Abstract
Objective
Exaggerated cardiovascular reactivity to acute psychological stress has been associated with increased carotid intima-media thickness (IMT). However, inter-study variability in this relationship suggests the presence of moderating factors. The current study aimed to test the hypothesis that poor nocturnal sleep, defined as short total sleep time or low slow-wave sleep (SWS), would moderate the relationship between cardiovascular reactivity and IMT.
Methods
Participants (N=99, 65.7% female, age = 59.3 ± 9.3 years) completed a 2-night laboratory sleep study and cardiovascular exam where sleep and IMT were measured. The multisource interference task was used to induce acute psychological stress while systolic and diastolic blood pressure and heart rate were monitored. Moderation was tested using the PROCESS framework in SPSS.
Results
Slow-wave sleep significantly moderated the relationship between all cardiovascular stress reactivity variables and IMT (all pinteraction ≤ .048, all ΔR2interaction ≥ .027). Greater stress reactivity was associated with higher IMT values in the low SWS group and lower IMT values in the high SWS group. No moderating effects of total sleep time were observed.
Conclusion
The results provide evidence that nocturnal slow-wave sleep moderates the relationship between cardiovascular stress reactivity and IMT and may buffer the effect of daytime stress-related disease processes.
Keywords: Sleep, Intima-media thickness, Cardiovascular Reactivity, Stress, Cardiovascular Disease
Introduction
Carotid intima-media thickness (IMT) is considered an accessible, reliably measured subclinical marker of cardiovascular disease (CVD) and has been shown to be a robust predictor of future CVD events, especially when considered alongside other traditional CVD risk factors (1–4). Exaggerated cardiovascular reactivity to laboratory-based acute psychological stress has been associated with greater IMT, independent of traditional CVD risk factors, in several independent cross-sectional (5–10) and prospective studies (11, 12) and, consequently, has been conceptualized as a pathway through which psychological stress increases risk of CVD. Large magnitude, stress-induced surges in blood pressure damage endothelial function and promote vascular hypertrophy, thereby comprising the two mechanisms responsible for short-term blood pressure regulation: flow-mediated dilation and the baroreflex. Compromised short-term regulation of blood pressure then supports future large-magnitude changes in blood pressure, establishing a feed-forward biological pathway through which psychological stress increases risk of CVD (13).
While there is reasonable evidence to accept that exaggerated cardiovascular reactivity is associated with greater IMT, closer inspection of this body of research reveals a level of variability across reported findings that suggests the presence of biopsychosocial effect modifiers. Indeed, this variation has been noted in qualitative (14) and quantitative (15) reviews and it has been suggested that exploration of moderating factors might enhance the clinical utility of cross-sectional and prospective findings by identifying subgroups in which the psychological stress-cardiovascular reactivity-IMT pathway might be particularly relevant. In support, associations between cardiovascular reactivity and IMT have been found to be moderated by age (10, 16), gender (16), workplace demands (17), self-reported exhaustion (16), and socioeconomic status (SES; 18). Relationships between stress reactivity and IMT are relatively stronger in younger, male participants, those reporting greater workplace demands and exhaustion, and lower SES individuals.
Whereas health psychology research has emphasized biopsychosocial disease processes during wakefulness, comparatively less is known about how sleep, a biobehavioral state characterized by marked cardiovascular changes, interacts with daytime disease processes (19) to modify disease risk. For example, one of the hypothesized functions of sleep is, in a general sense, restoration (20–23). Blood pressure naturally declines during sleep, eventually reaching its 24-hour nadir during slow-wave sleep (SWS), allowing for cardiovascular quiescence, and in theory, repair (20, 24). Thus, it is reasonable to speculate that an individual engaging in longer nocturnal total sleep time (TST) or greater amounts of SWS would afford his/her cardiovascular system a greater opportunity to recuperate from daytime insults, including stress-induced cardiovascular reactivity. The current study tested this idea in a secondary analysis of archival data using moderation analysis. It was hypothesized that the strength of the relationship between cardiovascular reactivity and IMT would be relatively weaker in individuals engaging in longer TST and greater amounts of SWS and relatively stronger in individuals exhibiting relatively short TST and low amounts of SWS.
