Abstract
Background
Olfactory impairments are prominent in both schizophrenia and the preceding at-risk state. Their presence prior to illness predicts poor functional outcome. In schizophrenia, these impairments reflect peripheral olfactory structural abnormalities, which are hypothesized to arise during early embryonic development. If this is correct, then similar structural anomalies should be apparent among clinical high-risk subjects.
Methods
Thirty-nine clinical high-risk (CR) subjects (24M/15F) were compared to 36 low-risk (LR) subjects (19M/17F). Olfactory measures derived from 3T MRI scans included olfactory bulb volume, primary olfactory cortical gray matter volume, and the depth of the olfactory sulcus overlying the bulb. Additionally, nasal cavity volumes were assessed with acoustic rhinometry.
Results
Male CR subjects exhibited bilateral reductions in olfactory bulb volume and abnormal asymmetries of the posterior nasal cavities and olfactory sulci (left reduced relative to right). Post-hoc contrasts also indicated reduced left, but not right, olfactory cortical gray matter volume. Female CRs had no significant abnormalities, although they exhibited similar trend effects. Left olfactory bulb volume correlated, across all CR subjects, with negative, but not positive, symptoms. In a classification analysis, with 80% target specificity, olfactory measurements distinguished male CR from male LR subjects with 93% sensitivity. Among females, the comparable sensitivity was 69%.
Conclusion
Psychosis-risk youths exhibit an array of sexually dimorphic and laterally asymmetric anomalies of the peripheral olfactory system. These are consistent with a developmental disruption primarily affecting male fetuses. These structural biomarkers may enhance the identification of at-risk subjects with poor prognosis, before their clinical trajectory is apparent.
Keywords: Psychosis Risk, Olfaction, Nasal Cavity, Olfactory Bulb, Olfactory Sulcus, Negative Symptoms
1. Introduction
Olfactory deficits are a prominent feature of schizophrenia (Moberg et al., 1999). A growing body of evidence indicates that olfactory performance impairments precede the onset of overt psychosis (Kamath et al., 2012, 2014) and these may, among individuals who are at clinical high risk, be predictive of those who will develop frank psychosis (Brewer et al., 2003; Woodberry et al., 2010) or otherwise progress to a poor functional outcome (Lin et al., 2015). It is also clear that olfactory deficits are not merely specific exemplars of the relatively diffuse cognitive impairment that characterizes schizophrenia. Rather they are the consequence, at least in part, of structural and functional abnormalities of the peripheral olfactory system. These abnormalities include reduced nasal cavity volumes (Moberg et al., 2004; Turetsky et al., 2007), reduced olfactory bulb volumes (Nguyen et al., 2011; Turetsky et al., 2000), physiological and molecular anomalies of olfactory receptor neurons in the nose (Borgmann-Winter et al., 2016; Turetsky et al., 2009b), shallow olfactory sulci in the prefrontal cortex (Takahashi et al., 2013; Turetsky et al., 2009a), and reduced gray matter volumes in the primary olfactory cortex (Prasad et al., 2004; Sim et al., 2006; Turetsky et al., 2003). While olfactory deficits have been observed in both men and women, there is evidence to suggest that they may be more pronounced in males (Malaspina et al., 2012; Seidman et al., 1997; Turetsky et al., 2007).
We have hypothesized that these anomalies are markers of aberrant intrauterine neurodevelopmental processes occurring during the late first and early second trimesters of pregnancy (Turetsky et al., 2007, 2009a). This is when the neural architecture of olfactory system, the earliest and most primitive sensory network, is constructed (Farbman, 1994; Kostovic et al., 1993). This also coincides with a period of heightened fetal developmental risk for schizophrenia. Environmental stressors (e.g., maternal infection, famine) during this period increase the incidence of adult schizophrenia, presumably by altering brain development and increasing susceptibility to a subsequent “second hit” (Brown, 2006; van Os and Selten, 1998). The coincidental development of the olfactory system during this risk period makes it especially sensitive to these developmental perturbations. Olfactory anomalies may therefore serve as neural markers of an early fetal developmental disturbance. If this hypothesis is correct, it implies that structural anomalies of the olfactory system will be evident prior to the onset of clinically diagnosable signs and symptoms of schizophrenia.
There is now some evidence to support this assertion (Roalf et al., 2016; Takahashi et al., 2014). We recently examined the volumes of several temporal lobe regions in a large community-based cohort of youths who met screening criteria for psychosis spectrum features without overt psychosis (Roalf et al., 2016). The only region that differentiated this young at-risk cohort from both healthy adolescents and those with psychopathology outside the psychosis spectrum (e.g., anxiety, mood disorders) was the left entorhinal/perirhinal cortex, which is the target for primary afferent neurons from the olfactory bulb. Importantly, volume reductions in this region were associated with greater negative symptomatology and cognitive impairment, but not positive symptomatology, which is consistent with observations regarding olfactory deficits in schizophrenia.
This suggests, but does not actually establish, the existence of developmental anomalies in peripheral olfactory structures. A thorough investigation of the structural integrity of the peripheral olfactory system has never been conducted in clinical high-risk subjects, despite the evidence for olfactory behavioral impairments in this population. Here, we present a comprehensive examination of these peripheral structures in a new sample of high-risk subjects. Measures of interest include the volumes of the nasal cavities, the olfactory bulbs and the olfactory cortex, and the depths of the olfactory sulci – which develop in tandem with the underlying olfactory tracts. We consider the relationship of these structural measures to olfactory behavioral performance, and the real-world utility of these measures as biomarkers that can aid in identifying at-risk individuals. Since there is evidence supporting sexual disparities in both normal olfactory ability and schizophrenia olfactory impairments, as well as illness-related developmental anomalies in other brain regions, we focus especially on the presence or absence of anomalies compared to same-sex healthy comparison subjects. Our primary hypotheses are: 1) structural anomalies of the peripheral olfactory system will be evident in individuals at clinical high risk for psychosis, prior to the onset of an overt psychotic illness; 2) these abnormalities will be more prominent among male at-risk subjects, rather than females.
2. Methods and Materials
2.1 Subjects
Young adults and adolescents were recruited into one of two groups: 1) Clinical Risk (CR) individuals who exhibited sub-psychotic symptoms (n = 39), but did not meet DSM-IV criteria for a psychotic disorder, and 2) Low Risk (LR) comparison subjects who were symptom free, without an Axis II Cluster A diagnosis or a family history of psychosis (n = 36). Subjects ranged in age from 15 to 28. Written informed consent was obtained from all participants over the age of 18. Parental consent and the subject’s assent were obtained for those under 18.
Consensus diagnoses for all subjects were established using data gathered from either the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994) or the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al., 1997), the Family Interview for Genetic Studies (FIGS) (NIMH Genetics Initiative, 1992), and any available information from medical records, family, and care providers. Collateral information was obtained from a parent or caregiver for all participants under the age of 18. All participants were administered the Structured Interview for Prodromal Syndromes (SIPS), which included the Scale of Prodromal Symptoms (SOPS) (Miller et al., 2003).
