Abstract
The aversive effect of nicotine withdrawal is greater in female versus male rats, and we postulate that this sex difference is mediated in the nucleus accumbens (NAc). Nicotine withdrawal induces decreases in NAc dopamine and increases in acetylcholine (ACh) levels in male rats. To our knowledge, these neurochemical markers of nicotine withdrawal have not been compared in female versus male rats. Given the role of amino acids in modulating NAc dopaminergic and cholinergic transmission, concomitant measures of gamma-aminobutyric acid (GABA) and glutamate levels were also compared across sex. Rats received continuous nicotine exposure for 14 days, and then NAc dialysate was collected during baseline and following administration of the nicotinic receptor antagonist mecamylamine to precipitate withdrawal. Chronic nicotine exposure was associated with larger increases in baseline dopamine, GABA and glutamate levels in the NAc of female versus male rats, whereas baseline ACh was only increased in male rats. During withdrawal, both sexes displayed equivalent increases in NAc ACh levels. As expected, male rats displayed decreases in dopamine, coupled with increases in GABA and decreases in glutamate levels, suggesting the possibility of increased inhibitory tone in the NAc during withdrawal. Relative to males, female rats displayed larger decreases in NAc dopamine and related increases in GABAergic transmission. As female rats also showed elevated glutamate levels that persist during withdrawal, it is suggested that sex differences may arise from increased glutamatergic drive of inhibitory tone in the NAc. The findings provide a potential mechanism whereby the aversive effects of nicotine withdrawal are enhanced in female rats.
Keywords: dependence, female, male, mecamylamine, microdialysis, tobacco
INTRODUCTION
Nicotine is the main compound that has been shown to motivate tobacco use. The positive reinforcing effects of nicotine have been characterized in humans and various rodent and primate species (Corrigall & Coen 1989; Le Foll & Goldberg 2009; Duke et al. 2015; Goodwin, Hiranita, & Paule 2015; Kohut & Bergman 2016). During abstinence from prolonged tobacco use, a nicotine withdrawal syndrome emerges that consists of physical signs and negative affective states (McLaughlin, Dani, & De Biasi 2015; Piper 2015). Nicotine withdrawal has been shown to play a central role in the maintenance of tobacco use and relapse behavior, and as a result, cessation therapies focus on alleviating withdrawal symptoms to avoid relapse (Benowitz 2008; Hall et al. 2015; Bruijnzeel 2016).
Epidemiological evidence has suggested that women are more susceptible to tobacco use than men. For example, women consume more tobacco products, exhibit lower quit rates and are less likely to benefit from nicotine replacement therapy as compared with men (Cepeda-Benito, Reynoso, & Erath 2004; Perkins & Scott 2008; Piper et al. 2010). Females also display reduced relief from withdrawal and lower abstinence rates with nicotine replacement therapy as compared to males (Pomerleau et al. 1994). During smoking abstinence, women also report greater levels of depression and anxiety than men (Schnoll, Patterson, & Lerman 2007; Xu et al. 2008). A study comparing physiological responses to fear-potentiated startle during smoking abstinence revealed that women display greater cue-induced fear responses and cortisol release than men (Hogle & Curtin 2006). Although the high rates of smoking likely contribute to health disparities in women, there is little information regarding the neural mechanisms that enhance vulnerability to tobacco use in females.
Pre-clinical rodent studies have revealed that the behavioral effects of nicotine are mediated, in large part, via the mesolimbic pathway, which originates in the ventral tegmental area (VTA) and projects to a series of forebrain structures, including the shell of the nucleus accumbens (NAc; Koob 2000). While acute administration of nicotine increases dopamine levels in the NAc, withdrawal from chronic nicotine exposure decreases dopamine in the NAc of male rats (Hildebrand et al. 1998; Di Chiara 2000; Rada, Jensen, & Hoebel 2001; Natividad et al. 2010). Nicotine withdrawal also produces an increase in acetylcholine (ACh) levels in the NAc of male rats (Rada et al. 2001; Carcoba et al. 2014). The decrease in dopamine and increase in ACh levels in the NAc have also been observed during withdrawal from other drugs of abuse, including ethanol and morphine (Pothos et al. 1991; Rossetti, Hmaidan, & Gessa 1992; Rada et al. 2004). These findings suggest that a decrease in dopamine and an increase in ACh levels in the NAc serve as a neurochemical marker of drug withdrawal in male rodents. However, sex differences in this biomarker profile have not been examined during nicotine withdrawal.
