Abstract
Despite having normal hearing sensitivity, patients with chronic tinnitus may experience more difficulty recognizing speech in adverse listening conditions as compared to controls. However, the association between the characteristics of tinnitus (severity and loudness) and speech recognition remains unclear. In this study, the Quick Speech-in-Noise test (QuickSIN) was conducted monaurally on 14 patients with bilateral tinnitus and 14 age- and hearing-matched adults to determine the relation between tinnitus characteristics and speech understanding. Further, Tinnitus Handicap Inventory (THI), tinnitus loudness magnitude estimation, and loudness matching were obtained to better characterize the perceptual and psychological aspects of tinnitus. The patients reported low THI scores, with most participants in the slight handicap category. Significant between-group differences in speech-in-noise performance were only found at the 5-dB signal-to-noise ratio (SNR) condition. The tinnitus group performed significantly worse in the left ear than in the right ear, even though bilateral tinnitus percept and symmetrical thresholds were reported in all patients. This between-ear difference is likely influenced by a right-ear advantage for speech sounds, as factors related to testing order and fatigue were ruled out. Additionally, significant correlations found between SNR loss in the left ear and tinnitus loudness matching suggest that perceptual factors related to tinnitus had an effect on speech-in-noise performance, pointing to a possible interaction between peripheral and cognitive factors in chronic tinnitus. Further studies, that take into account both hearing and cognitive abilities of patients, are needed to better parse out the effect of tinnitus in the absence of hearing impairment.
Keywords: signal-to-noise ratio loss, QuickSIN, left-ear disadvantage, bilateral tinnitus
INTRODUCTION
Tinnitus is the perception of sound in the absence of external acoustic stimuli (Jastreboff 1990; Moller 2007). The prevalence of tinnitus in adults ranges from 5.1 to 42.7 % in various studies (McCormack et al. 2016), likely influenced by the phrasing of the question. Most often, tinnitus co-occurs with hearing loss; tinnitus patients with normal hearing sensitivity, typically defined as having pure-tone thresholds of less than 25 dB HL from 250 to 8000 Hz, comprise only 10 % of the tinnitus population (Davis and El Rafaie 2000; Theodoroff and Folmer 2013). The mechanisms of tinnitus in individuals with hearing loss usually can be related to a functional loss of hair cells in the inner ear and resulting changes in neuronal activities in the peripheral or central auditory systems (Norena 2015); however, the mechanisms and the effects of tinnitus are understudied in the normal hearing population. In this study, individuals with tinnitus and normal hearing sensitivity were included to better understand the effect of perceptual and psychological factors of tinnitus on speech recognition.
The presence of tinnitus may have a variable impact on an individual. Approximately 20 % of people who experience tinnitus consider it “clinically significant,” whereas the remaining population shows some habituation to it (Cuny et al. 2004b; Henry et al. 2009). Patients reporting bothersome tinnitus may have disturbed sleep, anxiety, depression, and other cognitive deficits depending on the severity of tinnitus (Moller 2007). Psychological reaction to tinnitus is measured via self-report questionnaires, which include the Tinnitus Questionnaire (TQ: Goebel and Hiller 1994), the Tinnitus Handicap Inventory (THI: Newman et al. 1996), the Tinnitus Functional Index (TFI: Meikle et al. 2012), or the Tinnitus Primary Function Questionnaire (TPFQ: Tyler et al. 2014). In addition to using subjective questionnaires, an estimation of tinnitus severity may be performed by assessing tinnitus loudness with a visual analog scale (Basile et al. 2013; Figueiredo et al. 2009); these measures are moderate to highly correlated with each other. Psychoacoustic measurements such as tinnitus loudness matching are also used to estimate tinnitus severity (Tyler 2000).
