Abstract
Alzheimer’s disease is an irreversible, progressive brain disorder that slowly destroys cognitive skills and the ability to perform the simplest tasks. More than 5 million Americans are afflicted with Alzheimer’s; a disorder which ranks third, just behind heart disease and cancer, as a cause of death for older people. With no real cure and in spite of enormous efforts worldwide, the disease remains a mystery in terms of treatment. Importantly, African Americans are two times as likely as Whites to develop late-onset Alzheimer’s disease and less likely to receive timely diagnosis and treatment. Dopamine function is linked to normal cognition and memory and carriers of the DRD2 Taq1A A1 allele have significant loss of D2 receptor density in the brain. Recent research has shown that A1 carriers have worse memory performance during long-term memory (LTM) updating, compared to non-carriers or A2-carriers. A1-carriers also show less blood oxygen level dependent (BOLD) activation in left caudate nucleus which is important for Long Term Memory (LTM) updating. This latter effect was only seen in older adults, suggesting magnification of genetic effects on brain functioning in the elderly. Moreover, the frequency of the A1 allele is 0.40 in African-Americans, with an approximate prevalence of the DRD2 A1 allele in 50% of an African American subset of individuals. This is higher than what is found in a non-screened American population (≤28%) for Reward Deficiency Syndrome (RDS) behaviors. Based on DRD2 known genetic polymorphisms, we hypothesize that the DRD2 Taq1A A1 allele magnifies the risk of Alzheimer’s in aging African Americans. Research linking this high risk for Alzheimer’s in the African American population, with DRD2/ANKK1-TaqIA polymorphism and neurocognitive deficits related to LTM, could pave the way for novel, targeted pro-dopamine homeostatic treatment.
Keywords: Dopamine, Alzheimer’s Disease, Reward Deficiency Syndrome, DRD2 gene, Long-term Memory (LTM), Early Life Stress, African Americans
Introduction
Based on the current literature, we hypothesize that the DRD2 Taq1A A1 allele, heretofore referred to as A1, may magnify the risk of Alzheimer’s disease (AD) in aging African Americans. This hypothesis is based on growing scientific evidence linking the minor DRD2 allele with memory problems and with structural changes in reward centers of elderly patients. This hypothesis is presented to stimulate further research of this concept, which will provide genetic information that could result in precision medicine targeted to potentially high risk African Americans.
Following the initial work of Noble, Blum and associates showing the first association of the DRD2 A1 allele and severe alcoholism [1], there have been 4,374 articles published on this gene in Pubmed (7-30-17). A carrier of this A1 allele of the DRD2 gene may display many risky behaviors, neurological disorders, and cognitive impairment associated with altered circuitry, and these may be manifested in the form of impulsivity, decision-making problems, addiction, mood disorders and other reward deficiencies [2–16]. These clinical presentations include alcoholism, drug dependence (opioids, cocaine, nicotine, marijuana, glucose sedative – hypnotics); pathological gambling, internet gaming, post-traumatic stress disorder, inability to cope with stress, aggression, antisocial behavior; Parkinson Disease; spectrum disorder (ADHD, Tourette’s, Autism) magnification of aging, early onset intercourse, juvenile delinquency, anger issues, obesity, lower education status, higher hospitalization rates, higher mortality rates, suicide ideation, depression, anxiety disorder, anorexia nervosa, binge eating behavior, schizophrenia, paranoia, avoidant behavior, general cognition, temporal cognition, working memory and motivation problems.
DRD2 Gene and Polymorphisms
DA receptor D2, also known as DRD2, is a G protein-coupled receptor protein encoded by the DRD2 gene which encodes the D2 subtype of the DA receptor in humans. Two transcript variants encode different isoforms and a third variant that has been described are the result of alternative splicing of the gene. The Taq1A is a single nucleotide polymorphism (SNP) (rs: 1800497), originally thought to be located in the 3′-untranslated region of the DRD2 but has since been shown to be located within exon 8 of an adjacent gene, the ankyrin repeat and kinase domain containing 1 (ANKK1). Importantly, while there may be distinct differences in function, the mislocation of the Taq1A allele may be attributable to the ANKKI and the DRD2 being on the same haplotype or the ANKKI being involved in reward processing through a signal transduction pathway [17]. However, it is also possible that the ANKKI and the DRD2 gene polymorphisms may have distinct and different actions with regard to brain function [18] Presence of the A1+ genotype (A1/A1, A1/A2) compared to the A1− genotype (A2/A2) is associated with reduced receptor density [1,19–21]. This reduction causes hypodopaminergic functioning in the DA reward pathway. The Taq1 A allele is a predictive risk allele in families [22]. The association between ANKK1/DRD2 Taq1A A2/A2-genotype and higher novelty seeking and lower reward dependence was shown in men but not in women. Finally the binding potential of the DRD2 gene as reported by others suggest an altered dopamine release across the brain reward circuitry, with evidence that carriers of the DRD2 A1 allele are at high relapse risk for alcoholism compared to carriers of DRD2 A2 allele [23].
The neurological and neuropsychiatric manifestation of the DRD2 A1 allele in any given population is influenced by co-expressed enhancing or repressive genetic factors, chemical and psychosocial environment of individuals, their history, ancestry, and co-morbid medical conditions.
Alzheimer’s Disease
Alzheimer’s disease (AD) is an irreversible, progressive brain disorder that slowly destroys memory and cognitive skills, eventually impairing the ability to carry out the simplest tasks. In most people with Alzheimer’s, symptoms first appear in their mid-60s. Estimates vary, but experts suggest that more than 5 million Americans may have Alzheimer’s. AD is currently ranked as the sixth leading cause of death in the United States [24], but recent estimates indicate that the disorder may rank third, just behind heart disease and cancer, as a cause of death for older people. The March 2014 issue of Alzheimer’s and Dementia, reported that approximately 600,000 people aged ≥65 years with Alzheimer’s died in 2010, estimated to rise to 900,000 deaths in 2030 and to 1.6 million by 2050 [25]. Therefore, in 2050 the percentage of AD deaths of individuals ≥65 years will increase to 43% of this population compared to 32% for 2010. Moreover, a concurrent 2014 Neurology [25] article found that the actual number of deaths due to AD in people ≥75 years could be six times higher than the official count. The projected number of people age 65 and older (total and by age group) in the U.S. population with AD by 2050 is projected at 13.8 million [25–27]. Table 1 presents a number of facts regarding the epidemiology and societal costs of worldwide AD.
Table 1.
Worldwide Alzheimer’s disease:
|
The cost of AD Care:
|
Alzheimer’s in the United States
|
Alzheimer’s Incidence by Age and Ethnicity
Here, in addition to a thorough discussion of the current state of the disparity in identifying AD in different ethnic groups, we also review disparate genetic allelic states and social factors that render African Americans more susceptible than Whites to AD. We also review dopamine function in memory and in aging individuals. Based on evaluation of the literature, we propose that the abnormally high prevalence of a DRD2 minor allele previously associated with addiction, may account for the increased incidence of AD in African Americans.