Work in our laboratory is focused on studying the underlying mechanisms that modulate sex differences in the behavioral effects of nicotine. We recently observed that female rats experiencing nicotine withdrawal display greater negative affective states and a larger upregulation of stress-associated genes in the NAc as compared with male rats (Torres et al. 2013, 2015). The pattern of increases in stress-associated gene expression was largest in the NAc of female rats, as compared with modest changes observed in the hippocampus and amygdala (Torres et al. 2013). These findings led to a neurobiological hypothesis that sex differences in nicotine withdrawal are likely modulated within the local circuits of the NAc (Torres & O’Dell, 2016). In order to test our hypothesis, the present study compared dopamine and ACh levels in the NAc of female and male rats experiencing nicotine withdrawal. Changes in the amino acid neurotransmitters, gamma-aminobutyric acid (GABA) and glutamate were also assessed, given that dopamine release in the NAc is modulated via inhibitory GABAergic innervation from local interneurons (Kalivas, Churchill, & Klitenick 1993; Melchior et al. 2015) and excitatory glutamatergic input from the prefrontal cortex (Del Arco & Mora 2008; Britt et al. 2012). Moreover, recent reports have revealed that glutamatergic inputs from the VTA to the NAc elicit aversive states via enhanced GABAergic tone in the NAc (Qi et al. 2016). Thus, we expected that potential sex differences in the neurochemical effects of nicotine withdrawal would be modulated via amino acid regulation of dopamine transmission in the NAc.
MATERIALS AND METHODS
Subjects and drugs
Adult male and female Wistar rats (n = 6–8 per group) were used. Animals were postnatal day 60–75 at the time of the pump implantation surgery. Rats were pair-housed in a humidity-controlled and temperature-controlled (20–22°C) vivarium using a reverse 12-hour light/dark cycle with lights on at 8:00 PM. The rats were given free access to food and water throughout the study. The animals were bred from a stock of outbred rats from Envigo (Indianapolis, IN, USA). All procedures were approved by the Institutional Animal Care and Use Committee and followed the guidelines of the NIH Guide for the Care and Use of Laboratory Animals.
The drugs used in the following experiments were (−) nicotine hydrogen tartrate salt and mecamylamine (Sigma Inc., St Louis, MO). Mecamylamine was dissolved in 0.9 percent sterile saline and administered in a volume of 1 mL/kg. The mecamylamine doses were chosen based on previous studies showing that these doses produce neurochemical changes in the NAc in male rats experiencing nicotine withdrawal (Natividad et al. 2010; 2012). The nicotine in the osmotic pump was dissolved in 0.9 percent sterile saline. The dose of nicotine was selected based on previous research showing that this concentration produces similar nicotine levels in female and male adult rats (Torres et al. 2013). All drugs were administered at physiological pH of 7.2–7.4.
Surgical procedures
Rats were anesthetized with an isoflurane/oxygen mixture (1–3 percent Isoflurane) and were prepared with osmotic pumps (model 2ML2 14-day; Alzet, Inc.) that were placed subcutaneously on the back of the animal parallel to the spine. Following pump implantation, or sham surgery in control rats, the surgical wound was closed with 9-mm stainless steel wound clips and treated topically with antibiotic ointment. Following surgery, all rats received subcutaneous administration of the analgesic flunixin (2.5 mg/kg; Vedco, St Joseph, MO). Thirteen days after the pump surgery, the rats were prepared with a dialysis probe in the NAc with an active membrane length of 2 mm (model CMA 11, Holliston, MA). The probe was perfused for at least 1 hour prior to implantation at a rate of 1.0 μL/minute using artificial cerebral spinal fluid adjusted to a pH of 7.2–7.4 with 0.1 M NaOH. The dialysis probes were implanted into the NAc using the following stereotaxic coordinates from bregma: AP = +1.7, ML = ±1.4, DV = −8.1. The hemisphere that was implanted with the probe was randomized to control for possible hemispheric differences across groups. Following surgery, animals were transferred to test cages, with food and water available ad libitum throughout testing.
Dialysis procedures
Approximately 6–8 hours after probe implantation, the perfusate flow rate was reduced to 0.6 μL/minute for 1 hour to allow for equilibration. Samples were then collected in 20-minute intervals for a 1-hour baseline period and then for two additional 1-hour periods following intraperitoneal administration of the non-selective nicotinic receptor antagonist mecamylamine (1.5 and 3.0 mg/kg) to precipitate withdrawal. After collection, the samples were frozen on dry ice and then stored at −80°C until analysis. At the end of testing, the rats’ brains were extracted, frozen and then sectioned for verification of probe placement using the Paxinos & Watson (2014) atlas. As an additional elimination criterion, each animals’ baseline values had to fall within a range that was less than two standard deviations from the group mean to be included in the final analysis. Based on these criteria, three rats were excluded from the study.
