8):1315–24. doi: 10.2174/1871527313666141023100618. [DOI] [PubMed] [Google Scholar]
- 99.Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S. Resveratrol: review on therapeutic potential and recent advances in drug delivery. Exp Opin Drug Deliv. 2014;11(8):1285–98. doi: 10.1517/17425247.2014.919253. [DOI] [PubMed] [Google Scholar]
- 100.Kim YA, Lim SY, Rhee SH, Park KY, Kim CH, Choi BT, et al. Resveratrol inhibits inducible nitric oxide synthase and cyclooxy genase-2 expression in beta-amyloid-treated C6 glioma cells. Int J Mol Med. 2006;17(6):1069–75. [PubMed] [Google Scholar]
- 101.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005;280(45):37377–82. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 102.Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide related toxicity in cultured hippocampal neurons. Br J Pharmacol. 2000;131(4):711–20. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu X, Ji C, Xu H, Li X, Ding H, Ye M, et al. Resveratrol-loaded polymeric micelles protect cells from Aβ-induced oxidative stress. Int J Pharm. 2009;375(1–2):89–96. doi: 10.1016/j.ijpharm.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 104.Frozza RL, Bernardi A, Hoppe JB, Meneghetti AB, Matte A, Battastini AMO, et al. Neuroprotective effects of resveratrol against Aβ administration in rats are improved by lipid-core nanocapsules. Mol Neurobiol. 2013;47(3):1066–80. doi: 10.1007/s12035-013-8401-2. [DOI] [PubMed] [Google Scholar]
- 105.da Rocha Lindner G, Khalil NM, Mainardes RM. Resveratrol-loaded polymeric nanoparticles: validation of an HPLC-PDA method to determine the drug entrapment and evaluation of its antioxidant activity. Sci World J. 2013;2013:506083. doi: 10.1155/2013/506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bush AI. Drug development based on the metals hypothesis of Alzheimer's disease. J Alzheimer's Dis. 2008;15(2):223–40. doi: 10.3233/jad-2008-15208. [DOI] [PubMed] [Google Scholar]
- 107.Cui Z, Lockman PR, Atwood CS, Hsu CH, Gupte A, Allen DD, et al. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer's and other CNS diseases. Eur J Pharm Biopharm. 2005;59(2):263–72. doi: 10.1016/j.ejpb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 108.Curtain C, Ali F, Volitakis I, Cherny R, Norton R, Beyreuther K, et al. Alzheimer's disease amyloid- binds Cu and Zn to generate an allosterically-ordered membrane-penetrating structure containing SOD-like subunits. J Biol Chem. 2001;276(23):20466–73. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- 109.Liu G, Men P, Harris PL, Rolston RK, Perry G, Smith MA. Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci Lett. 2006;406(3):189–93. doi: 10.1016/j.neulet.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 110.Liu G, Men P, Kudo W, Perry G, Smith MA. Nanoparticle-chelator conjugates as inhibitors of amyloid-β aggregation and neurotoxicity: A novel therapeutic approach for Alzheimer disease. Neurosci Lett. 2009;455(3):187–90. doi: 10.1016/j.neulet.2009.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60(12):1685–91. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 112.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30(3):665–76. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 113.Mufamadi MS, Choonara YE, Kumar P, Modi G, Naidoo D, Ndesendo VM, et al. Surface-engineered nanoliposomes by chelating ligands for modulating the neurotoxicity associated with β-amyloid aggregates of Alzheimer's disease. Pharm Res. 2012;29(11):3075–89. doi: 10.1007/s11095-012-0770-0. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad J, Akhter S, Rizwanullah M, Amin S, Rahman M, Ahmad MZ, et al. Nanotechnology-based inhalation treatments for lung cancer: state of the art. Nanotechnol Sci Appl. 2015;8:55–66. doi: 10.2147/NSA.S49052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rahman M, Ahmad MZ, Ahmad J, Firdous J, Ahmad FJ, Mushtaq G, et al. Role of graphene nano-Composites in cancer therapy: Theranostic applications, metabolic fate and toxicity issues. Curr Drug Metab. 2015;16(5):397–409. doi: 10.2174/1389200215666141125120633. [DOI] [PubMed] [Google Scholar]
