Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that controls numerous virulence factors through intercellular signals. This bacterium has two quorum-sensing systems (las and rhl), which act through the intercellular signals N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL), respectively. P. aeruginosa also produces a third intercellular signal that is involved in virulence factor regulation. This signal, 2-heptyl-3-hydroxy-4-quinolone [referred to as the Pseudomonas quinolone signal (PQS)], is a secondary metabolite that is part of the P. aeruginosa quorum-sensing hierarchy. PQS can induce both lasB (encodes LasB elastase) and rhlI (encodes the C4-HSL synthase) in P. aeruginosa and is produced maximally during the late stationary phase of growth. Because PQS is an intercellular signal that is part of the quorum-sensing hierarchy and controls multiple virulence factors, we began basic studies designed to elucidate its biosynthetic pathway. First, we present data that strongly suggest that anthranilate is a precursor for PQS. P. aeruginosa converted radiolabeled anthranilate into radioactive PQS, which was bioactive. We also found that an anthranilate analog (methyl anthranilate) would inhibit the production of PQS. This analog was then shown to have a major negative effect on elastase production by P. aeruginosa. These data provide evidence that precursors of intercellular signals may provide viable targets for the development of therapeutic treatments that will reduce P. aeruginosa virulence.
Pseudomonas aeruginosa is an opportunistic Gram-negative bacterium that causes serious infections in plants, animals, and humans. This pathogen is a common cause of nosocomial infections and is responsible for chronic lung infections in over 90% of cystic fibrosis patients (1, 2). The effectiveness of this organism as an opportunistic pathogen is due to an arsenal of well-regulated virulence factors. One method by which P. aeruginosa controls a large number of genes, many of which are important for virulence, is through the mechanism known as quorum sensing (for reviews see refs. 3 and 4). Quorum sensing systems use a cell-to-cell signal to sense cell density and subsequently activate a transcriptional regulator that induces specific genes. In P. aeruginosa, two signals function to activate the las and rhl quorum-sensing systems and thereby regulate an estimated 3–5% of the genes in the genome (5). The las and rhl signals are N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL), respectively (6, 7). In addition to these acylated homoserine lactone signals, P. aeruginosa also produces a secondary metabolite that functions as an intercellular signal. This unique signal was identified as 2-heptyl-3-hydroxy-4-quinolone and is referred to as the Pseudomonas quinolone signal (PQS) (8). PQS can activate the genes for both LasB elastase (lasB) and the C4-HSL synthase (rhlI) in P. aeruginosa (8, 9). This signal was also shown to be part of the quorum-sensing hierarchy in P. aeruginosa, inasmuch as its production and activity depended on LasR and RhlR, respectively (8). However, the production of this signal during the growth cycle occurs much later than the activation of the quorum-sensing cascade of which it is a part (9). This finding led to the conclusion that PQS may be involved in signaling at a time of increased cell stress.
It could be conservatively estimated that P. aeruginosa produces more than 50 secondary metabolites, many of which were discovered because they possess antibiotic activity (10). Antibiotic activity has not been demonstrated by PQS (8), even though it is very similar to a class of P. aeruginosa-produced antibiotics referred to as “pyo compounds” (4-hydroxyquinolines). The 4-quinolone base structure of PQS also suggests that it can be synthesized through a mechanism similar to that for pyo compounds. Previous studies on pyo compound synthesis provided evidence that they were produced through the condensation of anthranilate and a β-keto-fatty acid (11).
In this study, we set out to learn more about the synthesis of PQS. We began by determining that anthranilate is a precursor for PQS. We also show that an anthranilate analog caused a decrease in PQS production and elastase activity in P. aeruginosa. The data presented allow us to suggest that a fundamental metabolite such as anthranilate may be targeted to interrupt cell-to-cell signaling and thereby prevent virulence factor production.
Materials and Methods
Bacterial Strains, Plasmids, and Media.
The following P. aeruginosa strains were used in this study: PAO1 (wild type) (12), PAO-R1 (lasR) (13), PAO-JP2 (lasI, rhlI) (14), and PAO-JP3 (lasR, rhlR) (14). Plasmid pECP39 (8) encodes a truncated form of LasR that is capable of activating LasR-controlled genes in the absence of autoinducer. All strains were maintained in 10% (wt/vol) skim milk (Becton Dickinson) at −70°C. Freshly plated cells from skim milk stocks were used to begin all experiments. Unless indicated otherwise, P. aeruginosa strains were grown in peptone trypticase soy broth (15) supplemented with 200 μg/ml carbenicillin when necessary to maintain a plasmid. Liquid cultures were grown at 37°C and shaken at 250 rpm.