Observations and Discussion
Awareness and Diagnosis of Alzheimer’s Disease in African Americans
It is puzzling that the rate of AD in the African-American population between the ages of 65–74 is 3.13 times more prevalent compared to Whites; is 1.82 times more prevalent between ages 75–84, and remains 1.95 (2 times) more prevalent by ages 85 and above [27,28]. Notably, although this health concern is increasing for African Americans, a study by Jett [29] suggested that many African-American communities consider AD to be a normal part of the aging process. Thus, the actual incidence may be higher and under-reported if dementia is accepted as a normal part of aging. Importantly, medical care professionals must themselves be aware of cultural attitudes, as well as the bias (cultural insensitivity) of cognitive tests, to properly diagnose AD in African Americans, to help bring focus to the incidence disparity and to advocate allocation of medical resources and attention to the care of the aging African-American patient. In 2002, the Alzheimer’s Association released an assessment of the so-called “silent epidemic” of dementia within the African-American community. It was reported that African Americans were not well represented at Memory Disorder Centers or Alzheimer’s Disease Centers. There is emerging recognition of the disproportionately high rate of Alzheimer’s disease within the African-American community [26] and an increasing attitude that dementia within the family unit must be met with care and support for patients and their caretakers alike. Still, many African American communities receive reduced treatment options because they do not self-report due to a lack of trust toward mental health care professionals, and their use of unconventional non-medical terms to describe the condition may not be understood by physicians. To assist in bridging the trust and communication gap between provider and patient, it may be important to adopt a more sensitive approach to capture the diagnosis. For example, Jett [29] has suggested to change the terms used during interactions: “instead of cognitive dysfunctioning, we can ask about mind slippage”.
Rovner et al. [30] reported that most African American subjects (56.7%) were unaware that African Americans were at higher risk for AD than Whites. They suggested that cultural diversity within older African Americans may contribute to disparities in the detection and treatment of AD in this high-risk population. Although this hypothesis paper focuses on the disparity of diagnosis and potential risk-conferring alleles in African-Americans, Table 2 shows that other ethnicities including Hispanics have disparate risks for Alzheimer’s.
Table 2.
Incidence of Alzheimer’s Disease by Age and Ancestry [26]
| Age of Onset (yrs.) | African American* | European American | Hispanic |
|---|---|---|---|
| 65–74 | 9.1% | 2.9% | 7.4% |
| 75–84 | 19.9% | 10.9% | 27.9% |
| 85+ | 58.6% | 30.2% | 62.9% |
African American includes people of African and Caribbean descent living in America whether or not born in the US.
Genetic Associations of Alzheimer’s Disease
Reitz et al. [31] studied 5,896 African Americans; 1,968 with AD, and 3,928 control participants 60 years or older, using datasets collected between 1989 and 2011 at multiple sites. The association of Alzheimer’s disease with genotyped and imputed single-nucleotide polymorphisms (SNPs) was assessed in case-control and in family-based data sets. They concluded that AD in African Americans was significantly associated with variants in rs115550680, allele = G of the ABCA7 (ATP-binding cassette transporter), with the APOE e4 allele and with certain other genes. However, many susceptibility loci associated with late onset Alzheimer’s disease in European Americans did not reach significance in African Americans even after multiple corrections. The most significant SNPs in African Americans differed from the top-ranked SNPs in Europeans or European Americans [31]. This is concomitant with decidedly higher incidences of AD in African Americans nationwide [26] and when compared to Whites in the same communities [32]. Along these lines, Logue et al. [33] carried a comprehensive genetic association study of AD in African Americans by analyzing a genome-wide set of 2.5 million imputed markers. Genetic risk association was observed with SNPs in Clusterin (CLU), phosphatidylinositol binding clathrin assembly protein (PICALM), bridging integrator 1 (BIN1), ephryn type A (EPHA1), membrane-spanning 4A (MS4A), ABCA7, and myeloid-associated antigen CD33 (CD33), although the effect direction for some SNPs and the most significant SNPs differed from findings in data sets consisting of Europeans. Interestingly, Ghani et al. [34] found an association of recessive long runs (ROHs) of homozygosity with AD among African-Americans. However, the researchers suggest that sequencing is required to uncover AD variants in these individuals. In terms of neurogenetic antecedents to cognitive functioning, although one study suggests the presence of 1 or 2 ApoE epsilon(ε)4 alleles is a determinant of AD risk in African-Americans and Whites [35]; others reported evidence suggesting the ApoE ε4 allele was not associated with cognitive functioning in African Americans diagnosed with AD [36]. Adjusting for sex and education, in the presence of ApoE(ε)4, the risk was approximately equal for African-Americans and Whites, whereas in the absence of ApoE(ε)4 the risk was 4- and 2-fold for African-Americans and Hispanics, respectively, compared to Whites. This allows inference that traditional risk associations associated with European Americans are not applicable to African Americans or Hispanics [37]. Our interpretation of these results do not allow for a simplistic explanation concerning the African American prevalence disparity as genetic associations with memory loss may not hold for African-American
Early Life Adversity and Cognition Disparity in Ethnic Groups
Barnes et al. [38] provided some evidence that in models stratified by ethnic groups, and adjusted for age and sex, early-life adversity was differentially related to cognitive decline in African Americans and Whites. They found in African Americans, unlike Whites, early-life adversity, both food deprivation and being thinner than average in early life, were associated with a slower rate of cognitive decline in older African Americans. It is not yet known if or how other life stressors such as the experience of violence, socioeconomic status, abuse, or neglect differentially impact different ethnic groups. Consistent with reports for hippocampal structures in Whites with AD [39], in 2001, Sencakova et al. [40] reported that hippocampi of African American AD patients were atrophic with respect to those of healthy African American subjects. Moreover, it was found that there were significant direct correlations between hippocampal volumes and performance on several different neuropsychological tests when patients and healthy subjects were combined. These findings are in agreement with that found for non-African American populations in Braverman et al. [41] who used 3T MRI with sophisticated NeuroQuant® software to measure both evoked potentials and memory/cognition in a mixed gender and age group of cognitively impaired patients. The study revealed hippocampal, central and temporal lobe atrophy, with delayed latency of evoked potentials marginally associated with temporal lobe atrophy. Additionally, reduced fractional anisotropy (FA) in frontal lobes correlated with aging, delayed P300 latency, and decreased visual and working memory. Moreover, the higher the P300 amplitude, the lower the bilateral atrophy, and the higher the immediate memory, the lower the central atrophy. Hippocampal atrophy negatively affected auditory memory, especially in males.
Structural magnetic resonance imaging (MRI) provides key biomarkers to predict onset and track progression of Alzheimer’s disease (AD). Most published reports of relationships between MRI variables and cognition in older adults include racially, ethnically, and socioeconomically homogenous samples. However, recent work by Zahodne et al. [42] using structural MRI as a predictor of late–life cognition compared African-Americans, Hispanics and Whites. They found white matter hyper-intensity (WMH) volumes associated with worse language and speed i.e., executive functioning among African Americans, but not among non-Hispanic Whites. In addition, they also found that larger hippocampal volume was more strongly associated with better memory among non-Hispanic Whites compared with Hispanics.