Classification of a subject as CR required at least one positive symptom (rated 3–5 in severity) or two negative and/or disorganized symptoms (rated 3–6 in severity) on the SOPS in the six months prior to testing. Among CR subjects, 23 met inclusion criteria for both positive and negative/disorganized symptoms, 14 exhibited solely positive symptoms, while 2 exceeded threshold only for negative/disorganized symptoms.
Individuals were excluded for lack of English proficiency, any medical condition that could affect brain function, significant loss of consciousness or head trauma, current substance abuse, past substance dependence, positive urine drug screen, or any medical condition that could alter olfactory functioning (e.g., upper respiratory infection, allergies, obvious craniofacial abnormality). Individuals with an estimated IQ less than 70 (WRAT-3R) (Wilkinson, 1993) were also excluded. No subjects had a history of past or current treatment with psychiatric medications.
Descriptive clinical and demographic measures are presented in Table 1. CR and LR groups did not differ in age [t(73)=1.67, p=.10], sex [χ2(1)=0.59, p=.44], racial composition [χ2(2)=3.73, p=.16] or smoking status [χ2(1)=3.04, p=.08]. When males and females were examined separately, however, there was a significant difference in smoking status for females [χ2(1)=3.94, p=.05] but not males [χ2(1)=0.37, p=.54]. CRs had lower levels of education [t(72)=3.50, p=.0008] and parental education [mothers: t(72)=2.00, p=0.049; fathers: t(67)=2.94, p=.004]. Not surprisingly, they also differed in levels of clinical symptomatology, as denoted by both SOPS and Global Assessment of Functioning (GAF) ratings. Within the CR group, there were no differences in clinical symptomatology between males and females.
Table 1.
Demographic and Clinical Characteristics
| Clinical Risk (n = 39) Mean ± SD (Range) |
Low Risk (n = 36) Mean ± SD (Range) |
|
|---|---|---|
|
|
|
|
| Age (years) | 19.9 ± 2.64 (16 – 24) | 21.0 ± 2.5 (15 – 25) |
| Sex (Male : Female) | 24 : 15 | 19 : 17 |
| Race (Cauc., Afr. Amer., Other) | 10 : 21 : 8 | 16 : 12 : 8 |
| Education level (years)a | 11.9 ± 2.0 | 13.8 ± 2.6 |
| Mother Education (years)a | 14.2 ± 2.3 | 15.3 ± 2.4 |
| Father Education (years)a | 13.7 ± 2.8 | 15.7 ± 2.7 |
| Smoker : Nonsmoker (#) | 12 (7M/5F) : 27 (17M/10F) | 5 (4M/1F) : 31 (15M/16F) |
| SOPS:b | ||
| Total Scorea | 33.3 ± 15.5 | 3.2 ± 4.9 |
| Positive Subscalea | 12.6 ± 5.4 | 1.0 ± 2.0 |
| Neg./Disorg. | 15.0 ± 8.6 | 1.5 ± 2.0 |
| Subscalesa | ||
| GAFc Scorea | 56.0 ± 11.2 | 85.5 ± 6.5 |
Significant group difference (p < 0.05)
SOPS = Scale of Prodromal Symptoms
GAF = Global Assessment of Functioning
2.2 Olfactory Psychophysical Assessments
2.2.1 Odor Detection Thresholds
Odor detection ability was assessed using two different odorants, lyral and citralva. Odors were presented birhinally in a single reversing staircase, forced-choice task format, with the order counterbalanced across subjects. Two vials, one containing mineral oil and the other containing odorant diluted in mineral oil, were presented sequentially to the subject’s nares. The subject was asked to identify the vial that “smells stronger.” Odor concentrations ranged from 10−1 M (strongest) to 10−9 M (weakest) in 0.5 log step dilution increments. The test began at the 10−5 M step, and odor concentration was increased in full-molar steps until correct detection occurred on five consecutive trials at a given concentration. Odor concentration was then increased or decreased in half-molar increments, depending upon performance on two trials at each concentration step (i.e., odor concentration was decreased after two correct trials and increased after an incorrect trial). The geometric mean of the last four staircase reversal points (out of seven total) was taken as the estimate of odor detection threshold sensitivity (i.e., the weakest odor concentration that could be reliably identified as stronger than mineral oil). Results for the two odorants were averaged to yield a composite detection threshold measure. Test data were unavailable for four CR and four LR subjects.
2.2.2 Odor Identification and Discrimination
Participants were administered the Sniffin’ Sticks Odor Identification and Discrimination Test (Hummel et al., 1997; Kobal et al., 1996). In the identification task, subjects were presented with 16 odor-impregnated markers which they smelled and then identified in a four-alternative multiple choice format. In the 16-trial discrimination test, subjects were presented with three odors on each trial. They then indicated which one odor differed from the other two. The score for each task was the number correct (0–16). These were combined to yield a composite odor processing score. Data were unavailable for three CR and three LR subjects.
2.3 Nasal Cavity Volume
Morphologic measurements of each nasal cavity were acquired using a Sleep Group Solutions Eccovision™ Acoustic Rhinometer. This device transmits an acoustic pulse which is reflected off walls of the nasal cavity and transmitted back to the device. The time-lagged reflected wave provides a measurement of acoustic impedance, which is proportional to cross-sectional area. By summing cross-sectional area measurements along the length of the nasal cavity, a volume measurement is obtained. Two independent measurements were averaged for each nostril. Consistent with standard practice (Clement and Gordts, 2005; Hilberg and Pedersen, 2000), total nasal volume was divided into anterior (0–3.5 mm) and posterior (3.5–5.5 mm) compartments. This break-point typically denotes the anterior end of the inferior turbinate. The nasal cavity anterior to this location is vulnerable to mucosal congestive changes caused by infection, pollutants or smoke irritation; the posterior region, being more cartilaginous, is relatively resistant to such changes. The posterior cavity is similarly less sensitive to racial and/or ethnic differences in nasal structure.
