Abstract
Background
This study’s objectives were to describe community oncologists’ beliefs about and confidence with geriatric care and to determine whether geriatric-relevant information influences cancer treatment decisions.
Methods
Community oncologists were recruited to participate in two multi-site geriatric oncology trials. Participants shared their beliefs about and confidence with caring for older adults. They were also asked to make a first-line chemotherapy recommendation (combination vs. single-agent vs. no chemotherapy) for a hypothetical vignette of an older patient with advanced pancreatic cancer. Each oncologist received one randomly-chosen vignette that varied on three variables: age (72/84 years), impaired function (yes/no), and cognitive impairment (yes/no). Other patient characteristics were held constant. Logistic regression models were used to identify associations between oncologist and vignette-patient characteristics with treatment decisions.
Results
Oncologist response rate was 61% (n=305/498). The majority of oncologists agreed that “the care of older adults with cancer needs to be improved” (89%) and that “geriatrics training is essential” (72%). However, less than 25% were “very confident” in recognizing dementia or conducting a fall risk or functional assessment, and only 23% reported using the geriatric assessment (GA) in clinic. Each randomly varied patient characteristic was independently associated with the decision to treat: younger age (adjusted OR: 5.01; 95% CI: 2.73–9.20), normal cognition (5.42; 3.01–9.76), and being functionally intact (3.85; 2.12–7.00). Accounting for all vignettes across all scenarios, 161 (52%) said they would offer chemotherapy. All variables were independently associated with prescribing single-agent over combination chemotherapy (older age: 3.22; 1.43–7.25; impaired cognition: 3.13, 1.36–7.20; impaired function: 2.48; 1.12 –5.72). Oncologists’ characteristics were not associated with decisions about providing chemotherapy.
Conclusion
Geriatric-relevant information, when available, strongly influences community oncologists’ treatment decisions.
INTRODUCTION
As the baby boomer generation in the United States ages, the numbers of older patients with cancer is also rising. A 67% increase in cancer incidence in those 65+ years of age is projected, compared to an 11% increase among younger adults.1,2 Oder patients with cancer have a higher prevalence of comorbidities, geriatric syndromes, and disabilities than younger patients and older patients without cancer.3,4 Older patients with conditions outside of cancer also carry a high risk of developing significant chemotherapy toxicity, functional and cognitive loss, and physical decline while on treatment.5,6 The under-representation of older adults in clinical trials places them at risk of receiving inappropriate under- or over-treatment for their cancer, leading to disparities in outcomes.5,7–9 For example, fit older patients are less likely to receive evidence-based standard of care cancer treatment than younger patients, while older patients with both cancer and comorbid conditions are too often treated with therapies with high toxicity rates and low likelihoods of benefit.10
A recent Institute of Medicine (IOM) report acknowledged that our current systems are ill-prepared to care for the most vulnerable patients with cancer—those who are older (especially patients who are age 80+) and those who have health conditions other than cancer.11 Because older patients with cancer receiving treatment are often seen by their oncology teams more frequently than by their primary care providers,12 community oncology practices should be equipped to recognize common age-related concerns. Despite the rapidly increasing numbers of older patients with cancer, most oncologists have received little geriatrics training, so common aging-related conditions that influence outcomes are rarely detected.13–16
In this study, community oncologists were recruited to take part of two nationwide, geriatric oncology clinical trials in the University of Rochester Cancer Center NCI Community Oncology Research Program (URCC NCORP). During enrollment, they completed a survey regarding their beliefs about and confidence in providing geriatric care.17 Similar to other studies,18–21 randomized vignettes were utilized to assess whether clinical factors influenced their cancer treatment decision making. This study, however, is the first that assesses how common geriatrics factors (i.e., function and cognition) affects decisions related to first-line chemotherapy in older patients with advanced cancer.
METHODS
Participants
Participants were community oncologists recruited for two geriatric oncology studies (URCC 13059, clinicaltrials.gov NCT02054741 and/or URCC 13070, clinicaltrials.gov NCT02107443). Both studies involve a GA, which is a battery of validated tools to evaluate health status in multiple domains including function, physical performance, depression, falls, and cognition, 22 and evaluate whether providing a GA summary and targeted recommendations to community oncologists can improve outcomes of older patients with cancer.
Community oncologists were eligible to participate if they practiced within an NCI-funded NCORP community affiliate site, their NCORP affiliate had IRB-approval for either study, and they were not planning on leaving the practice. Oncologists were provided with a link to a survey through email, using REDCap, a secure web-based electronic data capture tool. If not completed, a paper survey option was offered. Oncologists were required to complete the baseline survey prior to participating in procedures of the main study. “Waiver of consent” was approved by the IRB for enrollment of oncologists.