Neurochemical analysis
Most chemicals were purchased from Sigma Aldrich (St. Louis, MO), except Optima™ mass spectrometry (MS) grade water and acetonitrile, which were purchased from Fisher Chemical (Pittsburgh, PA, USA). Stock solutions of each neurotransmitter were prepared in MS grade water and kept at −80°C. The standard mixture was diluted from stock solutions using artificial cerebral spinal fluid from dialysis testing. Calibration curves were made using standards in a range of 5, 50, 100, 500 and 1000 nM for GABA and glutamate and 0.5, 5, 10, 50 and 100 nM for dopamine and ACh. The internal standard and sample derivatization procedures followed the methods described in Buczynski et al. 2016 and Song et al. (2012). Quantification of neurotransmitters in dialysate samples was performed using an approach involving liquid chromatography-mass spectrometry LC-MS/MS analysis. The neurochemical analyses were performed using a Thermo Scientific UltiMate™ 3000 Standard Quaternary System with a Waters BEH C18 column (1 mm × 100 mm, 1.7 μm, 130 Å pore size) for separation. The autosampler was coupled to a TSQ Endura triple quadrupole MS. All dialysate data were acquired and processed with THERMO SCIENTIFIC TRACEFINDER™ software version 3.2.512. The peaks were visually inspected to detect artifacts. Peak areas were obtained and analyzed for each neurotransmitter with their respective internal standard. Standard curves were used to calculate the concentration of all neurotransmitters in each sample.
Statistical analyses
Each neurotransmitter was analyzed independently using multi-factorial ANOVA. Two levels of analysis were employed in order to examine: (1) neurochemical effects of nicotine withdrawal in control versus nicotine-treated rats; and (2) sex differences produced by nicotine withdrawal in female versus male rats. The first level of analysis assessed nicotine withdrawal in female and male rats separately, using treatment group as a between-subject factor (control versus nicotine-treated) and time as a within-subject factor (20-minute samples). In instances where a significant interaction was observed, post hoc analyses compared between (control versus nicotine-treated; *P ≤ 0.05) or within groups (each timepoint during withdrawal versus average baseline value; †P ≤ 0.05) using Fisher’s protected least significant difference tests. The second level of analysis first compared sex differences in dialysate levels using repeated measures ANOVA with sex (female versus male) and treatment group (control versus nicotine-treated) as between-subject factors. In order to provide a comparison of sex differences that accounted for potential differences in baseline levels produced by nicotine exposure, the data were converted to percent change from respective control levels [(dialysate value/average control value) × 100]. These data were then analyzed using sex (female versus male) as a between-subject factor and time (20-minute samples) as a within-subject factor. In instances where a significant interaction was observed, post hoc analyses compared between groups (female versus male rats; #P ≤ 0.05) using Fisher’s protected least significant difference tests.
RESULTS
Overall, baseline ACh levels were increased in nicotine-treated male rats relative to controls, and withdrawal produced an increase in ACh levels that was similar in female and male rats (Fig. 1). The analysis of ACh levels in female rats revealed an interaction between group and time F8,96 = 6.5, P = 0.0001, with nicotine-treated female rats displaying higher ACh levels than controls following each dose of mecamylamine (*P ≤ 0.05). Also, nicotine-treated female rats displayed an increase in ACh levels relative to baseline at each timepoint following both doses of mecamylamine (†P ≤ 0.05). The analysis of ACh levels in male rats revealed an interaction between group and time F8,112 = 4.6, P = 0.0001, with nicotine-treated male rats displaying higher ACh levels than controls during all timepoints (*P ≤ 0.05), except the last baseline sample. Also, nicotine-treated male rats displayed an increase in ACh levels relative to baseline following each dose of mecamylamine (†P ≤ 0.05), with the exception of the first sample following the 1.5 mg/kg dose of mecamylamine. With regard to sex differences, an initial analysis of ACh levels revealed that there was no interaction between sex, treatment group and time F8,208 = 0.86, P = 0.55. A subsequent analysis of the data expressed as a percent change from controls revealed an interaction between sex and time F8,112 = 2.5, P = 0.02, with nicotine-treated female and male rats displaying an increase in ACh levels relative to baseline following mecamylamine administration that was similar across groups.
Figure 1.
