Abstract
Background
Singleton infants conceived using assisted reproductive technology have lower average birthweights than naturally-conceived infants and are more likely to be born low birthweight (<2500 grams). Lower birthweights are associated with increased infant and child mortality and poor adult health outcomes, including cardiovascular disease, hypertension, and diabetes. Data from registry and single center studies suggest that frozen/thawed embryo transfer may be associated with larger birthweights. To date, however, a nationwide, full-population study on United States infants born using frozen/thawed embryo transfer has not been reported.
Objectives
To compare the effect of frozen/thawed versus fresh embryo transfer on birthweight outcomes for singleton, term infants conceived using in vitro fertilization in the United States between 2007 and 2014, including average birthweight and the risks of both macrosomia (>4000 grams) and low birthweight (<2500 grams).
Study Design
We used data from the Centers for Disease Control and Prevention’s National Assisted Reproductive Technology Surveillance System to compare birthweight outcomes of live-born singleton, autologous oocyte, term (37–43 weeks) infants. Generalized linear models for all infants and stratified by infant sex were used to assess the relationship between frozen/thawed embryo transfer and birthweight, in grams. Infertility diagnosis, year of treatment, maternal age, maternal obstetric history, maternal and paternal race, and infant gestational age and sex were included in the models. Missing race data were imputed. The adjusted relative risks for macrosomia and low birthweight were evaluated using multivariable predicted marginal proportions from logistic regression models.
Results
In total, 180,184 singleton, term infants were included, with 55,898 (31.02%) having been conceived from frozen/thawed embryos. Frozen/thawed embryo transfer was associated with, on average, a 142.34 gram increase in birthweight compared with infants born after fresh embryo transfer (p<0.001). An interaction between infant sex and embryo transfer type was significant (p<0.0001), with FET having a larger effect on male infants by 16 grams. The adjusted risk of a macrosomic infant was 1.70 (95%CI 1.64–1.76) times higher following frozen/thawed embryo transfer than fresh embryo transfer. However, adjusted risk of low birthweight following frozen/thawed embryo transfer was 0.52 (95%CI 0.48–0.56) compared to fresh embryo transfer.
Conclusions
Frozen/thawed embryo transfer, in comparison with fresh embryo transfer, was associated with increased average birthweight in singleton, autologous oocytes, term infants born in the United States, with a significant interaction between frozen/thawed embryo transfer and infant sex. The risk of macrosomia following frozen/thawed embryo transfer was greater than that following fresh embryo transfer but the risk of low birthweight among frozen/thawed embryo transfer infants was significantly decreased in comparison with fresh embryo transfer infants.
Keywords: Birthweight, Fresh Embryo Transfer, Frozen/Thawed Embryo Transfer, In Vitro Fertilization, Low Birthweight, Macrosomia, National ART Surveillance System
Introduction
The number of infants born following conception using assisted reproductive technologies (ART) has increased by over 20% in the past decade, with 70,354 ART-conceived infants born in the United States in 2014 (1). During this time, ART practice and technology has improved, with significant improvements in slow freeze and vitrification technology making embryo freezing increasingly more successful (2) and allowing for increased use of embryo banking (3). There are therefore now many new treatment options for clinicians and their patients when deciding how to approach an in vitro fertilization (IVF) cycle. Frozen/thawed embryo transfer (FET), where embryos are retrieved and then frozen to allow for transfer at a later date, has seen an 82.5% increase in use in the US between 2006 and 2012 (4).
FET appears to result in similar pregnancy outcomes in comparison with fresh embryo transfer (5,6) and has also been found to be associated with birthweight increases ranging from 50–250 grams (7). IVF-conceived infants are more likely to be of lower birthweights than their naturally conceived peers (8). Given the importance of birthweight in short- and long-term health outcomes, especially infant, child, and adolescent morbidity and mortality (9), improving birthweights in ART infants is essential to improving overall ART outcomes. Single- or multi-site studies both internationally (10–12) and in the US (13) and international registry studies (10–12,14) have found increased infant birthweights among singletons following conception using FET, and a corresponding decrease in small for gestational age and low birthweight (LBW, <2,500 grams) (11,13,15). However, FET has also been linked to large singleton infants, with increased rates of macrosomia and large for gestational age (LGA, >90th percentile of birthweight for gestational age) identified in international single- or multi-site studies (10,16) and registry studies (5,17–19). Larger birthweights have been found to increase risk for morbidity due to stillbirth and sudden infant death syndrome (20), increased systolic blood pressure in adulthood (21), and increased risk of being overweight as children and adults (22).
In the US, a recent study by Dunietz et al. found fresh transfer infant birthweights for singletons were below the national mean, whereas singleton FET infants’ birthweights were above the mean. FET infants also had significantly lower risk of being small for gestational age (SGA, <10th percentile) than their naturally conceived peers (23). This study, though, was limited to three states. The most recent registry-based study of FET birthweight outcomes in the US included data from 2004–2006 (24) and found decreased risk of LBW and increased risk of both macrosomia and LGA following FET in comparison with fresh embryo transfer (ET) singleton infants. Since this time, however, embryo culture to blastocyst and blastocyst cryopreservation has changed (13) and, in turn, significantly improved the FET pregnancy outcomes. Additionally, ART procedures may have differing effects on infants depending on infant sex (25), indicating a need for new exploration of birthweight outcomes following FET. As FET is used more frequently, clarification of how it affects birthweight can help guide clinician practice and help patients make informed decisions regarding their IVF procedures.
In this study, we used the Centers for Disease Control and Prevention’s (CDC’s) National ART Surveillance System (NASS) data to explore infant birthweight outcomes for term, singleton infants born in the US between 2007 and 2014 following either fresh ET or FET, including rates of macrosomia and LBW by transfer type. We also examined the interaction between infant sex and frozen/thawed embryo transfer to determine if birthweight effects varied based on infant sex.