Experiments with Radioactive Anthranilate.
Freshly plated cells of P. aeruginosa strains PAO1, PAO-R1, PAO-JP2, and PAO-JP2 (pECP39) were used to inoculate 10-ml cultures for overnight growth. Overnight cultures were washed and used to inoculate a 10-ml subculture to an OD660 of 0.05. Subcultures were incubated until they reached the mid-logarithmic phase of growth and then were washed and added to flasks (starting OD660 = 0.05) containing 2 μCi of 12.5 mCi/mmol [ring-U-14C]anthranilic acid (Sigma-Aldrich). Cultures were then grown for 19 h in the presence of radiolabeled anthranilic acid. Total radioactivity of the cultures was calculated by measuring an aliquot of each culture at the end of the growth period. [Note: To measure radioactivity, triplicates of all samples were assayed for 2 min in 3 ml of Becton Dickinson ScintiVerse scintillation fluid with a Beckman LS5000TD scintillation counter, and results are presented as the mean ± one standard deviation (σn−1).] Cells were then harvested by centrifugation at 10,000 × g for 10 min at room temperature in a Sorvall SA-600 rotor. Cell pellets were resuspended in deionized H2O, and aliquots were assayed to determine the amount of radioactivity associated with the P. aeruginosa cells. Culture supernatants were then extracted twice with the use of 1 vol of HPLC-grade ethyl acetate. (Note: All ethyl acetate used in these studies was supplemented with glacial acetic acid to a final concentration of 435 μM.) After centrifugation as before, the organic and aqueous phases of the extract were placed in separate flasks and water was removed from the ethyl acetate phase with sodium sulfate. Aliquots from the aqueous and ethyl acetate fractions of the extract were assayed to determine the amount of radioactivity contained by each fraction. Ethyl acetate extracts were then dried by rotary evaporation and stored at −20°C until further analysis.
PQS Analysis and Purification by TLC.
All TLC plates (20 × 20 cm silica gel 60 F254) (EM Science) were soaked in 5% KH2PO4 and activated at 100°C for 1 h as described (8). Culture supernatant extracts were separated with the use of 17:2:1 methylene chloride/acetonitrile/dioxane as the solvent. This process was determined to be optimal for purification of PQS (8). Plates were then air dried and visualized under UV light and/or exposed to Kodak X-Omat x-ray film.
Purification of PQS from TLC plates was performed as described (8). Upon the completion of ethyl acetate extractions, samples were reconstituted in a small volume of a 1:1 mixture of acetonitrile and ethyl acetate and loaded onto TLC plates. After development, PQS was visualized with long-wave UV light and scraped from the plate. PQS was eluted from the silica gel with two 3-ml washes of 1:1 acetonitrile/ethyl acetate, and silica gel was removed by centrifugation at 10,000 × g for 10 min. The resulting sample was dried by rotary evaporation and reconstituted in 50 μl of 1:1 acetonitrile/ethyl acetate. This sample was applied again to a TLC plate and separated by two-dimensional TLC with the same solvent conditions as above. PQS was visualized and purified as described before. This technique has been shown to provide pure PQS (8).
For experiments that included methyl anthranilate (ICN), P. aeruginosa strain PAO1 was grown in the presence of increasing concentrations of methyl anthranilate. Culture supernatants were extracted with ethyl acetate, and extracts were loaded onto TLC plates. Plates were photographed under long-wave UV light, and photographs were analyzed with the use of the imagequant program (Molecular Dynamics) to determine relative amounts of PQS.
Elastase Assay.