Other work by Gibbons et al. [43] involving the impact of stress (environment) on cognitive life history strategies (LHS), genetic moderation and the role of discrimination of African-American adolescents helps dissects resilience related to risky behaviors. There is evidence that early risky/impulsive behavior, regardless of race or ethnicity, during critical periods of brain development can influence brain wiring and thus adult cognition and behavior. The role of stress and racial discrimination and African-American risk-taking behavior or development of resilience can be considered in the context of carrying the risk (“sensitivity”) alleles of 2 monoamine-regulating genes, the serotonin transporter gene (5HTTLPR) and the dopamine D4 receptor gene (DRD4). These alleles have been shown to mediate the impact of early stress and perceived racial discrimination in African-American adolescents on LHS cognition. These same parameters were not investigated in Whites or Hispanics. We are proposing that the combination of risky reward gene alleles, impacted by life conditions, could induce unwanted epigenetic effects that may confer risk for AD.
It is known that many genetic polymorphisms are linked to individual differences in cognitive performance [44]. For example, striatal dopamine functions, associated with cognitive performance, are linked to the DRD2 Taq A1 allele polymorphism. The role of dopamine and memory has been extensively explored and we provide an abridged synopsis in the following section of this article. A Pubmed search (04-01-17) using the search terms “DRD2 gene and Alzheimer’s” retrieved 15 publications loosely related to the topic, and “DRD2 gene and Alzheimer’s in African Americans” retrieved no results.
Dopamine Function and Cognition
Neuroscientists have investigated dopamine and memory for many years, and this information has filled textbooks and review articles [45–48]. In fact, the Nobel Prize in Medicine was awarded to Greengard and Kandel (2000) for their seminal studies of learning and memory, relating to dopamine signaling. Therefore, this section is not exhaustive of the topic, but rather allows for a brief summary to contextualize this review. Dopamine neurons are found in the arcuate nucleus of the hypothalamus, as well as the ventral mesencephalon from which they project to multiple targets in the forebrain. Although dopamine neurons constitute a small sample of the brain’s neuronal population (<1/100,000), they are involved in neuroendocrine regulation, mood, motivation, and psychological processes, such as memory [44].
Flood, et al. [49] had previously demonstrated that the formation of long-term memories was impaired by protein synthesis inhibitors which interfered with the biosynthetic pathways that underlie long-term memory formation. While the specific mechanisms are not clear, protein synthesis inhibitors that induced amnesia, also inhibited tyrosine hydroxylase activity and the conversion of tyrosine to dopamine and norepinephrine [49].
Subsequent research showed that neuropeptides and neurotransmitters influence the actions of dopamine. Vasopressin, a hypothalamic neuropeptide, facilitates memory through its interaction with dopamine in the amygdala, and serotonin in the dentate gyrus of the hippocampus [50]. Excitatory N-methyl-D-aspartate neurotransmitter enhances the release of dopamine in rat hippocampal neurons, suggesting a relationship between dopamine activity, synaptic long-term potentiation (LTP) in the hippocampus and long-term memory [51]. Likewise, reductions in acetylcholinesterase (AChE) in rat hippocampus and increased brain acetylcholine and serotonin levels improved learning and memory and were associated with LTP [52]. Lastly, spatial novelty lowered the threshold for LTP in rat CA1 hippocampal synapses, and this facilitation of LTP was dependent upon dopamine activity [53], a finding supported by rodent studies demonstrating that dopamine and norepinephrine release within the hippocampus (not cortex) is necessary for novel contextual learning independently of object recognition [54].
Dopaminergic Mechanisms of Memory
Seminal studies continue to be added to the literature strengthening the importance of dopamine and receptor subtypes in memory formation [55–66]. Aversive, long-term memories were recently shown to be mediated by activation of medial prefrontal cortex localized D1 and D5 receptor activation [67]. Yamagata et al. [68] found that distinct groups of neurons, in Drosophila, support reward for short and long-term memories. The traces of long-term memory, that is, the abstract memory that provides the rules or strategies that organize behavior over time, are stored in the prefrontal cortex (PFC) [69]. Otani et al. [69] have suggested that long-term synaptic plasticity might provide the cellular mechanism for this type of memory and that dopamine facilitates long-term, synaptic plasticity. Reichenbach, Hermann, Kahne et al. [70] reported that reduced dopamine signaling in alpha-synuclein deficient mice was associated with reduced memory consolidation. These results are consistent with other findings that blocking dopamine signaling, after learning, reduces memory consolidation in guinea pigs [71]. Work in Drosophila demonstrated the importance of a feedback circuit with reward signaling of dopamine neurons in the transformation of a short-term olfactory memory trace into a long-term memory [72]. Finally, Kim et al. [73] described a unique type of dopamine neuron in the monkey substantia nigra pars compacta that stores a stable representation of the past reward value of visual stimuli. The responses of these neurons are strengthened by learning, and are evoked by presentation of the visual stimuli, after the learning has been completed. Thus, this group of neurons supports learning and the retention of learned behavior.
Aging and Dopamine Function Decline
The role of neurogenetics in memory consolidation has been controversial [74]. Individual differences in cognitive performance have been related to genetic polymorphisms. The role of D2 in memory formation, and motivation is well documented in rodent studies which suggest that the D2 receptor surface expression is regulated by interacting proteins like the calcium sensor NCS-1 which can control motivation, exploration, synaptic plasticity, and memory formation [75–77]. The activity of striatal dopamine (DA), which influences cognitive function, is linked to the Taq1A polymorphism of the DRD2/ANKK1 gene. The A1 allele of this genetic polymorphism, in humans, relates to reduced density of striatal DA D2 receptors [1]. Recent research suggests that SNPs in dopamine receptor genes (DRD1, DRD2Taq1A A1, DRD3/Ser9Gly) may impair associative memory specifically, whereas SNPs that confer risk for AD induce general disruption of episodic memory in aging individuals [78].
The A1 allele of the Taq1A polymorphism was associated with an age-dependent reduction in memory performance, LTM updating, and reduced blood oxygen level dependent (BOLD) activation in the left caudate nucleus [79]. This result, which was only seen in older adults, suggests that effects of the Taq1A genetic polymorphism on memory functions are accentuated with age. Moreover, non-1A carriers demonstrated a positive association between BOLD activation of the caudate nucleus and memory updating, supporting the relevance of caudate activation to memory processes. These findings support an association between the DRD2/ANKK1-Taq1A genetic polymorphism and deficits in LTM, a relationship that is amplified in the aging brain.