- 116.Rahman M, Akhter S, Ahmad MZ, Ahmad J, Addo RT, Ahmad FJ, et al. Emerging advances in cancer nanotheranostics with graphene nanocomposites: opportunities and challenges. Nanomedicine (Lond) 2015;10(15):2405–22. doi: 10.2217/nnm.15.68. [DOI] [PubMed] [Google Scholar]
- 117.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304(2):845–54. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 118.Parhamifar L, Larsen AK, Hunter AC, Andresen TL, Moghimi SM. Polycation cytotoxicity: a delicate matter for nucleic acid therapy-focus on polyethylenimine. Soft Matter. 2010;6(17):4001–9. [Google Scholar]
- 119.Kabanov AV, Batrakova EV, Alakhov VY. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J Control Rel e. 2003;91(1–2):75–83. doi: 10.1016/s0168-3659(03)00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhabra G, Sood A, Fisher B, Cartwright L, Saunders M, Evans WH, et al. Nanoparticles can cause DNA damage across a cellular barrier. Nat Nanotechnol. 2009;4(12):876–83. doi: 10.1038/nnano.2009.313. [DOI] [PubMed] [Google Scholar]
- 121.Moghimi SM, Andersen AJ, Hashemi SH, Lettiero B, Ahmadvand D, Hunter AC, et al. Complement activation cascade triggered by PEG-PL engineered nanomedicines and carbon nanotubes: the challenges ahead. J Cont Rel. 2010;146(2):175–81. doi: 10.1016/j.jconrel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 122.Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM. Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere-serum interface: implications for stealth nanoparticle engineering. ACS Nano. 2010;4(11):6629–38. doi: 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- 123.Itagaki S, Akiyama H, Saito H, McGeer PL. Ultrastructural localization of complement membrane attack complex (MAC)-like immunoreactivity in brains of patients with Alzheimer's disease. Brain Res. 1994;645(1–2):78–84. doi: 10.1016/0006-8993(94)91640-3. [DOI] [PubMed] [Google Scholar]
- 124.Rozemuller JM, Bots GT, Roos RA, Eikelenboom P. Acute phase proteins but not activated microglial cells are present in parenchymal β/A4 deposits in the brains of patients with hereditary cerebral hemorrhage with amyloidosis-Dutch type. Neurosci Lett. 1992;140(2):137–40. doi: 10.1016/0304-3940(92)90087-n. [DOI] [PubMed] [Google Scholar]
- 125.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer's disease brain. Am J Pathol. 1999;154(3):927–36. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Webster S, Bonnell B, Rogers J. Charge-based binding of complement component C1q to the Alzheimer amyloid β-peptide. Am J Pathol. 1997;150(5):1531–36. [PMC free article] [PubMed] [Google Scholar]
- 127.McGeer PL, McGeer EG. The possible role of complement activation in Alzheimer disease. Trends Mol Med. 2002;8(11):519–23. doi: 10.1016/s1471-4914(02)02422-x. [DOI] [PubMed] [Google Scholar]
- 128.Yang FY, Lin YS, Kang KH, Chao TK. Reversible blood-brain barrier disruption by repeated transcranial focused ultrasound allows enhanced extravasation. J Cont Rel. 2011;150(1):111–16. doi: 10.1016/j.jconrel.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 129.Choi JJ, Feshitan JA, Baseri B, Wang S, Tung YS, Borden MA, et al. Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo. IEEE Trans Biomed Eng. 2010;57(1):145–54. doi: 10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hwang JH, Brayman AA, Reidy MA, Matula TJ, Kimmey MB, Crum LA. Vascular effects induced by combined 1-MHz ultrasound and microbubble contrast agent treatments in vivo. Ultrasound Med Biol. 2005;31(4):553–64. doi: 10.1016/j.ultrasmedbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 131.