Materials and Methods
Cohort selection
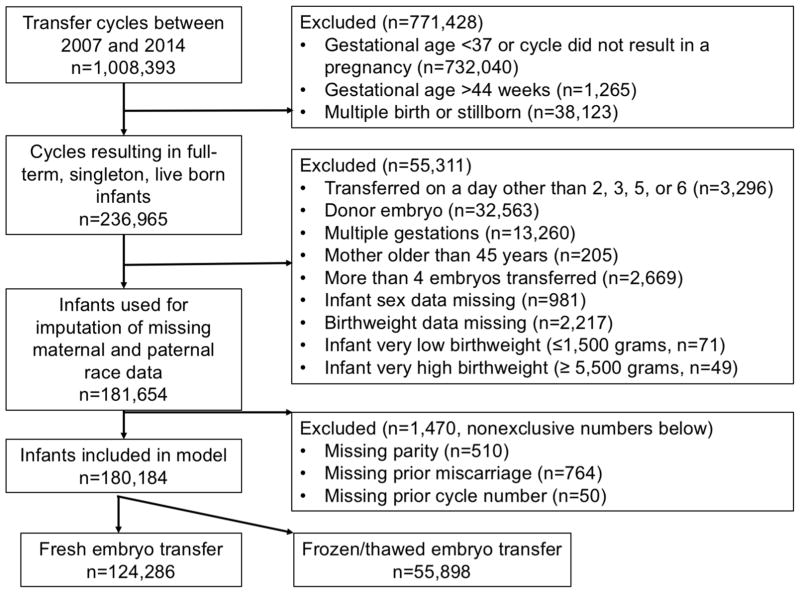
The CDC’s NASS data contains a record of ART cycles performed in the US, with more recent data containing an estimated >98% of cycles performed each year (26). We included all autologous oocyte ART cycles performed between 2007 and 2014. Cycles prior to 2007 were not included because BMI and maternal smoking data were not collected. In total, 1,008,393 ART cycles excluding embryo banking and gestational carrier cycles were performed between 2007 and 2014 (Figure 1). To establish a cohort of infants with low risk for adverse perinatal outcomes, we restricted to cycles that resulted in the birth of at least one term infant (37 to 43 weeks gestational age, n=275,088). Likewise, we further excluded cycles that resulted in multiple births (n=38,123), cycles using donor oocytes or embryos (n=32,563), those in which the female patient was >45 (n=205) at cycle start, those involving the transfer of >4 embryos (n=2,669), and multiple gestations that resulted in only one infant born (n=13,260). Cycles resulting in infants with birthweights ≥ 5,500g (n=49) or ≥ 1,500g (n=71) were excluded as these likely represented extreme outliers for term infants. Also excluded were cycles with missing data on infant sex (n=981), birthweight (n=2,217) and fresh transfers that occurred on days other than cleavage stage (day 2, 3) or blastocyst stage (day 5 or 6) (n=3,296). This resulted in total sample size of 181,654 autologous oocyte cycles resulting in live-born, singleton, term infants.
Figure 1.
Inclusion and exclusion criteria for term, live-born, autologous infants born following fresh and frozen/thawed embryo transfer cycles performed in the U.S. between 2007 and 2014, National ART Surveillance System (NASS).
Of this population of infants, 36.8% were missing maternal race/ethnicity and 40.6% were missing paternal race/ethnicity (see Figure 1 for exclusions). Under the assumption that data were missing at random, we employed multiple imputation to estimate missing values using a non-parametric method, HOTDECK (SUDAAN 11.0.0). Based on variations in the race/ethnicity distribution between US states, we used state as the clustering variable and included all other variables used in the linear models. Imputation was performed five times to obtain five datasets for analysis. Following imputation, 1,470 infants were removed due to missing maternal obstetric history data (prior ART cycles, parity, or prior miscarriage), resulting in a final cohort size of 180,184 infants.
Relationship between fresh/frozen/thawed embryo transfer and birthweight
All models were generated using SAS version 9.3 and SUDAAN 11 (Research Triangle Park, NC) statistical software. Generalized linear models were used to examine the impact of embryo transfer type (fresh or frozen/thawed) on infant birthweight in grams as the dependent variable. Demographic, treatment, and obstetric history variables were compared between the FET and fresh ET groups using chi-square tests or t-tests as appropriate. Variables which had been a priori established as related to birthweight but where less than 10% of data were missing were included in the models. Models therefore included the following covariates: infant sex, gestational age (days), maternal age (years), year of treatment, non-exclusive infertility diagnosis (male factor, diminished ovarian reserve, uterine factor, history of endometriosis, ovulatory disorder, tubal factor, other cause, and unexplained), whether the mother had undergone previous ART cycles (none, 1–2, or 3 or more), whether the mother had previous miscarriages (yes, no), whether the mother had previous births (none, 1–2, or 3 or more), and maternal and paternal race (including imputed values). Most IVF treatment variables were not reported for the frozen/thawed transfer infants, including transfer stage (blastocyst, day 5–6, or cleavage, day 2–3), number of oocytes retrieved, use of intracytoplasmic sperm injection (ICSI), and use of preimplantation genetic diagnosis/screening (PGD/S) and so these variables were unable to be included in the model.
To test for a differential effect of embryo transfer type by infant sex, an interaction term was included in the model. Sex-stratified models were generated to improve interpretability. The adjusted relative risk (RR) of both macrosomia and low birthweight by treatment was evaluated using multivariable predicted marginal proportions from logistic regression models and included the same covariates as the linear models. Because maternal BMI was missing for 22% of births, it was not included as a covariate in the adjusted models. To evaluate the extent to which BMI may confound our estimates, we conducted a sensitivity analysis in which BMI was included as a covariate in the linear and logistic regression models; cases with missing BMI values were excluded from these models. Additionally, to ensure that there were no differences in those whose race/ethnicity data was missing, we performed a sensitivity analysis using participants not missing that data, and also performed an analysis with missing race/ethnicity as a variable. We also performed an interaction test and sensitivity analysis for single embryo transfer (SET) to ensure that there was not an effect of number of embryos transferred and that this variable was not an effect modifier or confounder. All results were considered significant at an alpha of 0.05. This project was approved by the Institutional Review Board at the CDC.