Apart from tinnitus-specific measures, standard audiologic test battery can serve as a diagnostic or counseling tool. These assessments are efficacious in the differential diagnosis when the tinnitus is related to specific diseases, conditions, or hearing impairments. However, in tinnitus patients with normal hearing sensitivity, the results of such assessments are usually obtained in the normative range and therefore yield limited information regarding tinnitus etiology. Nevertheless, some clinical tests, such as those related to speech understanding, may be helpful in characterizing the impact of tinnitus even in those with normal hearing sensitivity. For instance, tinnitus patients report hearing and speech comprehension difficulties (Tyler and Baker 1983; Tyler et al. 2014), with such difficulties increasing in adverse listening environments (Vielsmeier et al. 2016). As summarized in Table 1, studies focusing on speech recognition ability in noise have demonstrated that tinnitus patients performed poorly on this task, even when they reported having normal hearing sensitivity. Compared to speech recognition in quiet, speech-in-noise recognition relies more on factors that involve interactions between peripheral and cognitive processes (Anderson and Kraus 2010). The listener needs to develop a perceptual object in order to distinguish the target voice from other competing sounds. Accordingly, tinnitus patients might find this task challenging, because their tinnitus sound may act as a distractor competing with the target speech.
Table 1.
Summary of speech-in-noise studies in tinnitus patients with normal hearing sensitivity
| Study | N | Age (years) | Tinnitus characteristics | Speech-in-noise test | ||
|---|---|---|---|---|---|---|
| Range | Mean (SD) | Laterality | Severity | |||
| Huang et al. (2007) | 20 | 22–62 | 40.75 (8.59) | Unilateral or bilateral | Mean THI: 30.4 | MSPIN |
| Hennig et al. (2011) | 19 | 21–59 | – | Unilateral or bilateral | – | LSP recognition in noise |
| Ryu et al. (2012) | 20 | 20–35 | 28.2 (6.3) | Unilateral | Mean THI: 30.2 | K-HINT |
| Jain and Sahoo (2014) | 20 | 18–55 | 38.1 | − | − | Kannada QuickSIN |
| Moon et al. (2015) | 9 | – | 28.22 (9.22) | Unilateral | Mean THI: 49 | SRT in noise |
| Gilles et al. (2016) | 19 | < 30 | – | Unilateral or bilateral | Mean TQ: 27.2 | LIST in noise |
N: number of tinnitus patients; THI: Tinnitus Handicap Inventory; TQ: Tinnitus Questionnaire; MSPIN: Mandarin Speech Perception in Noise Test; LSP: Lists of Sentences in Portuguese; K-HINT: Korean Hearing in Noise Test; QuickSIN: Quick Speech-in-Noise test; SRT: speech reception threshold; LIST: Leuven Intelligibility Sentence test
The aim of the study was to investigate how tinnitus interferes with speech recognition ability in noise, particularly in those without any confounding hearing loss, using an easily available clinical test. Additionally, we aimed to examine the impact of various characteristics of tinnitus (severity or loudness) on speech recognition. We hypothesized, based on previous studies shown in Table 1, that the mean speech-in-noise performance in the tinnitus group would be significantly worse than that in the control group. Further, we hypothesized that both tinnitus severity and loudness would have a negative effect on speech-in-noise ability in the tinnitus group.
We chose the Quick Speech-in-Noise test (QuickSIN: Killion et al. 2004) for the speech-in-noise measure as it contains sentence-level materials for obtaining individual ear information. Both the words-in-noise test (WIN: Wilson 2003) and the QuickSIN provide better separation of performance between normal hearing and hearing loss groups than the Hearing in Noise Test (HINT: Nilsson et al. 1994) according to McArdle et al. (2005) and Wilson et al. (2007). However, some word lists for WIN were already being used for our speech-in-quiet tests; therefore, to avoid duplication, we used the QuickSIN for speech-in-noise measure.
METHODS
Participants
Written consent was collected prior to data collection from all participants, who were recruited from the surrounding Urbana-Champaign area. Participants were tested using identical paradigms under the University of Illinois at Urbana-Champaign Institution Review Board protocols 15955 and 16784. To eliminate other variables, individuals who had the following history were excluded before the initiation of the study: history of traumatic brain injury, Meniere’s disease, transmandibular joint disease, posttraumatic stress disorder, other psychological disorders except for currently managed depression and/or anxiety, or a history of neurological disorders. Participants were categorized into the tinnitus group if they reported chronic tinnitus longer than 1 year, or in the control group if they presented with no history of tinnitus. All participants in the study reported having American English as their native language.
