Abstract
Background
Alcohol use is reported accurately among pregnant women in some populations.
Methods
Self-reported alcohol use via the AUDIT and 90-day recall for 193 women from antenatal clinics was compared to biomarker results: phosphatidylethanol (PEth) from bloodspots and ethyl glucuronide (EtG) in fingernails.
Results
AUDIT was positive for 67.9% of respondents, and 65.3% directly reported drinking. Individual biomarkers detected less drinking (PEth = 57.0%, EtG = 38.9%) than self-report. But 64.8% had drinking-positive values (>8ng) on one or both biomarkers, which was not significantly different from self-report. Biomarkers indicated that 3.1% – 6.8% of drinkers denied drinking. Combined biomarker sensitivity was 95% – 80% and specificity 49% –76% for drinking in the previous 7 to 90 days. Combined biomarker results have their best yield (89.6%) and accuracy (78.8%) when measuring 90 day drinking.
Conclusions
Women reported their alcohol use accurately, and the combined use of PEth and EtG is supported.
1. Introduction
In parts of the Western Cape Province (WCP) of South Africa (SA) there is a subculture of regular binge drinking. It is common for 35 to 50% of women of childbearing age to drink 2 to 9 alcoholic beverages each night on most Fridays and Saturdays [1,2]. This is the major factor creating a high prevalence of fetalalcohol spectrum disorders (FASD) in the general population of some communities of the WCP. These communities have the highest documented prevalence of FASD anywhere in the world; 17 to 28% of children in first grade classes have been found to have FASD [3–6].
Over the past twenty years, members of our SA research team have judged the local reporting of alcohol use to be extremely candid and forthright among women and men in the WCP [1,2,7,8]. Furthermore, we have found that reports of alcohol use, childbearing, and other personal information across various datasets collected in these populations were reliable. Associations between self-reported alcohol use data and specific alcohol-related outcomes, specifically diagnoses within the continuum of FASD, correlated significantly with seemingly credible levels of alcohol exposure in multiple samples and studies [3–6,8,9], yet the accuracy of the basic alcohol reporting had not been tested against biomarkers of alcohol use. Therefore, we embarked on this study to assess the accuracy of alcohol-use reporting in these SA communities.
In studies of alcohol use reporting carried out in some populations, women are believed to be less than honest and accurate when providing alcohol-use information, [10–12] especially in prenatal clinic settings in Western Europe. This finding has been reported when sensitive alcohol-specific biomarkers were employed using appropriate biological specimens [13–16]. However, there is also ample evidence that many populations report quite accurately if proper interviewing techniques are used, rapport is built, and multiple measures of alcohol use over time are used [16–23].
1.1 Study Objectives: Cross Validation for a Utilitarian Understanding
There were two objectives in this study. The first objective was to assess whether the maternal population of the WCP of SA is accurate in the overall reporting of alcohol use during pregnancy by utilizing objective biomarkers of drinking. Second, we sought to estimate how accurate, sensitive, and specific each of the two biomarkers was for detecting any level of alcohol use in this population through comparison of the biomarker results with self-report. It is a comparative validity study of the two methods to determine their utility for use in both antenatal clinic applications and for research purposes.
This manuscript compares positive and negative results from two self-reported alcohol-use measurements with results from two alcohol-use biomarkers. The self-report measures are the World Health Organization Alcohol Use Disorders Identity Test (AUDIT) [21], and standard measures of alcohol use by quantity and frequency (Q-F) [7]. The two biomarkers are ethyl glucuronide (EtG) and phosphatidylethanol (PEth), two metabolites of alcohol consumption that can be measured in various biological specimens (e.g. urine, blood, or cutaneous substances). They both have been found to be specific to alcohol use and are sensitive to moderate to heavy intake of alcohol over specific windows in time [25,26].
2. Methods
2.1 Measures and Sampling
The two biomarkers were measured from different biological materials. Phosphatidylethanol (PEth) was measured in bloodspots from finger pricks and ethyl glucuronide (EtG) was measured in fingernail clippings totalling 50 to 100mg or more. Both specimens were collected from 193 pregnant women attending community health care antenatal clinics that serve the vast majority of the local community population. The average gestation of the respondents at the time of bloodspot collection and simultaneous interview was 19.7 (±SD of 7.5) weeks. These participant interviews contained an array of maternal risk factors encountered during the index pregnancy, with an emphasis on dietary intake and alcohol consumption. The questionnaire contained two techniques for collecting and summarizing self-reported alcohol use. The AUDIT [21] was used with a cut off score of 4 for a high degree of sensitivity for measuring current alcohol use at the light to moderate range and above.Also well-established quantity/frequency (Q-F) questions were used that covered alcohol use at time of interview and specific time periods up to three months prior to the interview. All participants lived in one of two small towns and surrounding rural areas of the WCP.
2.2 Key Time Periods for Measuring Prior Alcohol Use
The three categories and time periods of particular interest for the biomarker analyses were: a) those using alcohol seven days prior to the interview, b) those consuming alcohol 30 to 90 days prior to the interview, and c) those who were self-reported abstainers throughout the previous 90 days. Both PEth and EtG are reported to be sensitive, direct, alcohol-specific biomarkers for most individuals [24–26]. PEth as a biomarker in blood samples has a half-life of five to seven days, and is accurate for measuring moderate consumption in the past seven days, and sustained, heavy consumption up to three weeks [27]. EtG collected from fingernails is purported to be accurate for detecting moderate to heavy drinking up to three months prior to sample collection [28,29]. Because most drinking occurs over the weekends for over 90% of alcohol users in this particular SA population [2,7], bloodspots and interviews for the PEth biomarker analyses were collected only on Monday or Tuesday clinics to provide accurate measures of drinking. Furthermore, since the fingernail samples record longer-term alcohol use, they were collected at either first contact, at the same time as the blood samples and interviews were collected, or at a scheduled return visit to the participant’s home 1 to 2 weeks later, once the nails had grown to an appropriate length (3 mm).