2.4 Olfactory Bulb Volume
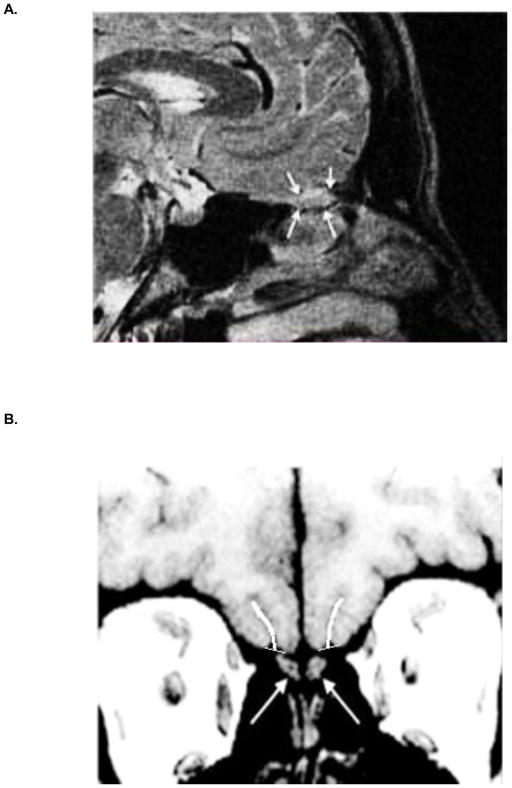
MRIs were acquired on a 3T Siemens Tim Trio whole-body scanner equipped with a 32-channel head coil. High-resolution images of the olfactory bulbs were acquired using a T2-weighted three dimensional multi-slice turbo-spin echo sequence: TR 9000 ms; TE 63 ms; bandwidth 190 Hz/pixel; FOV, 256 × 256 mm; 60 slices; flip angle, 180°; effective voxel resolution, 0.8 × 0.8 × 0.8 mm. An automated process was employed to select the FOV during acquisition, based on a previously acquired whole-brain scan. This was accomplished in real-time using the Imscribe tool, which was designed to allow reproducible selection of the same anatomical FOV within and between subjects (cmroi.med.upenn.edu/imscribe). When necessary, manual adjustments were made to ensure coverage of bilateral olfactory bulbs. Scan time was approximately 4 minutes. Bilateral olfactory bulb volumes were extracted by manual tracing of the bulb contour on multiple slices in the sagittal plane (Figure 1A). Inter-rater reliability >0.85 was established on a set of ten training scans, after which all tracings were completed by a single operator blind to diagnosis, using manual drawing and statistics tools in FSL (http://fsl.fmrib.ox.ac.uk/fsl/).
Figure 1.
(A) Sagittal magnetic resonance image through the olfactory bulb (arrows) sitting beneath the basal forebrain. (B) Coronal MR slice showing left and right olfactory bulbs (arrows) and the overlying olfactory sulci, with depth tracings.
2.5 Olfactory Sulcal Depth
Whole brain structural images were acquired using a T1-weighted MPRAGE sequence: TR 1850 ms; TE 4.0 ms; FOV, 192 × 256 mm; matrix, 180 × 240; 160 slices; slice thickness/gap, 1/0 mm; flip angle, 9°; effective voxel resolution, 0.9375 × 0.9375 × 1 mm. Scan time was approximately 5 minutes. All MPRAGE images were AC-PC aligned and resampled for quantification. Resampled images were viewed in the coronal plane and left and right olfactory sulci were traced using ImageJ software (https://imagej.nih.gov), beginning just caudal to the eyeballs and progressing posteriorly to the tip of the temporal horn. A single voxel-width line that followed the contour of each sulcus was drawn by a single operator trained to an inter-rater reliability >0.85 on a set of ten training scans. All drawings were made blind to diagnosis, beginning at a tangent line connecting the most superficial surfaces of the gyrus rectus and medial orbital gyrus and ending at the deepest point of the sulcus (Figure 1B). The length of this line, averaged across all slices, was computed as the measure of sulcal depth.
2.6 Olfactory Cortex Volume
Cortical reconstruction and measurement of the whole brain T1-weighted structural images was performed using FreeSurfer 5.3 (http://surfer.nmr.mgh.harvard.edu/). Regions of interest (ROIs) were automatically segmented from each subject’s MPRAGE (Fischl et al., 2004). Intra-cranial volume (ICV) was obtained using FreeSurfer’s process of estimation based on linear transformation to MNI305 space (Buckner et al., 2004). Automated quality assurance procedures and visual inspection of the resulting gray-white matter segmentation were performed on all images (Vandekar et al., 2015). Measures of left and right hemisphere olfactory cortical gray matter volume were extracted for further analysis (ROI designated as entorhinal cortex in Freesurfer but, as delineated, also incorporates perirhinal cortex).
2.7 Data Analysis
Statistical analyses were conducted using STATISTICA version 13 (https://software.dell.com/products/statistica/). Differences in age, education, parental education and clinical ratings were assessed using independent group t-tests. Pearson’s chi-squares assessed group differences for sex, race and smoking status. Multivariate analyses of variance (MANOVA) were used to assess group differences for all continuous variables, with sex and clinical group (CR vs. LR) as between-subjects factors. Olfactory task (identification vs. discrimination) was a within-subject factor for the psychophysical behavioral tests. Odorant (lyral vs. citralva) was a within-subject factor for threshold detection sensitivity. Laterality (left vs. right) was a within-subject factor for each structural measure, and total intracranial volume was a covariate. Compartment (anterior vs. posterior) was an additional within-subject factor for nasal cavity volume. Post-hoc analyses examined CR vs. LR contrasts on individual measures, separately for males and females. The relationships between clinical and olfactory measures, within the CR sample, were examined via Pearson correlation coefficients.
To assess the “real-world” utility of these measures to classify subjects as either CR or LR, logistic regression analyses were conducted separately within male and female subsamples. The initial analysis included the four psychophysical test measures as independent predictors of group status. A second analysis assessed the utility of the olfactory structural measures, and a third examined the combined effect of both behavioral and structural measures. A ROC curve, which plots true positive rate (sensitivity) against false positive rate (1 – specificity), was generated for each analysis, and the area under the curve (AUC) was computed. Prediction accuracy of a parameter set increases as the AUC approaches 1.0 (Zweig and Campbell, 1993). AUC provided a quantitative index of each parameter set’s ability to distinguish clinical high-risk from low-risk youth.
3. Results
3.1 Psychophysical Tests
3.1.1 Odor Discrimination and Identification
There was a marginally significant group difference in identification and discrimination performance (CR<LR) [F(1,67)=3.87, p=0.05]. Scores were better, overall, on the identification task [F(1,67)=21.06, p<0.0001] but there was no interaction between task and group [F(1,67)=0.58, p=0.45]. Separate within-sex contrasts revealed that olfactory performance was impaired in male CRs [F(1,39)=4.62, p=0.04], but not females [F(1,26)=0.005, p=0.95] (Table 2).
Table 2.