Survey Design
The “Physician Baseline Survey” had three components: 1) oncologist demographics and practice characteristics, 2) oncologist ratings of their beliefs about and confidence with management of common geriatric issues, and 3) one of eight randomly-assigned clinical vignettes. The beliefs and confidence questions were developed by Cancer and Aging Research Group (CARG) investigators (Magnuson, Mohile, Dale) and were modeled on a previously published survey.17 In accordance with prior studies,18,21 a vignette with a shared scenario was created describing an older patient with metastatic pancreatic cancer presenting to her oncologist for a decision on first-line chemotherapy. A vignette of a patient with metastatic disease was selected to assess how geriatric factors may influence the weighing the risks and benefits of chemotherapy for frail older patients with limited life expectancies. The patient was an older female who lived alone with a history of well-controlled hypertension, hyperlipidemia, and osteoarthritis, moderate fatigue (ECOG PS =1), and an estimated life expectancy of six months or less with no other symptoms from her cancer. Using this information as a base, eight vignette-patients were created that varied three factors: age (72 vs. 84 years), cognitive status (no impairment vs. moderate impairment requiring assistance with finances and low Mini-Mental Status Examination (MMSE score of 15), and functional status (no impairment vs. impairment that included falls and deficits in instrumental activity of daily living activities (IADLs)). These factors were chosen because they are among the most important predictors of poor outcomes in older patients and are associated with frailty.5,23–27 In order to reduce bias (e.g., physician answer for one vignette influences responses to others), a randomization scheme was developed so that each enrolled physician would receive one of the eight vignettes.
Statistical Analysis
Descriptive statistics were used to describe physician demographics. Descriptive statistics were also used for the Likert-scale questions regarding beliefs about and confidence with geriatrics, with inter-quartile range (IQR), mean, and median reported for each item. Bivariate associations between patient and physician characteristics and decision to treat with chemotherapy were analyzed with chi-square tests for categorical variables and t-tests for continuous variables. A total summary score was calculated for physician beliefs and physician confidence, and each score was categorized into tertiles due to a skewed distribution.
Logistic regression was performed to determine the independent association of the three varied vignette-patient characteristics (age, cognitive status, functional status) with primary outcome, namely, whether oncologists would recommend treatment with first-line chemotherapy (yes or no) (Model A). In cases when chemotherapy was recommended, a second regression was conducted, predicting whether oncologists recommended single-agent chemotherapy or combination chemotherapy (Model B). Both models controlled for physician characteristics. Physician characteristics included gender (male/female), race (white/non-white), number of patients seen per day, and years in practice. A p-value of <0.05 was considered significant for all analyses. Analyses were performed using SAS software version 9.4.
RESULTS
Of 498 surveys sent to eligible community oncologists in the UR NCORP network, 305 consented to one or both of the studies (61% response rate). The oncologists were associated with 58 individual practice sites.
Oncologist Demographics and Practice Characteristics
Participants (n=305) had a mean age of 49 years, and the majority were male (71%), white (65%), and non-Hispanic (94%) (Table 1). The majority were board certified in oncology (95%) and had a mean of 15 years in practice post-oncology fellowship. On average, oncologists saw 17 patients per day and were clinically active 4 days of the week.
Table 1.
Physician Characteristics (n=305)*
| Characteristic | |
|---|---|
| Mean Age (range) | 48.6 years (29–76) |
| Gender | |
| Male | 70.8% |
| Female | 29.2% |
| Race | |
| White | 65.0% |
| African American/Black | 2.7% |
| American Indian/Alaskan Native | 0.3% |
| Asian | 31.3% |
| Native Hawaiian/Other Pacific Islander | 0.7% |
| Ethnicity | |
| Hispanic/Latino | 2.0% |
| Non-Hispanic | 94.4% |
| Unknown | 3.6% |
| Board certified in oncology | |
| Yes | 95.1% |
| Mean years in practice (range) | 14.6 years (0.5–44) |
| Mean number of patients seen per day (range)** | 17.3 patients (2–45) |
Oncologists’ Perspectives Regarding Geriatrics Care
The vast majority of oncologists agreed that “there should be more clinical trials designed specifically for the elderly” (90%) and “the medical care of older adults with cancer needs to be improved” (89%) (Table 2). Many agreed that they would “appreciate additional training in topics related to the care of older adults with cancer” (79%). Most reported routinely asking patients about falls (70%). Much less commonly, oncologists agreed that they “frequently order home safety evaluations” (41%) or “enlist the help of a social worker with specialized geriatrics training” (31%). Only 23% agreed they “use standardized GA tools to help make decisions about treatment.”
