Abstract
Gaucher disease (GD) is an autosomal recessive disease caused by GBA mutations that is especially common in the Ashkenazi Jewish (AJ) population. The link between GBA mutations and Parkinson disease (PD), a later-onset neurodegenerative condition, is well established, and studies have shown that GBA carriers have an increased lifetime risk of developing PD. Carrier screening for GD is frequently offered to couples during or prior to pregnancy, especially to those of AJ descent. However, no studies have been performed to assess if prospective parents would want to learn about their risk of developing PD incidentally through carrier screening. It is also unknown if pre-test counseling on this topic would affect screening uptake. In order to answer these questions, a survey was administered to individuals who screened negative for GBA mutations. Of the 75 participants, 86.7% believed that patients should be informed about the increased risk of PD prior to having GD carrier screening, and 93.3% responded that this information would not have changed their decision to have carrier screening. These results indicate that healthcare providers should take into consideration patient preferences when determining how to counsel about GD carrier screening. Additionally, these results have implications for genetic counseling about other later-onset conditions that may be incidentally ascertained through carrier screening.
Keywords: Gaucher disease, Parkinson disease, carrier screening, reproductive genetic counseling
Introduction
Gaucher disease (GD) is an autosomal recessive condition caused by homozygous or compound heterozygous mutations in the β-glucocerebrosidase gene (GBA). The most common form of the condition, type 1 (GD1), has a highly variable phenotype that can include cytopenia, skeletal involvement and hepatosplenomegaly. There is significant clinical variability, and patients present across a spectrum of disease severity, with some who have early onset, severe manifestations and others who may be asymptomatic and never diagnosed (Balwani, Fuerstman, Kornreich, Edelmann, & Desnick, 2010; Bennett & Mohan, 2013; Thomas, Mehta, & Hughes, 2014).
GD1 was described as non-neuronopathic; however, it was later discovered that individuals with GD1 have an increased risk of Parkinson’s disease (PD), a common neurodegenerative disorder affecting about 2% of adults over the age of 80 (Beavan & Schapira, 2013; Pringsheim, Jette, Frolkis, & Steeves, 2014). Furthermore, PD risk is also increased among heterozygous GBA carriers (Sidransky et al., 2009). When comparing the age-specific risk of PD among Ashkenazi Jews, results showed a 2.1% risk in non-carriers, 7.7% risk in GBA heterozygotes and 9.1% risk in GD1 patients. Furthermore, PD age-at-onset was younger in GD1 patients than GBA heterozygotes (Alcalay et al., 2014).
The actual risk of PD in GBA heterozygotes was estimated in three studies, with estimates ranging from 7.7% to 29.7% (Alcalay et al., 2014; Anheim et al., 2012; McNeill, Duran, Hughes, Mehta, & Schapira, 2012; Rana, Balwani, Bier, & Alcalay, 2013). The highest estimated prevalence of almost 30% was likely influenced by ascertainment bias; the participants had two PD risk factors—they each had a GBA mutation and a first degree relative with PD (Anheim et al., 2012; Rana et al., 2013). A study performed at the Icahn School of Medicine at Mount Sinai (ISMMS), which included 224 obligate GBA carriers, most of whom (87.4%) had at least one Ashkenazi Jewish (AJ) grandparent, found a PD risk of 10.9% by age 85 (Rana et al., 2013). This study was expanded to include more data from an Israeli site, which showed a 7.7% risk of developing PD by age 80 (Alcalay et al., 2014). A similar study performed in the United Kingdom found the risk to be higher – 15% by age 80 (McNeill et al., 2012). While there is variability in the data, each study shows that GBA mutation carriers have a higher risk of PD compared to the general population. The increased risk is associated with all GBA mutations.
GD is the most common genetic condition in the AJ population, with a carrier frequency in this population between 1 in 18 and 1 in 15 (ACOG Committee on Genetics, 2009; Bennett & Mohan, 2013; Gross, Pletcher, Monaghan, & Professional Practice Guidelines Committee, 2008; Scott et al., 2010). GD carrier screening has been available for individuals of AJ descent since the 1990s, and it has been recommended by the American College of Medical Genetics and Genomics (ACMG) that this screening be offered to this population since 2008 (Gross et al., 2008). According to one study that surveyed prenatal healthcare providers across the United States, over 99% of genetic counselors offer Jewish carrier screening to couples with at least one AJ partner, and of those, 96% offer GD carrier screening as part of AJ carrier screening. Although general population carrier screening for GD is not recommended by any professional organization, GD is included on clinically available pan-ethnic carrier screening panels, and 23% of genetic counselors said they would offer GD carrier screening to any patient who requested all available prenatal screening tests (Falcone, Wood, Mennuti, Xie, & Van Deerlin, 2012). Based on these trends, it is reasonable to expect that GD carrier screening will become increasingly available to individuals of both AJ and non-AJ descent.