Behavioral Procedures
Tinnitus Laterality and Loudness Magnitude Estimation
Tinnitus patients were asked to report the laterality of their tinnitus by choosing one of the ten options (e.g., from right or left ear, both ears equally, both ears but worse in right or left ear, in the head, etc.). They were also asked to judge the general loudness of their tinnitus by using a scale from 0 to 100, with 0 meaning “very faint” and 100 meaning “very loud.” Both questions were included in the in-house tinnitus healthcare questionnaire.
Tinnitus Handicap Inventory
Tinnitus patients were asked to fill out the Tinnitus Handicap Inventory (Newman et al. 1996). The THI is a widely used self-report tinnitus questionnaire including 25 questions with three possible answers of “yes” (4 points), “sometimes” (2 points), and “no” (0 points). The range of scores is from 0 to 100, and the severity of tinnitus increases with the score. The THI scores were used for estimating the severity of tinnitus; there were no inclusion/exclusion criteria according to the scores.
Audiological Assessments
All participants underwent otoscopic inspection to make sure there was a clear visualization of the eardrum. Tympanometry and acoustic reflexes were conducted to ensure all participants had a normal middle ear function and to rule out possible retrocochlear pathologies. Participants were then evaluated with pure-tone audiometry from 250 to 16,000 Hz in each ear. Pure-tone average (PTA) in each ear was calculated using the mean thresholds of 500, 1000, and 2000 Hz. Speech audiometry including speech perception threshold (SRT) and word recognition score (WRS) with the Northwestern University Auditory Test No. 6 (NU-6: Tillman and Carhart 1966) lists were obtained from participants to verify their speech recognition ability in quiet. Further, we ruled out obvious outer hair cell dysfunction of participants by using the distortion product otoacoustic emissions (DPOAEs).
Normal hearing sensitivity was defined as pure-tone thresholds less than or equal to 25 dB HL from 250 to 8000 Hz and WRS greater than or equal to 80 % in each ear. To maintain a more balanced age and gender distribution in each group, one participant in the tinnitus group with a threshold of 30 dB HL at 2000 Hz in the left ear and normal hearing sensitivity at other frequencies was still included; we verified that the results did not change with the exclusion of this participant. Participants with asymmetrical hearing, which is defined as a discrepancy of hearing threshold greater than 10 dB between ears in more than two frequencies measured from 250 to 8000 Hz (Moon et al. 2015), were excluded from the study. Moreover, participants with binaural difference of speech recognition in quiet (as in WRS) greater than 12 % were also excluded (Ryu et al. 2012).
Quick Speech-in-Noise Test
The QuickSIN test was developed using the Institute of Electrical and Electronics Engineers (IEEE) sentences, which are constructed with proper syntax without strong semantic cues (Killion et al. 2004; Wilson et al. 2007). The test consists of 12 lists of sentences, with six sentences in each list spoken by a female speaker and five target words in each sentence. Each list was presented monaurally to participants through the ER-3A insert earphones (Etymotic Research) with built-in wave files in the Interacoustics Equinox 2.0 audiometer. Participants were given two lists in each ear: lists 1 and 2 presented to the right ear first and then lists 3 and 4 presented to the left ear. The presentation level was held constant at 70 dB HL during the testing; however, the signal-to-noise ratio (SNR) decreased gradually from 25 to 0 dB SNR after each sentence, with increased intensity of competing speech in 5-dB increments. Therefore, the task becomes increasingly difficult for the listeners as SNR decreases. Participants were instructed to repeat back the sentences from the target female talker and ignore the four-talker babble in the background; they were also encouraged to guess if they were not sure about the content. The SNR loss was calculated by subtracting the number of target words (total 25 words in one list) repeated correctly by the participant from 25.5, as advised in the QuickSIN user manual. The SNR loss in each ear was represented by averaging the SNR loss of the two lists.
To examine the effect of testing order on the SNR loss between the ears, a subgroup of participants with tinnitus were recruited for a follow-up testing of QuickSIN, with lists 5 and 6 presented to the left ear first and then lists 7 and 8 presented to the right ear.