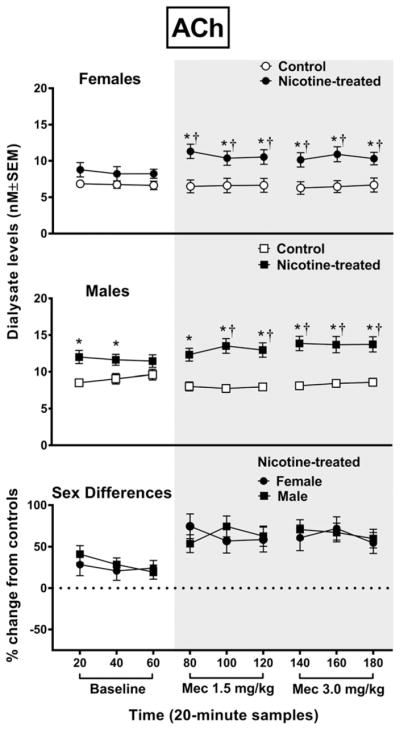
The top panel reflects acetylcholine (ACh) levels (nM ± SEM) in the NAc of female rats (control n = 6; nicotine-treated n = 8) and the middle panel reflects male rats (control n = 8; nicotine-treated n = 8). The bottom panel reflects percent change in ACh (±SEM) in nicotine-treated female and male rats from their respective controls. Each point reflects a 20-minute sampling period during baseline and then following mecamylamine administration (shaded area). Asterisks (*) denote a significant difference from controls, and daggers (†) denote a difference from average baseline values (P ≤ 0.05)
Overall, baseline dopamine levels were increased in nicotine-treated female rats relative to controls, and withdrawal produced a decrease in dopamine levels that was larger in female versus male rats (Fig. 2). The analysis of dopamine levels in female rats revealed an interaction between group and time F8,88 = 16.48, P = 0.0001, with nicotine-treated female rats displaying higher dopamine levels than controls during the first and last baseline sampling period (*P ≤ 0.05). Also, nicotine-treated female rats displayed a decrease in dopamine levels relative to controls following both doses of mecamylamine (*P ≤ 0.05). Moreover, nicotine-treated female rats displayed a decrease in dopamine levels relative to baseline at each timepoint following both doses of mecamylamine (†P ≤ 0.05). The analysis of dopamine levels in male rats revealed an interaction between group and time F8,96 = 4.57, P = 0.0001, with male rats displaying a decrease in dopamine levels relative to controls following the 3.0 mg/kg dose of mecamylamine (*P ≤ 0.05). Also, nicotine-treated male rats displayed a decrease in dopamine levels relative to baseline following each dose of mecamylamine (†P ≤ 0.05), except the third timepoint following the 1.5 mg/kg dose of mecamylamine. With regard to sex differences, an initial analysis of dopamine levels revealed that there was a strong trend for an interaction between sex, treatment group and time F8,184 = 1.88, P = 0.06. A subsequent analysis of the data expressed as a percent change from controls revealed an interaction between sex and time F8,104 = 2.65, P = 0.0001, with nicotine-treated female rats displaying lower levels of dopamine than male rats following administration of both doses of mecamylamine (#P ≤ 0.05), except the first timepoint after administration of the 3.0 mg/kg dose of mecamylamine.
Figure 2.
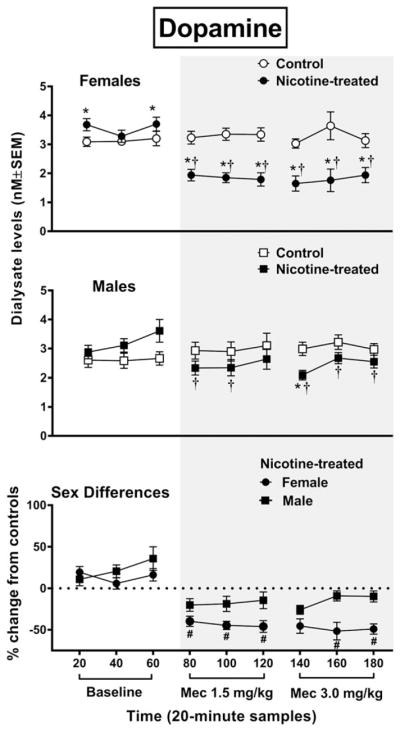
The top panel reflects dopamine levels (nM ± SEM) in the NAc of female rats (control n = 6; nicotine-treated n = 7) and the middle panel reflects male rats (control n = 6; nicotine-treated n = 8). The bottom panel reflects percent change in dopamine (±SEM) in nicotine-treated female and male rats from their respective controls. Each point reflects a 20-minute sampling period during baseline and then following mecamylamine administration (shaded area). Asterisks (*) denote a significant difference from controls (P ≤ 0.05), daggers (†) denote a difference from average baseline values and pound signs (#) denote a difference between female and male rats (P ≤ 0.05)
Overall, baseline GABA levels were increased in nicotine-treated female rats relative to controls, and withdrawal produced an increase in GABA levels that was larger in female versus male rats (Fig. 3). The analysis of GABA levels in female rats revealed an interaction between group and time F8,96 = 5.8, P = 0.0001, with nicotine-treated female rats displaying higher GABA levels than controls during baseline and following each dose of mecamylamine (*P ≤ 0.05). Also, nicotine-treated female rats displayed an increase in GABA levels relative to baseline at each timepoint following both doses of mecamylamine (†P ≤ 0.05), except the first timepoint after the 1.5 mg/kg dose of mecamylamine. The analysis of GABA levels in male rats revealed that there was no interaction between group and time. However, there was a main effect of group F2,24 = 188.7, P = 0.0001, with nicotine-treated male rats displaying higher overall GABA levels than controls (*P ≤ 0.05). With regard to sex differences, the initial analysis of GABA levels revealed that there was an interaction between sex, treatment group and time F8,192 = 4.1, P = 0.001. A subsequent analysis of the data expressed as a percent change from controls revealed an interaction between sex and time F8,112 = 9.5, P = 0.001, with nicotine-treated female rats displaying higher GABA levels than male rats at all timepoints following administration of the 3.0 mg/kg dose of mecamylamine (#P ≤ 0.05).