Although GD carrier screening is offered to provide information for reproductive planning, this testing could have the unintended consequence of revealing an increased susceptibility for PD. Genetic counselors and other healthcare providers who order GD carrier screening will be faced with the decision of whether to discuss this with patients and if so, when. This study aims to explore that question from the patient’s perspective.
Methods
Participants
Participants in this survey project were adults who had carrier screening and did not carry GBA mutations. Only non-carriers were surveyed in order to ensure that participants would not learn unwanted information about their own health by participating in the study. Participants included patients who came to ISMMS for reproductive genetic counseling or for carrier screening group genetic counseling within one year prior to the start of the project (one year prior to September 11th, 2014) or during the course of the study. Participants also included non-GBA carrier partners or relatives of patients with GD who are followed at the ISMMS GD clinic.
Procedures
Potential participants found to be eligible by chart review were given study packets, either in person or via mail. Study packets included a letter explaining the study and providing a link for an online version of the survey, a paper version of the survey, and a return envelope. Surveys were completed anonymously. Data was collected until April 5th, 2015.
Instrumentation
The survey (see supplementary materials) included a brief introduction that explains the following key points: (1) GD is a rare genetic disease with variable severity, (2) GBA carriers are not affected by the disease, but are at risk of having a child with the disease if their partner is also a carrier, (3) PD is a neurodegenerative disease with no prevention or cure, and (4) while the general population risk of developing PD is about 2%, GBA carriers have about a 10% chance of developing PD.
The survey included a total of thirty questions. There were ten demographic questions and eight questions related to participants’ experiences with and opinions of PD and GD. One question asked about participants’ experiences with genetic counseling for carrier screening. Four questions asked about participants’ feelings about genetic counseling on the risk for PD in GD carriers and how this counseling may affect their carrier screening choices. Five questions explored how this information being delivered in different ways or at different times would affect participants’ responses to the information. Finally, the last two questions were factual questions intended to assess the participants’ understanding of the background information.
Data Analysis
The results were analyzed using SPSS (IBM) version 20. Descriptive statistics, T-tests and ANOVA were performed.
Results
A total of 529 surveys were sent through the mail and at least 13 were undeliverable. Approximately 25 surveys were handed out by the ISMMS reproductive genetic counselors, and 28 surveys were sent to eligible spouses and relatives of GD patients. A total of 75 surveys were returned. The response rate is therefore approximately 13.2%.
The majority of participants were college-educated, white, non-Hispanic females. The majority of participants (72.0%) were of AJ descent and/or had a partner of AJ descent. 16% of participants had a relative with PD, and almost half (42.7%) had a personal relationship with someone with PD other than a relative. Five participants (6.7%) had a spouse with GD, and only one participant had a personal relationship with someone with GD other than a relative or spouse (1.3%).
None of the participants reported having been informed about the increased risk of PD among GD carriers prior to having carrier screening. A majority of participants (93.3%) reported they would still have been screened for all the disorders they were screened for had they been given this information.
A majority of participants (86.7%) agreed or strongly agreed that everyone should be informed prior to getting carrier screening that carriers of GD are at increased risk for PD (Figure 1). 70.7% of participants disagreed or strongly disagreed that only those people who are found to be carriers for GD should be informed about this correlation (Figure 2). Couples in which neither partner had any AJ ancestry disagreed more strongly with this statement (p = 0.015). 82.2% disagreed or strongly disagreed that it is not necessary that healthcare providers inform patients undergoing carrier screening about this correlation (Figure 3). The responses to these questions did not differ significantly based on gender, family history of PD or GD, or the participant’s impression of the disease severity of GD or PD.
Figure 1.
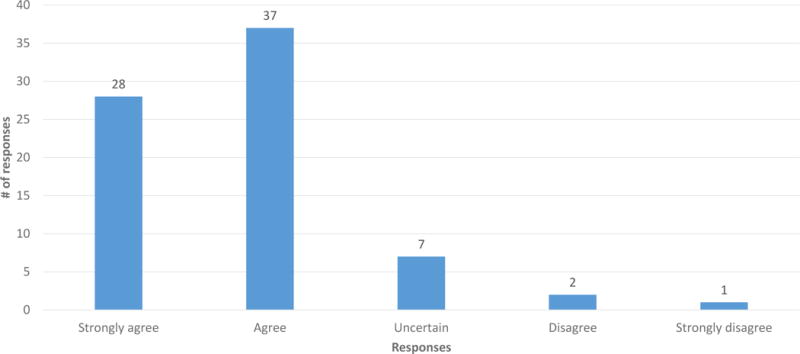
Responses to question Q21: “Everyone should be informed prior to getting carrier screening for GD that carriers of GD are at an increased risk for PD.” (n = 75)
Figure 2.