Psychoacoustic Measurement of Tinnitus
Tinnitus loudness matching was conducted by having tinnitus patients match the loudness of their tinnitus to both a 500-Hz pure tone (LD500) and a white noise (LDWN). The stimuli were presented to the ear with nondominant tinnitus for bilateral tinnitus. If bilateral tinnitus participants did not report a dominant ear, the test ear was the ear with the better PTA or to the right ear if there was no significant difference in PTA between the ears. A bracketing approach, with a step size of 2 dB, was used during the measurement. The results of the loudness matching were reported as the mean dB SL of three trials, which represents the loudness of tinnitus above the patient’s hearing threshold (Tyler 2000).
Equipment/Instrumentation
Instrumentation and Calibration
Threshold measurements and tinnitus loudness matching were obtained using an Interacoustics Equinox 2.0 clinical audiometer with the ER-3A insert earphones for thresholds from 250 to 8000 Hz and the Sennheiser HDA 200 headphones for thresholds from 9000 to 16,000 Hz. The audiometer and the transducers were calibrated annually according to the ANSI S3.6-2010 standard (American National Standards Institute 2010). Tympanometric tests were conducted by using the Interacoustics Titan Ver. 4.0 clinical tympanometer, and the DPOAEs were obtained with the Bio-Logic Scout OAE system; the tympanometer and the OAE system were also annually calibrated.
Test Environment and Calibration
Audiological assessments, QuickSIN test, and tinnitus loudness matching were conducted in a soundproof room, which satisfies the ANSI S3.1-1999 standard (American National Standards Institute 1999) to control the study’s validity and reliability.
Statistical Analysis
We confirmed that the data were normally distributed by using Kolmogorov-Smirnov tests. Repeated-measures analysis of variance (ANOVA) tests were used to evaluate between-group differences in pure-tone thresholds at each frequency, speech-in-noise performance (SNR loss) in each ear, and the speech-in-noise performance at various SNR conditions from 0 to 25 dB SNR. The relation between the SNR loss and tinnitus characteristics was examined with the Pearson correlation analyses. All statistical analyses were performed using the R statistical software version 3.4.0 at a critical value of α = 0.05.
RESULTS
Fourteen participants (mean age 43.86 years) with chronic tinnitus underwent behavioral testing and tinnitus-related questionnaire and measures. The control group contained 14 age-matched adults (mean age 44 years) with normal hearing thresholds. Table 2 shows a summary of tinnitus characteristics in the tinnitus group and Table 3 shows the characteristics and audiological findings in each group. The effect of dominant ear on tinnitus patients was not included in the analyses because only three of 14 patients reported having a dominant ear (one right, two left). The tinnitus and the control groups did not show significant differences in age distribution (t(26) = − 0.031, P = 0.98).
Table 2.
Tinnitus characteristics (N = 14)
| Mean (SD) | Min, max | |
|---|---|---|
| Duration (years) | 17.71 (10.55) | 3, 45 |
| THI | 17.29 (13) | 2, 48 |
| 0–16, slight handicap (n = 9) | ||
| 18–36, mild handicap (n = 4) | ||
| 38–56, moderate handicap (n = 1) | ||
| LME | 48.36 (25.71) | 2, 90 |
| LD500 (dB SL) | 31.83 (14.2) | 17, 59.67 |
| LDWN (dB SL) | 22.79 (14.12) | 6.33, 52 |
| Laterality | Bilateral, louder in the right ear (n = 1) | |
| Bilateral, louder in the left ear (n = 2) | ||
| Bilateral, equally loud (n = 11) | ||
| Tinnitus sounds | Ringing or whistling (n = 10) | |
| Buzzing (n = 3) | ||
| Hissing (n = 1) |
THI: Tinnitus Handicap Inventory; LME: loudness magnitude estimation; LD500: loudness matching at 500 Hz; LDWN: loudness matching with white noise
Table 3.