Elastase activity produced by P. aeruginosa strains grown in the absence or presence of methyl anthranilate was measured by a modified elastin Congo red (ECR) assay (16). Freshly plated cells of P. aeruginosa strains PAO-JP3 and PAO1 were used to inoculate 10-ml cultures for overnight growth. Overnight cultures were washed and used to inoculate a subculture to an OD660 of 0.05. Subcultures were grown to the mid-logarithmic phase, subcultured again to an OD660 of 0.05, and added to flasks containing increasing concentrations of methyl anthranilate. Cultures were then grown for 20 h at 37°C with shaking. Culture supernatants were recovered by centrifugation at 10,000 × g for 10 min at room temperature in a Sorvall SA-600 rotor. Supernatants were then passed through a 0.45-μm HT Tuffryn syringe filter (Gelman Sciences). One milliliter of filtrate was added to 1 ml of ECR buffer (0.1 M Tris⋅HCl/1 mM CaCl2, pH 7.2) containing 20 mg of ECR (Sigma-Aldrich). Tubes were incubated for 3 h at 37°C with shaking at 250 rpm. Elastolytic activity results in the cleavage of ECR, which releases a soluble red pigment. After incubation, 0.2 ml of 0.12 M Na2EDTA was added to stop the reaction. Insoluble ECR was separated by centrifugation at 3,500 × g for 10 min, and the absorbance of the supernatant at 495 nm was measured. The A495 of samples incubated in the absence of culture filtrate was considered background activity, and this value was subtracted from all samples.
Results
Anthranilate Is a Precursor of PQS.
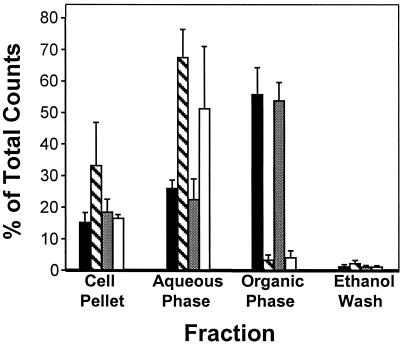
P. aeruginosa produces numerous secondary metabolites, including several 2-alkyl-4-hydroxyquinolines, which are referred to as “pyo compounds” (10). In previous work, it was proposed that 4-quinolones (such as PQS) are pyo compound precursors that result from the condensation of anthranilate and a β-keto-fatty acid (11). These results and the 4-quinolone base structure of PQS led us to believe that anthranilate is a PQS precursor. To begin to test this hypothesis, P. aeruginosa strains that produce PQS [PAO1 and PAO-JP2(pECP39)] (8) and non-PQS-producing strains (PAO-JP2 and PAO-R1) (8) were grown in the presence of 14C-ring-labeled anthranilate. The cells were harvested and culture supernatants were extracted with ethyl acetate. Radioactivity in the extracts, cell pellets, and culture supernatants from these cultures was counted to determine the percentage of radioactivity contained by each fraction. The percentages of radioactivity contained in the cell pellets from strains PAO1, PAO-JP2, and PAO-JP2 (pECP39) were similar (between 15% and 20%), and the cell pellet from strain PAO-R1 contained ≈33% of the total radioactivity added (Fig. 1). More interestingly, the majority of the total radioactivity added to the PQS-producing strains, PAO1 (56%) and PAO-JP2(pECP39) (54%), was found in the ethyl acetate extract fraction (Fig. 1). This finding was quite different from the percentage of total radioactivity found in the extracts from the quorum-sensing mutants, strains PAO-R1 and PAO-JP2. Culture supernatant extracts from strains PAO-R1 and PAO-JP2 contained an average of 3% and 4%, respectively, of the total radioactivity added (Fig. 1). The majority of radioactivity in these strains was found in the aqueous phase of the culture supernatant extraction, which contained 68% and 51% of the total radioactivity added to strains PAO-R1 and PAO-JP2, respectively (Fig. 1). These data indicate that quorum-sensing mutants (which do not produce PQS) do not convert anthranilate into compounds that can be extracted with ethyl acetate. This finding was a surprise because it has been proposed that numerous secondary metabolites produced by P. aeruginosa are derived from anthranilate (11). This hypothesis leads us to speculate that quorum sensing may be necessary for an early step in the utilization of anthranilate as a precursor for some secondary metabolites.
Figure 1.
Fate of [14C]anthranilate in P. aeruginosa. Strains PAO1 (solid bars), PAO-R1 (striped bars), PAO-JP2(pECP39) (stippled bars), and PAO-JP2 (unfilled bars) were grown in the presence of [14C]anthranilate. Cultures were centrifuged, and culture supernatant was extracted with acidified ethyl acetate. The percentage of total radioactivity contained by cell pellets and the aqueous and organic phases of culture supernatant extracts was determined. Data are the mean + σn−1 of triplicate counts from three separate experiments and are presented as the percentage of total radioactivity added to the cultures. As a control to ensure complete recovery of radioactivity, the empty culture flasks were washed with ethanol, which was then assayed for radioactivity.