Results
Demographics
A total of 180,184 singleton, live-born, term infants were included in the final models (Table 1). Of these, 55,898 (31.0%) were conceived from a frozen/thawed embryo transfer. Infants were born between 37 and 43 weeks gestation (M=39.3 weeks, SD 1.1) to mothers between 18 and 45 years old, with an average maternal age of 33.9 years (SD=4.2). Maternal BMI was predominantly normal or underweight (62.5%) and most mothers were non-Hispanic white (74.4%). Asian or Pacific Islander was the second most common maternal race (12.6%). Paternal race was similarly distributed, with 76.3% of fathers identifying as non-Hispanic white and 10.9% identifying as Asian or Pacific Islander. Very few mothers reported being lifetime smokers (4.6%), but 15.2% of lifetime smoking history data were missing. Of all mothers, 2.3% reported smoking during pregnancy.
Table 1.
Treatment, Parental, and Infant Characteristics for term, live-born, autologous, fresh and frozen/thawed embryo transfer infants born in the U.S. between 2007 and 2014, National ART Surveillance System (NASS).
| All Fresh or Frozen/Thawed Embryo Transfers | Fresh Embryo Transfers | Frozen/Thawed Embryo Transfers | |
|---|---|---|---|
|
| |||
| (N=180,184) | (N=124,286) | (N=55,898) | |
|
|
|||
| Treatment Characteristics | |||
| Blastocyst Transfer | 67,257 (37.33) | 67,257 (54.11) | NAa |
| Single Embryo Transfer | 49,496 (27.47) | 26,695 (21.48) | 22,801 (40.79) |
| Number of Embryos Transferred (Mean (SD)) | 1.94 (0.75) | 2.04 (0.74) | 1.72 (0.71) |
| Number of Oocytes Retrieved | 13.54 (7.54) | 13.50 (7.51) | NAa |
| ICSI Used | 94,605 (52.50) | 92,372 (74.32) | NAa |
| Preimplantation Genetics Testing/Screening Performed | 5,886 (3.27) | 5886 (4.75) | NAa |
| Assisted Hatching Used | 68,587 (38.06) | 40,696 (32.74) | 27,891 (49.90) |
| Maternal Demographics | |||
| Maternal Age (years) | 33.86 (4.17) | 33.72 (4.18) | 34.18 (4.11) |
| Maternal Weight | |||
| Normal or Underweight (BMI <= 25 kg/m2) | 87,869 (62.54) | 60,034 (61.62) | 27,835 (64.60) |
| Overweight (BMI > 25 and < 30 kg/m2) | 31,664 (22.53) | 22,239 (22.83) | 9,425 (21.87) |
| Obese (BMI > 30 kg/m2) | 20,987 (14.94) | 15,158 (15.56) | 5,829 (13.53) |
| Missing | 39,664 | ||
| Year of Cycle Initiation | |||
| 2007 | 18,204 (10.10) | 14,555 (11.71) | 3,649 (6.53) |
| 2008 | 19,708 (10.94) | 15,557 (12.52) | 4,151 (7.43) |
| 2009 | 20,100 (11.16) | 15,867 (12.77) | 4,233 (7.57) |
| 2010 | 20,934 (11.62) | 15,831 (12.74) | 5,103 (9.13) |
| 2011 | 21,877 (12.14) | 15,754 (12.68) | 6,123 (10.95) |
| 2012 | 24,167 (13.41) | 16,214 (13.05) | 7,953 (14.23) |
| 2013 | 26,123 (14.50) | 15,570 (12.53) | 10,553 (18.88) |
| 2014 | 29,071 (16.13) | 14,938 (12.02) | 14,133 (25.28) |
| Smoked >100 cigarettes in lifetime | 8,276 (4.59) | 5990 (4.82) | 2,286 (4.09) |
| Missing | 27,438 | ||
| Smoked during 3 months prior to cycled | 3,493 (2.31) | 2,524 (2.43) | 969 (2.05) |
| Missing | 173,651 | ||
| Infertility Diagnosis (nonexclusive) | |||
| Male Factor | 72,637 (40.31) | 50,699 (40.79) | 21,938 (39.25) |
| Diminished Ovarian Reserve | 26,124 (14.50) | 18,949 (15.25) | 7,175 (12.85) |
| Uterine Factor | 6,889 (3.82) | 4,457 (3.59) | 2,432 (4.35) |
| Endometriosis | 19,573 (10.86) | 13,761 (11.07) | 5,812 (10.40) |
| Ovulatory Disorder or Polycystic Ovaries | 32,446 (18.01) | 20,753 (16.70) | 11,693 (20.92) |
| Tubal Factor | 26,106 (14.49) | 18,280 (14.71) | 7,826 (14.00) |
| Hydrosalpinx c | 2,295 (1.27) | 1,584 (1.27) | 711 (1.27) |
| Tubal Ligation | 3,284 (1.82) | 2,479 (1.99) | 805 (1.44) |
| Other Tubal Cause c | 20,901 (11.60) | 14,462 (11.64) | 6,439 (11.52) |
| Other Cause | 23,273 (12.92) | 14,188 (11.42) | 9,085 (16.25) |
| Unexplained | 27,534 (15.28) | 19,757 (15.90) | 7,777 (13.91) |
| Maternal Pregnancy History | |||
| Number of Previous ART Cycles | |||
| No previous cycles | 82,836 (45.97) | 79,491 (63.96) | 3,345 (5.98) |
| 1–2 cycles | 72,699 (40.35) | 33,373 (26.85) | 39,326 (70.35) |
| 3 or more cycles | 24,649 (13.67) | 11,422 (9.19) | 13,227 (23.66) |
| One or More Previous Premature Birth(s) | 7,493 (4.17) | 3,331 (2.69) | 4,163 (7.46) |
| One or More Previous Miscarriage(s) | 51,972 (28.82) | 33,259 (26.76) | 18,713 (33.48) |
| Parity | |||
| One to two previous births | 56,286 (31.24) | 32,195 (25.90) | 24,091 (43.10) |
| No previous births | 120,071 (66.64) | 89,531 (72.04) | 30,540 (54.64) |
| 3 or more previous births | 3,827 (2.13) | 2,560 (2.06) | 1,267 (2.27) |
| Infant Variables | |||
| Birthweight (grams) | 3399.87 (472.37) | ||
| Low Birthweight (<2500 grams)) | 4,741 (2.63) | 3,855 (3.10) | 886 (1.59) |
| Normal Birthweight (2500–4000 grams)) | 157,721 (87.53) | 110,491 (88.90) | 47,230 (84.49) |
| Macrosomic (>4000 grams) | 17,722 (9.84) | 9,940 (8.00) | 7,782 (13.92) |
| Gestational Age (weeks) | 39.30 (1.08) | 39.35 (1.08) | 39.20 (1.06) |
| Female Sex | 89,007 (49.40) | 61,672 (49.62) | 27,335 (48.90) |
| Maternal Ethnicityb | |||
| Non-Hispanic White | 86,425 (74.43) | 60,329 (75.33) | 26,096 (72.42) |
| Non-Hispanic Black | 5,807 (5.00) | 3,937 (4.92) | 1,870 (5.19) |
| Asian or Pacific Islander | 14,631 (12.60) | 9,262 (11.57) | 5,369 (14.90) |
| Hispanic | 9,024 (7.77) | 6,389 (7.98) | 2,635 (7.31) |
| Other | 232 (0.20) | 169 (0.21) | 63 (0.17) |
| Missing | 64,065 | ||
| Paternal Ethnicityb | |||
| Non-Hispanic White | 81,703 (76.33) | 56,935 (77.11) | 24,768 (74.60) |
| Non-Hispanic Black | 5,732 (5.36) | 3,934 (5.33) | 1,798 (5.42) |
| Asian or Pacific Islander | 11,682 (10.91) | 7,333 (9.93) | 4,349 (13.10) |
| Hispanic | 7,724 (7.22) | 5,496 (7.44) | 2,228 (6.71) |
| Other | 193 (0.18) | 135 (0.18) | 58 (0.17) |
| Missing | 73,150 | ||
All data are presented as number (percent) of participants except where otherwise specified; SD = Standard Deviation
Data were not available for some treatment variables for infants conceived using frozen/thawed embryo transfers.