Methods
Participants
The current study represents a secondary analysis of data collected to assess the relationship between sleep, major depression, and subclinical markers of CVD. Participants were recruited from four separate studies conducted at the University of Pittsburgh between 1982–1999. One study [MH024652 (D. Kupfer, PI)] examined sleep in adults without personal or first-degree family history of psychiatric disorders; the remaining three studies assessed sleep in individuals with major depressive disorder [MH029618 (D. Kupfer, PI); MH049115 (E. Frank, PI); MH041884 (M. Thase, PI)]. It should be noted that cardiovascular stress reactivity, SWS, and IMT did not significantly differ as a function of lifetime depression history (all ps ≥.21) or scores on the Hamilton Rating Scale for Depression (all ps ≥.25). Finally, inclusion of scores on the Hamilton Rating Scale for Depression as a covariate did not significantly alter the results of the study (results not presented). Other psychiatric disorders and substance use were exclusion criteria across all four studies and inclusion was contingent upon participants being between 20 and 60 years of age and medically healthy, as confirmed by medical exam. For the current study, 339 participants previously enrolled in one of the above-noted studies were contacted between 2011–2014, and 177 consented to participate in the current study. The mental stress protocol was entered into the current study after data collection had already begun, so complete data on polysomnographically-assessed sleep, cardiovascular stress reactivity, and IMT were available in 99 participants (65.7% female, mean (SD) age = 59.26 (9.27) years). This study was approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent. Participants were compensated financially for their time.
Procedure
Participants completed a mental and physical health assessment and 2-week actigraphy and daily sleep diary period upon entry to the current study. A comprehensive cardiovascular assessment was undertaken in the morning following night 1 of a 2-night laboratory sleep study. To minimize the influence of cardiovascular circadian rhythms, the acute psychological stress task was administered during the laboratory sleep study by a trained research technician in the morning shortly after waking.
Psychological Stress
Prior to the stress task, participants engaged in a baseline period (25) where they were asked to count the number of times a colored square appeared on the computer screen in front of them. The multisource interference task (MSIT; 26) was used as an acute psychological stress task. Briefly, the 4-min task was comprised of two conditions: congruent and incongruent stimuli. During both conditions, participants were presented with three numbers, two of which were the same and one different. Participants were required to identify the different number by pressing the corresponding button on a keyboard. In the congruent condition, the different number aligned spatially with the button on the keyboard, meaning the position of the response button and number were congruent; in the incongruent condition, the different number and correct response button were misaligned spatially. In the incongruent condition, performance was titrated to 60% by adjusting intertrial intervals. Blood pressure (BP) and heart rate (HR) were measured during both the baseline and stress phases.
Cardiovascular Measurements
Blood pressure was measured using an automated oscillometric sphygmomanometer and BP cuff placed over the brachial artery (Critkon Inc., Tampa, FL). Measures of BP were taken at 2-min and 1-min intervals during the baseline and MSIT task, respectively. Serial measurements from each phase were averaged to create phase means for statistical analysis. Heart rate was measured using a 2-lead EKG configuration and Stellate Harmonie software (Natus Medical Inc, Pleasanton, CA; sampling rate = 1024 Hz). Continuous HR measures were averaged to create phase means. Stress reactivity was defined as the difference between baseline and stress phase means.
Participants underwent ultrasound assessment at the Vascular Clinical and Translational Research Center (V-CTRC). The timing of the ultrasound protocol was standardized to occur in the morning hours in a fasted state. All measurements were conducted with participants in a supine position following a standard 10-min rest period and were assessed by an experienced technician unaware of the participants’ sleep and stress reaction characteristics. Using B-mode ultrasound (GE Vingmed Ultrasounds A/S, Horten, Norway) and a linear array transducer (frequency 3–10 MHz), a series of common carotid artery (CCA) images were captured including the carotid bifurcation in the trans- and longitudinal-view. Measurements of IMT were made bilaterally, using digital calipers, on the far wall of the CCA 1–2 cm distal from the CCA bifurcation from images captured at the end of the T-wave derived from concurrent 3-lead electrocardiography (1). No differences were observed between right and left IMT measurements so the bilateral average IMT was used in statistical analyses. Sonographers at the V-CTRC periodically complete test-retest reliability assessments for IMT measures and maintain Pearson’s correlation values of ≥ .90.
Sleep Measurements
Participants completed a 2-night laboratory sleep study in the Neuroscience Clinical and Translational Research Center at Western Psychiatric Institute and Clinic. Laboratory sleep times were scheduled to align with participants’ self-reported habitual sleep times. The polysomnography montage consisted of bilateral frontal, central, and occipital electroencephalogram leads references to A1+A2, bipolar submentalis electromyogram, and right and left electrooculogram. Sleep stages were scored in 30 sec epochs according to American Academy of Sleep Medicine criteria (27). Total sleep time was calculated as the sum of all epochs on non-rapid eye movement and rapid eye movement sleep from bed time to wake time and percent SWS was defined as the minutes of N3 sleep divided by TST multiplied by 100. Percent SWS was used to distinguish it from our corresponding analyses of TST. The mean values for TST and SWS from both nights of laboratory sleep were used in analyses.