Figure 3.
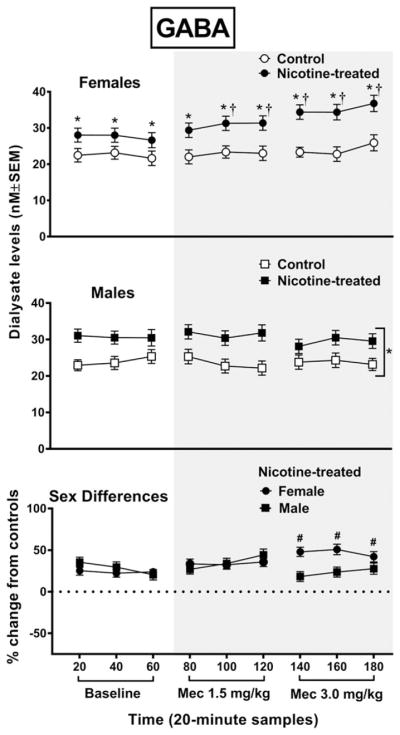
The top panel reflects gamma-aminobutyric acid (GABA) levels (nM ± SEM) in the NAc of female rats (tcontrol n = 6; nicotine-treated n = 8) and male rats (control n = 6; nicotine-treated n = 8). The bottom panel reflects percent change in GABA (±SEM) in nicotine-treated female and male rats from their respective controls. Each point reflects a 20-minute sampling period during baseline and then following mecamylamine administration (shaded area). Asterisks (*) denote a significant difference from controls (P ≤ 0.05), daggers (†) denote a difference from average baseline values and pound signs (#) denote a difference between female and male rats (P ≤ 0.05)
Overall, baseline glutamate levels were increased to a larger extent in nicotine-treated female versus male rats, and withdrawal produced an increase in glutamate in female rats and a decrease in glutamate levels in male rats (Fig. 4). The analysis of glutamate levels in female rats revealed an interaction between group and time F8,88 = 2.9, P = 0.0001, with nicotine-treated female rats displaying higher glutamate levels than controls during baseline and following each dose of mecamylamine (*P ≤ 0.05). Also, nicotine-treated female rats displayed an increase in glutamate relative to baseline during the second timepoint following the 3.0 mg/kg dose of mecamylamine (†P ≤ 0.05). The analysis of glutamate levels in male rats revealed an interaction between group and time F8,88 = 9.3, P = 0.0001, with nicotine-treated male rats displaying higher glutamate than controls during baseline, but lower glutamate levels following the 3.0 mg/kg dose of mecamylamine (*P ≤ 0.05). Also, nicotine-treated male rats displayed a decrease in glutamate relative to baseline following the 3.0 mg/kg dose of mecamylamine (†P ≤ 0.05). With regard to sex differences, the initial analysis of glutamate levels revealed that there was an interaction between sex, treatment group and time F8,176 = 10.1, P = 0.001. A subsequent analysis of the data expressed as a percent change from controls revealed an interaction between sex and time F8,88 = 20.7, P = 0.001, with nicotine-treated female rats displaying higher glutamate levels than male rats during baseline and following administration of both doses of mecamylamine (#P ≤ 0.05).
Figure 4.
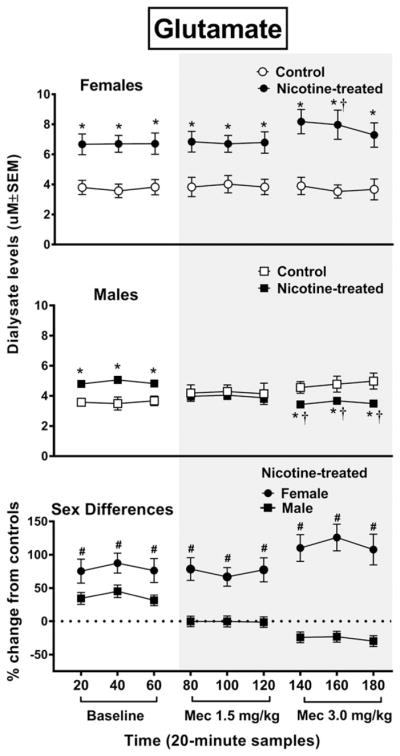
The top panel reflects glutamate levels (uM ± SEM) in the NAc of female rats (control n = 7; nicotine-treated n = 6) and male rats (control n = 6; nicotine-treated n = 7). The bottom panel reflects percent change in glutamate (±SEM) in nicotine-treated female and male rats from their respective controls. Each point reflects a 20-minute sampling period during baseline and then following mecamylamine administration (shaded area). Asterisks (*) denote a significant difference from controls (P ≤ 0.05), daggers (†) denote a difference from average baseline values and pound signs (#) denote a difference between female and male rats (P ≤ 0.05)
DISCUSSION
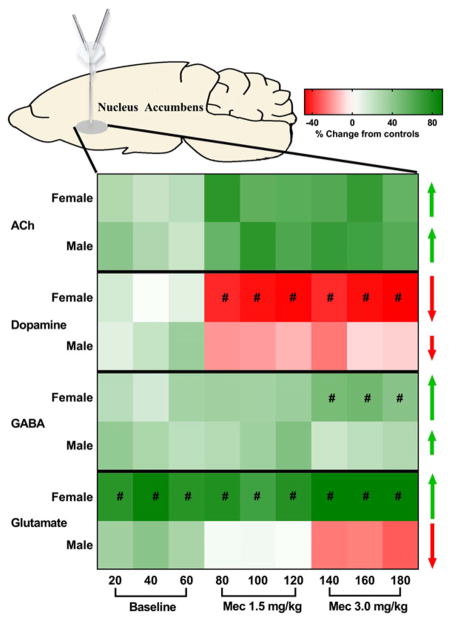
In order to summarize the pattern of sex differences produced by nicotine exposure and withdrawal, a heat map illustration is provided that denotes changes in each neurotransmitter relative to baseline values (Fig. 5). In summary, chronic nicotine exposure induced greater increases in baseline levels of GABA, glutamate and dopamine in the NAc of female relative to male rats, whereas baseline ACh levels were only enhanced in male rats. During precipitated withdrawal, an increase in ACh levels was observed that was similar across sex. However, female rats displayed a larger withdrawal-induced decrease in NAc dopamine than male rats. Concomitant measures of GABA and glutamate revealed critical differences in NAc amino acid transmission that may underlie sex-specific effects during nicotine withdrawal. Specifically, female rats displayed a larger withdrawal-induced increase in both glutamate and GABA levels as compared with male rats. Baseline levels of glutamate were also increased to a larger extent in female versus male rats. Below, a mechanistic hypothesis involving amino acid modulation of dopamine in the NAc is provided.