Table 2.
| Agree (%)a | Disagree (%)b | Neutral (%)c | |
|---|---|---|---|
| I believe there should be more clinical trials designed specifically for the elderly | 90% | 3% | 7% |
| I believe that the medical care of older adults with cancer needs to be improved | 89% | 3% | 8% |
| I strive to reduce the number of medications that my older patients are taking | 81% | 4% | 15% |
| I would appreciate additional training in topics related to the care of older adults with cancer | 79% | 4% | 17% |
| I believe that geriatric training is essential for the care of older adults with cancer | 72% | 9% | 18% |
| I routinely ask my patients if they have a history of recent falls | 70% | 14% | 16% |
| I frequently order home safety evaluations for my older patients | 41% | 35% | 25% |
| I frequently enlist the help of a social worker with specialized geriatrics training | 31% | 37% | 32% |
| I use standardized geriatric assessment tools to help me make decisions about my patients | 23% | 49% | 29% |
Based on a Likert scale, where 1=Strongly Disagree, 2=Somewhat Disagree, 3=Neutral, 4=Somewhat Agree and 5= Strongly Agree.
Some percentages may not add to 100 due to rounding
All questions with <5 missing values
Percent “agree” was calculated using the sum of physicians who chose a 4 or 5 on the scale
Percent “disagree” was calculated using the sum of physicians who chose a 1 or 2 on the scale
Percent “neutral” was calculated using physicians who chose a 3 on the scale
Oncologists’ Ratings of Confidence in Geriatric Care
The majority of oncologists felt “quite to very confident” when it comes to discussing advanced directives (84%), preventing and managing osteoporosis (72%), and determining patients’ social support/living experiences (53%) (Table 3). Confidence was lower for other skills; 25% or less were “quite to very confident” in the conducting and evaluating a functional assessment; recognizing, evaluating, and treating dementia; and conducting an assessment and intervention for falls.
Table 3.
| Quite to Very Confident (n)a | Slightly to Moderately Confident (n)b | Not at all Confident (n)c | Mean (median) | |
|---|---|---|---|---|
| Discuss advance directives | 84% | 15% | 0% | 4.3 (4) |
| Prevent and manage osteoporosis | 72% | 26% | 2% | 3.9 (4) |
| Determine patient’s social support/living experiences | 53% | 45% | 2% | 3.5 (4) |
| Recognize, evaluate, and treat depression | 47% | 49% | 4% | 3.4 (3) |
| Make recommendations for rehabilitation | 41% | 54% | 5% | 3.2 (3) |
| Recognize, evaluate, and treat delirium | 39% | 54% | 6% | 3.2 (3) |
| Assess nutritional status | 37% | 61% | 2% | 3.2 (3) |
| Conduct and evaluate a functional assessment | 25% | 65% | 10% | 2.8 (3) |
| Recognize, evaluate, and treat dementia | 23% | 69% | 8% | 2.8 (3) |
| Conduct an assessment of and an intervention for falls | 21% | 65% | 14% | 2.6 (3) |
| Recognize, evaluate, and treat urinary incontinence | 21% | 64% | 15% | 2.7 (3) |
Based on a Likert scale, where 1=Not at all confident, 2=Slightly confident, 3=Moderately confident, 4=Quite confident and 5= Very confident.
Some percentages may not add to 100 due to rounding
All values with <3 missing values
Percent “quite to very confident” was calculated using the sum of physicians who chose a 4 or 5 on the scale
Percent “slightly to moderately confident” was calculated using the sum of physicians who chose a 2 or 3 on the scale
Percent “not at all confident” was calculated using physicians who chose a 1 on the scale
Vignette Responses
Chemotherapy Choices
Accounting for all vignettes across all scenarios, 161 (52%) said they would offer at least some form of chemotherapy. Of the 161 oncologists who recommended chemotherapy, 64.6% (n=104) would offer single-agent chemotherapy such as gemcitabine or capecitabine; 35.4% (n=57) would offer multi-agent chemotherapy such as FOLFIRINOX or gemcitabine/nab-paclitaxel.
Bivariate Analyses
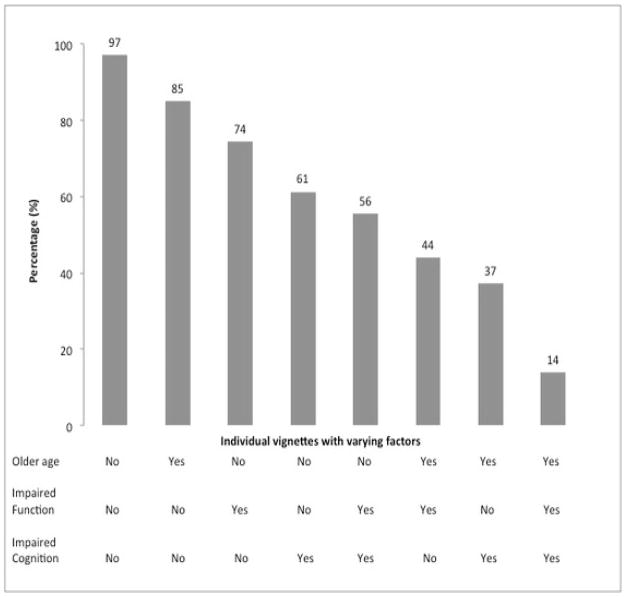
There was a consistent relationship between vignette-patient characteristics and a decision to recommend chemotherapy (Table 4 and Figure 1). The proportion of oncologists who recommended any chemotherapy decreased with older patient age, cognitive impairment, and functional impairment. At the extremes, the majority of oncologists (97%) randomized to receive vignette 1 (younger age and no functional or cognitive impairment) would recommend chemotherapy, while only a minority (14%) randomized to vignette 8 (older age, functional impairment, and cognitive impairment) would recommend chemotherapy. There was a general “dose-response” relationship, with older age and greater geriatrics deficits leading to less aggressive therapy choices.