2.3 Maternal Questionnaire
The self-report questionnaire was developed specifically for epidemiology studies of the prevalence and characteristics of FASD via active case ascertainment and the clinical diagnosis of FASD in the WCP. To establish rapport, nonthreatening questions about general maternal health and diet were asked first, and the interview moves to information on health, diet, and childbearing. Alcohol consumption responses are more accurate in such a format, especially embedded within the context of dietary questions [30]. Multiple measures of alcohol use in the previous 90 days were asked, paying special attention to alcohol brands and containers commonly used in this population (vessels measurement), as respondents were shown pictures of standard containers of local brands. This sequencing and vessels technique assists in accurate reporting and calibration of the amounts consumed [31,32]. Alcohol was measured in standard US units where one drink equals: a 340 ml can/bottle of beer (5 to 5.5% ethanol), 120 ml of wine (11% ethanol), 95 ml of wine (13.5% ethanol), or 44 ml of distilled spirits (43% ethanol). Experienced research staff employed by the grant, mainly nurses and social workers, conducted the maternal interviews.
2.4 Biomarker Analysis and Utilitarian Purposes
The biological specimens were shipped to the United States where the United States Drug Testing Laboratories of Des Plaines, Illinois prepared the samples for processing and performed the analyses for both of the biomarkers. Neither of these specimen types (blood spots and fingernails clippings) required special processing on site or refrigeration when shipping. The blood spots were squeezed from finger pricks onto paper blood collection cards and allowed to dry. Fingernails were clipped with a common fingernail clipper and placed in a small envelope for shipping. For both EtG and PEth, a liquid chromatography-tandem mass spectrometry method was used to analyse the specimens [26,33]. For the analyses presented in this paper, the cut-off level for positive or negative alcohol use was ≥8ng/mL for either EtG or PEth as past analyses have indicated both accuracy and sensitivity at this level. [24,26]
The results of the biomarker tests and the self-report measures, as analyzed and presented here, provide binary measures of positive or negative alcohol use. We call this analytic approach utilitarian because we focused the analysis on the validity or utility of one or both markers to detect any alcohol use that is meaningful for research and/or clinical purposes. For the clinic: does this person use alcohol (yes/no)? If yes, preventive measures might be instituted for this individual. For research purposes: can a positive result on one or both of the biomarkers be compared with self-reported use to determine accuracy of reporting and the case classified as an alcohol-exposed pregnancy?
2.5 Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) [34] was used for statistical analyses. Chi-square tests of significance were used for comparisons of the binary (positive/negative) data. Z-tests of proportions were used to compare positive results by various binary criteria. Analyses of strength of association were performed with squared phi coefficients. To compensate for inflation of Type I error rate due to the exploratory nature of the analyses and redundancies among them, criterion α has been set to .01 for all tests of statistical significance, as per Tabachnick and Fidell [35].
3. Results
3.1 Prevalence of Alcohol Use Measured Independently by Self-Report and Individual Biomarkers
One hundred ninety-five paired biomarker samples were collected (one of each biomarker for each woman) in the antenatal clinics, stored, and shipped across the Atlantic. And 193 pairs were processed successfully to yield results within a meaningful range of values. Each of these respondents completed the interview containing the self-reported alcohol use measures. Sixty-eight percent (67.9%) of the participants scored positive on the AUDIT when set at a level for high sensitivity (>4). The average AUDIT score for the entire sample was 11.1 (SD = 9.1), and 16.1 (SD = 6.6) for the drinkers only, and a value ≥8 is considered problem or heavy drinking [18]. On the Q-F measures, 65.3% of the participants reported drinking. The average overall standard drinks per drinking day (DDD) over the past 30 days was 2.7 (SD=3.8). Calculated for the drinkers only, the average DDD was 5.2 (SD = 3.8) drinks per drinking day.
Table 1 indicates that PEth detected alcohol use in 57.0% of the respondents. EtG detected drinking in 38.9% of the respondents. A positive detection on either one or both biomarkers was found in 125 women or 64.8% of the sample, and alcohol use was detected by both biomarkers in 62 women or 32.1%.
Table 1.
Positive Cases of Alcohol Usage as Indicated by Two Biomarkers among Females in the Antenatal Period (n=193)
| Significant EtOH Usage | PEth + (≥8ng/mL) | EtG + (≥8ng/mg) | Positive cases on one or both biomarkers | Positive on both biomarkers |
|---|---|---|---|---|
| Positive | 110 (57.0%) | 75 (38.9%) | 125 (64.8%) | 62 (32.1%) |
| Negative | 83 (43.0%) | 118 (61.1%) | 68 (35.2%) | 131 (67.9%) |
3.2 Comparison of Biomarker Results Utilizing Self-Reported Alcohol Use as the Standard
Table 2 compares self-reported drinking on the AUDIT measure with the percentage of drinkers detected by the two biomarkers. One hundred-thirty-one (131) cases were AUDIT positive (score >4). Only 83.9% as many drinking-positive cases were identified by PEth from bloodspots, for 75 drinkers or 57.3% of the sample were identified by EtG alone as measured in fingernails. Both of these individual biomarker detection rates were significantly lower than self-reported information on the AUDIT when measured by either a conventional chi-square test or a Z-test of proportions (p<.001), in which the proportion of positive results detected by the biomarker(s) is divided by the proportion of positive results reported by the AUDIT score. But when the results of the two biomarkers are combined, 125 were positive on one or both of the biomarkers. This represents 95.4% as many cases as were detected by AUDIT. Self-reported alcohol use via the AUDIT was not significantly different (z= 2.37, p=.018) from that detected by the two biomarkers used individually.