Missing maternal and paternal race data were imputed
No significant differences between the frozen/thawed embryo and fresh embryo groups for these two variables. All other variables were significantly different between the groups.
Question was only asked of those who reported having smoked >100 cigarettes in their lifetime
Parental infertility diagnosis was recorded nonexclusively, with male factor infertility being the most common diagnosis (40.3%). Among female infertility diagnoses, ovulatory disorder, including polycystic ovaries, was the most common (18.0%), followed by unexplained (15.3%) and tubal factor (14.5%) infertility. For most mothers, this was their first child (66.6%) and the case infant was most often conceived on the first round of ART (46.0%). Almost a third of mothers (28.8%) had had a previous miscarriage.
For the fresh transfer population, the majority of infants (54.1%) were conceived following blastocyst transfer, 74.4% were conceived using ICSI, and 4.8% had received PGD/S. An average of 13.5 oocytes were retrieved for fresh cycle patients. For the overall population, assisted hatching was used in 38.1% of cycles, and 27.5% of all cycles were performed using single embryo transfer. An average of 1.94 embryos were transferred per cycle.
The average birthweight of infants was 3,399.9 grams (SD = 472.4). Of these, 2.6% were LBW and 9.8% were macrosomic (>4,000 grams). Slightly fewer infants (49.4%) were female than male.
Association of fresh or frozen/thawed embryo transfer, other IVF treatment factors, obstetric history, and demographic factors with infant birthweight
FET was associated with a 142 gram increase in birthweight over fresh ET (p<0.001, Table 2). Independent of transfer type, female infants were 143 grams smaller than male infants (p≤0.001) and infant weight increased by 155 grams per each additional week of gestation. Birthweight was positively associated with maternal age and negatively associated with cycles having been completed more recently (treatment date by year). A diagnosis of diminished ovarian reserve was associated with a decrease in infant birthweight, while male factor infertility, endometriosis, and ovulatory disorder, were all associated with increased birthweight. Prior use of ART was also associated with increased birthweight, with infants whose mothers had previously had 1–2 cycles or 3 or more cycles being larger than those whose mothers had not had previous cycles. Infants whose mothers had had one or more prior miscarriages were also larger. Firstborn children weighed an average of 114 grams less than children born to mothers with previous children but there was no effect of the number of pervious children by category. Finally, infants born to non-Hispanic white mothers and to non-Hispanic white fathers were all larger than those born to any of the other race categories.
Table 2.
Difference in birthweight between infants born following fresh embryo transfer and those born following frozen/thawed embryo transfer for all variables included in the model, stratified by infant sex. Term, live-born, autologous embryo transfer infants born in the U.S. between 2007 and 2014 (n=180,184), National ART Surveillance System (NASS).