Olfactory Behavioral and Structural Measures Mean ± S.D.
| Males | Females | |||
|---|---|---|---|---|
| Clinical Risk | Low Risk | Clinical Risk | Low Risk | |
| Psychophysical Measurements | ||||
| Sniffin’ Sticks Tests (# correct) | ||||
| Odor Identification | 11.3 ± 2.8 | 12.9 ± 2.2 | 12.0 ± 2.2 | 12.5 ± 1.8 |
| Odor Discrimination | 9.8 ± 2.7 | 11.1 ± 2.3 | 11.2 ± 2.1 | 10.8 ± 2.3 |
| Composite Score | 21.2 ± 4.5 | 24.1 ± 3.8 | 23.2 ± 3.7 | 23.3 ± 2.7 |
| Threshold Detection (−log vol/vol) | ||||
| Citralva | 3.27 ± 0.89 | 3.52 ± 0.91 | 3.97 ± 1.19 | 3.91 ± 1.04 |
| Lyral | 2.69 ± 0.93 | 3.04 ± 1.00 | 2.60 ± 0.93 | 3.17 ± 1.15 |
| Composite Threshold | 2.98 ± 0.70 | 3.28 ± 0.65 | 3.29 ± 0.60 | 3.54 ± 0.74 |
| Structural Measurements | ||||
| Olfactory Bulb Volume (mm3) | ||||
| Left | 65.7 ± 34.2 | 104.0 ± 27.5 | 89.5 ± 38.2 | 100.1 ± 50.8 |
| Right | 71.2 ± 43.1 | 110.8 ± 45.9 | 88.3 ± 48.8 | 96.1 ± 51.0 |
| Olfactory Sulcal Depth (mm) | ||||
| Left | 10.06 ± 1.43 | 10.37 ± 1.34 | 9.21 ± 1.04 | 10.05 ± 1.19 |
| Right | 10.55 ± 1.13 | 10.44 ± 1.34 | 9.54 ± 0.84 | 10.07 ± 1.34 |
| Nasal Cavity Volume (cc3) | ||||
| Left Anterior | 3.10 ± 1.03 | 2.99 ± 0.94 | 2.83 ± 0.53 | 3.07 ± 0.87 |
| Right Anterior | 3.47 ± 1.39 | 2.87 ± 0.98 | 3.14 ± 0.96 | 3.15 ± 0.76 |
| Left Posterior | 2.88 ± 1.89 | 2.91 ± 1.57 | 2.32 ± 1.02 | 3.17 ± 1.65 |
| Right Posterior | 3.68 ± 2.11 | 2.74 ± 2.07 | 2.86 ± 1.06 | 2.86 ± 1.14 |
| Olfactory Cortex Volume (mm3) | ||||
| Left Hemisphere | 1793.9 ± 278.6 | 1982.7 ± 268.4 | 1578.3 ± 358.5 | 1634.2 ± 266.9 |
| Right Hemisphere | 1667.3 ± 324.4 | 1759.1 ± 404.0 | 1478.6 ± 352.0 | 1502.0 ± 292.6 |
3.1.2 Odor Threshold Detection Sensitivity
There was a marginally significant group difference in threshold detection sensitivity [F(1,65)=3.66, p=0.05]. This reflected, primarily, a CR decrement in the ability to detect lyral [F(1,65)=3.45, p=0.06], while the ability to detect citralva was preserved [F(1,65)=0.73, p=0.39]. Male and female CRs exhibited similar response profiles (Table 2).
3.2 Olfactory Bulb Volume
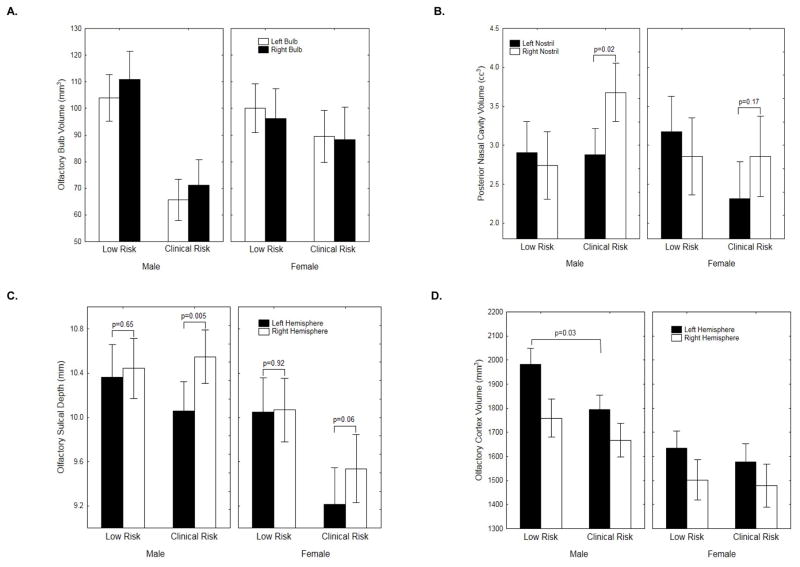
CRs exhibited significant bilateral reductions in olfactory bulb volume [F(1,73)=8.11, p=0.006]. Co-varying for total intracranial volume did not affect this. Within-sex comparisons revealed that bulb volumes were significantly smaller in male CRs [F(1,41)=12.55, p=0.001], but not females [F(1,30)=0.32, p=0.57]. On a percentage basis, male CR bulb volumes were 36% smaller than LR males (Figure 2A).
Figure 2.
Means and standard errors for the various olfactory structural measures, plotted by group, sex and laterality: (A) olfactory bulb volume; (B) posterior nasal cavity volume; (C) olfactory sulcal depth; (D) olfactory cortex gray matter volume.
3.3 Nasal Cavity Volume
There was no overall group difference in nasal cavity volume [F(1,63)=0.27, p=0.60]. There were, however, significant group X nostril [F(1,63)=5.86, p=0.018] and group X nostril X compartment [F(1,63)=4.53, p=0.037] interactions. CRs exhibited a prominent left-right asymmetry (left nostril volume smaller than right) [F(1,34)=8.30, p=0.006], which was more pronounced in the posterior compartment [nostril x compartment Interaction: F(1,34)=5.68, p=0.02]. LRs showed no evidence of a lateralized left vs. right nostril difference [F(1,29)=0.42, p=0.52] (Figure 2B). Co-varying for total intracranial volume did not alter these findings. Follow-up analyses revealed that this posterior left-right asymmetry was significant for CR males [t(22)=2.53, p=0.02] but not CR females [t(11)=1.45, p=0.17].
3.4 Olfactory Sulcal Depth
There was a significant hemispheric asymmetry [F(1,73)=7.47, p=0.008], with left olfactory sulcus shallower than right. There was also a significant group X hemisphere interaction [F(1,73)=4.64, p=0.03]. As illustrated (Figure 2C), this left-right asymmetry was significant in CRs [t(38)=3.73, p=0.0006] but not LRs [t(35)=0.38, p=0.71]. Within the CR sample, this asymmetry was again more robust in males [t(23)=3.11, p=0.005], though females also demonstrated a marginal effect [t(14)=2.02, p=0.06].