Figure 5.
Heat map illustration of neurochemical changes in the NAc of female and male rats during baseline and following mecamylamine administration. The scale is based on a percent change from their respective control values. The green shades reflect an increase, the red shades reflect a decrease in each neurotransmitter relative to baseline and the white color denotes no change relative to baseline values. Pound signs (#) denote where a significant difference between female and male rats was observed (P ≤ 0.05)
Previous work has revealed that dopaminergic fibers projecting from the VTA synapse onto GABAergic and cholinergic interneurons in the NAc that, together with innervating glutamatergic projections from the cortex, provide an extensive network that modulates the behavioral effects of nicotine and withdrawal from this drug (Di Chiara, Morelli, & Consolo 1994; de Rover et al. 2002). With regard to cholinergic transmission, the present study revealed that nicotine withdrawal produced an increase in ACh levels in the NAc, consistent with previous reports in male rats (Rada et al. 2004; Carcoba et al. 2014). However, the present study expands previous work by showing that the withdrawal-induced increase in ACh levels in the NAc is similar in female and male rats. This suggests that sex differences in the behavioral effects of nicotine withdrawal are not likely modulated via cholinergic systems in the NAc, or at minimum not via the precipitated withdrawal procedures used here. Previous work has also revealed that the increase in ACh levels in the NAc produced by nicotine withdrawal is similar in adolescent and adult male rats (Carcoba et al. 2014). Taken together, these findings suggest that the neurochemical systems modulating ACh release in the NAc may not play a role in age and/or sex differences in the behavioral effects of nicotine withdrawal.
Previous work has revealed that decrements in NAc dopamine transmission are inextricably linked to anhedonia and negative affect states, particularly those elicited with withdrawal from drugs of abuse (Koob 2009). With regard to dopamine transmission, the present study revealed that nicotine withdrawal produced a decrease in NAc dopamine levels within a range that has been previously observed in male rats (Hildebrand et al. 1998; Natividad et al. 2010). However, the withdrawal-induced decrease in dopamine levels in the NAc was larger in female versus male rats. A previous report revealed that female rats display a larger increase in dopamine D1 receptor messenger RNA levels in the NAcc during nicotine withdrawal than male rats (Torres et al. 2015). The increase in post-synaptic D1 receptor levels is believed to serve as a compensatory mechanism produced by withdrawal-induced decreases in synaptic levels of dopamine. Thus, the larger upregulation of D1 receptors in the NAc of female rats is believed to serve as indirect evidence that synaptic levels of dopamine are reduced to a larger extent in female versus male rats. Together, these studies provide converging lines of evidence suggesting that nicotine withdrawal produces a larger decrease in dopamine release in the NAc of female versus male rats.