Table 2.
Association between Self-Reported AUDIT score and Drinking Detected by PEth, EtG, and both biomarkers combined among Female Participants (N=193). Classification Tables and Statistics.
| AUDIT score | PEth Result | EtG Result | Positive cases on one or both biomarkers | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| Negative (0–4) | 46 (23.8%) | 16 (8.3%) | 56 (29.0%) | 6 (3.1%) | 42 (21.8%) | 20 (10.4%) |
| Positive (>4) | 37 (19.2%) | 94 (48.7) | 62 (32.1%) | 69 (35.8%) | 26 (13.5%) | 105 (54.4%) |
| χ2=36.250, df=1, p<.001, φ=.433 (+ by PEth) / (+ by AUDIT) = 83.9% Z=4.53, p<.001 |
χ2=32.742, df=1, p<.001, φ=.412 (+ by EtG)/(+ by AUDIT) = 57.3% Z= 7.40, p< .001 |
χ2=42.303, df=1, p<.001, φ=.468 (+ by 1 or both biomarkers)/(+ by AUDIT) = 95.4% Z=2.37, p=.018 |
||||
In Table 3, self-reported drinking prevalence of any drinking on the Q-F questions in the past 90 days was compared to the positive results from the two biomarkers used individually and in combination. Measured by both chi-square and Z-test of proportions (as described above), the detection rate of the individual biomarkers is significantly less than self-reported drinking (Q-F). PEth detected drinking in 87.3% as many of the participants as those who reported drinking in the past 90 days. EtG produced a positive result in 59.5% of the participants. And the two biomarkers combined indicated that 125 of the respondents drank, which is 99.2% as many as reported drinking by quantity and frequency. The combined difference of proportions is not statistically significantly different (z = 0.825, p=.409) from that reported in interviews.
Table 3.
Association between Self-Reported Prenatal Drinking Prevalence among Females and Prevalence Detected by PEth and EtG (N=193). Classification Tables and Statistics.
| Quantity- Frequency of Drinking Self-report drinking history |
PEth Result | EtG Result | Positive cases on one or both biomarkers | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| Did not drink | 53(27.5%) | 14(7.3%) | 59(30.6%) | 8 (4.1%) | 48 (24.9%) | 19 (9.8%) |
| Drank in the past 7 days | 5(2.6%) | 55(28.5%) | 21(10.9%) | 39(20.2%) | 3 (1.6%) | 57 (29.5) |
| Drank in the past 30 days | 15(7.8%) | 36(18.7%) | 32(16.6%) | 19 (9.8%) | 12 (6.2%) | 39 (4.7%) |
| Drank in the past 3 months | 10 (5.2%) | 5 (2.6%) | 6 (3.1%) | 9 (5.1%) | 5 (2.6%) | 10 (5.2%) |
| χ2=72.320, df=3, p<.001, Somer’s d=.549 (+ PEth)/(+Q-F) = 87.3% Z=3.94, p <.001 |
χ2=40.568, df=3, p<.001, Somer’s d=.382 (+ EtG)/(+Q-F) = 59.5% Z=6.78. p<.001 |
χ2=65.179, df=4, p<.001, Somer’s d=.517 (+ by 1 or both/ + by Q-F) =99.2% Z=0.825, p=.409 |
||||
Note: Symmetric form of Somer’s d
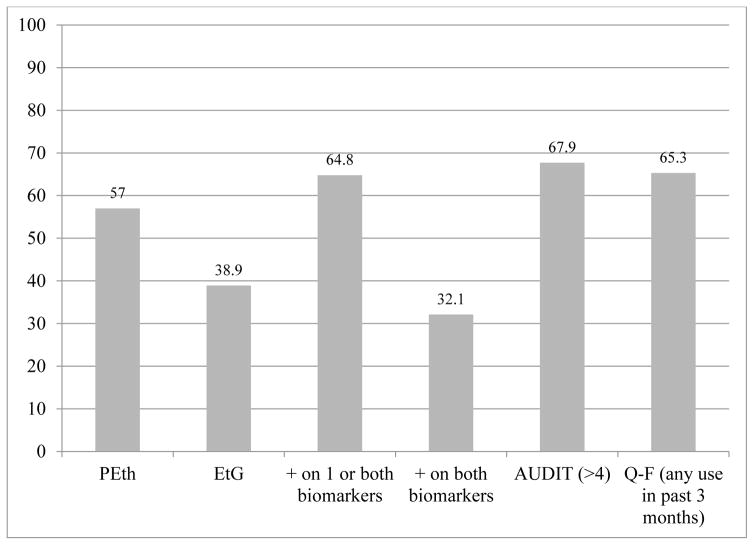
In Table 4, final, aggregate reports of drinking are compared by cases and percentages positive on one or both biomarkers. The AUDIT identified six more drinkers (4.8%), and the Q-F measures indicated one more case (0.8%) than the two biomarkers combined. A Z-test of proportions tested whether the proportion of cases identified as positive differed between detection by one or both biomarkers and self-report (separately tested for AUDIT and Q-F). Neither of these differences, was statistically significant (z =.664, p = .507 for AUDIT, and z=.103, p = .918 for Q-F). Therefore, self-report and combined biomarker results produce similar overall prevalence findings in these aggregate comparisons of proportion of drinkers. Figure 1 summarizes the various detection rates.
Table 4.