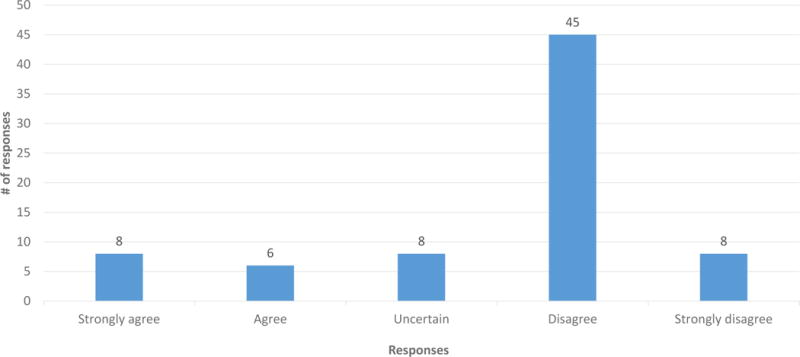
Responses to question Q22: “Only those people who are found to be carriers of GD should be informed that carriers of GD are at increased risk for PD.” (n = 75)
Figure 3.
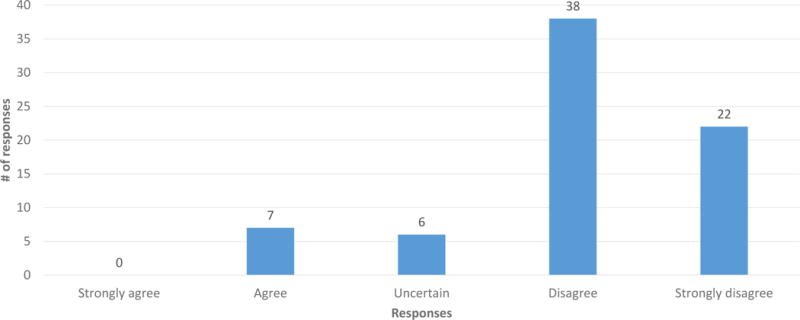
Responses to Q23: “It is not necessary that healthcare providers inform people undergoing carrier screening for GD that carriers of GD are at increased risk for PD.” (n = 73, 2 did not respond)
Participants were asked to rate scenarios, describing various timings (e.g. prior to versus after undergoing screening) and methods (e.g. from a healthcare provider versus online) of learning about increased PD risk, on 1–5 scales from beneficial to harmful, important to unimportant and anxiety relieving to anxiety causing. These scenarios and results are summarized in Table I. On average, the scenario that participants rated least beneficial was Q28, learning about PD risk from an online article (mean = 2.78). For each of the other scenarios, on average, participants described the information as beneficial (mean range = 1.42 to 1.88). Participants, on average, rated the information as important for each of the scenarios (mean range = 1.46 to 1.88). Participants (all of whom screened negative for GBA mutations) described their experience with GD carrier screening as slightly anxiety relieving (mean = 2.24; Q24). In every scenario where participants were asked to imagine that they were found to be GD carriers, the participants described the situation as anxiety causing (mean range = 3.46 to 4.21). Participants indicated that learning about PD risk prior to having carrier screening would be least anxiety-causing (mean = 3.46), while learning about PD risk afterwards by reading an article online would be most anxiety-causing (mean = 4.21).
Table I.