In addition, studies by Papenberg, Backman, Nagel et al. [80] found that older adults with SNPs of the D2 and D3 receptors, as well as the dopamine transporter gene (DAT1), forgot more information than older individuals with genotypes associated with increased dopamine availability and receptors. This effect was not observed in younger individuals with the same SNPs, supporting the hypothesis that genetic effects on memory are magnified by aging. This hypothesis is consistent with Positron Emission Tomography (PET) research that demonstrated an age-related decline in the dopamine system [81], possibly linked to structural defects in reward centers of aged individuals carrying a dopamine minor alleles. Roussotte et al. showed that the DRD2 polymorphism rs1076560 predicted an increase in volume of the lenticular nucleus, hippocampus, and other brain regions associated with reward processing, and individuals with this SNP were more susceptible to cognitive decline and dementia [82,83]. They did not however separate this cohort based on ethnicity. In a study of aging, Matuskey et al. [84] have found an age-related decrease in the availability of D2 receptors in the striatum, while D3 receptor availability in this area remained constant or increased with age. Another factor that can change dopamine metabolism in the brain is the chronic exposure to drugs of abuse and chronic stress during early life. These adverse environmental factors can influence hippocampal neuron development and connectivity [85,86] and decrease dopaminergic neurons in the midbrain [87] persistently in adulthood. Therefore, it is plausible that a reduction of dopamine terminals of the hippocampus due to these adverse life experiences could interact with abnormal regional brain architecture due to genetic risks, to enhance memory loss in AD.
Prevalence of the DRD2 Taq A1 allele as a function of Ethnicity
A very important question and even criticism of the early work on the DRDD2 Taq A1 allele frequency in many ethnic groups, centers around the suggestion of Barr and Kidd [88] that allelic differences using samples from Whites do not adequately take into account stratification based on ethnicity, and as such reflect a lack of heterogeneity and inclusiveness in research samples studied. While the studies of Finish Alcoholics [89] American Indian alcoholics [90] and German Alcoholics [91] would support the view of Barr and associates [88], deeper scrutiny reveals that the issue of appropriately categorizing subjects according to severity of alcoholism or dependence was not fully addressed. It is important to realize that just carrying the DRD2 A1 allele by itself may not translate to, for example, alcoholism. We must take into account the environment, especially epigenetic impact on gene expression. Along these lines, Goldman and associates [90] found a very high frequency of the A1 allele in Cheyenne Indians and failed to show an association with this allele of the DRD2 gene in subjects with alcoholism and drug abuse upon subjective questioning. This finding is not surprising since others, Levy & Kunitz [92] found a 60% abstention (i.e. “they took an oath not to use drugs”) rate among native Americans compared to 25% for the general population. However, Arinami et al. [93] found a significantly higher frequency of the DRD2 A1 allele in severe alcoholics than in less severe alcoholics. In fact, other work by the same group found that 100% of the alcoholics carrying the A1/A1 genotype were in the severe category [93].
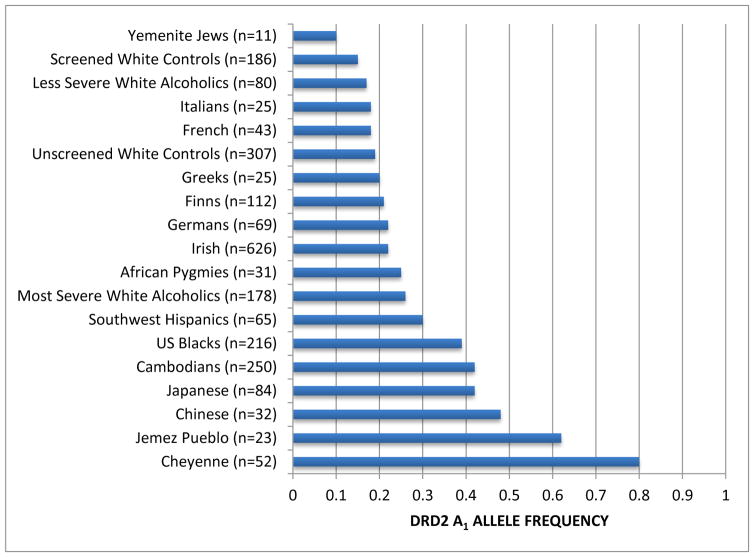
In order to appreciate the varying frequencies amongst various ethnic groups Barr & Kidd [88] produced a gene map revealing that for example, the frequency of the DRD2 A1 allele in Yemenite Jews is only 0.09 (known to have very low rates of alcoholism) compared to 0.75 in Cheyenne American Indians (known in most studies to have high rates of alcoholism). Of particular importance is that the frequency of the DRD2 A1 allele in African Pygmies is 0.25 compared to almost 0.40 in US African-Americans (Blacks) (see Figure 1, [94]). This difference could be explained by admixture and interracial impact on DRD2 A1 frequency rate. With this stated we are faced with the problem of “cultural disparity” whereby US African-Americans have a disproportional higher rate of a genotype that predisposes them to reduced dopamine function and as such a “magnification” of the aging process and possible higher rate of Alzheimer’s in this ethnic population. Future work will allow us to evaluate behaviors associated with the DRD2 TAq A1 Allele in African Americans, similar to what has been done for other populations.
Figure 1.
DRD2 frequency as a function of ethnicity. The DRD2 Taq 1 allele frequency as a function of ethnicity was derived from a number of independent investigations. The number in parentheses denotes the proband size. Modified from Barr & Kidd46
Prognosis
Unfortunately, there is no real cure for Alzheimer’s Disease, and in spite of enormous efforts worldwide the disease remains a mystery in terms of treatment. African Americans are two times more likely to develop late-onset Alzheimer’s disease than Whites and less likely to have a timely diagnosis resulting in less time for treatment and planning. Connel et al. [95] examined differences between African Americans and Whites with regard to their attitudes, beliefs, and knowledge about AD. They pointed out that the 2 groups differed in terms of the following: (1) their knowledge about the disease (e.g., recognizing that AD is not a part of normal aging); (2) concern about AD (e.g., worry about developing the disease); (3) beliefs about putative causes of AD (e.g., stress); and (4) beliefs about the effectiveness of various options for reducing risk of and treating AD (e.g., physical activity). A societal effort is needed to change these attitudes. An important, well-established fact is that dopamine function is linked to normal cognition and memory. Carriers of the DRD2 A1 allele have significant reduction of D2 receptor density in the brain. Most recently, it has been shown that A1 carriers have worse memory performance during Long-Term Memory (LTM) updating, compared to non-carriers or A2. It has also been shown that A1 carriers show less blood oxygen level dependent (BOLD) activation in the left caudate nucleus, an area important for LTM updating. This latter effect was only seen in older adults, suggesting magnification of genetic effects on brain functioning in the elderly. Tantamount to this finding is the fact that the prevalence of the DRD2 A1 allele is found with an allelic frequency of 0.40 or approximately 50% in the African-American community which is higher than what is found even in a non-screened American population, (≤28%) for Reward Deficiency Syndrome (RDS) behaviors.