Comparing Positive Self-Report with Positive Biomarker Data (PEth and EtG) (N=193)
| Self-Report AUDIT^ | Positive By 1 or Both biomarkers | Difference in Positive Cases Detected by Self- Report | Self-Report Q-F | Positive By 1 or Both Biomarkers | Difference in Positive Cases Detected by Self- Report | |
|---|---|---|---|---|---|---|
| Negative | 62 (32.1%) | 68 (35.3%) | +6 (4.8%) | 67 (34.7%) | 68 (35.2%) | +1 (0.8%) |
| Positive | 131 (67.9%) | 125 (64.8a | 126 (65.3%) | 125 (64.8%)b |
AUDIT positive: >4;
Z= .664, p=.507
Z= .103, p=.918
Figure 1.
positive result indicating any alcohol use by two biomarkers and two methods of self-report
3.3 Comparison of Self-Reported Alcohol Use with Biomarker Results as the Standard
Table 5 was constructed because the biomarkers identified some participants as drinkers who had not self-identified, and some participants who identified themselves as drinkers were not detected by a positive result on one or both of the biomarkers. Part A of Table 5 indicates that a positive value on one or both of the biomarkers identifies 112 (true positive) drinkers or 58.0% and 40 (20.7%) were identified as abstainers (true negatives). The remaining participants are possible deniers, for 13 cases (6.8%) may not have responded truthfully, by denying drinking when they were positive on one or both biomarkers. Finally, 28 or 14.5% were false negatives who were positive via self-report yet negative on both biomarkers. The test of proportions comparing participants who were positive on one or both biomarkers (n = 125, 64.8%), with those positive on one or more self-report measures (n= 140, 72.5%) is not statistically significant (z = 1.630, p=.103)
Table 5.
Positive and Negative Alcohol Use Case Detection Results for Two Biomarkers (PEth and EtG) Compared with Two Self-Report Measures (AUDIT and Quantity/Frequency in the Past 90 days) as the Standard
| 1 or Both biomarkers | ||
|---|---|---|
| Positive (≥8ng/ml) | Negative (<8ng/ml) | |
| A. On 1 or Both Self-Report Measures | ||
| Positive | 112 (58.0%) (true positives) |
28 (14.5%) (false negatives) |
| Negative |
n=13 (6.8%) (false positives/likely deniers) |
n=40 (20.7%) (true negatives) |
| On Both biomarkers | ||
|---|---|---|
| B. On both Self-Report Measures | Positive (≥8ng/ml) | Negative (<8ng/ml) |
| Positive | 55 (28.4%) (true positives) |
62 (32.1%) (false negatives) |
| Negative | 6 (3.1%) (false positives/likely deniers) |
70 (36.3%) (true negatives) |
Z-test of proportions comparing positive on 1 or both biomarkers (64.8%) to positive on 1 or more self-report measures (72.5%) : Z=1.630, p=.103 based on A portion of table.
Shown in part B of Table 5, there are 55 cases categorized as true positives (28.4%) on both biomarkers (true positives). Seventy were classified as true negatives (36.3%) on both biomarkers, for they reported that they abstained, and the biomarker results are negative. Furthermore, 62 (32.1%) were categorized as false negatives, for they reported drinking yet they were negative on the biomarkers. Finally, six cases (3.1%) reported no drinking on either self-report measure, but were positive for drinking on one or both biomarkers.
These two comparisons indicate that six to 13 biomarker positive/self-report negative respondents may have falsely denied drinking. It is therefore likely that at least 3.1% to 6.8% of the women participants have concealed their drinking. The other 28 (14.5%) from Table 5A, to 62 (32.1%) from Table 5B, were cases who reported drinking but were classified as positive by one or both of the biomarkers. They are false negatives for they reported drinking, yet the biomarkers did not classify them as drinkers. Were they light and/or occasional drinkers and therefore not detected by these two biomarkers? Table 5, therefore, raises the question of the sensitivity and specificity of these biomarkers.
3.4 Sensitivity/Specificity Analysis Using Self-Report as the Standard
Given that lying, or denial of drinking when it actually occurred, seems to have been minimal, one can conclude that drinking is reported quite accurately in this population. Therefore, sensitivity analysis is appropriate using self-report as the standard. In Table 6, PEth measured in bloodspots is 91.7% (0.9167) sensitive and 58.7% (0.5865) specific when drinking occurred in the past seven days, and 72.1% sensitive and 83% specific when drinking occurred sometime in the past 90 days. EtG measured in fingernails is less sensitive but more specific overall, for it is 65% sensitive and 72.9% specific for drinking in the past seven days, and 50.1% sensitive and 92.5% over the 90-day period.
Table 6.
Sensitivity and Specificity of Alcohol Use Biomarkers: Detection by individual test and with combined results by time period measured
| Sensitivity | Specificity | Positive Predictive Value (Yield) | Accuracy | |
|---|---|---|---|---|
| PEth* | ||||
| 7 days | 0.9167 | 0.5865 | 0.5000 | 0.6891 |
| 90 days | 0.7214 | 0.8302 | 0.9182 | 0.7513 |
| EtG** | ||||
| 7 days | 0.6500 | 0.7293 | 0.5200 | 0.7047 |
| 90 days | 0.5071 | 0.9245 | 0.9467 | 0.6218 |
| Positive by 1 or both biomarkers | ||||
| 7 days | 0.9500 | 0.4887 | 0.4560 | 0.6321 |
| 90 days | 0.8000 | 0.7547 | 0.8960 | 0.7876 |
Measured in blood spots
Measured in fingernail clippings
When the positive results of the two biomarkers are combined, the best sensitivity is obtained while maintaining reasonably high specificity. Positive on one or both biomarkers, seven-day sensitivity is 95% and specificity is 48.9%. Over the 90-day period, sensitivity for the combined biomarkers is 80% and specificity is 75.5%. The positive predictive value (yield) is best for all measures when used over the longer time frame, 90 days. Accuracy in this population is good for PEth when measuring drinking in either the past 7 or 90 days and for EtG at 7 days up to 90 days. Finally, using the biomarkers together is most sensitive for measuring use in the past seven days (95%), most specific at 90 days (75.5%), and combined results have the best yield at 90 days (89.6%) and most accurate at 90 days (78.8%).