With regard to GABA transmission, nicotine withdrawal produced a general increase in GABA levels in the NAc of female and male rats. With regard to sex differences, female rats displayed a larger withdrawal-induced increase in GABA levels in the NAc than male rats. This large increase in GABAergic inhibition in the NAc likely promotes the large decrease in dopamine in female rats. This pattern of changes may not be surprising based on previous reports showing that dopamine transmission in the NAc is under local inhibitory GABAergic control (Kalivas et al. 1993; Melchior et al. 2015). The pattern of enhanced GABA release in the NAc is consistent with the pattern of changes observed in the VTA of male rats experiencing withdrawal (Natividad et al. 2012). These studies suggest that nicotine withdrawal produces enhanced GABAergic inhibition of dopamine systems in the cell body and terminal regions of the mesolimbic pathway. The possibility also exists that sex differences in dopamine responses may be modulated in the cell body region, with extended modulation serving to buffer the microenvironment in the NAc in a manner that facilitates larger decreases in dopamine in female rats.
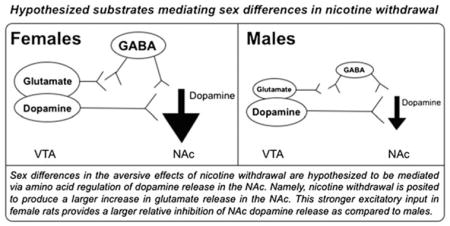
One of the largest sex differences observed in the present study was with regard to glutamate transmission. Specifically, nicotine exposure facilitated an increase in baseline glutamate levels that persisted across precipitated withdrawal procedures in the NAc of female rats. However, nicotine withdrawal produced an opposite pattern in male rats involving a decrease in glutamate levels in the NAc. Here, it is posited that the pattern of changes between female and male rats may be explained via glutamatergic innervation of NAc dopamine release (see diagram). Our hypothesis is based on a recent report showing that there is a glutamatergic input from the VTA to the NAc that modulates aversive states (Qi et al. 2016). Specifically, targeted activation of glutamatergic inputs to the NAc produced place aversion for the environment paired with activation of this pathway, an increase in wheel turning to remove stimulation of this pathway and a reduction in operant food responding. The induction of aversive states was attributed to excitatory inputs to the NAc that directly synapse onto GABAergic interneurons. Thus, in the present study, nicotine exposure produced a larger increase in glutamate release in the NAc of female versus male rats. This is posited to provide a larger relative activation of GABA interneurons in the NAc, which we hypothesize promotes a larger decrease in dopamine in female rats during withdrawal. In male rats, an opposite pattern was observed, with nicotine withdrawal producing a decrease in glutamate levels in the NAcc. This reduction in glutamate likely contributes to a lower relative activation of GABAergic inhibition of dopamine. As a result, male rats display a smaller decrease in dopamine during withdrawal as compared with female rats. We recognize that our hypothesis regarding sex differences in glutamate is based on a small fraction of the neuronal population in the NAc, as the large majority of cells in this region are GABAergic medium spiny neurons. Indeed, it has been posited that medium spiny GABAergic neurons in the NAc play a primary role in modulating the aversive effects of drugs of abuse (Carlezon & Thomas 2009). Future studies are needed to elucidate the relative contribution of different cell types in the NAc in modulating sex differences produced by withdrawal from chronic nicotine exposure.
Previous work has revealed that the aversive effects of nicotine withdrawal are heightened in female versus male rats. For example, female adult rats display more physical signs of nicotine withdrawal relative to male rats (Hamilton et al. 2009). Female rats also display a greater place aversion produced by nicotine withdrawal as compared with male rats (Torres et al. 2013). During nicotine withdrawal, female rats also display a larger increase in anxiety-like behavior and corticosterone release as compared with male rats (Gentile et al. 2011). Female rats also display a larger increase in stress-associated gene expression in the NAc as compared with male rats experiencing withdrawal from nicotine (Torres et al. 2013). The present study expands previous behavioral work by offering a hypothesis that sex-dependent differences in nicotine withdrawal are modulated via increased inhibitory tone in the NAc. Our work also speaks to the possibility that the recently discovered pathway involving glutamatergic inputs from the VTA to the NAc may play a critical role in modulating enhanced susceptibility to the aversive effects of nicotine withdrawal in female rats.
Acknowledgments
This study combined in vivo microdialysis procedures with advanced neurochemical approaches that were developed with our friend and mentor, Dr. Loren H. Parsons, who this special issue is dedicated to. We also appreciate the technical assistance provided by Mr. Tony Kerr. This research was supported by the National Institute on Drug Abuse (R01-DA021274 & HHSN271201600057C), the Diversity-promoting Institutions Drug Abuse Research Program (R24-DA029989) and the SMART:MINDS Research Experience for Undergraduates Program (R25-DA033613). LAN is supported by the National Institute on Alcohol Abuse and Alcoholism (K99-AA025393).