Conclusions
Based on the importance of dopamine on learning and memory, the role of the A1 allele in downregulating dopamine receptors and reducing function in memory-related tasks, the prevalence of the A1 SNP in African Americans, and the high, increased, prevalence of Alzheimer’s disease in African Americans, we hypothesize that the DRD2 Taq1A A1 allele magnifies the risk of Alzheimer’s in aging African Americans, and that this risk may be modified/amplified based on traumatic, stressful life experiences. Additional required research, further linking this disparaging high risk for Alzheimer’s in the African American population, with DRD2/ANKK1-TaqIA polymorphism and neurocognitive deficits related to LTM updating and magnification of aging, could pave the way for novel, targeted personalized medicine involving pro-dopamine homeostatic treatment, including Repetitive Transcranial Magnetic Stimulation (rTMS) [96].
Acknowledgments
Dr. Kenneth Blum is the owner of US and foreign patents related to genetic testing Dr. Rajendra D. Badgaiyan is supported by the National Institutes of Health (NIH) grants R01NS073884 and 1R21MH073624, and Dr. Marjorie C. Gondré-Lewis is supported by NIH/NIAAA grant # R01AA021262 and G12MD007597.
References
- 1.Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48(7):648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- 2.Fagundo AB, Fernandez-Aranda F, de la Torre R, Verdejo-Garcia A, Granero R, Penelo E, Gene M, Barrot C, Sanchez C, Alvarez-Moya E, Ochoa C, Aymami MN, Gomez-Pena M, Menchon JM, Jimenez-Murcia S. Dopamine DRD2/ANKK1 Taq1A and DAT1 VNTR polymorphisms are associated with a cognitive flexibility profile in pathological gamblers. Journal of psychopharmacology (Oxford, England) 2014;28(12):1170–1177. doi: 10.1177/0269881114551079. [DOI] [PubMed] [Google Scholar]
- 3.Hillemacher T, Frieling H, Buchholz V, Hussein R, Bleich S, Meyer C, John U, Bischof A, Rumpf HJ. Dopamine-receptor 2 gene-methylation and gambling behavior in relation to impulsivity. Psychiatry research. 2016;239:154–155. doi: 10.1016/j.psychres.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Voigt G, Montag C, Markett S, Reuter M. On the genetics of loss aversion: An interaction effect of BDNF Val66Met and DRD2/ANKK1 Taq1a. Behavioral neuroscience. 2015;129(6):801–811. doi: 10.1037/bne0000102. [DOI] [PubMed] [Google Scholar]
- 5.Nisoli E, Brunani A, Borgomainerio E, Tonello C, Dioni L, Briscini L, Redaelli G, Molinari E, Cavagnini F, Carruba MO. D2 dopamine receptor (DRD2) gene Taq1A polymorphism and the eating-related psychological traits in eating disorders (anorexia nervosa and bulimia) and obesity. Eating and weight disorders: EWD. 2007;12(2):91–96. doi: 10.1007/BF03327583. [DOI] [PubMed] [Google Scholar]
- 6.Thaler L, Groleau P, Badawi G, Sycz L, Zeramdini N, Too A, Israel M, Joober R, Bruce KR, Steiger H. Epistatic interactions implicating dopaminergic genes in bulimia nervosa (BN): relationships to eating- and personality-related psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):120–128. doi: 10.1016/j.pnpbp.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Doehring A, Hentig N, Graff J, Salamat S, Schmidt M, Geisslinger G, Harder S, Lotsch J. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics. 2009;19(6):407–414. doi: 10.1097/FPC.0b013e328320a3fd. [DOI] [PubMed] [Google Scholar]
- 8.Gluskin BS, Mickey BJ. Genetic variation and dopamine D2 receptor availability: a systematic review and meta-analysis of human in vivo molecular imaging studies. Translational psychiatry. 2016;6:e747. doi: 10.1038/tp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan A, Heckman MG, Ahlskog JE, Wszolek ZK, Serie DJ, Uitti RJ, van Gerpen JA, Okun MS, Rayaprolu S, Ross OA. Association of Parkinson disease age of onset with DRD2, DRD3 and GRIN2B polymorphisms. Parkinsonism & related disorders. 2016;22:102–105. doi: 10.1016/j.parkreldis.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasiewicz A, Samochowiec A, Samochowiec J, Malecka I, Suchanecka A, Grzywacz A. Suicidal behavior and haplotypes of the dopamine receptor gene (DRD2) and ANKK1 gene polymorphisms in patients with alcohol dependence--preliminary report. PLoS One. 2014;9(11):e111798. doi: 10.1371/journal.pone.0111798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazantseva A, Gaysina D, Malykh S, Khusnutdinova E. The role of dopamine transporter (SLC6A3) and dopamine D2 receptor/ankyrin repeat and kinase domain containing 1 (DRD2/ANKK1) gene polymorphisms in personality traits. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1033–1040. doi: 10.1016/j.pnpbp.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Myrga JM, Juengst SB, Failla MD, Conley YP, Arenth PM, Grace AA, Wagner AK. COMT and ANKK1 Genetics Interact With Depression to Influence Behavior Following Severe TBI: An Initial Assessment. Neurorehabilitation and neural repair. 2016;30(10):920–930. doi: 10.1177/1545968316648409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi H, Tomita H, Taki Y, Kikuchi Y, Ono C, Yu Z, Sekiguchi A, Nouchi R, Kotozaki Y, Nakagawa S, Miyauchi CM, Iizuka K, Yokoyama R, Shinada T, Yamamoto Y, Hanawa S, Araki T, Hashizume H, Kunitoki K, Sassa Y, Kawashima R. The associations among the dopamine D2 receptor Taq1, emotional intelligence, creative potential measured by divergent thinking, and motivational state and these associations’ sex differences. Frontiers in psychology. 2015;6:912. doi: 10.3389/fpsyg.2015.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voisey J, Swagell CD, Hughes IP, Morris CP, van Daal A, Noble EP, Kann B, Heslop KA, Young RM, Lawford BR. The DRD2 gene 957C>T polymorphism is associated with posttraumatic stress disorder in war veterans. Depression and anxiety. 2009;26(1):28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Simen A, Arias A, Lu QW, Zhang H. A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Human genetics. 2013;132(3):347–358. doi: 10.1007/s00439-012-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318(5856):1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 17.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23(6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, Li MD. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34(2):319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- 19.Montag C, Markett S, Basten U, Stelzel C, Fiebach C, Canli T, Reuter M. Epistasis of the DRD2/ANKK1 Taq Ia and the BDNF Val66Met polymorphism impacts novelty seeking and harm avoidance. Neuropsychopharmacology. 2010;35(9):1860–1867. doi: 10.1038/npp.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4(3):290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 21.Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3(3):256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 22.Hill SY, Hoffman EK, Zezza N, Thalamuthu A, Weeks DE, Matthews AG, Mukhopadhyay I. Dopaminergic mutations: within-family association and linkage in multiplex alcohol dependence families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):517–526. doi: 10.1002/ajmg.b.30630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlgren A, Wargelius HL, Berglund KJ, Fahlke C, Blennow K, Zetterberg H, Oreland L, Berggren U, Balldin J. Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? A pilot study. Alcohol Alcohol. 2011;46(5):509–513. doi: 10.1093/alcalc/agr045. [DOI] [PubMed] [Google Scholar]