3.5 Association Analysis
Table 7 summarizes analyses of association to assess the individual, case-by-case variation in the accuracy of measures of alcohol use employed here. The strongest association for an individual biomarker is between PEth and reported quantity and frequency of alcohol use in the past 90 days (φ2 = .301;, p<.001). The association between PEth and AUDIT was weaker although still statistically significant, φ 2 = .187, p<.001. The association between EtG and AUDIT was similar, φ2 = .170, p<.001.
Table 7.
Summary of Associations Among Biomarker Results and Self-Reported Drinking over the Past Three Months
| Variables | φ2 (strength of association* |
|---|---|
| PEth vs AUDIT | .187 |
| EtG vs AUDIT | .170 |
| PEth vs EtG | .154 |
| PEth vs Q-F | .301 |
| EtG vs Q-F | .146 |
| 1 or both biomarkers vs 1 or both self-report | .268 |
| Both biomarkers vs Both Self-Report | .169 |
All p < .001
The association between the two biomarkers (PEth vs. EtG) is statistically significant, φ2 = .154, p<.001. Finally the association between a positive value on one or both biomarkers and one or both self-report measures is second only to that between PEth and Q-F, φ 2 = .268, p<.001.
4. Discussion
The two questions posed in this study have been answered to a substantial degree by this utilitarian analysis. Are these respondents in the WCP of SA accurate reporters of alcohol use? And, are PEth and EtG, used individually or in combination, valid and useful binary (use/no use) measures of alcohol consumption, particularly in a population where a common drinking pattern is to engage in moderate to heavy consumption in a binge pattern on a weekly basis?
4.1 Accurate Reporting in This Population
The respondents in this sample of women recruited from antenatal clinics have been found to be accurate reporters of their alcohol use. A slightly higher percentage of the respondents reported alcohol use on both the AUDIT and by quantity and frequency than was detected by the biomarkers individually. And when positive results were combined for the two biomarkers, they were virtually the same (not statistically significant). This is particularly striking given the venue, for self-report studies from prenatal clinics in the United States and Europe have found that underreporting is frequently the norm when self-reported prevalence has been compared to a variety of biomarkers taken in the clinics (via urine and blood) and at birth via meconium, (e.g. fatty acid ethyl esters (FAEE), EtG, PEth, and others) [13,15,36–42]. One meta-analytic review found that biomarker testing of meconium for several different biomarkers produced 4.26 times more positive results than did matched self-reports [14].
Underreporting of the actual amounts of alcohol consumed in prenatal clinics has also been found to be significant in some populations when retrospective drinking reports, gathered up to 13 years later, are compared to the amounts and frequencies of drinking reported in prenatal clinics [18–20]. One other study in Africa, among Ugandans, also concluded that self-reported alcohol use often lacks agreement with PEth results, particularly among women [16]. Underreporting, however, was not the case in this study from SA, for the combined biomarker results and the self-reported data indicated a similar prevalence of drinking. In this study, the percentage of alcohol users reported by the respondents is higher than that detected by individual biomarkers, but similar to that reported as positive by the two biomarkers in combination. Other studies in other populations have also shown that a combination of biomarkers [33] and/or a combination of biomarkers and self-report yield the highest prevalence of alcohol use [44–45]. We maintain that accuracy of alcohol use reporting varies by population, particularly for quantity of drinking reported among heavy or binge drinkers. There are a number of populations where studies have shown reporting to be quite accurate [17–18,43,46,47]. And for Southern African populations accurate reporting has been noted and validated. For example, one in Lesotho [12], and two in the Western Cape Province using EtG and FAEE [46,47] also found self-reported use to be significantly higher than detected by biomarkers alone. Therefore, in this study where the reporting by the respondents overall was not significantly different from that detected by the combined results from the two biomarkers is another indicator that the predominately Coloured and Black populations of Southern Africa report accurately.
Additionally, a recent longitudinal study of the Inuit in Northern Canada also indicated accurate reporting of use/no use of alcohol during pregnancy in both prenatal clinics at 11 years after delivery [18]. An additional point can be made here. Accurate reporting in any population may be a function of the nature, setting, and conduct of the interviews. In studies carried out in two separate populations, Jacobson et al. [17–18,43] reported that accuracy of reporting may more consistently accurate when interviews are conducted by well-trained, experienced, and sensitive interviewers with well-worded and well-sequenced protocols. Therefore, women may report less to nurses and physicians in less formal interview settings, particularly in antenatal clinics where disclosure of drinking may lead to critical, corrective, or judgmental rhetoric. Additionally, there were very few participants in this sample who were suspected of lying about their drinking because of a negative report of drinking and a positive biomarker result. Between 3.1% and 6.8% were classified as false positive by the biomarkers on the other hand, many more (14.5% to 32.1%) were categorized as false negatives by the biomarkers. This may be an indication that the drinking of these individuals was light to moderate (likely not heavy or consumed in a binge-like fashion) and therefore escaped detection by these biomarkers which are reported to be best in detecting binge drinking and heavy consumption. Lack of self-report in these instances could also be a result of light drinkers forgetting instances of alcohol use in the past 90 days [17].