Characteristics and audiological findings in the tinnitus and the control groups: values are mean (SD) unless otherwise stated
| Tinnitus (N = 14) | Control (N = 14) | ||||
|---|---|---|---|---|---|
| Age (years) | 43.86 (13.13), range 23–63 | 44.00 (11.02), range 25–60 | |||
| Gender | 5 females | 8 females | |||
| Audiological findings | Right | Left | Right | Left | |
| Pure-tone threshold (dB HL) | 250 Hz | 11.07 (4.01) | 10.36 (4.99) | 8.21 (5.04) | 7.86 (5.79) |
| 500 Hz | 12.14 (5.08) | 9.64 (4.99) | 9.29 (5.14) | 8.93 (5.61) | |
| 1000 Hz | 11.07 (6.84) | 11.07 (5.61) | 9.29 (6.16) | 10.00 (4.39) | |
| 2000 Hz | 15.00 (6.50) | 13.21 (7.75) | 8.93 (5.61) | 7.86 (5.08) | |
| 4000 Hz | 12.14 (5.08) | 13.57 (6.63) | 10.00 (6.20) | 10.00 (7.34) | |
| 8000 Hz | 11.43 (7.70) | 10.36 (8.43) | 9.64 (8.87) | 8.93 (8.59) | |
| PTA (dB HL) | 12.74 (4.83) | 11.31 (5.20) | 9.17 (4.92) | 8.93 (4.27) | |
| WRS (%) | 99.14 (1.70) | 100.00 (0.00) | 100.00 (0.00) | 100.00 (0.00) | |
| SNR loss | 1.54 (1.51) | 2.57 (1.38) | 1.93 (1.05) | 1.57 (0.85) | |
PTA: pure-tone average of 500, 1000, and 2000 Hz; WRS: word recognition score; SNR loss: signal-to-ratio loss calculated based on the QuickSIN manual (Killion et al. 2004)
Comparison Between the Tinnitus and the Control Groups
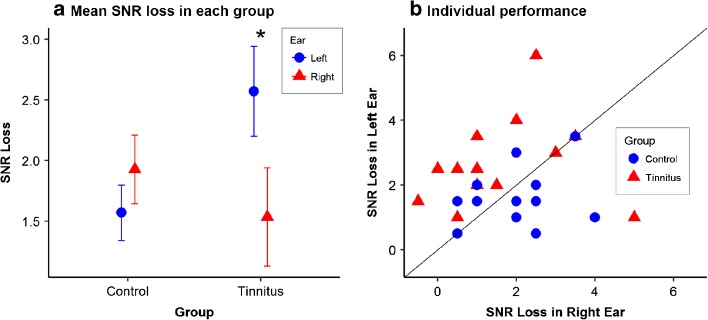
A two-way group × ear repeated-measures ANOVA was conducted to compare the SNR loss between the two groups. The results indicated a significant interaction effect of group × ear (F(1, 26) = 5.89, P = 0.022). Post hoc t tests with Bonferroni correction for two comparisons suggested that in the tinnitus group, the SNR loss was significantly higher in the left ear (mean 2.57; SD 1.38) than in the right ear (mean 1.54; SD 1.51) (t(26) = 2.55, P = 0.034). The between-ear difference in SNR loss was not observed in the control group (t(26) = − 0.88, P = 0.77). Figure 1 shows the mean and the standard error of the SNR loss in each group, as well as individual scores in SNR loss separated by ears. Both panels a and b of Fig. 1 demonstrate that tinnitus participants had significantly poorer speech-in-noise performance in the left ear than in the right ear.
Fig. 1.