Covariates
Due to statistical constraints associated with a limited sample size, covariates were chosen based on either established relationships in the literature or on their relationship to IMT in the current dataset at a threshold of p <.10. Based on these criteria, socioeconomic status (SES), sex, age, body mass index (BMI), resting systolic BP (SBP), fasting measures of glucose and insulin, and apnea-hypopnea index (AHI) were chosen. Fasting measures of high-density lipoprotein, hemoglobin A1c, C-reactive protein, low-density lipoprotein, triglycerides, and average number of periodic limb movements (PLMs) with arousal per hour of sleep were excluded (all ps ≥ .25). Subjective SES was measured using the 9-rung social standing ladder (28) with reference to the surrounding community; lower scores indicated higher social standing. Fasting blood samples were obtained during the physical health assessment. Height, weight, and resting SBP were measured prior to cardiovascular testing in the V-CTRC; BMI was calculated as weight (kg) divided by height (m2). Finally, AHI and PLMs were measured during the first night of laboratory sleep and scored according to current AASM criteria (27).
Statistical Approach
A repeated measures ANOVA was used to assess cardiovascular activation by the MSIT. Total sleep time and SWS were dichotomized using a median split to create high and low TST and SWS groups for moderation analysis; one-way ANOVAs were used to test for group differences on key sociodemographic and biological measures. It should be noted that results were not different when TST and SWS were used as continuous variables in moderation analyses. The PROCESS macro (29) in SPSS (version 24) was used to test the primary hypothesis that TST and SWS would statistically moderate the relationship between cardiovascular stress reactivity and IMT. In a model predicting IMT, the predictor (stress reactivity), moderator (TST or SWS) and stress reactivity×sleep interaction were assessed. Sex, age, SES, fasting glucose and insulin, BMI, resting SBP, and AHI were added as covariates in a subsequent model. Due to the cross-sectional nature of the data, sensitivity analyses were carried out using stress reactivity as the moderator of the relationship between TST, SWS and IMT. Null results were expected and, if found, would further support the hypothesis of sleep as a unique modifier. Heart rate data from two participants and fasting insulin data from one participant were unusable or missing, respectively. Discrepancies in N have been noted in the tables. Significance was operationalized at p <.05.
Results
Participants
Characteristics of the entire sample and high- and low-variable groups are presented in Table 1. No systematic differences were observed across any of the groupings, with exception of the variables on which they were grouped. In addition, participants in the low-TST group had higher BMI and insulin values than those in the high-TST group. Participants in the low- SWS group were more likely to be male and had higher IMT values.
Table 1.
Sample and Sleep Group Characteristics
| Whole Sample N = 99 |
Low TST N = 50 |
High TST N = 49 |
Low SWS N = 50 |
High SWS N = 49 |
||
|---|---|---|---|---|---|---|
| Age, years | 59.26 (9.27) | 60.55 (7.55) | 57.94 (10.66) | 60.56 (8.90) | 57.94 (9.54) | |
| Sex, %F | 65.7 | 56.0* | 75.5 | 44.0* | 87.8 | |
| SES | 3.55 (1.72) | 3.50 (1.69) | 3.59 (1.76) | 3.64 (1.88) | 3.45 (1.54) | |
| Glucose, mg/dL | 101.21 (33.42) | 104.94 (32.13) | 97.41 (34.61) | 105.78 (34.81) | 96.55 (31.62) | |