3.5 Olfactory Cortex Volume
There were no overall group [F(1,71)=1.93, p=0.17] or group X hemisphere [F(1,71)=0.74, p=0.39] effects. However, there were robust main effects of both sex [F(1,71)=15.2, p=0.0002] and hemisphere [F(1,71)=14.96, p=0.0002]. In paired contrasts, CR males had less olfactory gray matter volume on the left [F(1,41)=5.03, p=0.03] but not the right [F(1,41)=0.68, p=0.41]. CR females were indistinguishable from LR females [Left: F(1,30)=0.25, p=0.62; Right: F(1,30)=0.04, p=0.84]. (Figure 2D)
3.6 Relationship of Olfactory Structural Anomalies to Olfactory Behavioral Measures
There was a significant association, in CR subjects, between abnormal posterior nasal cavity asymmetry and impaired olfactory identification performance (r = .54, p=0.001). When segregated by gender, this relationship persisted for males (r = 0.57, p=0.004) but not females (r = 0.42, p=0.169). There were no other significant cross-modal correlations within the CR sample. However, among male LR subjects, there was a significant association between posterior nasal cavity asymmetry and lyral threshold detection performance (r = −0.56, p=0.031), with reduced (i.e., more normal) asymmetry associated with better lyral detection. This normative relationship was disrupted in male CRs (r = 0.07, p=0.77).
3.7 Relationship of Structural to Clinical Measures
Among the CR subjects, left olfactory bulb volume correlated significantly with both total score on the SOPS clinical rating scale (r= −0.34, p=0.036), and with the negative/disorganized subscale score (r = −0.36, p=0.024). Subjects with greater clinical symptomatology had smaller left bulb volumes. Right bulb volume exhibited similar, but non-significant, trends (total SOPS score: r= −0.27, p=0.100; negative/disorganized score: r= −0.28, p=0.082). SOPS positive symptom scores were unrelated to bulb volume (left bulb: r= −0.18, p=0.280; right bulb: r= −0.19, p=0.258). None of the other olfactory structural or behavioral measures correlated with clinical symptomatology.
3.8 Classification Sensitivity and Specificity of Olfactory Measures
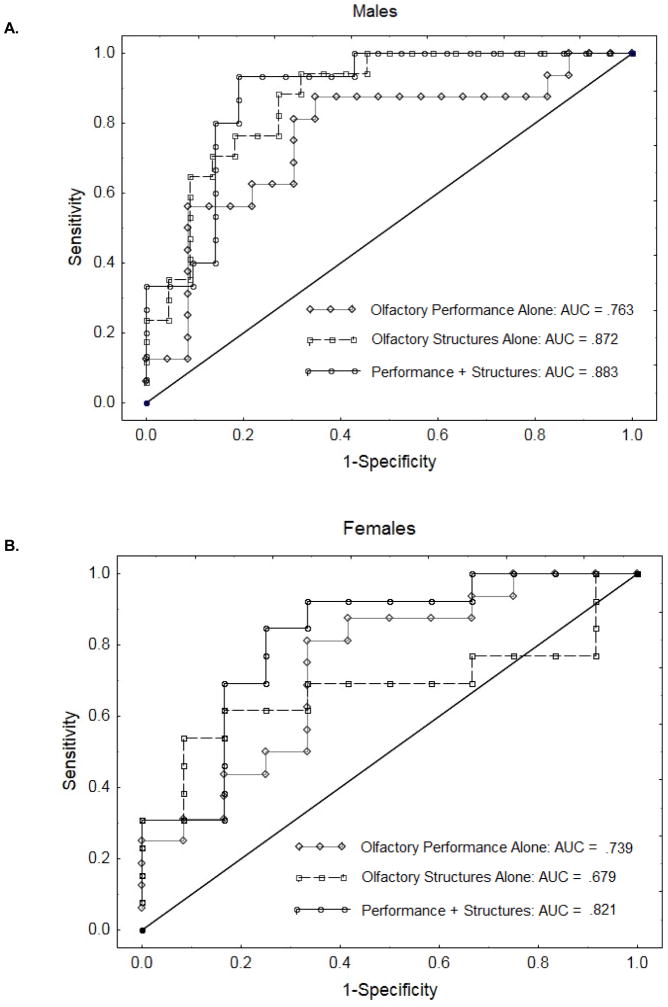
For male subjects, logistical regression analysis with the four behavioral olfactory measures (identification, discrimination, lyral threshold, citralva threshold) as predictors of group membership yielded an area under the curve (AUC) of 0.763. As illustrated (Figure 3A), with targeted specificity greater than 0.80, these behavioral measures could discriminate male CRs from male LRs with 0.56 sensitivity. A comparable model using four significant structural measures (mean olfactory bulb volume, left olfactory cortex volume, olfactory sulcal depth asymmetry (left – right), posterior nasal cavity asymmetry) as independent predictors yielded AUC = 0.872, with sensitivity = 0.76 for specificity > 0.80. Combining behavioral and structural measures in one model resulted in AUC = 0.883 and sensitivity = 0.93 for specificity >0.80.
Figure 3.
ROC curves (A) male subjects and (B) female subjects, illustrating the sensitivity vs. (1-specificity) for correct prediction of group membership, based on olfactory behavioral tests alone, olfactory structural measures alone, or the combination of both behavior and structure.
A parallel analysis in female subjects yielded AUC = 0.739 and sensitivity = 0.44 for the behavioral measures. For structural measures alone, AUC was 0.679 and sensitivity at 80% specificity was 0.61. Although sensitivity was higher, the overall fit for the structural model, as denoted by the AUC, was lower. The Receiver Operating Characteristic (ROC) curve (Figure 3B) illustrates why: any further increase in sensitivity beyond this level required a nearly complete loss of specificity. The combined behavioral plus structural model, however, yielded both a substantially higher AUC (0.821) and a higher sensitivity (0.69) for the targeted 0.80 specificity. This combined model outperformed both of the individual models at all points along the sensitivity-specificity continuum.
4. Discussion
In this study, adolescents at clinical high risk for psychosis exhibited a relatively robust and consistent profile of structural anomalies linked to development of the olfactory system. Abnormalities were observed at all levels of the ascending olfactory system, including the nasal cavities, the olfactory bulbs, the overlying olfactory sulcus in the forebrain, and the primary olfactory cortex in the medial temporal lobe. However, these abnormalities were more prominent in peripheral structures, such as the olfactory bulb, than the cortex. To illustrate, on a percentage basis, the left olfactory bulb was 36% smaller in male CR compared to male LR subjects. The left olfactory cortex decrement, in these same subjects, was only 10%. Clinically, olfactory bulb volume reduction was related specifically to negative/disorganized, but not positive, symptoms. These findings broadly mirror previous observations in established schizophrenia patients, who also exhibit abnormalities in each of these structures (Turetsky et al., 2000, 2003, 2007, 2009a), and whose olfactory impairments have been similarly linked to negative symptoms (Moberg et al., 1999). Similarly, among the established schizophrenia patients, olfactory bulb volume was reduced by 23% (Turetsky et al., 2000), while the olfactory cortex was reduced by 8% (Turetsky et al., 2003). This suggests that peripheral measures, such as bulb volume, may be more sensitive developmental risk markers than cortical volume measures. It is also notable that, in this high-risk sample, structural abnormalities were unequivocal despite the fact that there were only marginal decrements on olfactory psychophysical tests. This suggests that olfactory structural changes may be somewhat independent, and perhaps more sensitive, indicators of clinical risk status than olfactory behavioral decrements. This is borne out by the ROC analyses, which demonstrated enhanced classification sensitivity for structural, compared to performance, measures.