- 24.Tejada-Vera B. NCHS Data Brief. Vol. 116. National Center for Health Statistics; Hyattsville, MD: 2013. Mortality from Alzheimer’s disease in the United States: Data for 2000 and 2010. [PubMed] [Google Scholar]
- 25.Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the United States among persons with Alzheimer’s disease (2010–2050) Alzheimers Dement. 2014;10(2):e40–46. doi: 10.1016/j.jalz.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82(12):1045–1050. doi: 10.1212/WNL.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Association As. Alzheimer’s disease facts and figures, Alzheimer’s & dementia. Vol. 8. Alzheimer’s Association; 2012. [DOI] [PubMed] [Google Scholar]
- 28.Association As. 2017 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2017;13:325–373. [Google Scholar]
- 29.Jett KF. Mind-loss in the African American community: Dementia as a normal part of aging. J Aging Stud. 2006;20(1):1–10. doi: 10.1016/j.jaging.2005.05.002. [DOI] [Google Scholar]
- 30.Rovner BW, Casten RJ, Harris LF. Cultural diversity and views on Alzheimer disease in older African Americans. Alzheimer Dis Assoc Disord. 2013;27(2):133–137. doi: 10.1097/WAD.0b013e3182654794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, Valladares O, Lin CF, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, Buxbaum JD, Murrell JR, Raj T, Ertekin-Taner N, Logue M, Baldwin CT, Green RC, Barnes LL, Cantwell LB, Fallin MD, Go RC, Griffith P, Obisesan TO, Manly JJ, Lunetta KL, Kamboh MI, Lopez OL, Bennett DA, Hendrie H, Hall KS, Goate AM, Byrd GS, Kukull WA, Foroud TM, Haines JL, Farrer LA, Pericak-Vance MA, Schellenberg GD, Mayeux R Alzheimer Disease Genetics C. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E 4, and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483–1492. doi: 10.1001/jama.2013.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RCP, Griffith P, Obisesan TO, Shatz R, Borenstein A, Cupples LA, Lunetta KL, Fallin MD, Baldwin CT, Farrer LA, Grp MS. A Comprehensive Genetic Association Study of Alzheimer Disease in African Americans. Arch Neurol-Chicago. 2011;68(12):1569–1579. doi: 10.1001/archneurol.2011.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghani M, Reitz C, Cheng R, Vardarajan BN, Jun G, Sato C, Naj A, Rajbhandary R, Wang LS, Valladares O, Lin CF, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, Buxbaum JD, Murrell JR, Raj T, Ertekin-Taner N, Logue M, Baldwin CT, Green RC, Barnes LL, Cantwell LB, Fallin MD, Go RCP, Griffith PA, Obisesan TO, Manly JJ, Lunetta KL, Kamboh MI, Lopez OL, Bennett DA, Hendrie H, Hall KS, Goate AM, Byrd GS, Kukull WA, Foroud TM, Haines JL, Farrer LA, Pericak-Vance MA, Lee JH, Schellenberg GD, George-Hyslop PS, Mayeux R, Rogaeva E Genetics AsD. Association of Long Runs of Homozygosity With Alzheimer Disease Among African American Individuals. Jama Neurol. 2015;72(11):1313–1323. doi: 10.1001/jamaneurol.2015.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graff-Radford NR, Green RC, Go RC, Hutton ML, Edeki T, Bachman D, Adamson JL, Griffith P, Willis FB, Williams M, Hipps Y, Haines JL, Cupples LA, Farrer LA. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch Neurol. 2002;59(4):594–600. doi: 10.1001/archneur.59.4.594. [DOI] [PubMed] [Google Scholar]
- 36.Mount DL, Ashley AV, Lah JJ, Levey AI, Goldstein FC. Is ApoE epsilon4 associated with cognitive functioning in African Americans diagnosed with Alzheimer Disease? An exploratory study. South Med J. 2009;102(9):890–893. doi: 10.1097/SMJ.0b013e3181b21b82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang M, Stern Y, Marder K, et al. The apoe-ε4 allele and the risk of alzheimer disease among african americans, whites, and hispanics. JAMA. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 38.Barnes LL, Wilson RS, Everson-Rose SA, Hayward MD, Evans DA, Mendes de Leon CF. Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology. 2012;79(24):2321–2327. doi: 10.1212/WNL.0b013e318278b607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR, Jr, Petersen RC, Xu YC, O’Brien PC, Waring SC, Tangalos EG, Smith GE, Ivnik RJ, Thibodeau SN, Kokmen E. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43(3):303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sencakova D, Graff-Radford NR, Willis FB, Lucas JA, Parfitt F, Cha RH, O’Brien PC, Petersen RC, Jack CR., Jr Hippocampal atrophy correlates with clinical features of Alzheimer disease in African Americans. Arch Neurol. 2001;58(10):1593–1597. doi: 10.1001/archneur.58.10.1593. [DOI] [PubMed] [Google Scholar]
- 41.Braverman ER, Blum K, Hussman KL, Han D, Dushaj K, Li M, Marin G, Badgaiyan RD, Smayda R, Gold MS. Evoked Potentials and Memory/Cognition Tests Validate Brain Atrophy as Measured by 3T MRI (NeuroQuant) in Cognitively Impaired Patients. Plos One. 2015;10(8) doi: 10.1371/journal.pone.0133609. ARTN e0133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, Mayeux R, Brickman AM. Structural MRI Predictors of Late-Life Cognition Differ Across African Americans, Hispanics, and Whites. Curr Alzheimer Res. 2015;12(7):632–639. doi: 10.2174/1567205012666150530203214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbons FX, Roberts ME, Gerrard M, Li Z, Beach SR, Simons RL, Weng CY, Philibert RA. The impact of stress on the life history strategies of African American adolescents: cognitions, genetic moderation, and the role of discrimination. Dev Psychol. 2012;48(3):722–739. doi: 10.1037/a0026599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61(5):641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 45.Arnsten AF, Wang M, Paspalas CD. Dopamine’s Actions in Primate Prefrontal Cortex: Challenges for Treating Cognitive Disorders. Pharmacological reviews. 2015;67(3):681–696. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature reviews Neuroscience. 2016;17(8):524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirigu A, Duhamel JR. Reward and decision processes in the brains of humans and nonhuman primates. Dialogues in clinical neuroscience. 2016;18(1):45–53. doi: 10.31887/DCNS.2016.18.1/asirigu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westbrook A, Braver TS. Dopamine Does Double Duty in Motivating Cognitive Effort. Neuron. 2016;89(4):695–710. doi: 10.1016/j.neuron.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flood JF, Smith GE, Jarvik ME. A comparison of the effects of localized brain administration of catecholamine and protein synthesis inhibitors on memory processing. Brain Res. 1980;197(1):153–165. doi: 10.1016/0006-8993(80)90441-2. [DOI] [PubMed] [Google Scholar]