To determine whether the radiolabeled anthranilate was actually being converted to PQS, we examined the ethyl acetate extracts on TLC plates. The extracts from strains PAO1, PAO-R1, PAO-JP2(pECP39), and PAO-JP2 were separated by TLC and visualized under long-wave UV light. The extracts from strains PAO1 and PAO-JP2 (pECP39) possessed a fluorescent blue spot characteristic of PQS (Rf = 0.6), whereas extracts from strains PAO-R1 and PAO-JP2 produced no substance that was similar to PQS (data not shown). These results were in agreement with results from other PQS bioassays (8). The position of PQS on the TLC plates was marked, and plates were then exposed to x-ray film for 24 h. Multiple radiolabeled compounds were evident on the autoradiograph (Fig. 2). It is interesting to note here that very little (if any) radiolabeled anthranilate was present in the extracts. Control experiments showed that ≈73% of anthranilate added to culture media will partition into the organic phase of an ethyl acetate extract (data not shown). Therefore, had there been unused radiolabeled anthranilate, one would expect to find it in the ethyl acetate extracts of culture supernatants, which was not the case. When comparing lane 5 of Fig. 2, which contains only radiolabeled anthranilate, with lanes 1–4, it is apparent that the vast majority (or all) of the added radiolabeled anthranilate was altered by the growing cells. More importantly, when the autoradiograph and the TLC plate were aligned, the PQS on the TLC plate corresponded exactly to a radiolabeled compound on the autoradiograph (Rf = 0.6) (see arrowheads in Fig. 2). This radiolabeled compound was purified and shown to be active in our PQS bioassay (8) (data not shown). These results show that the radiolabeled anthranilate had been converted into PQS. This finding led us to conclude that anthranilate is a precursor of the intercellular signal, PQS. A proposed synthesis pathway for PQS is presented in Fig. 3.
Figure 2.
Autoradiograph of extracts from P. aeruginosa strains grown with [14C]anthranilic acid. Extracts from 5-ml cultures of P. aeruginosa strains were loaded onto the TLC plate as follows: lane 1, strain PAO1; lane 2, strain PAO-R1; lane 3, strain PAO-JP2(pECP39); lane 4, strain PAO-JP2; lane 5, [14C]anthranilic acid (0.067 μCi). PQS is indicated by arrowheads. Strains were grown for 19 h in the presence of 2 μCi of [14C]anthranilic acid. Cell-free supernatants were extracted with ethyl acetate and separated by TLC as described in Materials and Methods.
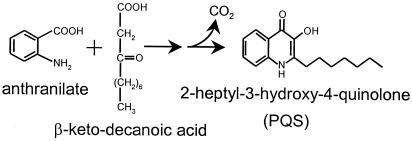
Figure 3.
Proposed synthesis scheme for PQS. In this synthetic pathway, anthranilate and β-ketodecanoic acid are condensed in a multistep reaction that would produce PQS. A one-carbon unit must be released at some stage of the reaction, which is indicated by CO2.
An Anthranilate Analog Represses PQS Production.
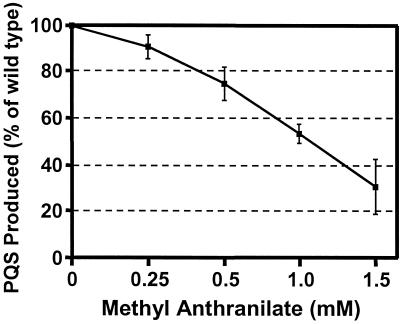
The inhibition or blocking of cell-to-cell signaling by bacteria is an obvious target area for the development of drugs that will reduce bacterial virulence. The identification of anthranilate as a likely precursor of PQS provided us the opportunity to attempt to interfere with PQS synthesis with the use of an anthranilate analog. Previous studies in Escherichia coli have shown that anthranilate analogs can inhibit anthranilate synthase activity (17, 18). We therefore decided to examine the effect of methyl anthranilate on PQS production by P. aeruginosa. P. aeruginosa strain PAO1 was grown in the presence of increasing amounts of methyl anthranilate, and culture supernatants were extracted with ethyl acetate and separated on TLC plates. PQS was visualized under long-wave UV light and photographed to quantify PQS densitometrically (see Materials and Methods). Our results showed that the amount of PQS present in culture supernatants decreased as the concentration of methyl anthranilate increased (Fig. 4). It is also important to note here that the growth of cultures was not affected by the presence of methyl anthranilate at the concentrations used in these experiments (data not shown). Taken together, these data indicated that the production of PQS was inhibited by the anthranilate analog methyl anthranilate.