There are two striking aspects of these findings. One is the extent to which they manifested not as overall volume decrements but as abnormal patterns of left-right asymmetries, consistent with focal left-sided disturbances. The only structures that did not exhibit clearly lateralized disruption were the olfactory bulbs, which are located adjacent to the midline and each other. Yet even in this case, the association with clinical severity was evident for the left, but not right, bulb. The second is the extent to which these abnormalities were confined, almost exclusively, to male CR subjects. Deficits in males were quite strong across all olfactory structures, while females showed only sporadic marginal effects that failed to reach statistical significance.
These two aspects – sex specificity and anomalous asymmetry – are both consistent with, and provide support for, the hypothesis that these structural anomalies are byproducts of an early developmental disturbance. It is a clinical truism that schizophrenia often presents quite differently in men and women, with some even questioning whether they share the same illness (Häfner, 2002). Likewise, the idea that schizophrenia is characterized by a disruption of the normal emergent pattern of cerebral asymmetry, which preferentially alters left hemisphere development (especially of the temporal lobes) and leads to more prominent left hemisphere dysfunction, is a long-standing one for which there is substantial evidence (Crow et al., 1989).
Normal developmental brain asymmetry is highly sexually dimorphic, with male brains exhibiting greater lateral asymmetry. Recent studies have suggested that sex differences in cortical development and plasticity may exist not only at the level of gross structure and circuitry, but even at the level of synaptic mechanisms (Dachtler and Fox, 2017). Sex differences can be detected in MRI scans of fetal brains as early as 21 weeks gestational age (Kyriakopoulou et al., 2016). In schizophrenia, structural abnormalities are also commonly sex-specific (Gur et al., 2004; Mendrek and Mancini-Marïe, 2016), with men exhibiting more numerous and more severe anomalies (Leung and Chue, 2000). Structural abnormalities are also more common in patients with prominent negative symptoms (Medkour et al., 2010), a clinical feature that is similarly more prevalent and more severe in men (Drake et al., 2016). Although these disease-related developmental anomalies have been linked to fetal and perinatal insults, such as prenatal infections and obstetrical complications, there is no evidence that these environmental events actually occur more frequently in male vs. female patients – though they may have a differential impact (Dalman et al., 1999; Debost et al., 2017). Rather, the regions that most often exhibit sex-specific abnormalities in schizophrenia tend to be the same ones that exhibit normal sexual dimorphisms (Abel et al., 2010; Goldstein et al., 2002). This suggests that the developmental factors that contribute to typical genetically and hormonally-mediated sex differences also modulate the development of brain abnormalities in the illness (Abel et al., 2010).
Consistent with this there is a growing body of literature, derived primarily from animal models, which clearly demonstrates that comparable prenatal insults have a greater negative impact on the developing male brain compared to the female brain (Llorente et al., 2009; Nunez et al., 2003. Santos-Galindo et al., 2011; Wischhof et al., 2015; Wynne et al., 2011). One intriguing aspect of these model systems is that the emerging effects of the prenatal insults are often not clearly evident until subsequent adolescent brain maturation. These prenatal insults appear to alter the normal trajectory of subsequent adolescent brain development. This delayed effect makes these models especially relevant as developmental models of emerging psychosis. Mechanistically, it is thought that prenatal insults can induce epigenetic changes which then alter subsequent genetically-mediated developmental changes. There is now evidence to support the idea that prenatal inflammatory processes, which are increasingly recognized as important mediators of psychosis risk (Miller and Goldsmith, 2017), can induce such epigenetic changes (Basil et al., 2014). While it in not clear, at this point, why the effect of these insults are enhanced in male offspring, recent data indicate that pro-inflammatory cytokines are enhanced in the brains of male fetuses following maternal infection (Makinson et al., 2017), thus providing a mechanistic link for the sex-specific impact of intrauterine insults.
It is important to note, in this context, that the normal developmental course of the peripheral olfactory system is both sexually dimorphic and laterally asymmetric. Olfactory structures and processes are typically enhanced in women compared to men (Alizadeh et al., 2015; Doty et al., 1984; Garcia-Falgueras et al., 2006; Oliveira-Pinto et al., 2014), and on the right side compared to the left (Brand et al., 2002; Frasnelli et al., 2010; Hummel et al., 2003; Zatorre et al., 1992). Our findings are thus consistent with a neurodevelopmental disease model in which olfactory structures emerge, coincidentally, during an established period of embryonic disease vulnerability. Their developmental trajectory is disrupted at that point, presumably by environmental stressors interacting with genetic susceptibility, with a more significant impact on male fetal development (Ursini et al., 2016). This results in a set of olfactory abnormalities that, like other disease-related brain anomalies, are consonant with their own sexually dimorphic and laterally asymmetric character and are associated with negative, rather than positive, symptoms.
Definitive proof of this early developmental hypothesis would require MRI scans acquired well before the emergence of clinical high-risk symptoms. However, as is true for total cranial size, it is difficult to explain differences in the bony/cartilaginous structure of the nasal cavity as anything other than a developmental anomaly. Without more longitudinal data, though, we cannot rule out the possibility that these abnormalities emerge during childhood growth periods, rather than prenatally. Nevertheless, our findings clearly establish that structural disruptions of the olfactory system are present during the psychosis risk period, prior to the emergence of any diagnosable psychotic illness, and that they are relatively more sensitive indicators of this clinical state than olfactory performance alone.
There are several “real world” implications of these findings. During the pre-psychosis risk period, young adults can present with a highly variable mélange of symptoms. In addition to sub-psychotic symptoms, they may exhibit depression, anxiety, attention deficit, and conduct disorder symptoms (Calkins et al., 2014). Reliable identification of a “true” psychosis prodrome that presages the onset of a psychotic illness, especially early in its course, can be a challenge. The ROC curves demonstrate that, among males at least, these olfactory measures – which are not subject to the temporal variability of clinical symptoms – are both sensitive and specific markers of clinical risk status. In cases where the diagnosis of clinical high-risk is unclear, these olfactory measures may serve as secondary or confirmatory classification tools which can be applied following a suggestive or inconclusive clinical assessment. The presence of robust abnormalities would increase confidence in the at-risk diagnosis, while an entirely normal profile might help to identify false positive cases and allay some clinical concerns.