- 50.van Wimersma Greidanus TB, Jolles J, De Wied D. Hypothalamic neuropeptides and memory. Acta Neurochir (Wien) 1985;75(1–4):99–105. doi: 10.1007/BF01406329. [DOI] [PubMed] [Google Scholar]
- 51.Schroder H, Reymann KG. N-methyl-D-aspartate stimulates the release of dopamine from rat hippocampal slices. Biomed Biochim Acta. 1990;49(4):281–284. [PubMed] [Google Scholar]
- 52.Sudha S, Lakshmana MK, Pradhan N. Changes in learning and memory, acetylcholinesterase activity and monoamines in brain after chronic carbamazepine administration in rats. Epilepsia. 1995;36(4):416–422. doi: 10.1111/j.1528-1157.1995.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6(5):526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 54.Moreno-Castilla P, Perez-Ortega R, Violante-Soria V, Balderas I, Bermudez-Rattoni F. Hippocampal release of dopamine and norepinephrine encodes novel contextual information. Hippocampus. 2017 doi: 10.1002/hipo.22711. [DOI] [PubMed] [Google Scholar]
- 55.Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci. 2015;18(12):1763–1771. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemppainen S, Lindholm P, Galli E, Lahtinen HM, Koivisto H, Hamalainen E, Saarma M, Tanila H. Cerebral dopamine neurotrophic factor improves long-term memory in APP/PS1 transgenic mice modeling Alzheimer’s disease as well as in wild-type mice. Behav Brain Res. 2015;291:1–11. doi: 10.1016/j.bbr.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Moraga-Amaro R, Gonzalez H, Ugalde V, Donoso-Ramos JP, Quintana-Donoso D, Lara M, Morales B, Rojas P, Pacheco R, Stehberg J. Dopamine receptor D5 deficiency results in a selective reduction of hippocampal NMDA receptor subunit NR2B expression and impaired memory. Neuropharmacology. 2016;103:222–235. doi: 10.1016/j.neuropharm.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 58.Karunakaran S, Chowdhury A, Donato F, Quairiaux C, Michel CM, Caroni P. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat Neurosci. 2016;19(3):454. doi: 10.1038/nn.4231. [DOI] [PubMed] [Google Scholar]
- 59.Broussard JI, Yang K, Levine AT, Tsetsenis T, Jenson D, Cao F, Garcia I, Arenkiel BR, Zhou FM, De Biasi M, Dani JA. Dopamine Regulates Aversive Contextual Learning and Associated In Vivo Synaptic Plasticity in the Hippocampus. Cell Rep. 2016;14(8):1930–1939. doi: 10.1016/j.celrep.2016.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiang PY, Janc O, Grochowska KM, Kreutz MR, Reymann KG. Dopamine agonists rescue Abeta-induced LTP impairment by Src-family tyrosine kinases. Neurobiol Aging. 2016;40:98–102. doi: 10.1016/j.neurobiolaging.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Moreno-Castilla P, Rodriguez-Duran LF, Guzman-Ramos K, Barcenas-Femat A, Escobar ML, Bermudez-Rattoni F. Dopaminergic neurotransmission dysfunction induced by amyloid-beta transforms cortical long-term potentiation into long-term depression and produces memory impairment. Neurobiol Aging. 2016;41:187–199. doi: 10.1016/j.neurobiolaging.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 62.Shetty MS, Sajikumar S. Differential involvement of Ca2+/calmodulin-dependent protein kinases and mitogen-activated protein kinases in the dopamine D1/D5 receptor-mediated potentiation in hippocampal CA1 pyramidal neurons. Neurobiol Learn Mem. 2017;138:111–120. doi: 10.1016/j.nlm.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 63.Shetty MS, Sharma M, Sajikumar S. Chelation of hippocampal zinc enhances long-term potentiation and synaptic tagging/capture in CA1 pyramidal neurons of aged rats: implications to aging and memory. Aging Cell. 2017;16(1):136–148. doi: 10.1111/acel.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du H, Deng W, Aimone JB, Ge M, Parylak S, Walch K, Zhang W, Cook J, Song H, Wang L, Gage FH, Mu Y. Dopaminergic inputs in the dentate gyrus direct the choice of memory encoding. Proc Natl Acad Sci U S A. 2016;113(37):E5501–5510. doi: 10.1073/pnas.1606951113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou W, Chang L, Fang Y, Du Z, Li Y, Song Y, Hao F, Lv L, Wu Y. Cerebral dopamine neurotrophic factor alleviates Abeta25-35-induced endoplasmic reticulum stress and early synaptotoxicity in rat hippocampal cells. Neurosci Lett. 2016;633:40–46. doi: 10.1016/j.neulet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Braren SH, Drapala D, Tulloch IK, Serrano PA. Methamphetamine-induced short-term increase and long-term decrease in spatial working memory affects protein Kinase M zeta (PKMzeta), dopamine, and glutamate receptors. Front Behav Neurosci. 2014;8:438. doi: 10.3389/fnbeh.2014.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez MC, Kramar CP, Tomaiuolo M, Katche C, Weisstaub N, Cammarota M, Medina JH. Medial prefrontal cortex dopamine controls the persistent storage of aversive memories. Front Behav Neurosci. 2014;8:408. doi: 10.3389/fnbeh.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamagata N, Ichinose T, Aso Y, Placais PY, Friedrich AB, Sima RJ, Preat T, Rubin GM, Tanimoto H. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc Natl Acad Sci U S A. 2015;112(2):578–583. doi: 10.1073/pnas.1421930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otani S, Bai J, Blot K. Dopaminergic modulation of synaptic plasticity in rat prefrontal neurons. Neurosci Bull. 2015;31(2):183–190. doi: 10.1007/s12264-014-1507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichenbach N, Herrmann U, Kahne T, Schicknick H, Pielot R, Naumann M, Dieterich DC, Gundelfinger ED, Smalla KH, Tischmeyer W. Differential effects of dopamine signalling on long-term memory formation and consolidation in rodent brain. Proteome Sci. 2015;13:13. doi: 10.1186/s12953-015-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee KN, Chirwa S. Blocking Dopaminergic Signaling Soon after Learning Impairs Memory Consolidation in Guinea Pigs. PLoS One. 2015;10(8):e0135578. doi: 10.1371/journal.pone.0135578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ichinose T, Aso Y, Yamagata N, Abe A, Rubin GM, Tanimoto H. Reward signal in a recurrent circuit drives appetitive long-term memory formation. Elife. 2015;4:e10719. doi: 10.7554/eLife.10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim HF, Ghazizadeh A, Hikosaka O. Dopamine Neurons Encoding Long-Term Memory of Object Value for Habitual Behavior. Cell. 2015;163(5):1165–1175. doi: 10.1016/j.cell.2015.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bartres-Faz D, Junque C, Serra-Grabulosa JM, Lopez-Alomar A, Moya A, Bargallo N, Mercader JM, Moral P, Clemente IC. Dopamine DRD2 Taq I polymorphism associates with caudate nucleus volume and cognitive performance in memory impaired subjects. Neuroreport. 2002;13(9):1121–1125. doi: 10.1097/00001756-200207020-00010. [DOI] [PubMed] [Google Scholar]