Responses to scenario questions
| Prompt: | Beneficial (1) to Harmful (5) n Mean (Stan. Dev.) |
Important (1) to Unimportant (5) n Mean (Stan. Dev.) |
Anxiety Relieving (1) to Anxiety Causing (5) n Mean (Stan. Dev.) |
|
|---|---|---|---|---|
| Q24: For me, undergoing screening to determine if I was a carrier for GD was: | n=66 1.42 (0.725) |
n=64 1.53 (0.959) |
n=63 2.24 (1.266) |
|
| For questions 25 to 28, imagine you were found to be a carrier for GD | Q25: Imagine that your results from carrier screening showed that you were a carrier for GD. Learning that you were a carrier for a mutation causing GD would have been: | n=71 1.48 (0.876) |
n=68 1.46 (0.905) |
n=68 4.03 (1.133) |
| Q26: Imagine that prior to deciding to have carrier screening, a health care provider informed you that carriers of GD have a 10% risk of getting PD during their lifetime. Getting that information in that way would have been: | n=70 1.86 (0.952) |
n=68 1.88 (1.127) |
n=69 3.46 (0.994) |
|
| Q27: Now imagine that after learning that you were a carrier for GD, a health care provider informed you that carriers of GD have a 10% risk of getting PD during their lifetime. Getting that information in that way would have been: | n=67 1.88 (1.094) |
n=69 1.59 (0.990) |
n=68 4.10 (0.964) |
|
| Q28: Now imagine that after learning that you are a carrier for GD, you read an article that says carriers of GD have a 10% risk of getting PD during their lifetime. Getting that information in that way would have been. | n=69 2.78 (1.533) |
n=67 1.76 (1.046) |
n=71 4.21 (1.133) |
Participants were asked to answer two true or false questions in order to gauge their understanding of the background information presented in the introduction. For each question, over three quarters (77.3% and 88.0%) of participants answered correctly.
Discussion
According to ACMG recommendations, patients being offered carrier screening should be counseled that “a carrier of a gene mutation for an autosomal recessive disorder is a healthy individual who is not at risk of developing the disease but has a risk of passing the gene mutation to his/her offspring” (Gross et al., 2008). However, learning that a patient is a carrier of a GBA mutation does not just provide information about reproductive risk for GD, it also gives information about the patient’s personal risk of developing PD. A majority of participants in this study felt that patients should be informed about the increased PD risk among GD carriers prior to screening. There was a high level of unanimity in these responses, and responses were consistent throughout the study population, regardless of gender, AJ ancestry, family history of GD or PD, or opinion of the severity of these conditions. These results may indicate that genetic counselors and other healthcare providers should consider incorporating this information into their pre-test counseling for GD carrier screening.
Some healthcare providers and genetic counselors may be concerned that providing information about PD risk in this context may cause psychological harm because PD is a late-onset condition without prevention or cure and because GBA mutations are neither necessary nor sufficient to cause PD. In this study, participants indicated that learning about PD risk before or after testing would be beneficial as opposed to harmful. In addition, while participants indicated that it would be anxiety causing to learn that they were GD carriers, they did not feel that learning about the associated PD risk prior to testing would cause any further anxiety. Furthermore, even if patients are not counseled on PD risk during the screening process, it is still possible that GD carriers will discover their increased PD risk on their own after testing, and participants rated this as the most harmful and anxiety provoking scenario. These results indicate that failing to discuss PD risk during the GD screening process could potentially cause greater psychological distress for patients, as the information learned through other sources may not be accurate or appropriately contextualized.
Because GBA screening offers the benefit of establishing GD carrier status and the potential risk of learning unwanted information about PD risk, it may be appropriate for healthcare providers to provide patients with the information so they can weigh the risks and benefits to determine if they are interested in this testing. Over 90% of participants in this study indicated that had they been given all this information prior to screening, they would have still undergone carrier screening for GD.
Healthcare providers and genetic counselors may also be less likely to discuss this topic with patients out of concern that it would be overwhelming or that there would not be enough time during the session. Given that most of the participants in this study had already undergone carrier screening, they could consider what it would mean to learn this additional information in the context of a genetic counseling session.
Study Limitations
Because of the low response rate, those who participated in the study may not be representative of the eligible study population. In addition, since this study was limited to one major academic medical center in New York City and the participants are fairly homogenous in terms of race, ethnicity and education level, the results of this study may not be representative of the opinions of patients in other regions, settings and populations. In the background information provided as part of the survey, it was stated that GBA carriers have about a 10% lifetime risk of developing PD compared to about 2% in the general population. This estimate is supported based on a study performed at ISMMS and an expansion of that study to include a center in Israel (Alcalay et al., 2014; Rana et al., 2013). However, there is a wide range of risk estimates reported in the literature, and it is unknown if or how the specific risk numbers impacted participant responses.
In order to complete this survey, participants, all of whom were not carriers of GD, were asked to imagine that they did not already know their carrier screening results. It is possible that their opinions would differ if they were asked these questions prior to knowing their carrier status.
Research Recommendations
Future directions could include a prospective study where healthcare providers provide this information at the time of pre-test counseling and evaluate how patients respond to their carrier screening results, which could overcome many of the limitations of this study. Additionally, it would be interesting to determine to what extent genetic counselors and other healthcare providers are already incorporating information about PD risk among GD carriers into counseling sessions and to investigate what barriers may be keeping them from doing so.