Table 4.
Percentage of Oncologists’ Recommending Chemotherapy for each Vignette Patient*
| Patient Vignette The patient (initials) is a ______year old female with a history of well-controlled hypertension, hyperlipidemia and osteoarthritis, who is referred for evaluation of metastatic pancreatic cancer. She has a 3 cm pancreatic adenocarcinoma with metastatic disease to the liver. Based upon her cancer diagnosis, her estimated life expectancy is six months or less. She currently reports moderate fatigue which is impacting her daily activities (ECOG PS =1) but denies any other symptoms from her cancer. She currently lives alone. | ||||
|---|---|---|---|---|
| Vignette Number (N=303)# | Varied Factors | Explanation of Varied Factors | % of Oncologists Recommending Chemotherapy^ | |
| Vignette 1 (n=34) | AM is a 72 year old female. She independently performs all activities of daily living and instrumental activities of daily living. She denies any memory problems or history of dementia. | -Younger -No functional impairment -No cognitive impairment |
97% | Multi-agent: 63% Monotherapy: 38% |
| Vignette 2 (n=39) | BL is a 72 year old female. She independently performs all activities of daily living but requires assistance with some instrumental activities of daily living including housekeeping and grocery shopping. She has had 3 falls in the past 6 months, and sustained an injury requiring an emergency room visit during one episode. She denies any memory problems or history of dementia. | -Younger -Functional impairment -No cognitive impairment |
74% | Multi-agent: 41% Monotherapy: 59% |
| Vignette 3 (n=31) | CK is a 72 year old female. She independently performs all activities of daily living and most instrumental activities of daily living. She requires assistance with managing household finances due to memory problems. Cognitive testing is performed and her cognition is found to be impaired (MMSE 15)**. | -Younger -No functional impairment -Cognitive impairment |
61% | Multi-agent: 39% Monotherapy: 61% |
| Vignette 4 (n=27) | DJ is a 72 year old female. She independently performs all activities of daily living but requires assistance with some instrumental activities of daily living including housekeeping, grocery shopping, and managing finances. She has had 3 falls in the past 6 months, and sustained an injury requiring an emergency room visit during one episode. Cognitive testing is performed and her cognition is found to be impaired (MMSE 15)**. | -Younger -Functional impairment -Cognitive impairment |
56% | Multi-agent: 13% Monotherapy: 87% |
| Vignette 5 (n=39) | EK is an 84 year old female. She independently performs all activities of daily living and instrumental activities of daily living. She denies any memory problems or history of dementia. | -Older -No functional impairment -No cognitive impairment |
85% | Multi-agent: 38% Monotherapy: 62% |
| Vignette 6 (n=41) | FH is an 84 year old female. She independently performs all activities of daily living, but requires assistance with some instrumental activities of daily living including housekeeping and grocery shopping. She has had 3 falls in the past 6 months, and sustained an injury requiring an emergency room visit during one episode. She denies any memory problems or history of dementia. | -Older -Functional impairment -No cognitive impairment |
44% | Multi-agent: 18% Monotherapy: 82% |
| Vignette 7 (n=43) | GS is an 84 year old female. She independently performs all activities of daily living and most instrumental activities of daily living. She only requires assistance with managing household finances due to memory problems. Cognitive testing is performed and her cognition is found to be impaired (MMSE 15)**. | -Older -No Functional impairment -Cognitive impairment |
37% | Multi-agent: 19% Monotherapy: 81% |
| Vignette 8 (n=49) | HT is an 84 year old female. She independently performs activities of daily living but requires assistance with some instrumental activities of daily living including housekeeping, grocery shopping and managing finances. She has had 3 falls in the past 6 months, and sustained an injury requiring an emergency room visit during one episode. Cognitive testing is performed and her cognition is found to be impaired (MMSE 15)**. | -Older -Functional impairment -Cognitive impairment |
14% | Multi-agent: 0% Monotherapy: 100% |
Bolded item are characteristics that were varied systematically between vignettes.
Each physician was randomized to one vignette as part of the survey; two physicians did not provide a response
Doublet vs monotherapy answer may not add to 100% due to missing data
A Mini-Mental State Exam Score (MMSE) of 15 is indicative of problems with learning new information, recognizing close relatives, personality changes, and behavior disorders.