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51(4):793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 132.Hynynen K. Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev. 2008;60(10):1209–17. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–80. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, et al. The "perivascular pump" driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70(21):1980–83. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 136.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–11. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 137.Skaat H, Margel S. Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-β fibrils detection and removal by a magnetic field. Biochem Biophys Res Commun. 2009;386:645–49. doi: 10.1016/j.bbrc.2009.06.110. [DOI] [PubMed] [Google Scholar]
- 138.Choi JS, Choi HJ, Jung DC, Lee JH, Cheon J. Nanoparticle assisted magnetic resonance imaging of the early reversible stages of amyloid β self-assembly. Chem Commun. 2008;19:2197–99. doi: 10.1039/b803294g. [DOI] [PubMed] [Google Scholar]
- 139.Hartig W, Kacza J, Paulke B, Grosche J, Bauer U, Hoffmann A, et al. In vivo labelling of hippocampal-amyloid in triple-transgenic mice with a fluorescent acetylcholinesterase inhibitor released from nanoparticles. Eur J Neurosci. 2010;31:99–109. doi: 10.1111/j.1460-9568.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- 140.Siegemund T, Paulke BR, Schmiedel H, Bordag N, Hoffmann A, Harkany T, et al. Thioflavins released from nanoparticles target fibrillar amyloid β in the hippocampus of APP/PS1 transgenic mice. Int J Dev Neurosci. 2006;24:195–201. doi: 10.1016/j.ijdevneu.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 141.Kulkarni PV, Roney CA, Antich PP, Bonte FJ, Raghu AV, Aminabhavi TM. Quinoline-n-butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer's disease. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(1):35–47. doi: 10.1002/wnan.59. [DOI] [PubMed] [Google Scholar]
- 142.Roney CA, Arora V, Kulkarni PV, Antich PP, Bonte FJ. Nanoparticulate radiolabelled quinolines detect amyloid plaques in mouse models of Alzheimer's disease. Int J Alzheimers Dis. 2009;2009:481031. doi: 10.4061/2009/481031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang D, Fa HB, Zhou JT, Li S, Diao XW, Yin W. The detection of β-amyloid plaques in an Alzheimer's disease rat model with DDNP-SPIO. Clin Radiol. 2015;70(1):74–80. doi: 10.1016/j.crad.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 144.Jaruszewski KM, Curran GL, Swaminathan SK, Rosenberg JT, Grant SC, Ramakrishnan S, et al. Multimodal Nanoprobes to target cerebrovascular amyloid in Alzheimer's disease brain. Biomaterials. 2014;35:1967–76. doi: 10.1016/j.biomaterials.2013.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang C, Wan X, Zheng X, Shao X, Liu Q, Zhang Q, et al. Dualfunctional nanoparticles targeting amyloid plaques in the brains of Alzheimer's disease mice. Biomaterials. 2014;35(1):456–65. doi: 10.1016/j.biomaterials.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 146.Agyare EK, Jaruszewski KM, Curran GL, Rosenberg JT, Grant SC, Lowe VJ, et al. Engineering theranostic nanovehicles capable of targeting cerebrovascular amyloid deposits. J Control Release. 2014;185:121–29. doi: 10.1016/j.jconrel.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Skaat H, Corem-Slakmon E, Grinberg I, Last D, Goez D, Mardor Y, et al. Antibody-conjugated, dual-modal, near-infrared fluorescent iron oxide nanoparticles for antiamyloidgenic activity and specific detection of amyloid-β fibrils. Int J Nanomedicine. 2013;8:4063–76. doi: 10.2147/IJN.S52833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dehvari K, Lin KS. Synthesis, characterization and potential applications of multifunctional PEO-PPOPEO- magnetic drug delivery system. Curr Med Chem. 2012;19(30):5199–204. doi: 10.2174/092986712803530584. [DOI] [PubMed] [Google Scholar]