Footnotes
Author Contributions
LMC and LEO were responsible for the study concept and design. LMC and RF contributed to the acquisition of animal data. LMC and RF performed the statistical analysis. All authors assisted with interpretation of findings. LMC, RF and LEO drafted the manuscript. LAN provided critical input in the development of the neurochemical analyses. All authors provided critical revision of the manuscript for important intellectual content, and they all approved the final version for publication. The authors thank Adriana Perez and Alejandra Lopez for their technical assistance, and Bryan Cruz and Kevin Uribe for their intellectual contribution to the final version of this work.
References
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J of Med. 2008;121:3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Boncil A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. Neuropeptide systems and new treatments for nicotine addiction. Psychopharmacol. 2016 Dec 28; doi: 10.1007/s00213-016-4513-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Herman MA, Hsu KL, Natividad LA, Irimia C, Polis IY, Pugh H, Chang JW, Niphakis MJ, Cravatt BF, Roberto M, Parsons LH. Diacylglycerol lipase disinhibits VTA dopamine neurons during chronic nicotine exposure. Proc Natl Acad Sci. 2016;113(4):1086–1091. doi: 10.1073/pnas.1522672113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcoba LM, Orfila JE, Natividad LA, Torres OV, Pipkin JA, Ferree PL, Castañeda E, Moss DE, O’Dell LE. Cholinergic transmission during nicotine withdrawal is influenced by age and pre-exposure to nicotine: implications for teenage smoking. Dev Neurosci. 2014;36:347–355. doi: 10.1159/000360133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;1:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacol. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;30:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Duke AN, Johnson MW, Reissig CJ, Griffiths RR. Nicotine reinforcement in never-smokers. Psychopharmacol. 2015;232:4243–4252. doi: 10.1007/s00213-015-4053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic-pituitary-adrenal HPA responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull. 2011;853–4:145–152. doi: 10.1016/j.brainresbull.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AK, Hiranita T, Paule MG. The reinforcing effects of nicotine in humans and nonhuman primates: a review of intravenous self-administration evidence and future directions for research. Nicotine Tob Res. 2015;17:1297–1310. doi: 10.1093/ntr/ntv002. [DOI] [PubMed] [Google Scholar]
- Hall FS, Der-Avakian A, Gould TJ, Markou A, Shoaib M, Young JW. Negative affective states and cognitive impairments in nicotine dependence. Neurosci Biobehav Rev. 2015;58:168–185. doi: 10.1016/j.neubiorev.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Berger SS, Perry ME, Grunberg NE. Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacol Biochem Behav. 2009;92:51–59. doi: 10.1016/j.pbb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;434:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neurosci. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J. Reinforcing effectiveness of nicotine in nonhuman primates: effects of nicotine dose and history of nicotine self-administration. Psychopharmacol. 2016;233:2451–2458. doi: 10.1007/s00213-016-4293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handbook of Exp Pharmacol. 2009;192:335–367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin I, Dani JA, De Biasi M. Nicotine withdrawal. Curr Top Behav Neurosci. 2015;24:99–123. doi: 10.1007/978-3-319-13482-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior JR, Ferris MJ, Stuber GD, Riddle DR, Jones SR. Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. J Neurochem. 2015;134:833–844. doi: 10.1111/jnc.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Parsons LH, Torres OV, O’Dell LE. Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. J Neurochem. 2012;123:578–588. doi: 10.1111/j.1471-4159.2012.07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Tejeda HA, Torres OV, O’Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7. Academic Press; New York: 2014. [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;107:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Piper ME. Withdrawal: expanding a key addiction construct. Nicotine Tob Res. 2015;17:1405–1415. doi: 10.1093/ntr/ntv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;126:647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Tate JC, Lumley MA, Pomerleau OF. Gender differences in prospectively versus retrospectively assessed smoking withdrawal symptoms. J Subst Abuse. 1994;6:433–440. doi: 10.1016/s0899-3289(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;6:348–350. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang HL, Barker DJ, Miranda-Barrientos J, Morales M. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat Neurosci. 2016;19:725–733. doi: 10.1038/nn.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacol. 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rada P, Johnson DF, Lewis MJ, Hoebel BG. In alcohol-treated rats, naloxone decreases extracellular dopamine and increases acetylcholine in the nucleus accumbens: evidence of opioid withdrawal. Pharmacol Biochem Behav. 2004;79:599–605. doi: 10.1016/j.pbb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB. Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci. 2002;16:2279–2290. doi: 10.1046/j.1460-9568.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. Womens Health. 2007;16:1211–1218. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- Song P, Mabrouk OS, Hershey ND, Kennedy RT. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Analytical Chem. 2012;3:412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front Psychiatry. 2013;4:1–18. doi: 10.3389/fpsyt.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, O’Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry. 2016;4:260–268. doi: 10.1016/j.pnpbp.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Pipkin JA, Ferree P, Carcoba LM, O’Dell LE. Nicotine withdrawal increases stress-associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine Tob Res. 2015;17:422–430. doi: 10.1093/ntr/ntu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, London ED. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;8:1653–1661. doi: 10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]