Additionally, these measures may be useful even when the psychosis risk diagnosis is unambiguous. Diagnostic criteria for the clinical risk state typically focus on subthreshold positive psychosis symptoms. However, in this study, we included an additional set of diagnostic criteria based solely on negative/disorganized symptoms. Twenty five subjects (64%) exceeded this diagnostic threshold, all but two of whom also exceeded the positive symptom threshold. This indicates that negative symptoms, which have been associated with greater functional impairment and poorer clinical prognosis, are variably present during the clinical risk period. Olfactory deficits during this period have also been associated with poorer long-term outcome (Lin et al., 2015). The link between these two sets of measures – olfactory abnormalities and negative symptoms – is well established in schizophrenia. Consistent with this, we observed an association between negative symptoms and olfactory bulb volume in the clinical risk sample, despite the relatively reduced range of negative symptoms in subthreshold psychosis. If the association between olfactory measures and poor functional prognosis is valid, then a measure such as olfactory bulb volume could serve as an objective anatomical marker to stratify individuals, even in the very early stages of psychosis risk, before behavioral symptom trajectories are established, to identify those with especially worrisome pre-clinical presentations.
Ultimately, the predictive utility of these measures will require longitudinal follow-up to determine subjects’ long-term clinical outcomes. The absence of outcome data, at this point, is the major limitation of this cross-sectional study. Additionally, although we found no difference in the relative number of smokers in the male CR and LR groups, we cannot rule out the possibility that male CRs smoked more heavily than male LRs, and that this may have had a differential impact on their olfactory structures. However, we are impressed by the consistent parallels between what we observe here in the pre-psychotic risk period and what has been reported in schizophrenia patients, independent of smoking. Identifying these neurobiological markers prior to the emergence of super-threshold symptomatology may enhance our predictive capability and facilitate earlier intervention.
Acknowledgments
Role of the Funding Source
The sponsors had no influence on the design of the study, on the analysis and interpretation of the data, or on the writing and submission of this article for publication.
This work was supported by National Institute of Mental Health grants R01MH099156 (BIT), K01MH102609 (DRR), and RC2MH089983 (REG).
Footnotes
Conflict of Interest
All authors report that they have no biomedical financial interests or potential conflicts of interest.
Contributors
BIT, PJM and DRR designed the study. MEC and REG designed the clinical assessment protocol, oversaw subject recruitment and assessment, and established clinical diagnoses. MQ, ED, KR and KP collected and processed the data. BIT, PJM and DRR analyzed the data and interpreted the results. BIT wrote the manuscript. All authors critically revised the manuscript and approved it for submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Alizadeh R, Hassanzadeh G, Soleimani M, Joghataei MT, Siavashi V, Khorgami Z, Hadjighassem M. Gender and age related changes in number of dopaminergic neurons in adult human olfactory bulb. J Chem Neuroanat. 2015;69:1–6. doi: 10.1016/j.jchemneu.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Basil P, Li Q, Dempster EL, Mill J, Sham PC, Wong CC, McAlonan GM. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry. 2014;4:e434. doi: 10.1038/tp.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmann-Winter KE, Wang HY, Ray R, Willis BR, Moberg PJ, Rawson NE, Gur RE, Turetsky BI, Hahn CG. Altered G protein coupling in olfactory neuroepithelial cells from patients with schizophrenia. Schizophr Bull. 2016;42:377–385. doi: 10.1093/schbul/sbv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand G, Millot JL, Saffaux M, Morand-Villeneuve N. Lateralization in human nasal chemoreception: differences in bilateral electrodermal responses related to olfactory and trigeminal stimuli. Behav Brain Res. 2002;133:205–210. doi: 10.1016/s0166-4328(01)00474-0. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, Anderson V, Copolov DL, Singh B, Velakoulis D, Pantelis C. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, Ruparel K, Chiavacci R, Wolf DH, Mentch F, Qiu H, Connolly JJ, Sleiman PA, Hakonarson H, Gur RC, Gur RE. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement PAR, Gordts F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology. 2005;43:169–179. [PubMed] [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia as an anomaly of development of cerebral asymmetry. Arch Gen Psychiatry. 1989;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Dachtler J, Fox K. Do cortical plasticity mechanisms differ between males and females? J Neurosci Res. 2017;95:518–526. doi: 10.1002/jnr.23850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman C, Allebeck P, Cullberg J, Grunewald C, Köster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- Debost JP, Larsen JT, Munk-Olsen T, Mortensen PB, Meyer U, Petersen L. Joint effects of exposure to prenatal infection and peripubertal psychological trauma in schizophrenia. Schizophr Bull. 2017;43:171–179. doi: 10.1093/schbul/sbw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Sikorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Drake RJ, Addington J, Viswanathan AC, Lewis SW, Cotter J, Yung AR, Abel KM. How age and gender predict illness course in a first-episode nonaffective psychosis cohort. J Clin Psychiatry. 2016;77(3):e283–289. doi: 10.4088/JCP.14m09369. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Developmental biology of olfactory sensory neurons. Semin Cell Biol. 1994;5:3–10. doi: 10.1006/scel.1994.1002. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Lundström JN, Boyle JA, Djordjevic J, Zatorre RJ, Jones-Gotman M. Neuroanatomical correlates of olfactory performance. Exp Brain Res. 2010;201:1–11. doi: 10.1007/s00221-009-1999-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Junque C, Giménez M, Caldú X, Segovia S, Guillamon A. Sex differences in the human olfactory system. Brain Res. 2006;1116:103–111. doi: 10.1016/j.brainres.2006.07.115. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS, Jr, Faraone SV, Tsuang MT. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59(2):154–64. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler C, Turetsky BI, Siegel SJ, Kanes SJ, Bilker WB, Brennan AR, Gur RC. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol Psychiatry. 2004;55(5):512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Häfner H. Schizophrenia: do men and women suffer from the same disease? Rev Psiq Clin. 2002;29:267–292. [Google Scholar]
- Hilberg O, Pedersen OF. Acoustic rhinometry: recommendations for technical specifications and standard operating procedures. Rhinology. 2000;16:3–17. [PubMed] [Google Scholar]