Future analysis of these data will address the association between the specific levels of each biomarker detected in a subject, and the specific quantity and frequency of drinking that she reported. This will shed further light on the accuracy question in the future. But, from this binary analysis and the results of three previous studies comparing biomarker results with self-report in Southern Africa [12,46,47], we can conclude that alcohol use reporting in this region is generally accurate, much more so than that reported in most comparative biomarker/self-report studies from Europe [40–45] and North America [13]. There has traditionally been a lack of stigma surrounding recreational binge drinking in this population and this may also make accurate reporting more likely [2].
4.2 The Biomarkers, When Used in Combination, Are Sensitive and Valid
Taking these two biomarker comparisons as the gold standard of validation, we might surmise that as many as 93.8% to 96.9% of the biomarker positive/self-report negative cases are truly drinkers. And one might also conclude that 14.5% to 32.1% of the drinkers have been missed (i.e. biomarker negative) because of light to moderate drinking. Or they may be individuals with particular metabolic characteristics that make detection by one or both of the specific biomarkers unlikely in a single sample collection.
Furthermore, the sensitivity analysis indicated that PEth was, in this population, the more sensitive measure when measured from bloodspots, but both PEth from bloodspots and EtG from fingernails were substantially specific. The highest sensitivity was achieved when the two biomarkers were used in combination (95%) to measure drinking in the past seven days and specificity was relatively high (76% over 90 days) with a positive predictive value of 90% and accuracy of 79% to detect drinking in the past 90 days. As utilitarian measures for antenatal clinic use, the combined results of PEth and EtG are certainly sensitive enough for most research applications for behavioral studies and in clinics to trigger preventive measures such as providing information on abstaining from alcohol during pregnancy and explanations of fetal vulnerability. They may further be utilized to justify offers of referral to alcohol treatment or case management.
Association analysis revealed that the biomarkers correlated significantly, although the magnitude of association utilizing only a binary measure of drinking detection (both self-report and biomarker results were treated as simply positive or negative) was not very strong. Again, further analysis on this topic is needed to decisively determine association by magnitude of each measure, self-report versus each biomarker.
4.3 Strengths and Limitations
The strengths of this study are these. First, we utilized a conventional technique of comparing the performance of biomarkers to self-reported alcohol use provided by the same participants. And this is one of a few studies to utilize both two different biomarkers to measure drinking in antenatal clinics and also two complimentary self-report measures to determine the accuracy of alcohol use measurement in a population believe to be candid and accurate in their reporting of alcohol consumption. Second, the two different self-report techniques were used for cross validation of the reporting. Third, of the two different biomarkers used, one was a marker for detecting recent use and the other a marker to detect moderate to heavy use over a three-month period. Each marker had its strengths and weaknesses, and the two in combination worked quite well. Using these mixed techniques in a population that has been studied previously and extensively, we were able to reliably assess or estimate both whether this was a population that is relatively accurate in their reporting, and to assess the accuracy and utility of the two biomarkers. We believe these multiple comparisons served both objectives well. The biomarkers proved to be efficacious and therefore can have utilitarian applications for both clinical and research use in populations where moderate to heavy drinking exists among women of childbearing age. The weaknesses are these. First, similar studies should be undertaken in populations characterized by different drinking patterns, for this population is somewhat unique in the pattern of regular binge drinking on weekends. When this biomarker study was designed, we believed from prior experience and research that this was likely to be an accurate reporting population.
Therefore, testing the biomarkers against self-report in this population may render the results valid only in regards to drinking in this particular population. Second, we analyzed the data for this manuscript on a binary basis, drinking versus not drinking. We did not correlate the magnitude of the results with one another (self-report quantities versus biomarker concentrations), for such assessment requires further exploration, analysis, and discernment which is underway.
5. Conclusion
Used in combination, these two biomarkers are particularly good for confirming that this is an accurate alcohol-use reporting population and for measuring alcohol use in a binary fashion. If we use the biomarkers in combination as the standard by which to judge the accuracy of reporting, only 3.1% to 6.8% of respondents denied significant alcohol use during the prenatal period. Therefore, these biomarkers can be used for accurate estimation of moderate to heavy prenatal alcohol use in both individuals and in entire populations. Each self-report measurement and each of these two biomarkers has its particular strengths and weaknesses. But, when used in combination, they provide a strong set of tools for accurate measurement of alcohol use.
HIGHLIGHTS.
Pregnant women in this population report alcohol use accurately.
The AUDIT interview tool identified 67.9% as alcohol users.
Alcohol use via reported quantity/frequency measures was 65.3%.
PEth in blood spots identified 57% as drinkers.