Figure 1.
Percentage of Oncologists who Recommended Chemotherapy by Varied Factors in Vignettes
For the patients for whom chemotherapy was recommended, doublet chemotherapy was preferred over monotherapy only for the vignette patient who was 72 years old without functional or cognitive impairment (63% vs. 38%). For the rest of the vignette-patients, monotherapy was strongly preferred.
Older age (84 years), impaired function, and cognitive impairment were all associated with the decision to not recommend chemotherapy (p’s<0.01 for all). For vignette-patients for whom chemotherapy was recommended, there was a significant relationship between older age and a higher likelihood of recommending single-agent therapy (p<0.01). There was also a significant association between impaired functional and cognitive status of the vignette-patient and the likelihood of recommendation for single-agent therapy (p’s<0.01).
There was no association found between physician beliefs about and confidence in caring for older adults with decision to treat with chemotherapy. Total summary scores of beliefs (Table 2) and confidence (Table 3) were not associated with chemotherapy decisions (decision to treat with chemotherapy or intensity of treatment in those for whom chemotherapy was recommended).
Multivariable Analyses
Oncologists’ demographic and practice characteristics were not associated with the decision to treat with chemotherapy (Table 5). Each varied patient characteristics were independently and strongly associated with the decision to give chemotherapy: younger age (adjusted OR: 5.01; 95% CI: 2.73–9.20), no cognitive impairment (5.42; 3.01–9.76), and no functional impairment (3.85; 2.12–7.00). Older age (adjusted OR: 3.22; 1.43–7.25), impaired cognition (3.13; 1.36–7.20), and functional impairment (2.48; 1.12–5.46) were independently associated with prescribing single-agent over multi-agent chemotherapy.
Table 5.
Multivariable Models Evaluating the Associations between Physician and Vignette Patient Characteristics with the Decision to Recommend Chemotherapy (Model A) and the Decision to Recommend Single Agent vs Combination Therapy (Model B)
| Variables | Adjusted Odds Ratio | 95% Confidence Interval |
|---|---|---|
|
Model A** Decision to Recommend Chemotherapy vs No Chemotherapy |
||
|
| ||
| Physician Characteristics | ||
|
| ||
| Age (years) | 1.00 | 0.93–1.08 |
|
| ||
| Gender | ||
| Female | 1 (ref) | |
| Male | 0.85 | 0.43–1.66 |
|
| ||
| Race | ||
| Non-white | 1 (ref) | |
| White | 0.76 | 0.39–1.46 |
|
| ||
| Number of years in practice | 1.01 | 0.94–1.09 |
|
| ||
| Number of patients seen per day | 1.01 | 0.97–1.06 |
|
| ||
| Number of days per week seeing patient | 1.24 | 0.87–1.76 |
|
| ||
| Vignette Patient Characteristics | ||
|
| ||
| Age (years) | ||
| 72 | 5.01* | 2.73–9.20 |
| 84 | 1 (ref) | |
|
| ||
| Cognitive impairment | ||
| No | 5.42* | 3.01–9.76 |
| Yes | 1 (ref) | |
|
| ||
| Functional impairment | ||
| No | 3.85* | 2.12–7.00 |
| Yes | 1 (ref) | |
|
| ||
|
Model B^ Decision to Recommend Single Agent vs Combination Therapy |
||
|
| ||
| Physician Characteristics | ||
|
| ||
| Age (years) | 1.01 | 0.92–1.11 |
|
| ||
| Gender | ||
| Female | 1 (ref) | |
| Male | 1.00 | 0.39–2.60 |
|
| ||
| Race | ||
| Non-white | 1 (ref) | |
| White | 1.28 | 0.54–3.08 |
|
| ||
| Number of years in practice | 1.00 | 0.91–1.10 |
|
| ||
| Number of patients seen per day | 1.01 | 0.95–1.08 |
|
| ||
| Number of days per week seeing patient | 0.75 | 0.46–1.24 |
|
| ||
| Vignette Patient Characteristics | ||
|
| ||
| Age (years) | ||
| 72 | 1 (ref) | |
| 84 | 3.22* | 1.43–7.25 |
|
| ||
| Cognitive impairment | ||
| No | 1 (ref) | |
| Yes | 3.13* | 1.36–7.20 |
| Functional impairment | ||
| No | 1 (ref) | |
| Yes | 2.48* | 1.12–5.46 |
p<0.05
Model A, n=279; 26 observations not included due to missing information for response and exploratory variables
Model B, n=161; model includes only those observations where chemotherapy was recommended
DISCUSSION
In this study, we found that community oncologists incorporate patient age, functional impairment, and cognitive impairment into decision making for cancer treatment for older adults. Despite the high prevalence of cognitive and functional decline in older adults with cancer,4 ≤25% of community oncologists rated themselves as “very confident” in assessment and interventions for function, falls, and dementia. To our knowledge, this is the first study to show that, while only a minority of community oncologists feels confident in assessing and intervening on geriatric issues, the majority utilize this information in clinical decision making. However, this study also shows that there is significant variability in how geriatric issues are incorporated into decision making for older patients who are not clearly fit or frail.