Figure 4.
An anthranilate analog partially blocks PQS production. PQS production was monitored in P. aeruginosa strain PAO1 grown in the presence of methyl anthranilate. Extracts from cultures grown in the presence of increasing amounts of methyl anthranilate were separated by TLC, and PQS was quantified by computer densitometry. Data are the mean ± σn−1 from three separate experiments and are reported as a percentage of PQS produced by the wild-type P. aeruginosa strain PAO1 grown without methyl anthranilate.
Methyl Anthranilate Decreases P. aeruginosa Elastase Activity.
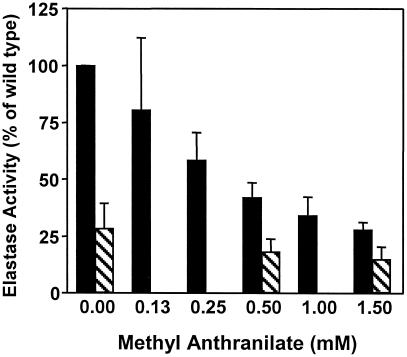
PQS has been shown to induce the lasB elastase gene in P. aeruginosa (8). We hypothesized that a reduction in the amount of PQS produced by P. aeruginosa, as shown in the experiments above, would lead to a concomitant reduction in elastase production. To determine whether this reduction in elastase production had occurred, elastase activity from P. aeruginosa grown in the presence of methyl anthranilate was determined. Cell-free supernatants from cultures grown in the presence of increasing concentrations of methyl anthranilate were assayed for elastase activity as described in Materials and Methods. The results of this experiment showed that increasing concentrations of methyl anthranilate caused a concentration-dependent decrease in elastase activity produced by P. aeruginosa strain PAO1 (Fig. 5). The elastase activity produced by cells in the presence of 1.5 mM methyl anthranilate was similar to the minimal amount produced by P. aeruginosa strain PAO-JP3, a lasR, rhlR double mutant that synthesizes no LasB elastase (14). These exciting results show that a compound that is an analog of a precursor for a cell-to-cell signal can inhibit the synthesis of the signal molecule and cause a decrease in the expression of a virulence factor controlled by the signal molecule. The implications of this finding are discussed below.
Figure 5.
Methyl anthranilate inhibits elastase production by P. aeruginosa. Elastase activities were determined for cell-free supernatants of P. aeruginosa strains PAO1 (solid bars) and PAO-JP3 (striped bars) grown in the presence of increasing amounts of methyl anthranilate. Activity is reported as a percentage of activity seen from the wild-type P. aeruginosa strain PAO1 grown without methyl anthranilate. Data are the mean + σn−1 from duplicate assays performed during three separate experiments.
Discussion
A common theme in secondary metabolite synthesis is the condensation of primary metabolites to form compounds that can then be enzymatically altered to obtain different activities. We have determined that the primary metabolite anthranilate is a precursor for the P. aeruginosa intercellular signal PQS. We presented data that showed that radiolabeled anthranilate is converted to PQS and have suggested a possible synthesis pathway for PQS (Figs. 1–3). It is interesting to note that the majority of radiolabeled anthranilate added to growing cultures of P. aeruginosa strains that produce PQS was converted into compounds that could be extracted by ethyl acetate (Fig. 1). This finding suggests that exogenous anthranilate enters specific pathways responsible for the production of extracellular secondary metabolites, which is surprising when one considers that anthranilate is an intermediate in the tryptophan synthetic pathway. Had the exogenous anthranilate been incorporated into tryptophan, it would have been found in proteins, which are usually not soluble in organic solvents such as ethyl acetate. In cultures of quorum-sensing mutants that do not make PQS, the radiolabeled anthranilate partitioned to the aqueous phase of the organic extracts. These data suggest that exogenous anthranilate is metabolized differently in quorum-sensing mutants that do not make PQS.