| Birthweight for All Infants (grams) | Birthweight for Female Infants (grams) | Birthweight for Male Infants (grams) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| N=180,184 | N=89,007 | N=91,177 | |||||||
|
|
|
|
|||||||
| Obstetric and Infant variables | Est. | Std. Error | P | Est. | Std. Error | P | Est. | Std. Error | P |
| Fresh Embryo Transfer | −142.34 | 2.68 | <0.001 | −134.09 | 3.73 | <0.001 | −150.28 | 3.85 | <0.001 |
| Female Sex | −142.51 | 2.02 | <0.001 | – | – | – | – | – | – |
| Gestational Age in Days | 154.71 | 0.99 | <0.001 | 152.35 | 1.39 | <0.001 | 157.03 | 1.42 | <0.001 |
| Maternal Demographics | |||||||||
| Maternal Age in Years | 1.04 | 0.27 | <0.001 | 1.23 | 0.37 | 0.001 | 0.83 | 0.38 | 0.029 |
| Year of Treatment | −4.40 | 0.45 | <0.001 | −4.29 | 0.63 | <0.001 | −4.50 | 0.65 | <0.001 |
| Infertility Diagnosis (nonexclusive) | |||||||||
| Male Factor | 14.58 | 2.64 | <0.001 | 16.57 | 3.68 | <0.001 | 12.53 | 3.80 | 0.001 |
| Diminished Ovarian Reserve | −13.47 | 3.31 | <0.001 | −14.57 | 4.59 | 0.002 | −12.41 | 4.78 | 0.009 |
| Uterine Factor | −9.50 | 5.28 | 0.072 | −8.05 | 7.38 | 0.276 | −11.27 | 7.56 | 0.136 |
| Endometriosis | 12.06 | 3.43 | <0.001 | 10.43 | 4.73 | 0.028 | 13.43 | 4.97 | 0.007 |
| Ovulatory Disorder or Polycystic Ovaries | 18.73 | 3.09 | <0.001 | 24.22 | 4.32 | <0.001 | 13.19 | 4.43 | 0.003 |
| Tubal Factor | 1.51 | 3.3 | 0.648 | 10.36 | 4.60 | 0.024 | −7.42 | 4.75 | 0.118 |
| Other Cause | 2.59 | 3.42 | 0.447 | 13.27 | 4.77 | 0.005 | −7.81 | 4.89 | 0.110 |
| Unexplained | 0.35 | 3.85 | 0.929 | 7.42 | 5.38 | 0.168 | −6.70 | 5.51 | 0.224 |
| Maternal Pregnancy History | |||||||||
| Number of Previous ART Cycles* | |||||||||
| No previous cycles | Ref. | Ref. | Ref. | ||||||
| 1–2 cycles | 14.05 | 2.58 | <0.001 | 12.76 | 3.60 | <0.001 | 15.13 | 3.70 | <0.001 |
| 3 or more cycles | 11.63 | 3.56 | 0.001 | 8.66 | 4.91 | 0.078 | 14.57 | 5.14 | 0.005 |
| One or More Previous Miscarriage(s)* | 9.90 | 2.32 | <0.001 | 1.98 | 3.23 | 0.539 | 17.37 | 3.33 | <0.001 |
| Parity* | |||||||||
| One to two previous births | Ref. | Ref. | Ref. | ||||||
| No previous births | −114.57 | 2.36 | <0.001 | −107.91 | 3.28 | <0.001 | −121.47 | 3.38 | <0.001 |
| 3 or more previous births | 3.02 | 7.38 | 0.683 | −3.82 | 10.31 | 0.711 | 9.48 | 10.52 | 0.367 |
| Maternal Ethnicity** | |||||||||
| Non-Hispanic White | Ref. | Ref. | Ref. | ||||||
| Non-Hispanic Black | −65.72 | 7.79 | <0.001 | −68.44 | 10.56 | <0.001 | −62.73 | 11.71 | <0.001 |
| Asian or Pacific Islander | −53.31 | 4.71 | <0.001 | −52.04 | 7.13 | <0.001 | −54.01 | 7.30 | <0.001 |
| Hispanic | −29.15 | 5.46 | <0.001 | −27.37 | 8.10 | 0.001 | −30.72 | 7.74 | <0.001 |
| Other | −56.97 | 23.31 | 0.015 | −59.13 | 34.85 | 0.090 | −51.37 | 32.70 | 0.116 |
| Paternal Ethnicity** | |||||||||
| Non-Hispanic White | Ref. | Ref. | Ref. | ||||||
| Non-Hispanic Black | −12.82 | 7.59 | 0.092 | −4.92 | 10.76 | 0.647 | −20.80 | 11.91 | 0.084 |
| Asian or Pacific Islander | −67.04 | 5.01 | <0.001 | −54.73 | 7.79 | <0.001 | −78.84 | 7.47 | <0.001 |
| Hispanic | −27.32 | 5.63 | <0.001 | −22.19 | 8.02 | 0.006 | −32.51 | 8.18 | <0.001 |
| Other | −37.82 | 16.22 | 0.023 | −60.40 | 21.24 | 0.005 | −15.57 | 22.14 | 0.485 |
Model is controlled for all variables in the table. Participants with missing data for variables indicated with an asterisk (*) were excluded. Missing data for maternal and paternal race were imputed(**). No other data was missing. Est.= Estimate; Std. Error=Standard Error; Ref.=Reference
Modification of the effect of fresh or frozen/thawed embryo transfer on birthweight outcomes and stratification by sex
A significant interaction between infant sex and fresh/frozen/thawed embryo transfer was also identified (p<0.0001). Sex stratified models revealed that frozen/thawed embryo transfer had a greater effect on male infants’ birthweights than it did on female infants’ birthweights; male infants born from FET were 150 grams heavier than male infants born following fresh embryo transfers, whereas female infants born following FET were 134 grams larger than female infants born following fresh embryo transfer. Most of the additional variables in the model that previously had been found to be associated with birthweight were still significant in the stratified models. However, for male infants the association between the mother or father identifying as “other” in the race categories was no longer significant. For female infants, the mother having had three or more prior ART cycles or a prior miscarriage was no longer significantly associated with birthweight, nor was the mother identifying race as “other” or the father identifying as non-Hispanic Black. For female infants, a maternal diagnosis of tubal factor infertility or another cause of infertility was significantly associated with increased birthweight.
The effect of frozen/thawed embryo transfer on low birthweight and macrosomia
Infants born following FET were 1.7 times more likely to be macrosomic than infants born following fresh embryo transfer (p≤0.001, Table 3) after adjustment for infertility diagnosis, race/ethnicity, and infant and obstetric characteristics. Likewise, there were significantly fewer LBW infants following FET as compared to fresh transfer (aRR 0.5, p<0.001).
Table 3.
Rates of macrosomia and low birthweight (LBW) among infants conceived with Assisted Reproductive Technology following fresh and frozen/thawed embryo transfer, and relative risks for frozen/thawed versus fresh embryo transfer. National ART Surveillance System (NASS).