| Insulin, pmol/L | 91.06 (91.28) | 112.25 (112.55)* | 69.88 (57.05) | 93.48 (77.17) | 88.55 (104.75) | |
| HbA1c, % | 6.00 (0.91) | 6.12 (1.00) | 5.88 (0.80) | 6.04 (0.97) | 5.96 (0.86) | |
| CRP, mg/L | 0.35 (0.50) | 0.38 (0.57) | 0.31 (0.43) | 0.39 (0.60) | 0.30 (0.39) | |
| Triglycerides, mg/dL | 117.91 (63.46) | 119.08 (62.84) | 116.71 (64.72) | 123.54 (65.54) | 112.16 (61.41) | |
| BMI, kg/m2 | 30.54 (7.55) | 32.35 (8.41)* | 28.69 (6.10) | 31.14 (6.78) | 29.92 (8.29) | |
| AHI | 8.55 (11.35) | 10.17 (11.73) | 6.89 (10.83) | 10.55 (13.72) | 6.50 (7.91) | |
| IMT, mm | 0.844 (0.146) | 0.872 (0.148) | 0.816 (0.139) | 0.883 (0.144)* | 0.806 (0.138) | |
| SBP, mmHg | 129.36 (17.27) | 131.76 (17.77) | 126.92 (16.56) | 131.30 (18.33) | 127.39 (16.05) | |
| DBP, mmHg | 76.27 (9.88) | 78.04 (9.92) | 74.47 (9.62) | 76.50 (10.50) | 76.04 (9.32) | |
| TST, min | 381.46 (54.45) | 337.89 (35.48)* | 425.93 (27.78) | 377.03 (51.93) | 385.98 (57.09) | |
| SWS, % | 10.23 (8.29) | 9.44 (9.15) | 11.03 (7.33) | 3.64 (2.68)* | 16.96 (6.45) | |
| Acute Psychological Stress | ||||||
| SBP, mmHg | Baseline | 124.99 (16.01) | 126.60 (15.23) | 123.34 (16.00) | 127.23 (15.04) | 122.71 (16.78) |
| Stress | 129.10 (15.67) | 130.89 (14.02) | 127.27 (17.13) | 131.69 (14.84) | 126.45 (16.19) | |
| DBP, mmHg | Baseline | 75.01 (9.50) | 75.66 (9.79) | 74.34 (9.25) | 75.65 (9.32) | 74.36 (9.74) |
| Stress | 76.46 (10.39) | 77.20 (11.00) | 75.71 (9.77) | 78.19 (10.74) | 74.71 (9.81) | |
| HR, bpm | Baseline | 65.54 (10.85) | 66.43 (11.79) | 64.63 (9.84) | 63.81 (11.15) | 67.37 (10.33) |
| Stress | 67.46 (11.60) | 68.10 (13.11) | 66.81 (9.91) | 65.21 (11.65) | 69.86 (11.17) | |
| Stress Rating | Baseline | 1.40 (2.05) | 1.56 (2.26) | 1.24 (1.81) | 1.46 (2.31) | 1.35 (1.76) |
| Stress | 3.55 (2.69) | 3.86 (2.80) | 3.22 (2.55) | 3.36 (2.72) | 3.73 (2.66) | |
Note: All data are presented as mean (standard deviation).
SES = socioeconomic status, HbA1c = hemoglobin A1c protein, CRP = C-reactive protein, BMI = body mass index, AHI = apnea-hypopnea index, IMT = intima-media thickness, SBP = systolic blood pressure, DBP = diastolic blood pressure, TST = total sleep time, SWS = slow wave sleep.
denotes significantly different (ANOVA, p <.05) from other corresponding high-, low-variable group
Cardiovascular Stress Reactivity
Baseline and stress task values for SBP, diastolic BP (DBP), and HR are presented in Table 1. The acute psychological stress task elicited a significant increase in SBP, F(1,98) = 26.78, p <.001, η2 = .215, DBP, F(1,98) = 7.42, p =.008, η2 = .070, and HR, F(1,96) = 34.48, p <.001, η2 = .264. Importantly, no systematic differences in cardiovascular stress reactivity were observed across the high- and low-TST or SWS groups (all p >.10).
Moderation Analyses: Intima-Media Thickness
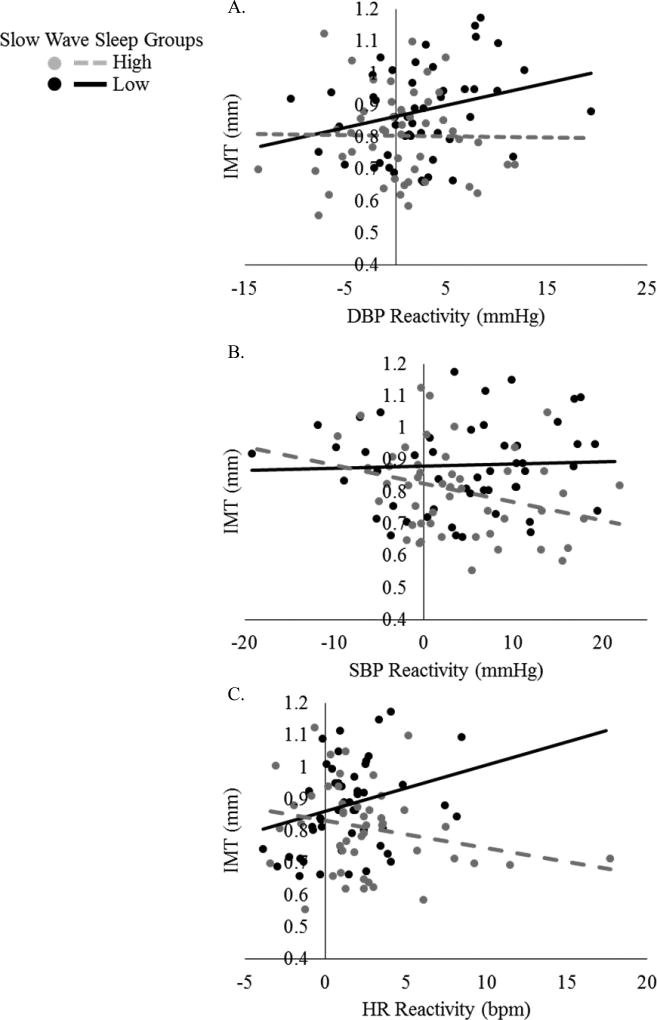
Univariate Spearman correlations between IMT, cardiovascular reactivity, and sleep for the whole sample and TST and SWS subgroups are presented in Tables S1–S5, Supplemental Digital Content. Slow wave sleep significantly moderated the relationship between cardiovascular stress reactivity and IMT. In unadjusted models, SWS was found to only moderate the relationship between HR reactivity and IMT (pinteraction = .017, ΔR2interaction = .056; Table 2). However, after adjustment for sociodemographic and biological covariates, SWS significantly moderated the relationships between all cardiovascular stress reactivity variables and IMT (all p ≤ .048; Table 2). Inspection of Figure 1 shows that, in the low SWS group a notable positive relationship between HR reactivity and IMT was observed and that greater BP reactivity was weakly related to higher IMT. In the high SWS group, greater HR reactivity was associated with lower IMT values; greater BP reactivity was weakly related to lower IMT values. No evidence of moderation by TST was found (all pinteraction ≥ .09).