Anthranilate has been proposed or shown to be a precursor for numerous secondary metabolites in P. aeruginosa (10, 11), and this finding is illustrated by the presence of several radioactive compounds in culture supernatant extracts (Fig. 2). However, it is interesting to point out that PQS appeared to be one of the major end products (constituting ≈12% of radioactivity in the extract) for exogenous anthranilate (Fig. 2). Essar et al. (19) speculated that some anthranilate synthases may be a part of multienzyme complexes that cannot accept exogenous anthranilate. If this hypothesis were true, exogenous anthranilate would not be incorporated into all compounds synthesized from anthranilate. The exogenous anthranilate would only enter into certain synthetic pathways, which could explain why a large portion of the radiolabeled anthranilate was found in a relatively small number of compounds. Alternatively, the addition of anthranilate could cause cells to up-regulate the production of specific secondary metabolites that contain anthranilate. Nevertheless, P. aeruginosa converted a large portion of radiolabeled anthranilate into exported compounds that were extracted by ethyl acetate, and one of these compounds was PQS.
One approach for the development of novel antibacterial treatments is to first identify a viable target. Considering this approach, bacterial cell-to-cell signals have been an enticing point at which to aim. In E. coli, specific analogs of the Vibrio fischeri N-3-oxohexanoyl homoserine lactone signal and the P. aeruginosa 3-oxo-C12-HSL signal have been shown to compete for binding to LuxR and LasR, respectively, thereby acting as antagonists of LuxR or LasR activity (20, 21). It has also been found that a marine macroalga produced furanones (which are similar to acyl-homoserine lactone molecules) that can inhibit acyl-homoserine lactone signaling by Serratia liquefaciens and Vibrio fischeri (22). In addition, an antibacterial compound has been shown to have an effect on the production of an intercellular signal. Triclosan, a common antibacterial additive, inhibits FabI, an acyl carrier protein reductase that is important for the synthesis of C4-HSL and 3-oxo-C12-HSL by P. aeruginosa (23).
The studies presented here were started with the hope of learning more about metabolic events that lead to the control of virulence factor expression by intercellular signaling. Our long-term goal is to learn how to exploit bacterial cell-to-cell communication to develop treatments that will decrease bacterial virulence. With this goal in mind, we viewed our determination that anthranilate was a PQS precursor as an opportunity to alter the production of an intercellular signal (PQS). In E. coli, anthranilate is synthesized from chorismate through the enzymatic action of at least TrpE (see ref. 24 for a review). TrpE is an anthranilate synthase that can act alone or in conjunction with TrpG, which is a glutamine amidotransferase. TrpE contains a tryptophan-binding site that is responsible for feedback inhibition of the tryptophan pathway in E. coli. Two separate anthranilate synthase genes, trpE and phnA, have been identified and characterized in P. aeruginosa (19, 25). The trpE gene was shown to be part of the tryptophan synthetic pathway, and phnA was important for pyocyanin production (19, 25). To interrupt anthranilate metabolism in P. aeruginosa, we grew cells in the presence of an anthranilate analog, methyl anthranilate. Our results showed that this compound had a concentration-dependent negative effect on the production of PQS (Fig. 4). We presume that methyl anthranilate affects PQS production by competing with anthranilate in the proposed reaction in which anthranilate and β-ketodecanoic acid are condensed (see Fig. 3). Alternatively, methyl anthranilate could be converted into a compound that leads to feedback inhibition of an anthranilate synthase (or other enzyme) that is necessary for PQS production.
Considering the results in Fig. 4, the obvious question that arose was whether methyl anthranilate would have an effect on a PQS-controlled virulence factor. PQS has been shown to control lasB in P. aeruginosa (8), so we performed assays to determine the effect of methyl anthranilate on P. aeruginosa elastase activity. These studies showed that elastase activity from P. aeruginosa decreased as cells were grown in the presence of increasing concentrations of methyl anthranilate (Fig. 5). This finding was an exciting result because it meant that an analog of a precursor for a cell-to-cell signal could be used to inhibit the expression of a virulence factor controlled by the signal. Such a finding indicated that the PQS synthetic pathway may be a worthwhile target for the development of drugs that reduce P. aeruginosa virulence.
Intercellular signaling is required for P. aeruginosa virulence in several models of infection (26–28). It has also been shown that C4-HSL and 3-oxo-C12-HSL are produced in the lungs of mice (29) and cystic fibrosis patients (30) infected with P. aeruginosa. There is even evidence that indicates that an increase in the ratio of C4-HSL to 3-oxo-C12-HSL occurs during biofilm growth in vitro, and similar signal ratios were found in sputum from cystic fibrosis patients infected with P. aeruginosa (30). Those results add importance to a previous report that showed PQS can induce rhlI (encodes the C4-HSL synthase) in P. aeruginosa (9). Considering all of these findings in conjunction with the data presented in this report, the inhibition of PQS synthesis may help to hinder the chain of events that leads to the establishment of a chronic P. aeruginosa infection. As we learn more about the intercellular signaling systems of bacteria, it becomes apparent that multiple avenues of attack based on these systems can be pursued in the search for new and effective therapies for bacterial infections.