a Mean and one standard error of the SNR loss in each group. The higher the SNR loss, the worse the speech-in-noise performance. In the tinnitus group, the SNR loss in the left ear was significantly higher than that in the right ear (P = 0.034). b A scatter plot of the individual performance. Higher SNR loss represents poorer speech-in-noise performance. Points that fall below the diagonal indicate higher SNR loss in the right ear compared to that in the left ear. Only one of 14 tinnitus patients showed a score below the diagonal
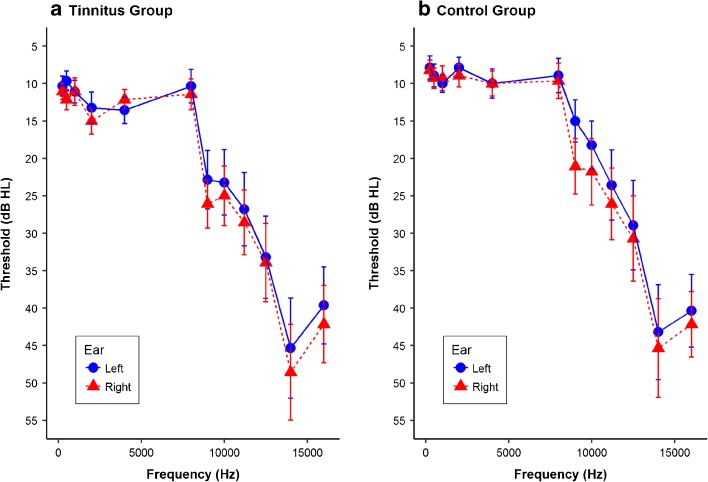
As shown in Fig. 2, the mean thresholds from 250 to 16,000 Hz in each group suggested symmetrical hearing sensitivity (no greater than 10 dB difference between ears). A three-way group × ear × frequency (from 250 to 8000 Hz) repeated-measures ANOVA showed no significant main effects of group, ear, or frequency and no significant interaction effects. The results indicated no significant between-group differences in pure-tone thresholds up to 8000 Hz in each ear, implicating that the two groups did not differ in audiological profiles.
Fig. 2.
Mean hearing thresholds from 250 to 16,000 Hz in a the tinnitus group and b the control group. Error bars indicate one standard error of the mean. Although thresholds were significantly different between ears in the extended high-frequency range (above 8000 Hz) in the control group with the right ear thresholds significantly worse than left ear thresholds (F(1, 13) = 5.307, P = 0.038), the mean thresholds shown in the figure suggested symmetrical hearing sensitivity (no greater than 10 dB difference between ears) in both groups
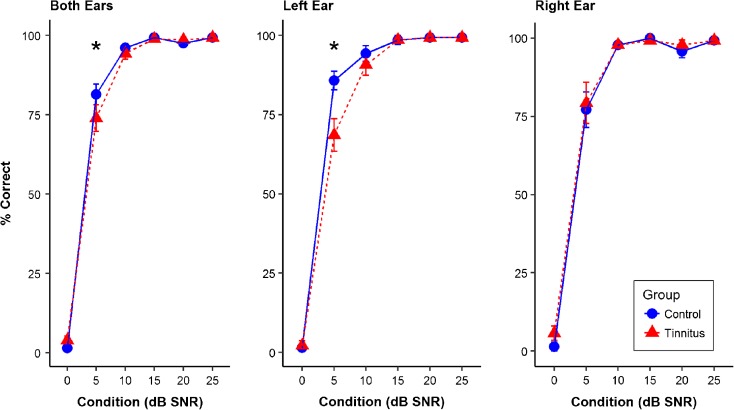
To determine whether a group-level or an ear-level difference was present, QuickSIN performance at each SNR condition was further examined. The QuickSIN performance was calculated as percent correct, depending on the number of key words repeated correctly divided by the total five key words in each sentence. A three-way group × ear × SNR condition repeated-measures ANOVA showed a significant main effect of SNR condition (F(5, 130) = 981.23, P < 0.0001) and a significant interaction effect of group × ear (F(1, 26) = 5.89, P = 0.022). Post hoc t tests with Bonferroni correction for two comparisons suggested that in the tinnitus group, the QuickSIN performance in the left ear (mean 76.43 %; SD 38.26 %) was significantly worse compared to that of the right ear (mean 79.88 %; SD 37.15 %) (t(26) = − 2.55, P = 0.034). Statistical trends were also observed on the interaction effect of group × SNR condition (F(5, 130) = 2.053, P = 0.075) and the interaction effect of group × ear × SNR condition (F(5, 130) = 2.093, P = 0.070). Post hoc t tests with Bonferroni correction for multiple comparisons indicated that the tinnitus group performed significantly worse compared to the control group at 5 dB SNR condition (t(26) = 2.96, P = 0.021 adjusted for six comparisons). At 5 dB SNR condition, the performance of the left ear in the tinnitus group (mean = 68.57 %; SD = 19.16 %) was significantly worse than that in the control group (mean = 85.71 %; SD = 10.89 %) (t(26) = 4.80, P = 0.0001 Bonferroni correction adjusted for 36 comparisons). The between-group difference was only observed at the 5-dB SNR condition, possibly due to the ceiling effect at favorable SNR conditions from 10 to 25 dB SNR (mean performance greater than 90 % correct at each condition) and the floor effect at 0 dB SNR (mean performance less than 6 % correct). This is similar to the findings of Wong et al. (2010) where the 0-dB SNR was the only condition that showed significant between-group differences. The change from 0 to 5 dB SNR is likely due to the inclusion of individuals with clinically defined hearing loss in the Wong et al. (2010) study. Figure 3 shows the performance at various SNR conditions in each ear as well as in both ears.