Table 2.
Moderation of the relationship between cardiovascular stress reactivity and intima-media thickness by slow-wave sleep
| SBP Reactivity (N = 99) | DBP Reactivity (N = 99) | HR Reactivity (N = 97)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B(SE) | p | ΔR2 | B(SE) | p | ΔR2 | B(SE) | p | ΔR2 | |
| Unadjusted | |||||||||
| Reactivity | .001(.002) | .77 | .007(.004) | .06 | .014(.008) | .07 | |||
| SWS | −.080(.028) | .006 | −.071(.029) | .016 | −.074(.029) | .011 | |||
| Interaction | −.006(.004) | .08 | .029 | −.007(.006) | .18 | .017 | −.023(.010) | .017 | .056 |
| Adjustedb | |||||||||
| Reactivity | .003(.002) | .21 | .009(.003) | .009 | .018(.007) | .007 | |||
| SWS | −.036(.028) | .20 | −.040(.027) | .15 | −.040(.027) | .15 | |||
| Interaction | −.006(.003) | .048 | .027 | −.013(.005) | .011 | .043 | −.026(.008) | .002 | .064 |
Note: B values are unstandardized regression coefficients. Adjusted analyses include sex, age, socioeconomic status, fasting glucose and insulin, body mass index, resting SBP, and apnea-hypopnea index as covariates.
N = 97 due to loss of HR data.
N= 98 for SBP and DBP reactivity and N = 96 for HR reactivity because of participant with missing insulin data point.
SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate, SWS = slow-wave sleep, Interaction = SWS × Reactivity
Figure 1.
Raw cardiovascular stress reactivity and intima-media thickness data. Slow-wave sleep significantly moderates the relationship between diastolic (A), and systolic (B) blood pressure, and heart rate (C) reactivity and intima-media thickness. HR = heart rate, SBP = systolic blood pressure, DBP = diastolic blood pressure, IMT = intima-media thickness
Sensitivity Analysis: Reverse Moderation Model
No evidence was found to support the role of any cardiovascular reactivity variable in moderating the relationship between SWS and IMT in unadjusted (all pinteraction ≥ .09) or adjusted (all pinteraction ≥ .23) analyses.
Discussion
Nocturnal sleep, especially slow-wave sleep, is a period of cardiovascular quiescence and may represent an opportunity for the cardiovascular system to recuperate from daytime insults, such as stress-induced blood pressure surges. As such, it was hypothesized that the strength of the relationship between cardiovascular reactivity and IMT would be relatively weaker in individuals engaging in longer TST and greater amounts of SWS and relatively stronger in individuals exhibiting relatively short TST and low amounts of SWS. In line with the hypothesis, in participants with relatively large amounts of SWS, greater HR reactivity was related to smaller IMT values; BP reactivity was weakly associated with smaller IMT values. In individuals with relatively low amounts of SWS, greater HR reactivity was associated with higher IMT values; greater BP reactivity weakly related to greater IMT. All results were independent of relevant sociodemographic and biological covariates. Importantly, no consistent significant effects were found when cardiovascular reactivity was tested as a statistical moderator of the relationship between SWS and IMT. These results provide initial support for the hypothesis that SWS may buffer the psychological stress-cardiovascular reactivity-IMT pathway and show that nocturnal sleep can interact with daytime disease processes to modify disease risk.