| Fresh Embryo Transfer | Frozen/Thawed Embryo Transfer | |
|---|---|---|
| Normal Birthweight (>2,500 to ≤4,000 grams) | ||
| N (%) | 110491 (88.9) | 47230 (84.5) |
| Low Birthweight (1500–2500 grams) | ||
| N (%) | 3855 (3.1) | 886 (1.6) |
| RR (95% CI) | Ref. | 0.51 (0.48–0.55) |
| aRR (95% CI) | Ref. | 0.52 (0.48–0.56) |
| Macrosomic (>4000 grams) | ||
| N (%) | 9940 (8.0) | 7782 (13.9) |
| RR (95% CI) | Ref | 1.74 (1.69–1.79) |
| aRR (95% CI) | Ref. | 1.70 (1.64–1.76) |
RR= relative risk; aRR = adjusted relative risk (adjusted for infertility diagnosis, maternal and paternal race, obstetric history, infant sex, year of transfer, gestational age, and maternal age)
Sensitivity analyses
In models where BMI was included as a covariate, FET was associated with a decrease of 142.3 grams. When stratified by infant sex, a 156.2 gram decrease in birthweight was observed in males and a 136.7-gram decrease was observed in females. The aRR for macrosomia was 1.75 (95%CI 1.64–1.76) and the aRR for LBW was 0.52 (95% CI 0.47–0.57). Missingness for race/ethinicity data was not significantly associated with birthweight, and estimates were not meaningfully different when the analysis was restricted to those without missing race/ethnicity. Finally, there was no significant interaction between FET and single embryo transfer. Including SET in the model did not meaningfully change the relationship between birthweight and FET (effect size 141.98 grams). When infants born using SET were examined alone (n=49,496), the effect of FET was 138.45 grams, and when double embryo transfer infants were examined alone (n=98,063) the effect of FET was 146.80 grams.
Comment
In this study, we found a positive association between the use of FET and infant birthweight, with singleton infants born following FET being 142 grams heavier than those born following fresh ET. We also found a significant interaction between infant sex and birthweight, indicating that the effect of FET differs depending on infant sex. Our data indicate that FET has a slightly greater impact on male infants than it does on female infants, with a 16-gram difference in effect between the two groups. Although this difference is small, and unlikely to be clinically significant in terms of the long-term effects of birthweight, the significant interaction is indicative of a potential sex-specific mechanism by which FET affects birthweight, and therefore warrants further exploration.
Keane et al. (2016) recently found an opposite effect of infant sex in their single-clinic study in Australia, with stratification indicating that the effect of FET on birthweight was only significant for female infants, with no apparent effect of FET on birthweight for male infants (10). Previous work by Kaartinen et al., also in a single clinic, found a greater effect size of FET on female infants than male infants for embryos transferred on day 2/3, and a trend toward greater birthweights in male infants for day 5/6 transfers but no difference in female infants for the later transfer group (25). In contrast, we found a greater effect on male infants and a significant effect for both sexes in a population-level study. Day of transfer data, however, was not available for FET infants in our cohort and further work is needed to elucidate the differing effects of IVF treatments based on sex and transfer stage.
Our results also indicate that there was a significantly increased risk of macrosomia among FET infants, and a concomitant decrease in the risk for LBW. In 2014, the macrosomia rate for the US term infant population was 86 infants per 1,000 and the rate of LBW was 31 per 1,000 (27). The rate of macrosomia among term fresh ET infants in our study was comparable, at 80 per 1,000, as was the rate of LBW in this population, 31 per 1,000 infants. In contrast, the rate of macrosomia among FET infants was 130 per 1,000, much higher than the national rates, but the rate of LBW was only 16 infants per 1,000. Our results correspond with those from international studies, with risk ratios ranging from 1.29 to 2.05 for macrosomia (18,19,28) and from 0.73 to 0.81 for LBW (19,29,30) for FET infants in comparison with fresh ET infants. Several US studies have similarly found decreased LBW or SGA (13,23,31) in infants conceived following FET.
Additionally, our results support recent sibling-pair findings by Luke et al. (2017) (32), who used Society for Reproductive Technology (SART) CORS data to evaluate the effect of FET on US infants in comparison with siblings conceived using fresh ET. They similarly found an increased risk of macrosomia and decreased risk of low birthweight among infants conceived using FET in comparison with those conceived using fresh ET. Luke et al.’s findings, however, are generalizable only to those infants whose mothers used IVF for two conceptions, resulting in about 15,000 infants. Our aim, on the other hand, was to expand this understanding to the entire IVF population across United States, and to that end we used a different methodological approach via a larger cohort study. Although SART-CORS data is very comprehensive, fertility clinic participation is voluntary, and those clinics that do participate (around 80% of fertility programs) may be inherently different from the 20% that choose not to participate, resulting in selection bias. NASS data, in contrast, contains data from clinics that report SART-CORS, but also those that choose not to report to SART-CORS. Finally, our study provides evidence of an interaction between FET and infant sex; our results indicate that FET affects male and female infants differently. Participant sex is a critical confounder in all studies and is increasingly being recognized as central to the mechanism of effect of many prenatal exposures.
Limitations of our study include missing treatment information for frozen/thawed embryo transfers, most notably embryo stage, as these data are not routinely collected for these cycles. As a result, we were unable to include variables such as use of intracytoplasmic sperm injection, preimplantation genetic diagnosis/screening, and number of oocytes retrieved in this analysis. We also cannot compare rates of blastocyst or cleavage stage embryo transfer between the fresh and frozen/thawed infants due to missing data for the infants conceived using FET. Although it is possible that more frozen/thawed embryos are transferred at blastocyst stage, previous work by our group found only a 5.3-gram effect of blastocyst stage transfer which is unlikely to be sufficient to create the effect size seen in this study.
We also do not have data on the cryopreservation method used to freeze each embryo, though recent data indicate that freezing approach may not have an effect on birthweight (33). Type of cycle (natural or with hormone replacement) is also not available for FET cycles, but given that our cohort contains almost all US infants, we are confident that our effect estimates accurately reflect the true effect in this population. Future work on FET cycle types and cryopreservation is needed to better elucidate what component of FET affects birthweight. Additionally, due to the amount of missing maternal BMI and smoking history data, both known predictors of birthweight, neither variable was included in the model. Finally, paternal age is not currently available in NASS, and could not be included in models.
Although our data indicate differences in birthweight outcomes between fresh and frozen/thawed ET, we cannot determine whether these differences are due to the freezing procedure or to underlying characteristics of the patients. For example, FET is also used more often in patients who are at risk for ovarian hyper-stimulation syndrome (34). We also do not have data on chronic or gestational diabetes, a risk factor for macrosomia, nor for other pregnancy-related complications that could play a role in the increased macrosomia seen in FET infants. Although there were significant differences in many demographic, obstetric history, and treatment characteristics between the fresh and frozen/thawed embryo groups, inclusion of many of these variables in the model controls for some of the variability in prognosis between these patients. However, we were also unable to control for variation in clinic use of FET compared with fresh ET, as some clinics use differing criteria to determine which patients should freeze all embryos and only be offered FET.