In addition to having implications for counseling about GD, this study establishes a survey method that could be applicable to other conditions for which being a carrier has consequences for patient health, for example, screening for fragile X ataxia syndrome or ataxia telangiectasia. Additionally, as our knowledge of genetics expands, other examples where carriers of recessive conditions have increased susceptibility to medical problems may be uncovered, and results from this study may help providers consider how to counsel in these situations.
Conclusions
Participants indicated with a high level of unanimity that they believe that healthcare providers should inform patients about the increased risk of PD among GBA carriers prior to screening. In addition, participants indicated that had they been given this information, they would have still undergone the same carrier screening that they had. They also expressed that learning this information from a healthcare professional would be important and beneficial, and would not cause more anxiety than receiving news of being a GBA carrier alone. These results suggest that genetic counselors and other healthcare providers should consider the possibility of incorporating information about increased risk of PD among GBA carriers into their counseling sessions in order to align practice with patient preference.
Supplementary Material
Acknowledgments
This study was performed as part of a thesis project to fulfill a degree requirement at the Icahn School of Medicine at Mount Sinai.
Author Dr. Alcalay is supported by the Parkinson’s Disease Foundation, the National Institutes of Health (K02NS080915 and P50HG007257), and the Michael J Fox Foundation. He consulted and received travel support from Genzyme/Sanofi, Prophase and Denali Therapeutics.
Author Dr. Balwani is a member of the International Collaborative Gaucher Group North American board of advisors. She has received honoraria and travel reimbursement from Genzyme and Shire for advisory board participation.
Footnotes
Comments
This manuscript is being submitted solely to the Journal of Genetic Counseling and has not been published elsewhere.
Conflict of Interest Statements
Authors Maureen Mulhern and Louise Bier declare that they have no conflict of interest.
Human Studies and Informed Consent
This project was approved and determined to be exempt by the ISMMS Institutional Review Board on September 11th, 2014. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
References
- ACOG Committee on Genetics. ACOG Committee Opinion No. 442: Preconception and prenatal carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet Gynecol. 2009;114(4):950–953. doi: 10.1097/AOG.0b013e3181bd12f4. [DOI] [PubMed] [Google Scholar]
- Alcalay RN, Dinur T, Quinn T, Sakanaka K, Levy O, Waters C, Zimran A. Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol. 2014;71(6):752–757. doi: 10.1001/jamaneurol.2014.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anheim M, Elbaz A, Lesage S, Durr A, Condroyer C, Viallet F, French Parkinson Disease Genetic, G Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology. 2012;78(6):417–420. doi: 10.1212/WNL.0b013e318245f476. [DOI] [PubMed] [Google Scholar]
- Balwani M, Fuerstman L, Kornreich R, Edelmann L, Desnick RJ. Type 1 Gaucher disease: significant disease manifestations in “asymptomatic” homozygotes. Arch Intern Med. 2010;170(16):1463–1469. doi: 10.1001/archinternmed.2010.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavan MS, Schapira AH. Glucocerebrosidase mutations and the pathogenesis of Parkinson disease. Ann Med. 2013;45(8):511–521. doi: 10.3109/07853890.2013.849003. [DOI] [PubMed] [Google Scholar]
- Bennett LL, Mohan D. Gaucher disease and its treatment options. Ann Pharmacother. 2013;47(9):1182–1193. doi: 10.1177/1060028013500469. [DOI] [PubMed] [Google Scholar]
- Falcone D, Wood EM, Mennuti M, Xie SX, Van Deerlin VM. Prenatal healthcare providers’ Gaucher disease carrier screening practices. Genet Med. 2012;14(10):844–851. doi: 10.1038/gim.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SJ, Pletcher BA, Monaghan KG, Professional Practice Guidelines Committee Carrier screening in individuals of Ashkenazi Jewish descent. Genet Med. 2008;10(1):54–56. doi: 10.1097/GIM.0b013e31815f247c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Duran R, Hughes DA, Mehta A, Schapira AH. A clinical and family history study of Parkinson’s disease in heterozygous glucocerebrosidase mutation carriers. J Neurol Neurosurg Psychiatry. 2012;83(8):853–854. doi: 10.1136/jnnp-2012-302402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med. 2013;15(2):146–149. doi: 10.1038/gim.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Edelmann L, Liu L, Luo M, Desnick RJ, Kornreich R. Experience with carrier screening and prenatal diagnosis for 16 Ashkenazi Jewish genetic diseases. Hum Mutat. 2010;31(11):1240–1250. doi: 10.1002/humu.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AS, Mehta A, Hughes DA. Gaucher disease: haematological presentations and complications. Br J Haematol. 2014;165(4):427–440. doi: 10.1111/bjh.12804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