Since SWS is considered the physiologically restorative segment of sleep (20–23), it makes sense conceptually that individuals with relatively large amounts of SWS would not display the same adverse relationship between cardiovascular stress reactivity and IMT seen in individuals with low amounts of SWS. The frequently reported relationship between blood pressure reactivity and IMT is likely the result of upward structural resetting of the vasculature (13,30). In a feed-forward fashion, stress-induced surges in blood pressure promote vascular hypertrophy which comprise the processes associated with short-term blood pressure regulation: flow-mediated dilation and the baroreflex (31–33). Once comprised, subsequent stress-induced blood pressure surges cannot be effectively buffered, promoting further vascular hypertrophy and thickening of the intima-media layers of the vasculature. However, blood pressure reaches its 24-hour nadir during SWS and is accompanied by a decrease in HR, baroreflex set point, vascular resistance, and sympathetic activity; parasympathetic activity and baroreflex sensitivity increase during SWS (24, 34–36). This provides a window for vascular rest (20) that, if not experienced, can lead to endothelial dysfunction as seen in experimental studies of full and partial sleep deprivation (37–39). Thus, it may be the case that obtaining adequate amounts of SWS allows for nocturnal cardiovascular quiescence, staving off the vascular damage and increased IMT associated with exaggerated blood pressure responses to acute psychological stress.
The present results must be interpreted in the context of several limitations. First, on average, cardiovascular reactions to the psychological stress task were modest, albeit statistically significant in size. However, a notable amount of inter-individual variability was observed for BP and HR reactivity with range values of 33.12 mmHg, 41.1 mmHg, and 21.55 bpm for DBP, SBP, and HR reactivity, respectively. Thus, we feel confident in drawing conclusions about the relationship between IMT and individual differences in cardiovascular reactivity. A second limitation is that the cross-sectional nature of the data precludes making inference about the progression of IMT or the causative role of SWS in the relationship between cardiovascular reactivity and IMT. The development of CVD occurs over the course of years, as does intima-media thickening. Because of this, it would be instructive to know whether displaying exaggerated BP and HR responses to psychological stress in combination with low amounts of SWS would enhance the progression of intima-media thickening and increase CVD risk longitudinally. Based on available evidence, one can speculate that this would be the case since individual differences in cardiovascular stress reactivity and SWS have both been shown to be relatively stable over time (40–42). If demonstrated, such a relationship might provide support for two modifiable intervention targets for behavioral treatment against the development and progression of CVD. Finally, the present sample was composed of mostly non-Hispanic white participants, limiting the generalizability of current results to other racial populations. Of particular relevance, Blacks have been found to carry disproportionately high CVD risk compared to whites (43), and display increased blood pressure stress reactivity (44–46) and decreased SWS (47–50). Given the results of the current study, attempts to replicate this finding in other racially-diverse samples may be particularly instructive. Individuals displaying exaggerated cardiovascular stress reactivity and relatively low amounts of SWS appeared to have the greatest IMT values. It is precisely Blacks and males that have been shown to display this stress- and sleep-phenotype, and carry a disproportionately high CVD burden.
In summary, in a cross-sectional study of generally healthy adults, the quantity of SWS was found to moderate the relationship between psychological stress-induced cardiovascular reactivity and carotid IMT, a subclinical marker of CVD risk. In individuals with relatively low amounts of SWS, greater cardiovascular reactivity was associated with greater IMT, while in individuals with relatively greater amounts of SWS, greater stress reactivity was related to generally lower IMT values. These data show that SWS significantly buffers stress-related cardiovascular disease risk and underscores the need to consider nocturnal sleep in studies of, what traditionally are considered, daytime disease processes.
Supplementary Material
Acknowledgments
Funding Source: Supported by grants from the National Institutes of Health: Primary T2 study was R01 HL104607 (M. Hall). T1 studies were R01 MH024652 and R01 MH029618 (D. Kupfer), R01 MH049115 (E. Frank), and R01 MH041884 (M. Thase). Partial support for investigator effort was also provided by R01 GM113243 (R. Krafty, M. Hall), and T32 HL082610 (R. Brindle, M. Cribbet), T32 HL007560 (K. Duggan), F32 HL137227 (R. Brindle), and K23 HL118318 (C. Kline). Infrastructure support was provided by UL1 TR000005 and UL1 RR024153.
Potential Conflicts of Interest: Drs. Hall, Brindle, Duggan, Cribbet, Thayer, Krafty, Kline, report grants from the National Institutes of Health.