- Hummel T, Damm M, Vent J, Schmidt M, Theissen P, Larsson M, Klussmann JP. Depth of olfactory sulcus and olfactory function. Brain Res. 2003;975(1–2):85–89. doi: 10.1016/s0006-8993(03)02589-7. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Kamath V, Moberg PJ, Calkins ME, Borgmann-Winter K, Conroy CG, Gur RE, Kohler CG, Turetsky BI. An odor-specific threshold deficit implicates abnormal cAMP signaling in youths at clinical risk for psychosis. Schizophr Res. 2012;138(2–3):280–284. doi: 10.1016/j.schres.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Turetsky BI, Calkins ME, Kohler CG, Conroy CG, Borgmann-Winter K, Gatto DE, Gur RE, Moberg PJ. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World J Biol Psychiatry. 2014;15(3):209–218. doi: 10.3109/15622975.2011.615862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- Kostovic I, Petanjek Z, Judas M. Early areal differentiation of the human cerebral cortex: entorhinal area. Hippocampus. 1993;4:447–458. doi: 10.1002/hipo.450030406. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulou V, Vatansever D, Davidson A, Patkee P, Elkommos S, Chew A, Martinez-Biarge M, Hagberg B, Damodaram M, Allsop J, Fox M, Hajnal JV, Rutherford MA. Normative biometry of the fetal brain using magnetic resonance imaging. Brain Struct Funct. 2016 doi: 10.1007/s00429-016-1342-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand. 2000;401(Supplementum):3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lin A, Brewer WJ, Yung AR, Nelson B, Pantelis C, Wood SJ. Olfactory identification deficits at identification as ultra-high risk for psychosis are associated with poor functional outcome. Schizophr Res. 2015;161:156–162. doi: 10.1016/j.schres.2014.10.051. [DOI] [PubMed] [Google Scholar]
- Llorente R, Gallardo ML, Berzal AL, Prada C, Garcia-Segura LM, Viveros MP. Early maternal deprivation in rats induces gender-dependent effects on developing hippocampal and cerebellar cells. Int J Dev Neurosci. 2009;27:233–241. doi: 10.1016/j.ijdevneu.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Makinson R, Lloyd K, Rayasam A, McKee S, Brown A, Barila G, Grissom N, George R, Marini M, Fabry Z, Elovitz M, Reyes TM. Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.07.016. S0889-1591(17)30232-5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Keller A, Antonius D, Messinger JW, Goetz DM, Harkavy-Friedman J, Goetz RR, Harlap S. Olfaction and cognition in schizophrenia: sex matters. J Neuropsychiatry Clin Neurosci. 2012;24(2):165–175. doi: 10.1176/appi.neuropsych.11070154. [DOI] [PubMed] [Google Scholar]
- Medkour T, Walden AT, Burgess AP, Strelets VB. Brain connectivity in positive and negative syndrome schizophrenia. Neuroscience. 2010;169:1779–1788. doi: 10.1016/j.neuroscience.2010.05.060. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Mancini-Marïe A. Sex/gender differences in brain and cognition in schizophrenia. Neurosci Biobehav Rev. 2016;67:57–78. doi: 10.1016/j.neubiorev.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Goldsmith DR. Towards an immunophenotype of schizophrenia: Progress, potential mechanisms, and future directions. Neuropsychopharmacology. 2017;42:299–317. doi: 10.1038/npp.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Roalf DR, Gur RE, Turetsky BI. Smaller nasal volumes as stigmata of aberrant neurodevelopment in schizophrenia. Am J Psychiatry. 2004;161:2314–2316. doi: 10.1176/appi.ajp.161.12.2314. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Pelavin PE, Shenton ME, Chilakamarri P, McCarley RW, Nestor PG, Levitt JJ. Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: an MRI study. Brain Imaging Behav. 2011;5(4):252–261. doi: 10.1007/s11682-011-9129-0. [DOI] [PubMed] [Google Scholar]
- NIMH Genetics Initiative. Family Interview for Genetic Studies (FIGS) National Institute of Mental Health; Rockville MD: 1992. [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A novel model for prenatal brain damage. II Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exp Neurol. 2003;181:270–280. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Oliveira-Pinto AV, Santos RM, Coutinho RA, Oliveira LM, Santos GB, Alho AT, Leite RE, Farfel JM, Suemoto CK, Grinberg LT, Pasqualucci CA, Jacob-Filho W, Lent R. Sexual dimorphism in the human olfactory bulb: females have more neurons and glial cells than males. PLoS One. 2014;9(11):e111733. doi: 10.1371/journal.pone.0111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Calkins ME, Satterthwaite TD, Ruparel K, Elliott MA, Moore TM, Gur RC, Gur RE, Moberg PJ, Turetsky BI. Temporal lobe volume decrements in psychosis spectrum youths. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw112. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ. 2011;2:7. doi: 10.1186/2042-6410-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner WM, Faraone SV, Tsuang MT. Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol Psychiatry. 1997;42(2):104–115. doi: 10.1016/S0006-3223(96)00300-9. [DOI] [PubMed] [Google Scholar]
- Sim K, DeWitt I, Ditman T, Zalesak M, Greenhouse I, Goff D, Weiss AP, Heckers S. Hippocampal and parahippocampal volumes in schizophrenia: a structural MRI study. Schizophr Bull. 2006;32(2):332–340. doi: 10.1093/schbul/sbj030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nakamura Y, Nakamura K, Ikeda E, Furuichi A, Kido M, Kawasaki T, Noguchi K, Seto H, Suzuki M. Altered depth of the olfactory sulcus in first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:167–172. doi: 10.1016/j.pnpbp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Nelson B, Lin A, Yücel M, Phillips LJ, Nakamura Y, Suzuki M, Brewer WJ, Proffitt TM, McGorry PD, Velakoulis D, Pantelis C. Altered depth of the olfactory sulcus in ultra high-risk individuals and patients with psychotic disorders. Schizophr Res. 2014;153(1–3):18–24. doi: 10.1016/j.schres.2014.01.041. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Crutchley P, Walker J, Gur RE, Moberg PJ. Depth of the olfactory sulcus: a marker of early embryonic disruption in schizophrenia? Schizophr Res. 2009a;115:8–11. doi: 10.1016/j.schres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Glass CA, Abbazia J, Kohler CG, Gur RE, Moberg PJ. Reduced posterior nasal cavity volume: a gender-specific neurodevelopmental abnormality in schizophrenia. Schizophr Res. 2007;93:237–44. doi: 10.1016/j.schres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Hahn CG, Arnold SE, Moberg PJ. Olfactory receptor neuron dysfunction in schizophrenia. Neuropsychopharmacology. 2009b;34:767–774. doi: 10.1038/npp.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003;60:1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- Ursini G, Punzi G, Chen Q, Marenco S, Robinson JF, Porcelli A, Hamilton EG, Maddalena G, Jaffe AE, Berman KF, Egan MF, Straub RE, Colantuoni C, Blasi G, Hashimoto R, Bertolino A, Weinberger DR. Placental gene expression and the interaction between obstetrical history and genetic risk for schizophrenia. Neuropsychopharmacology. 2016;41:S223. [Google Scholar]
- van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- Vandekar SN, Shinohara RT, Raznahan A, Roalf DR, Ross M, DeLeo N, Ruparel K, Verma R, Wolf DH, Gur RC, Gur RE, Satterthwaite TD. Topologically dissociable patterns of development of the human cerebral cortex. J Neurosci. 2015;35(2):599–609. doi: 10.1523/JNEUROSCI.3628-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test (WRAT3) Administration Manual. Wide Range; Wilmington DE: 1993. [Google Scholar]
- Wischhof L, Irrsack E, Osorio C, Koch M. Prenatal LPS-exposure – a neruodevelopmental rat model of schizophrenia – differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:17–30. doi: 10.1016/j.pnpbp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res. 2010;123:188–198. doi: 10.1016/j.schres.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne O, Horvat JC, Osei-Kumah A, Smith R, Hansbro PM, Clifton VL, Hodgson DM. Early life infection alters adult BALB/c hippocampal gene expression in a sex specific manner. Stress. 2011;14:247–261. doi: 10.3109/10253890.2010.532576. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman E, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]