The goal of this study was to establish the relationship between FET and birthweight while controlling for as many confounding factors as possible to be able to isolate that relationship. Because both preterm birth and multiple gestations are linked with many causes and complications that would impair the ability to evaluate the effect of FET on birthweight, we did not include preterm or multiple infants in our analysis. Preterm birth has also been associate with FET, and so outcomes in preterm infants should be examined separately due to the possibility of differing effects on preterm infants. Outcomes in preterm and multiple infants are of course important and further work is needed to understand the relationship between IVF procedures and their birthweight outcomes, but that was beyond the scope of our aims with this project.
To our knowledge, this is the first study to explore fresh and frozen/thawed embryo transfer birthweight outcomes for a national population of ART infants for this time-period, as well as the interaction between sex and FET. Additionally, the wealth of data available in the NASS data allowed for a comprehensive model, controlling for many variables related to birthweight that are not often available. Overall, our data indicate an increased birthweight following FET when compared with fresh ET. FET infants had an increased risk of macrosomia and a corresponding decrease in risk of LBW. Given the increasing trend toward freeze-all cycles, where all embryos are frozen for future use, understanding the perinatal risks of FET is essential for selecting patients who are good candidates for this treatment. However, the long-term effects of macrosomia are less well-established than those of LBW, and so determining whether the apparent trade-off related to FET—fewer LBW infants but more macrosomic infants—is beneficial is not yet possible. Additional studies are needed to fully understand the long-term implications of these risk on ART infants.
Implications and Contributions.
This study used comprehensive, US national data to examine the relationship between frozen/thawed embryo transfer (FET) and birthweight outcomes with the goal of verifying, in a US full-term, singleton, autologous population, previous findings indicating that infants conceived using FET may have larger birthweights than those conceived using fresh embryo transfer (ET). FET infants were 142 grams heavier than those conceived using fresh ET, with an even greater effect on male infants. Importantly, FET infants had a 70% increased risk of macrosomia (>4,000 grams) but a 50% decreased risk of being born low birthweight (LBW, <2,500 grams). Since the long-term effects of macrosomia are less well-established than those of LBW, determining the benefit of decreased LBW infants at the expense of increased macrosomic infants is not yet possible.
Acknowledgments
Financial Support: NIH-NIMH R01MH094609, NIH-NIEHS R01ES022223, P01 ES022832, and EPA RD8354420
Role of Funding Source: Funding sources were not involved in the design of the study or in the analysis or interpretation of the data.
Footnotes
Disclaimer for authors employed by federal government: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Paper presentation information: This work was presented at the American Society for Reproductive Medicine meeting on Oct. 30th, 2017.
Disclosures/Conflicts of Interest: R.N.T. receives research funding from Bayer (Leverkusen, Germany) for work unrelated to assisted reproduction. Authors have no other funding or conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Assisted Reproductive Technology (ART) Data. [Internet] [cited 2017 Jul 27]. Available from: https://nccd.cdc.gov/drh_art/rdPage.aspx?rdReport=DRH_ART.ClinicInfo&ClinicId=9999&ShowNational=1.
- 2.Gosden R. Fertil Steril [Internet] 2. Vol. 96. Elsevier Ltd; 2011. Cryopreservation: A cold look at technology for fertility preservation; pp. 264–8. Available from: http://dx.doi.org/10.1016/j.fertnstert.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Kissin DM, Crawford S, Boulet SL, Kushnir VA, Vidali A, Barad DH, et al. Fertil Steril [Internet] 3. Vol. 100. Elsevier Inc; 2013. The status of public reporting of clinical outcomes in assisted reproductive technology; pp. 736–41. Available from: http://dx.doi.org/10.1016/j.fertnstert.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Pereira N, Rosenwaks Z. Fertil Steril [Internet] 2. Vol. 106. American Society for Reproductive Medicine; 2016. A fresh(er) perspective on frozen embryo transfers; pp. 257–8. Available from: http://dx.doi.org/10.1016/j.fertnstert.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Maheshwari A, Raja EA, Bhattacharya S. Fertil Steril [Internet] 7. Vol. 106. Elsevier Inc; 2016. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset; pp. 1703–8. Available from: http://dx.doi.org/10.1016/j.fertnstert.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–62. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro BS, Daneshmand ST, Bedient CE, Garner FC. Fertil Steril [Internet] 2. Vol. 106. Elsevier Inc; 2016. Comparison of birth weights in patients randomly assigned to fresh or frozen-thawed embryo transfer; pp. 317–21. Available from: http://dx.doi.org/10.1016/j.fertnstert.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Palomba S, Homburg R, Santagni S, La Sala GB, Orvieto R. Risk of adverse pregnancy and perinatal outcomes after high technology infertility treatment: a comprehensive systematic review. Reprod Biol Endocrinol [Internet] Reproductive Biology and Endocrinology. 2016;14(1):76. doi: 10.1186/s12958-016-0211-8. Available from: http://rbej.biomedcentral.com/articles/10.1186/s12958-016-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins WJ, Kotecha SJ, Kotecha S. All-Cause Mortality of Low Birthweight Infants in Infancy, Childhood, and Adolescence: Population Study of England and Wales. PLoS Med [Internet] 2016;13(5):1–20. doi: 10.1371/journal.pmed.1002018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862683/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keane KN, Mustafa KB, Hinchliffe P, Conceicao J, Yovich JL. Reprod Biomed Online [Internet] 2. Vol. 33. Elsevier Ltd; 2016. Higher beta-HCG concentrations and higher birthweights ensue from single vitrified embryo transfers; pp. 149–60. Available from: http://dx.doi.org/10.1016/j.rbmo.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Nishi O, Miyata H, Kinoshita Y, Tominaga T. At What Stage Does the Embryo Begin to Grow Larger in Frozen-Thawed Embryo Transfers?: Fetal Development from the Standpoint of Gestational Sac Diameter and Birth Weight. J Mamm Ova Res [Internet] 2015;32(3):109–13. Available from: http://www.bioone.org/doi/10.1274/jmor.32.109. [Google Scholar]