Acknowledgments
We thank S. L. McKnight for technical assistance and C. J. Smith and C. S. Pesci for help in manuscript preparation and thoughtful insight. This work was supported by research grants from the Cystic Fibrosis Foundation (Grant PESCI99I0), the American Lung Association of North Carolina, the North Carolina Biotechnology Center, and the National Institutes of Health (Grant R01-AI46682).
Abbreviations
- PQS
Pseudomonas quinolone signal
- 3-oxo-C12-HSL
N-(3-oxododecanoyl)-l-homoserine lactone
- C4-HSL
N-butyryl-l-homoserine lactone
- ECR
elastin Congo red
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Van Delden C, Iglewski B H. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch C, Hoiby N. Lancet. 1993;341:1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 3.Fuqua W C, Winans S C, Greenberg E P. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 4.Pesci E C, Iglewski B H. In: Cell-Cell Signaling in Bacteria. Dunny G, Winans S C, editors. Washington, DC: Am. Soc. Microbiol.; 1999. pp. 147–155. [Google Scholar]
- 5.Whiteley M, Lee K M, Greenberg E P. Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson J P, Passador L, Iglewski B H, Greenberg E P. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesci E C, Milbank J B J, Pearson J P, McKnight S, Kende A S, Greenberg E P, Iglewski B H. Proc Natl Acad Sci USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKnight S L, Iglewski B H, Pesci E C. J Bacteriol. 2000;182:2702–2708. doi: 10.1128/jb.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leisinger T, Margraff R. Microbiol Rev. 1979;43:422–442. doi: 10.1128/mr.43.3.422-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornforth J W, James A T. Biochem J. 1956;63:124–130. doi: 10.1042/bj0630124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holloway B W, Krishnapillai V, Morgan A F. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambello M J, Iglewski B H. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson J P, Pesci E C, Iglewski B H. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohman D E, Cryz S J, Iglewski B H. J Bacteriol. 1980;142:836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorn M J, Sokol P A, Iglewski B H. J Bacteriol. 1979;138:193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyed H S. J Biol Chem. 1960;235:1098–1102. [PubMed] [Google Scholar]
- 18.Held W A, Smith O H. J Bacteriol. 1970;101:209–217. doi: 10.1128/jb.101.1.209-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essar D W, Eberly L, Hadero A, Crawford I P. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer A L, Hanzelka B L, Eberhard A, Greenberg E P. J Bacteriol. 1996;178:2897–2901. doi: 10.1128/jb.178.10.2897-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline T, Bowman J, Iglewski B H, de Kievit T, Kakai Y, Passador L. Bioorg Med Chem Lett. 1999;9:3447–3452. doi: 10.1016/s0960-894x(99)00626-5. [DOI] [PubMed] [Google Scholar]
- 22.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P D, Kjelleberg S. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoang T T, Schweizer H P. J Bacteriol. 1999;181:5489–5497. doi: 10.1128/jb.181.17.5489-5497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols B P. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, Curtis R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, M, Schaecter A, Umbarger H E, editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 2638–2648. [Google Scholar]
- 25.Essar D W, Eberly L, Han C Y, Crawford I P. J Bacteriol. 1990;172:853–866. doi: 10.1128/jb.172.2.853-866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan M, Rahme L G, Sternberg J A, Tompkins R G, Ausubel F M. Proc Natl Acad Sci USA. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson J P, Feldman M, Iglewski B H, Prince A. Infect Immun. 2000;68:4331–4334. doi: 10.1128/iai.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darby C, Cosma C L, Thomas J H, Manoil C. Proc Natl Acad Sci USA. 1999;96:15202–15207. doi: 10.1073/pnas.96.26.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Song Z, Hentzer M, Andersen J B, Heydorn A, Mathee K, Moser C, Eberl L, Molin S, Hoiby N, et al. Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]
- 30.Singh P K, Schaefer A L, Parsek M R, Moninger T O, Welsh M J, Greenberg E P. Nature (London) 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]