Older age was independently associated with chemotherapy decisions, which may result from limited evidence of the risks and benefits of chemotherapy for older patients. For advanced pancreatic cancer, multi-drug chemotherapy regimens (e.g., FOLFIRINOX, gemcitabine/nab-paclitaxel) have shown survival benefits.28–30 The phase III trial of FOLFIRINOX vs. gemcitabine alone only included patients with an ECOG score of 0 or 1 and excluded those aged 76 and older28 with age over 65 years being significantly associated with worse survival.29 Although the phase III trial of gemcitabine/nab-paclitaxel vs. gemcitabine alone did not have an upper age limit (42% of patients enrolled were ≥65 years with only 10% of patients aged 75 and older), older age was associated with worse survival.31 In addition, the grade 3–4 toxicity rate for these regimens in the clinical trial population is over 50%.28–31 Toxicities are more severe and prevalent in the non-clinical trial population; in one study of 46 patients who received FOLFIRINOX, 54% were hospitalized for sepsis and 7% died from treatment.32 Hesitancy to provide multi-agent chemotherapy regimens to older patients, even those who are fit, likely stem from oncologists’ concerns about the ability of older adults to tolerate these regimens.33 Conversely, many oncologists continue to offer single-agent regimens to older patients with cognitive or functional impairments (often unrecognized without formal GA)15 despite modestbenefit. This study demonstrates that lack of evidence-based data to support cancer treatment plans in older patients leads to significant variability in treatment decision making.21,34–37
In this study, physician beliefs about or confidence in evaluation of management of age-related health issues did not influence chemotherapy decisions. However, the majority of oncologists believe that geriatric training is essential for the care of older cancer patients and would appreciate additional training in age-related topics. The majority of oncologists reported lower levels of confidence in assessing and intervening in certain geriatric syndromes—particularly dementia, functional decline, and risk for falls—precisely the areas that were found in the vignettes to influence treatment choices. These results mirror those from other studies. Among 758 primary care physicians, there was significant interest in learning more about dementia, urinary incontinence, and functional assessment.17 A study by Maggiore et al. investigated perceptions towards geriatrics among University of Chicago hematology/oncology fellows.16 Under-recognition of geriatric syndromes was identified as a gap in knowledge, as well as under-appreciation of the complexity of geriatric oncology cases. The majority perceived a lack of dedicated formal instruction on older patients with cancer during their fellowship. In a study by Moy et al., oncologist members of American Society of Clinical Oncology (ASCO) reported that the mandatory integration of key principles of geriatrics into oncology training was a high priority.14 The investigators made recommendations to include geriatric training in the fellowship curriculum and to develop geriatric oncology modules for maintenance of certification training.
Only 23% of community oncologists report using standardized GA tools in clinical practice. GA assists with the capture of age-related factors (such as cognitive impairment and functional status) known to affect morbidity and mortality in older patients with cancer that often are not recognized in clinical practice.15,22,38 In addition, GA has been shown to predict tolerance to treatment and overall survival, and specific variables captured by GA can predict chemotherapy toxicity in older cancer patients.5,26,39,40 Consequently, multiple guidelines, including the National Comprehensive Cancer Network (NCCN) guidelines, support the use of a GA in older patients with cancer to identify patients at risk for adverse outcomes.41 Falls and cognitive impairment are associated with chemotherapy toxicity in older patients.5,26 Although GA has demonstrated feasibility in the clinical oncology setting,42–45 oncologists have been slow to adopt GA, which may reflect lack of knowledge, training, and systematic barriers.
In this study, GA information (e.g., IADL impairments, falls, low MMSE score indicating significant cognitive impairment) when provided in vignettes was utilized to guide cancer treatment recommendations. Other studies have demonstrated that GA information can influence an oncologist’s treatment decisions in older cancer patients.46–48 In six of the ten studies in a systematic review by Hamaker et al., the initial cancer treatment plan was modified in 39% of patients after GA evaluation.47 Non-oncological interventions based on the GA were recommended for a median of 83% of patients.47 Non-oncological interventions included nutritional interventions, further evaluation and management of cognitive status, interventions for mobility and falls, as well as interventions for minimizing polypharmacy.47 Oncologists use of geriatric factors in treatment decisions for patients in the vignettes, despite their limited confidence in assessing for functional and cognitive issues in clinical practice, suggest the importance of routine use of GA in clinical practice to guide management decisions for cancer treatment and non-oncological interventions.