- 12.Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Fertil Steril [Internet] 4. Vol. 104. Elsevier Inc; 2015. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: Retrospective analysis; pp. 899–907.pp. e3 Available from: http://dx.doi.org/10.1016/j.fertnstert.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Maas K, Galkina E, Thornton K, Penzias AS, Sakkas D. No change in live birthweight of IVF singleton deliveries over an 18-year period despite significant clinical and laboratory changes. Hum Reprod. 2016;31(9):1987–96. doi: 10.1093/humrep/dew173. [DOI] [PubMed] [Google Scholar]
- 14.Roy TK, Bradley CK, Bowman MC, McArthur SJ. Fertil Steril [Internet] 5. Vol. 101. Elsevier Inc; 2014. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers; pp. 1294–1301.pp. e2 Available from: http://dx.doi.org/10.1016/j.fertnstert.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 15.Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, et al. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161(1):46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Korosec S, Frangez HB, Steblovnik L, Verdenik I, Bokal EV. Independent factors influencing large-for-gestation birth weight in singletons born after in vitro fertilization. J Assist Reprod Genet. 2016;33(1):9–17. doi: 10.1007/s10815-015-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Fertil Steril [Internet] 1. Vol. 101. Elsevier Inc; 2014. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: An analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan; pp. 128–33. Available from: http://dx.doi.org/10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): Is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29(3):618–27. doi: 10.1093/humrep/det440. [DOI] [PubMed] [Google Scholar]
- 19.Wennerholm UB, Henningsen AKA, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: A Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28(9):2545–53. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Decker A, Platt RW, Kramer MS. How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol [Internet] 2008;198:517.e1–517.e6. doi: 10.1016/j.ajog.2007.12.005. [cited 2017 May 9] Available from: w http://ac.els-cdn.com/S0002937807022703/1-s2.0-S0002937807022703-main.pdf?_tid=d1dfb45a-3530-11e7-afb1-00000aab0f6b&acdnat=1494387125_57928bf8d0f8b723028c1d89355addb5. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Wu J, Yu J, Gao E, Meads C, Afnan M, et al. Is fetal macrosomia related to blood pressure among adolescents? A birth cohort study in China. J Hum Hypertens [Internet] 2013;27(10):686–92. doi: 10.1038/jhh.2013.31. [cited 2017 May 9] Available from: http://media.proquest.com.dartmouth.idm.oclc.org/media/pq/classic/doc/3093512931/fmt/pi/rep/NONE?cit%3Aauth=Li%2C+Y%3BWu%2C+J%3BYu%2C+J%3BGao%2C+E%3BMeads%2C+C%3BAfnan%2C+M%3BRen%2C+J%3BRong%2C+F&cit%3Atitle=Is+fetal+macrosomia+related+to+blood+pressure+a. [DOI] [PubMed] [Google Scholar]
- 22.Schellong K, Schulz S, Harder T, Plagemann A. Birth Weight and Long-Term Overweight Risk: Systematic Review and a Meta-Analysis Including 643,902 Persons from 66 Studies and 26 Countries Globally. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunietz GL, Holzman C, Zhang Y, Talge NM, Li C, Todem D, et al. Assisted Reproductive Technology and Newborn Size in Singletons Resulting from Fresh and Cryopreserved Embryos Transfer. PLoS One [Internet] 2017;12(1):e0169869. doi: 10.1371/journal.pone.0169869. Available from: http://dx.plos.org/10.1371/journal.pone.0169869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian Stimulation and Low Birth Weight in Infants Conceived Through In Vitro Fertilization. Obs Gynecol. 2011;118(4):863–71. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaartinen NM, Kananen KM, Rodriguez-Wallberg KA, Tomas CM, Huhtala HS, Tinkanen HI. Male gender explains increased birthweight in children born after transfer of blastocysts. Hum Reprod [Internet] 2015;30(10):2312–20. doi: 10.1093/humrep/dev174. Available from: https://academic.oup.com/humrep/article-lookup/doi/10.1093/humrep/dev174. [DOI] [PubMed] [Google Scholar]
- 26.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted Reproductive Technology Surveillance - United States, 2013. MMWR Surveill Summ [Internet] 2015;64(11):1–28. doi: 10.15585/mmwr.ss6411a1. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6411a1.htm. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Mathews T. National Vital Statistics Reports Births : Final Data for 2014. Statistics (Ber) [Internet] 2015;64(1):1–104. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_12.pdf. [PubMed] [Google Scholar]
- 28.Sazonova A, Kllen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod. 2012;27(5):1343–50. doi: 10.1093/humrep/des036. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari A, Hamilton M, Bhattacharya S. Reprod Biomed Online [Internet] 2. Vol. 32. Elsevier Ltd; 2016. Should we be promoting embryo transfer at blastocyst stage? pp. 142–6. Available from: http://dx.doi.org/10.1016/j.rbmo.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Schwarze JE, Crosby JA, Zegers-Hochschild F. Reprod Biomed Online [Internet] 1. Vol. 31. Elsevier Ltd; 2015. Effect of embryo freezing on perinatal outcome after assisted reproduction techniques: lessons from the Latin American Registry of Assisted Reproduction; pp. 39–43. Available from: http://dx.doi.org/10.1016/j.rbmo.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Kalra SK, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Fertil Steril [Internet] 2. Vol. 95. Elsevier Ltd; 2011. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer; pp. 548–53. Available from: http://dx.doi.org/10.1016/j.fertnstert.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luke B, Brown MB, Wantman E, Stern JE, Toner JP, Coddington CC. Increased risk of large-for-gestational age birthweight in singleton siblings conceived with in vitro fertilization in frozen versus fresh cycles. J Assist Reprod Genet. 2017;34(2):191–200. doi: 10.1007/s10815-016-0850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaartinen N, Kananen K, Huhtala H, Keränen S, Tinkanen H. The freezing method of cleavage stage embryos has no impact on the weight of the newborns. J Assist Reprod Genet. 2016;33(3):393–9. doi: 10.1007/s10815-015-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legro RS. Introduction: Evidence-based in vitro fertilization treatment of fresh versus frozen embryo transfer: peeling away the layers of the onion. Fertil Steril. 2016;106(2):239–40. doi: 10.1016/j.fertnstert.2016.06.031. [DOI] [PubMed] [Google Scholar]