- 75.Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic PS, Lidow MS. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci U S A. 2003;100(1):313–317. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura TY, Nakao S, Nakajo Y, Takahashi JC, Wakabayashi S, Yanamoto H. Possible Signaling Pathways Mediating Neuronal Calcium Sensor-1-Dependent Spatial Learning and Memory in Mice. PLoS One. 2017;12(1):e0170829. doi: 10.1371/journal.pone.0170829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng E, Varaschin RK, Su P, Browne CJ, Hermainski J, Le Foll B, Pongs O, Liu F, Trudeau LE, Roder JC, Wong AH. Neuronal calcium sensor-1 deletion in the mouse decreases motivation and dopamine release in the nucleus accumbens. Behav Brain Res. 2016;301:213–225. doi: 10.1016/j.bbr.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 78.Papenberg G, Becker N, Ferencz B, Naveh-Benjamin M, Laukka EJ, Backman L, Brehmer Y. Dopamine Receptor Genes Modulate Associative Memory in Old Age. J Cogn Neurosci. 2017;29(2):245–253. doi: 10.1162/jocn_a_01048. [DOI] [PubMed] [Google Scholar]
- 79.Persson J, Rieckmann A, Kalpouzos G, Fischer H, Backman L. Influences of a DRD2 polymorphism on updating of long-term memory representations and caudate BOLD activity: magnification in aging. Hum Brain Mapp. 2015;36(4):1325–1334. doi: 10.1002/hbm.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Papenberg G, Backman L, Nagel IE, Nietfeld W, Schroder J, Bertram L, Heekeren HR, Lindenberger U, Li SC. Dopaminergic gene polymorphisms affect long-term forgetting in old age: further support for the magnification hypothesis. J Cogn Neurosci. 2013;25(4):571–579. doi: 10.1162/jocn_a_00359. [DOI] [PubMed] [Google Scholar]
- 81.Makman MH, Ahn HS, Thal LJ, Sharpless NS, Dvorkin B, Horowitz SG, Rosenfeld M. Aging and monoamine receptors in brain. Fed Proc. 1979;38(5):1922–1926. [PubMed] [Google Scholar]
- 82.Roussotte FF, Gutman BA, Hibar DP, Madsen SK, Narr KL, Thompson PM Alzheimer’s Disease Neuroimaging I. Carriers of a common variant in the dopamine transporter gene have greater dementia risk, cognitive decline, and faster ventricular expansion. Alzheimers Dement. 2015;11(10):1153–1162. doi: 10.1016/j.jalz.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roussotte FF, Jahanshad N, Hibar DP, Thompson PM Alzheimer’s Disease Neuroimaging I. Altered regional brain volumes in elderly carriers of a risk variant for drug abuse in the dopamine D2 receptor gene (DRD2) Brain Imaging Behav. 2015;9(2):213–222. doi: 10.1007/s11682-014-9298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matuskey D, Worhunksy P, Correa E, Pittman B, Gallezot JD, Nabulsi N, Ropchan J, Sreeram V, Gudepu R, Gaiser E, Cosgrove K, Ding YS, Potenza MN, Huang Y, Malison RT, Carson RE. Age-related changes in binding of the D2/3 receptor radioligand [(11)C](+)PHNO in healthy volunteers. Neuroimage. 2016;130:241–247. doi: 10.1016/j.neuroimage.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gondre-Lewis MC, Warnock KT, Wang H, June HL, Jr, Bell KA, Rabe H, Tiruveedhula VV, Cook J, Luddens H, Aurelian L, June HL., Sr Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress. 2016;19(2):235–247. doi: 10.3109/10253890.2016.1160280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Gondre-Lewis MC. Prenatal nicotine and maternal deprivation stress deregulate the development of CA1, CA3, and dentate gyrus neurons in hippocampus of infant rats. PLoS One. 2013;8(6):e65517. doi: 10.1371/journal.pone.0065517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gondre-Lewis MC, Darius PJ, Wang H, Allard JS. Stereological analyses of reward system nuclei in maternally deprived/separated alcohol drinking rats. J Chem Neuroanat. 2016;76(Pt B):122–132. doi: 10.1016/j.jchemneu.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barr CL, Kidd KK. Population frequencies of the A1 allele at the dopamine D2 receptor locus. Biol Psychiatry. 1993;34(4):204–209. doi: 10.1016/0006-3223(93)90073-m. [DOI] [PubMed] [Google Scholar]
- 89.Adamson MD, Kennedy J, Petronis A, Dean M, Virkkunen M, Linnoila M, Goldman D. DRD4 dopamine receptor genotype and CSF monoamine metabolites in Finnish alcoholics and controls. Am J Med Genet. 1995;60(3):199–205. doi: 10.1002/ajmg.1320600306. [DOI] [PubMed] [Google Scholar]
- 90.Goldman D, Brown GL, Albaugh B, Robin R, Goodson S, Trunzo M, Akhtar L, Lucas-Derse S, Long J, Linnoila M, et al. DRD2 dopamine receptor genotype, linkage disequilibrium, and alcoholism in American Indians and other populations. Alcohol Clin Exp Res. 1993;17(2):199–204. doi: 10.1111/j.1530-0277.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 91.Kraschewski A, Reese J, Anghelescu I, Winterer G, Schmidt LG, Gallinat J, Finckh U, Rommelspacher H, Wernicke C. Association of the dopamine D2 receptor gene with alcohol dependence: haplotypes and subgroups of alcoholics as key factors for understanding receptor function. Pharmacogenet Genomics. 2009;19(7):513–527. doi: 10.1097/fpc.0b013e32832d7fd3. [DOI] [PubMed] [Google Scholar]
- 92.Levy JE, Kunitz SJ. Indian drinking: Navajo practices and Anglo-American theories. Wiley; New York: 1974. [Google Scholar]
- 93.Arinami TIM, Komiyama T, et al. Association between severity of alcoholism and the A1 allele of the dopamine D2 receptor gene TaqI A RFLP in Japanese. Biological Psychiatry. 1993;33(2):104–114. doi: 10.1016/0006-3223(93)90309-2. doi: http://dx.doi.org/10.1016/0006-3223(93)90309-2. [DOI] [PubMed] [Google Scholar]
- 94.Blum KSPJ, Chen TCH, et al. Handbook of Psychiatric Genetics. CRC Press; Boca Raton, Fl., USA: 1996. The dopamine D2 receptor gene locus in reward deficiency syndrome: Meta –Analyses. [Google Scholar]
- 95.Connell CM, Scott Roberts J, McLaughlin SJ, Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(2):110–116. doi: 10.1097/WAD.0b013e318192e94d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bagherzadeh Y, Khorrami A, Zarrindast MR, Shariat SV, Pantazis D. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex enhances working memory. Exp Brain Res. 2016;234(7):1807–1818. doi: 10.1007/s00221-016-4580-1. [DOI] [PubMed] [Google Scholar]