EtG in fingernails identified 38.9% as drinkers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.May PA, Gossage JP, Brooke LE, Snell CL, Marais AS, Hendricks LS, Croxford JA, Viljoen DL. Maternal risk factors for fetal alcohol syndrome in the Western Cape province of South Africa: a population-based study. Am J Public Health. 2005;95(7):1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May PA, Gossage JP, Marais AS, Hendricks L, Snell C, Tabachnick BG, Stellavate C, Buckley DG, Brooke L, Viljoen DL. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. 2008;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 3.May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalenceof the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013 May;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May PA, de Vries MM, Marais AS, Kalberg WO, Adnams CM, Hasken JM, Robinson LK, Manning M, Jones KL, Hoyme D, Seedat S, Parry CD, Hoyme HE. The continuum of fetal alcohol spectrum disorders in four rural communities in South Africa: prevalence and characteristics. Drug Alcohol Depend. 2016 Feb;159:207–218. doi: 10.1016/j.drugalcdep.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May PA, Marais AS, de Vries MM, Kalberg WO, Buckley D, Hasken JM, Adnams CM, Barnard R, Joubert B, Cloete M, Tabachnick B, Robinson LK, Manning MA, Jones KL, Bezuidenhout H, Seedat S, Parry CD, Hoyme HE. The continuum of fetal alcohol spectrum disorders in a community in South Africa: Prevalence and characteristics in a fifth sample. Drug Alcohol Depend. 2016 Nov;168:274–286. doi: 10.1016/j.drugalcdep.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May PA, de Vries MM, Marais AS, Kalberg WO, Buckley D, Adnams CM, Hasken JM, Tabachnick B, Robinson LK, Manning MA, Bequidenhout H, Adam MP, Jones KL, Seedat S, Parry CDH, Hoyme HE. Replication of high fetal alcohol spectrum disorders prevalence rates, child characteristics, and maternal risk factors in a second sample of rural communities in South Africa. Int J Environ Res Public Health. 2017;14(5) doi: 10.3390/ijerph14050522. pii:E522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, de Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CD, Hoyme HE, Tabachnick B, Seedat S. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013 Dec;133:502–12. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croxford J, Viljoen D. Alcohol consumption by pregnant women in the Western Cape. S Afr Med J. 1999;89(9):962–965. [PubMed] [Google Scholar]
- 9.Viljoen D, Croxford J, Gossage JP, Kodituwakku PW, May PA. Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: a case control study. J Stud Alcohol. 2002;63:6–17. [PubMed] [Google Scholar]
- 10.Wetterling T, Kanitz R-D, Rumpf H-J, Hapke U, Fisher D. Comparison of CAGE and MAST with the alcohol markers CDT, GGT, ALAT, ASAT, and MCV. Alcohol & Alcoholism. 1998;33:424–430. doi: 10.1093/oxfordjournals.alcalc.a008414. [DOI] [PubMed] [Google Scholar]
- 11.Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res. 1988;12:506–511. doi: 10.1111/j.1530-0277.1988.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 12.Siegfried N, Parry CDH, Morojele NK, Wason D. Profile of drinking behavior and comparison of self-report with the CAGE questionnaire and carbohydrate-deficient transferrin in a rural Lesotho community. Alcohol & Alcoholism. 2001;36(3):243–248. doi: 10.1093/alcalc/36.3.243. [DOI] [PubMed] [Google Scholar]
- 13.Bakhireva LN, Sharkis J, Shrestha S, Miranda-Sohrabji TJ, Williams S, Miranda RC. Prevalence of prenatal alcohol exposure in the state of Texas as assessed by phosphatidylethanol in newborn dried blood spot specimens. Alcohol Clin Exp Res. 2017;41(5):1004–1011. doi: 10.1111/acer.13375. Epub 2017 Apr 5. [DOI] [PubMed] [Google Scholar]
- 14.Lange S, Shield K, Koren G, Rehm J, Popova S. A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self-reports versus meconium testing: a systematic literature review and meta-analysis. BMC Pregnancy and Childbirth. 2014;14:127. doi: 10.1186/1471-2393-14-127. doi:10:1186/1471-2393-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gareri J, Lynn H, Handley M, Rao C, Koren G. Prevalence of fetal ethanol exposure in a regional population-based sample by meconium analysis of fatty acid ethyl esters. Ther Drug Monit. 2008;30(2):239–245. doi: 10.1097/FTD.0b013e318167cfe5. [DOI] [PubMed] [Google Scholar]
- 16.Papas R, Gakinya BN, Mwaniki MM, Keter AK, Lee H, Loxley M, Klein DA, Sidle JE, Martino S, Baliddawa JB, Schlaudt K, Maisto SA. Associations between the phosphatidylethanol alcohol biomarker and self-reported alcohol use in a sample of HIV-infected outpatient drinkers in western Kenya. Alcohol Clin Exp Res. 2016;40(8):1779–1787. doi: 10.1111/acer.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to Neurobehavioral outcome. Pediatrics. 2002;L109(5):815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 18.Fortin M, Muckle G, Jacobson S, Jacobson J, Bélanger R. Alcohol use among Inuit pregnant women: Validity of alcohol ascertainment measures over time. Neurotoxicol Teratol. 2017;64:73–78. doi: 10.1016/j.ntt.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Czarnecki DM, Russell M, Cooper ML, Salter D. Five-year reliability of self-reported alcohol consumption. J Stud Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- 20.Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwalk MK, Delaney-Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44:583–594. doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. 2 Geneva: World Health Organization; 2001. [Google Scholar]
- 22.Howlett H, Abernethy S, Brown NW, Rankin J, Gray W. How strong is the evidence for using blood biomarkers alone to screen for alcohol consumption during pregnancy? A systematic review. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2017;213:45–52. doi: 10.1016/j.ejogrb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin AE, Jones J, Jones M, Plate C, Lewis D. Retrospective assessment of prenatal alcohol exposure by detection of phosphatidylethanol in stored dried blood spot cards: An objective method for determining prevalence rates of alcohol consumption during pregnancy. International Journal of Alcohol and Drug Research. 2015;4(2):131–137. doi: 10.7895/ijadr.v4i2.209. [DOI] [Google Scholar]