Abbreviations
- AHI
Apnea-Hypopnea Index
- BMI
Body mass index
- BP
Blood pressure
- CCA
Common carotid artery
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- EKG
Electrocardiogram
- HR
Heart rate
- IMT
Intima-media thickness
- MSIT
Multisource interference task
- PLM
Period Limb Movement
- SBP
Systolic blood pressure
- SES
Socioeconomic status
- SWS
Slow-wave sleep
- TST
Total sleep time
- V-CTRC
Vascular clinical and translational research center
Footnotes
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
References
- 1.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandex-Hernandex R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th, and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conference, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–6. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb. 2012;23:18–31. doi: 10.5551/jat.31989. [DOI] [PubMed] [Google Scholar]
- 3.Van den Oord SCH, Sijbrands EJG, ten Kate GL, van Klaveren D, van Domburg RT, van der Steen AFW, Schinkel AFL. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis. 2013;228:1–11. doi: 10.1016/j.atherosclerosis.2013.01.025. [DOI] [PubMed] [Google Scholar]; Carpenter M, Sinclair H, Kunadian V. Carotid intima media thickness and its utility as a predictor of cardiovascular disease: A review of evidence. Cardiol Rev. 2016;24:70–5. doi: 10.1097/CRD.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 5.Gianaros PJ, Bleil ME, Muldoon MF, Jennings JR, Sutton-Tyrrell K, McCaffery JM, Manuck SB. Is cardiovascular reactivity associated with atherosclerosis among hypertensives? Hypertension. 2002;40:742–47. doi: 10.1161/01.hyp.0000035707.57492.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambiase MJ, Dorn J, Roemmich JN. Metabolic and cardiovascular adjustments during psychological stress and carotid artery intima-media thickness in youth. Physiol Behav. 2012;105:1140–47. doi: 10.1016/j.physbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Lassila HC, Wolfson SK. Stress-induced pulse pressure change predicts women’s carotid atherosclerosis. Stroke. 1998;29:1525–30. doi: 10.1161/01.str.29.8.1525. [DOI] [PubMed] [Google Scholar]
- 8.Roemmich JN, Lobarinas CL, Joseph PN, Lambiase MJ, Archer FD, Dorn J. Cardiovascular reactivity to psychological stress and carotid intima-media thickness in children. Psychophysiology. 2009;46:293–9. doi: 10.1111/j.1469-8986.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 9.Roemmich JN, Feda DM, Seelbinder AM, Lambiase MJ, Kala GK, Dorn J. Stress-induced cardiovascular reactivity and atherogenesis in adolescents. Atherosclerosis. 2011;215:465–70. doi: 10.1016/j.atherosclerosis.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamarck TW, Everson SA, Kaplan GA, Manuck SB, Jennings JR, Salonen R, Salonen JT. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 1997;96:3842–48. doi: 10.1161/01.cir.96.11.3842. [DOI] [PubMed] [Google Scholar]
- 11.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- 12.Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. J Hypertens. 1997;15:49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Folkow B. “Structural factors” in primary and secondary hypertension. Hypertension. 1990;16:89–101. doi: 10.1161/01.hyp.16.1.89. [DOI] [PubMed] [Google Scholar]
- 14.Treiber FA, Kamarck R, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010(55):1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 16.Chumaeva N, Hintsanen M, Ravaja N, Puttonen S, Heponiemi T, Pulkki-Råback L, Juonala M, Raitakari OT, Viikari JSA, Keltikangas-Järvinen L. Interactive effect of long-term mental stress and cardiac stress reactivity on carotid intima-media thickness: The Cardiovascular Risk Young Finns Study. Stress. 2009(12):283–93. doi: 10.1080/10253890802372406. [DOI] [PubMed] [Google Scholar]
- 17.Everson SA, Lynch JW, Chesney MA, Kaplan GA, Goldberg DE, Shade SB, Cohen RD, Salonen R, Salonen JT. Interaction of workplace demands and cardiovascular reactivity in progression of carotid atherosclerosis: Population based study. Brit Med J. 1997;314:553–8. doi: 10.1136/bmj.314.7080.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch JW, Everson SA, Kaplan GA, Salonen R, Salonen JT. Does low socioeconomic status potentiate the effects of heightened cardiovascular responses to stress on the progression of carotid atherosclerosis? Am J Public Health. 1998(88):389–94. doi: 10.2105/ajph.88.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall MH, Brindle RC, Buysse DJ. Sleep and cardiovascular disease: Emerging opportunities for health psychology. Am Psychol. doi: 10.1037/amp0000362. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trinder J, Waloszek J, Woods MJ, Jordan AS. Sleep and cardiovascular regulation. Pflugers Arch. 2012;463:161–68. doi: 10.1007/s00424-011-1041-3. [DOI] [PubMed] [Google Scholar]
- 21.Tononi G, Cirelli C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyazovskiy VV, Harris KD. Sleep and the single neuron: The role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14:443–51. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, IIiff JJ, Takano T, Dean R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–77. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javaheri S, Redline S. Sleep, slow-wave sleep, and blood pressure. Curr Hypertens Rep. 2012;14:442–48. doi: 10.1007/s11906-012-0289-0. [DOI] [PubMed] [Google Scholar]
- 25.Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment technique: Vanilla or resting baseline. Psychophysiology. 1992;29:742–50. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 26.Bush G, Shin LM. The multi-source interference task: An fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1:308–13. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.Operario D, Adler NE, Williams DR. Subjective social status: Reliability and predictive utility for global health. Psychol Health. 2004;19:237–46. [Google Scholar]
- 29.Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [white paper] 2012 Retrieved from http://www.afhayes.com/public/process2012.pdf.