Fig. 3.
Mean QuickSIN performance in percent correct at various SNR conditions. Error bars indicate one standard error of the mean. Significant between-group difference was found at 5 dB SNR condition in the left ear (P = 0.0001) as well as in both ears (P = 0.021)
Effect of Testing Order in the Tinnitus Subgroup
Participants in the tinnitus group were contacted for a follow-up testing to rule out the effect of testing order. Seven of 14 (4 females; mean age 47 years; SD = 13.9 years) responded and returned for repeated measures of THI, pure-tone audiometry from 250 to 16,000 Hz, and the QuickSIN test. A one-way repeated-measures ANOVA showed no significant change of the THI scores between the two visits (F(1, 6) = 0.28, P = 0.62), suggesting that tinnitus severity did not change significantly over time.
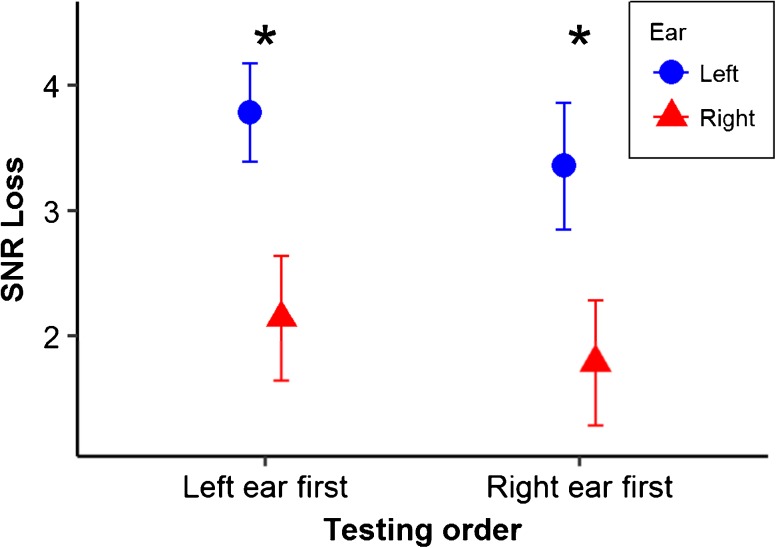
Due to the small sample size in the subgroup, the effect size was calculated by using partial eta-squared (ŋ p 2) following the ANOVA analysis. A two-way ear × testing order repeated-measures ANOVA indicated a significant main effect of ear (F(1, 6) = 15.26, P = 0.0079, ŋ p 2 = 0.7178), but no significant main effect of testing order (F(1, 6) = 2.49, P = 0.17, ŋ p 2 = 0.293) or interaction effect of testing order × ear (F(1, 6) = 0.013, P = 0.91, ŋ p 2 = 0.0021). The results in the tinnitus subgroup suggested that 71.78 % of the variation of the SNR loss was explained by the effect of the ear. The tinnitus subgroup showed a significantly higher SNR loss in the left ear than in the right ear, regardless of the testing order. Figure 4 shows the SNR loss in each ear presented with different testing orders.
Fig. 4.
Mean and one standard error of the SNR loss with different testing orders in the tinnitus subgroup (n = 7). The higher the SNR loss, the worse the speech-in-noise performance. Results indicated that the SNR loss in the left ear was significantly higher than that in the right ear (P = 0.0079), disregarding the testing order
Correlation of Variables in the Tinnitus Group
Table 4 shows the correlation between the SNR loss in each ear and tinnitus characteristics in the tinnitus group. Pearson correlation analyses indicated no significant correlation between the right SNR loss, left SNR loss, THI, loudness magnitude estimation (LME), and the two loudness measures, except between LD500 and LDWN (r = 0.89, P < 0.001) and between left SNR loss and LDWN (r = 0.64, P = 0.014). Not all the severity and loudness variables positively correlated with SNR loss in each ear. As shown in Table 4, a significant positive correlation was only found between the left SNR loss and LDWN, with a statistical trend between left SNR loss and LD500 (r = 0.47, P = 0.087). The results implied that psychoacoustic tinnitus loudness measures affected the QuickSIN performance in each ear differently.