Limitations should be considered when evaluating the results of this study. This was a decision making study using hypothetical vignettes, not decisions for real patients. Nevertheless, studies have shown that decisions made for vignettes were highly correlated with decisions made during patient encounters.18,49,50 Use of vignettes can help understand decision making processes that may not be easily studied in routine practice due to ethical or practical considerations.18,49,50 Systematic control of variables of interest provides insight into the specific role of these selected patient factors in the decision to initiate chemotherapy, but does limit inferences for actual practice. While the response rate for the survey was higher than that of other studies, it was still just over 60%. Because oncologists completed the baseline survey as part of the recruitment procedures for geriatric oncology trials, oncologists who participated may be more sensitive to geriatric issues than those who did not participate. We did not collect detailed information on practice characteristics (e.g., access to geriatricians). Despite limitations, this study has a significant strength in that it involved community oncologists from different practices and regions of the country, which improves generalizability.
CONCLUSIONS
With the use of randomized vignettes, we found chronologic age was associated with treatment decisions. Despite their lack of confidence in certain areas of geriatric assessment and evaluation, the oncologists incorporated geriatric factors into treatment decision making. Because the current investigation was nested in larger, ongoing multi-site geriatric oncology studies, future research will examine community oncologists’ decision making for treatment of “real-world” older patients recruited into the trials. Further work is necessary to evaluate and improve geriatrics education for oncologists. As our population ages, it is increasingly important for oncologists to be able recognize geriatric issues so that appropriate evidence-based treatment is provided to those patients who will be helped and not harmed.
Acknowledgments
Supported in part by: The work was also funded through a Patient-Centered Outcomes Research Institute (PCORI) Program contract (4634), UG1 CA189961 from the National Cancer Institute, and R01 CA177592 from the National Cancer Institute. This work was made possible by the generous donors to the WCI geriatric oncology philanthropy fund. All statements in this report, including its findings and conclusions, are solely those of the authors, do not necessarily represent the official views of the funding agencies, and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Footnotes
Author Contributions:
Study Conception and Design: Mohile, Magnuson, Pandya, Velarde, Hurria, Heckler, Dale
Data Acquisition: Mohile, Magnuson, Velarde, Hurria, Wells, Plumb, Gilmore, Hopkins, Liu, Peri, Dale
Data Analysis: All authors
Manuscript preparation: All authors
Manuscript approval: All authors
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32(24):2587–2594. doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohile SG, Fan L, Reeve E, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29(11):1458–1464. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101(17):1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34(20):2366–2371. doi: 10.1200/JCO.2015.65.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wildes TM, Depp B, Colditz G, Stark S. Fall-risk prediction in older adults with cancer: an unmet need. Support Care Cancer. 2016;24(9):3681–4. doi: 10.1007/s00520-016-3312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quipourt V, Jooste V, Cottet V, Faivre J, Bouvier AM. Comorbidities alone do not explain the undertreatment of colorectal cancer in older adults: a French population-based study. J Am Geriatr Soc. 2011;59(4):694–698. doi: 10.1111/j.1532-5415.2011.03334.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill CB, Baxi SS, Atoria CL, et al. Treatment-related toxicities in older adults with head and neck cancer: A population-based analysis. Cancer. 2015;121(12):2083–2089. doi: 10.1002/cncr.29262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1(1):88–96. doi: 10.1001/jamaoncol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dale W, Mohile SG, Eldadah BA, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst. 2012;104(8):581–589. doi: 10.1093/jnci/djs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurria A, Naylor M, Cohen HJ. Improving the quality of cancer care in an aging population: recommendations from an IOM report. JAMA. 2013;310(17):1795–1796. doi: 10.1001/jama.2013.280416. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029–1036. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurria A, Balducci L, Naeim A, et al. Mentoring junior faculty in geriatric oncology: report from the Cancer and Aging Research Group. J Clin Oncol. 2008;26(19):3125–3127. doi: 10.1200/JCO.2008.16.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moy B, Flaig TW, Muss HB, Clark B, Tse W, Windham TC. Geriatric oncology for the 21st century: a call for action. Journal of oncology practice / American Society of Clinical Oncology. 2014;10(4):241–243. doi: 10.1200/JOP.2013.001333. [DOI] [PubMed] [Google Scholar]
- 15.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 16.Maggiore RJ, Gorawara-Bhat R, Levine SK, Dale W. Perceptions, attitudes, and experiences of hematology/oncology fellows toward incorporating geriatrics in their training. J Geriatr Oncol. 2014;5(1):106–115. doi: 10.1016/j.jgo.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Robinson BE, Barry PP, Renick N, Bergen MR, Stratos GA. Physician confidence and interest in learning more about common geriatric topics: a needs assessment. J Am Geriatr Soc. 2001;49(7):963–967. doi: 10.1046/j.1532-5415.2001.49188.x. [DOI] [PubMed] [Google Scholar]