- 30.Obrist P. Cardiovascular Psychophysiology: A perspective. New York, New York: Plenum Press; 1981. [Google Scholar]
- 31.Dampney RAL, Coleman MJ, Fontes MAP, Hirooka Y, Horiuchi J, Li Y-W, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29:261–8. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 32.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2010;30:H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. Circ Res. 1969;24:109–21. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- 35.Legramante JM, Marciani MG, Placidi F, Aguilani S, Romigi A, Tombini M, Massaro M, Galante A, Iellam F. Sleep-related changes in baroreflex sensitivity and cardiovascular autonomic modulation. J Hypertens. 2003;21:1555–61. doi: 10.1097/00004872-200308000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 37.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, Bourrilhon C, Florence G, Chennaoui M. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 38.Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Cavasin de Souza SBP, Irigoyen MC, Mostarda C, Borile S, Krieger EM, Moreno H, Jr, Lorenzi-Filho G. Cardiovascular effect of partial sleep deprivation in healthy volunteers. J Appl Physiol. 2012;113:232–6. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- 39.Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, Wang S, Somers VK. Experimental sleep restriction causes endothelial dysfunction in healthy humans. J Am Heart Assoc. 2014;3:e001143. doi: 10.1161/JAHA.114.001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Long-term stability of cardiovascular and catecholamine responses to stress tests: An 18-year follow-up study. Hypertension. 2010;55:131–6. doi: 10.1161/HYPERTENSIONAHA.109.143164. [DOI] [PubMed] [Google Scholar]
- 41.Sherwood A, Turner JR, Light KC, Blumenthal JA. Temporal stability of the hemodynamics of cardiovascular reactivity. Int J Psychophysiol. 1990;10:95–8. doi: 10.1016/0167-8760(90)90050-n. [DOI] [PubMed] [Google Scholar]
- 42.Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: For some measures, one night is enough. Sleep. 2012;35:1285–91. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girdler SS, Hinderliter AL, Light KC. Peripheral adrenergic receptor contributions to cardiovascular reactivity: Influence of race and gender. J Psychom Res. 1993;37:177–93. doi: 10.1016/0022-3999(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 45.Murphy JK, Stoney CM, Alpert BS, Walker SS. Gender and ethnicity in children’s cardiovascular reactivity: 7 years of study. Health Psychol. 1995;14:48–55. doi: 10.1037//0278-6133.14.1.48. [DOI] [PubMed] [Google Scholar]
- 46.Light KC, Turner RC, Hinderliter AL, Sherwood A. Race and gender comparisons: I. Hemodynamic responses to a series of stressors. Healthy Psychol. 1993;12:354–65. doi: 10.1037//0278-6133.12.5.354. [DOI] [PubMed] [Google Scholar]
- 47.Mokhlesi B, Pannain S, Ghods F, Knutson KL. Predictors of slow wave sleep in a clinic-based sample. J Sleep Res. 2011;21:170–75. doi: 10.1111/j.1365-2869.2011.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuda N, Honma H, Kahsaka M, Kobayashi R, Sakakibara S, Kohsaka S, Koyama T. Gender differences in slow wave sleep in middle aged and elderly subjects. Psychiatry Clin Neurosci. 1999;53:151–53. doi: 10.1046/j.1440-1819.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- 49.Halder I, Matthews KA, Buysse DJ, Strollo PJ, Causer V, Reis SE, Hall MH. African genetic ancestry is associated with sleep depth in older African Americans. Sleep. 2015;38:1185–93. doi: 10.5665/sleep.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomfohr L, Pung MA, Edwards KM, Dimsdale JE. Racial differences in sleep architecture: The role of ethnic discrimination. Biol Psychol. 2012;89:34–8. doi: 10.1016/j.biopsycho.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.