- 24.Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788–812. doi: 10.3390/ijms131114788. doi:10:3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger L, Fendrich M, Jones J, Furhmann D, Plate C, Lewis D. Ethyl glucuronide in hair and fingernails as a long-term biomarker. Addiction Research Report. 2013 doi: 10.1111/add.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones J, Jones M, Plate C, Lewis D, Fendrich M, Berger L, Fuhrmann D. Liquid chromatography-tandem mass spectrometry assay to detect ethyl glucuronide in human fingernail: Comparison to hair and gender differences. Am J Analyt Chem. 2012;3(1):83–91. doi: 10.4236/ajac.2012.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones J, Jones M, Plate C, Lewis D. The detectio n of l-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011;3(5):1101–1106. [Google Scholar]
- 28.Morini L, Colucci M, Ruberto MG, Groppi A. Determination of ethyl glucuronide in nails by liquid chromatography tandem mass spectrometry as a potential new biomarker for chronic alcohol abuse and binge drinking behaviour. Anal Bioanal Chem. 2012;402:1865–1870. doi: 10.1007/s00216-011-5609-8. [DOI] [PubMed] [Google Scholar]
- 29.Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: Recent advances and future research opportunities. Alcohol Clin Exp Res. 2010;34(6):955–67. doi: 10.1111/j.1530-0277.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- 30.King AC. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am J Public Health. 1994;84:294–296. doi: 10.2105/ajph.84.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaskutas L, Graves K. Pre-pregnancy drinking: how drink size affects risk assessment. Addition. 2001;96:1199–1209. doi: 10.1046/j.1360-0443.2001.968119912.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaskutas L, Graves K. Accuracy of photographs to capture respondent-defined drink size. J Stud Alcohol Drugs. 2008;69:605–610. doi: 10.15288/jsad.2008.69.605. [DOI] [PubMed] [Google Scholar]
- 33.Marques PR. Levels and types of alcohol biomarkers in DUI and clinic samples for estimating workplace alcohol problems. Drug Test Anal. 2012;4(2):76–82. doi: 10.1002/dta.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IBM SPSS. IBM SPSS Statistics, Release 24. Somers, NY: IBM Corporation; 2016. [Google Scholar]
- 35.Tabachnick BG, Fidell LS. Using multivariate statistics. 6 Boston, MA: Pearson; 2013. [Google Scholar]
- 36.Bakhireva LN, Leeman L, Savich RD, Cano S, Gutierrez H, Savage DD, Rayburn WF. The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcohol Clin Exp Res. 2014;38(4):1078–1085. doi: 10.1111/acer.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Algar O, Kulaga V, Gareri J, Koren G, Vall O, Zuccaro P, Pacifici R, Pichini S. Alarming prevalence of fetal alcohol exposure in a Mediterranean city. Ther Drug Monit. 2008;30:249–254. doi: 10.1097/FTD.0b013e31816a8657. [DOI] [PubMed] [Google Scholar]
- 38.Morini L, Marchei E, Tarani L, Trivelli M, Rapisardi G, Elicio MR, Ramis J, Garcia-Algar O, Memo L, Pacifici R, Groppi A, Danesino P, Pichini S. Testing ethylglucuronide in maternal hair and nails for the assessment of fetal exposure to alcohol: comparison with meconium testing. Ther Drug Monit. 2013;35:402–407. doi: 10.1097/FTD.0b013e318283f719. [DOI] [PubMed] [Google Scholar]
- 39.Pichini S, Marchei E, Vagnarelli F, Tarani L, Raimondi F, Maffucci R, Sacher B, Bisceglia M, Rapisardi G, Elicio MR, Biban P, Zuccaro P, Pacifici R, Pierantozzi A, Morini L. Assessment of prenatal exposure to ethanol by meconium analysis: results of an Italian multicenter study. Alcohol Clin Exp Res. 2012;36:417–724. doi: 10.1111/j.1530-0277.2011.01647.x. [DOI] [PubMed] [Google Scholar]
- 40.Sanvisens A, Robert N, Hernández JM, Zuluaga P, Farré M, Coroleu W, Serra M, Tor J, Muga R. Alcohol consumption during pregnancy: Analysis of two direct metabolites of ethanol in meconium. Int J Mol Sci. 2016;17:417. doi: 10.3390/ijms17030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundström Poromaa I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT—a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol. 2008;198:407e1–407.e5. doi: 10.1016/j.ajog.2007.10.801. [DOI] [PubMed] [Google Scholar]
- 42.Eichler A, Grunitz J, Grimm J, Walz L, Rabbe E, Goecke TW, Beckmann MW, Kratz O, Heinrich H, Moll GH, Fasching PA, Kornhuber J. Did you drink alcohol during pregnancy? Inaccuracy and discontinuity of women’s self-reports: On the way to establish meconium ethyl glucuronide (EtG) as a biomarker for alcohol consumption during pregnancy. Alcohol. 2016;54:9–44. doi: 10.1016/j.alcohol.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW, Kaplan MG. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol Teratrol. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- 44.Symon A, Rankin J, Butcher G, Smith L, Cochrane L. Evaluation of a retrospective diary for peri-conceptual and mid-pregnancy drinking in Scotland: a cross-sectional study. Acta Obstetricia et Gynecologica. 2017 Jan;96:53–60. doi: 10.1111/aogs.13050. [DOI] [PubMed] [Google Scholar]
- 45.Symon A, Rankin J, Sinclair H, Butcher G, Barclay K, Gordon R, MacDonald M, Smith L. Peri-conceptual and mid-pregnancy drinking: a cross-sectional assessment in two Scottish health board areas using a 7-day retrospective diary. J Adv Nurse. 2017 Feb;73:375–385. doi: 10.1111/jan.13112. [DOI] [PubMed] [Google Scholar]
- 46.Kader R, Seedat S, Koch JR, Parry CD. A preliminary investigation of the AUDIT and DUDIT in comparison to biomarkers for alcohol and drug use among HIV-infected clinic attendees in Cape Town, South Africa. Afr J Psychiatry. 2012;16:346–351. doi: 10.4314/ajpsy.v15i5.43. [DOI] [PubMed] [Google Scholar]
- 47.Williams PP, Jordaan E, Mathews C, Lombard C, Parry CDH. Alcohol and other drug use during pregnancy among women attending midwife obstetric units in the Cape Metropole, South Africa. Adv Prev Med. 2014;2014:871427. doi: 10.1155/2014/871427. [DOI] [PMC free article] [PubMed] [Google Scholar]