- 18.Campbell KH, Smith SG, Hemmerich J, et al. Patient and provider determinants of nephrology referral in older adults with severe chronic kidney disease: a survey of provider decision making. BMC nephrology. 2011;12:47. doi: 10.1186/1471-2369-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilburt JC, Miller FG, Jenkins S, et al. Factors that influence practitioners’ interpretations of evidence from alternative medicine trials: a factorial vignette experiment embedded in a national survey. Med Care. 2010;48(4):341–348. doi: 10.1097/mlr.0b013e3181ca3ee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunins HV, Sohler NL, Roose RJ, Cunningham CO. HIV provider endorsement of primary care buprenorphine treatment: a vignette study. Family medicine. 2009;41(10):722–728. [PMC free article] [PubMed] [Google Scholar]
- 21.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26(15):2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 22.Mohile SG, Velarde C, Hurria A, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw. 2015;13(9):1120–1130. doi: 10.6004/jnccn.2015.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson M. Chemotherapy treatment decision making by professionals and older patients with cancer: a narrative review of the literature. Eur J Cancer Care (Engl) 2012;21(1):3–9. doi: 10.1111/j.1365-2354.2011.01294.x. [DOI] [PubMed] [Google Scholar]
- 24.Wan-Chow-Wah D, Monette J, Monette M, et al. Difficulties in decision making regarding chemotherapy for older cancer patients: A census of cancer physicians. Crit Rev Oncol Hematol. 2011;78(1):45–58. doi: 10.1016/j.critrevonc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Karuturi M, Wong ML, Hsu T, et al. Understanding cognition in older patients with cancer. J Geriatr Oncol. 2016;7(4):258–269. doi: 10.1016/j.jgo.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 27.Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013;4(3):227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 29.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31(1):23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 30.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. The oncologist. 2015;20(2):143–150. doi: 10.1634/theoncologist.2014-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amireault C, Beaudet J, Gaudet G, NR, Ayoub J, Letourneau R. FOLFIRINOX in the real-world setting: the multicentric experience of six Canadian institutions. ASCO Meeting. 2014;32(suppl 3):367. [Google Scholar]
- 33.Stone ME, Lin J, Dannefer D, Kelley-Moore JA. The Continued Eclipse of Heterogeneity in Gerontological Research. J Gerontol B Psychol Sci Soc Sci. 2017;72(1):162–167. doi: 10.1093/geronb/gbv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry MF, Worni M, Pietrobon R, D’Amico TA, Akushevich I. Variability in the treatment of elderly patients with stage IIIA (N2) non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8(6):744–752. doi: 10.1097/JTO.0b013e31828916aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright J, Doan T, McBride R, Jacobson J, Hershman D. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer. 2008;98(7):1197–1203. doi: 10.1038/sj.bjc.6604298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alliot C. Undertreatment of breast cancer in elderly women: contribution of a cancer registry. J Clin Oncol. 2005;23(21):4800–4801. doi: 10.1200/JCO.2005.01.4241. author reply 4801–4802. [DOI] [PubMed] [Google Scholar]
- 37.Lee IH, Hayman JA, Landrum MB, et al. Treatment recommendations for locally advanced, non-small-cell lung cancer: the influence of physician and patient factors. Int J Radiat Oncol Biol Phys. 2009;74(5):1376–1384. doi: 10.1016/j.ijrobp.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohile S, Dale W, Hurria A. Geriatric oncology research to improve clinical care. Nat Rev Clin Oncol. 2012;9(10):571–578. doi: 10.1038/nrclinonc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29(27):3620–3627. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 40.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurria A, Wildes T, Blair SL, et al. Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(1):82–126. doi: 10.6004/jnccn.2014.0009. [DOI] [PubMed] [Google Scholar]
- 42.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 43.Hurria A, Akiba C, Kim J, et al. Reliability, Validity, and Feasibility of a Computer-Based Geriatric Assessment for Older Adults With Cancer. J Oncol Pract. 2016;12(12):e1025–e1034. doi: 10.1200/JOP.2016.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCleary NJ, Wigler D, Berry D, et al. Feasibility of computer-based self-administered cancer-specific geriatric assessment in older patients with gastrointestinal malignancy. Oncologist. 2013;18(1):64–72. doi: 10.1634/theoncologist.2012-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol. 2014;5(3):245–251. doi: 10.1016/j.jgo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29(27):3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 47.Hamaker ME, Schiphorst AH, ten Bokkel Huinink D, Schaar C, van Munster BC. The effect of a geriatric evaluation on treatment decisions for older cancer patients--a systematic review. Acta Oncol. 2014;53(3):289–296. doi: 10.3109/0284186X.2013.840741. [DOI] [PubMed] [Google Scholar]
- 48.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. J Geriatr Oncol. 2016;7(4):242–248. doi: 10.1016/j.jgo.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283(13):1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 50.Peabody JW, Luck J, Glassman P, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141(10):771–780. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]