Table 4.
Pearson correlation between speech-in-noise performance and tinnitus characteristics
| THI | LME | LD500 | LDWN | SNR-R | SNR-L | |
|---|---|---|---|---|---|---|
| THI | 1 | |||||
| LME | 0.48 | 1 | ||||
| LD500 | − 0.40 | 0.07 | 1 | |||
| LDWN | − 0.20 | 0.29 | 0.89*** | 1 | ||
| SNR-R | 0.29 | 0.13 | 0.04 | 0.12 | 1 | |
| SNR-L | − 0.06 | 0.07 | 0.47 | 0.64* | 0.24 | 1 |
Note: Values with significant correlations are in boldface
THI: Tinnitus Handicap Inventory; LME: loudness magnitude estimation; LD500: loudness matching at 500 Hz; LDWN: loudness matching with white noise; SNR-R: signal-to-noise ratio loss in the right ear; SNR-L: signal-to-noise ratio loss in the left ear
***P < 0.001; *P < 0.05
DISCUSSION
The aims of the study were to investigate the effect of tinnitus on speech-in-noise recognition in tinnitus patients with normal hearing thresholds and to ascertain any association between various characteristics of tinnitus and their recognition scores. Across groups, we did not find significantly different performance, except at the 5-dB SNR condition (Fig. 3). Further, within-group tests revealed that tinnitus participants (but not controls) showed a significant between-ear difference (right-ear advantage) despite reporting bilateral tinnitus and symmetrical hearing thresholds (Fig. 1). With regard to other factors of tinnitus, loudness had some effect on speech-in-noise performance, primarily in the left ear, but severity did not have any impact.
The strength of the present study is the comparison of between-ear performance in patients and controls—in our study, this revealed a right-ear advantage for the patient group reporting bilateral tinnitus. Few studies have addressed this aspect in speech-in-noise testing and none that uses the QuickSIN test. The difference in between-ear performance has only been considered in cases with unilateral tinnitus (Ryu et al. 2012); in cases with bilateral tinnitus, researchers tend to test only one ear or to add tinnitus-affected ears for data analyses (Gilles et al. 2016; Hennig et al. 2011; Huang et al. 2007). Moon et al. (2015) found that bilateral tinnitus patients had similar SRTs in noise between ears; however, their bilateral tinnitus group contained nine patients with a mild to moderately severe hearing loss. It is unclear what impact hearing impairment has in combination with tinnitus on speech-in-noise ability.
Right-Ear Advantage in the Tinnitus Group
The fact that our tinnitus participants had a better speech-in-noise score in the right ear than in the left ear may be explained by two scenarios: as a result of listener’s fatigue due to a fixed testing order or a right-ear advantage in language. Hearing loss or tinnitus laterality did not seem to cause the between-ear difference because our patients showed symmetrical hearing sensitivity at frequencies from 250 to 16,000 Hz (Fig. 2), and only two of 14 reported having tinnitus louder in the left ear. In contrast, listener’s fatigue may result in between-ear differences in performance when the right ear is administered first. Fatigue is believed to be a moderator of the tinnitus sound and its related annoyance (Andersson and Westin 2008), and it is likely that patients with tinnitus become tired more easily in adverse listening environments. Initially, we tested all participants in a fixed order, as is typically done in a clinic. However, a follow-up QuickSIN test with the opposite testing order in a subgroup of the patients was conducted and it ruled out a possible effect of fatigue. Thus, the second scenario relating these performance differences to a right-ear advantage appears more likely.
A right-ear advantage has been used to explain the difference in between-ear performance for dichotic listening tasks and is believed to be related to the left-hemisphere dominance for speech and language and the efficient conduction of sounds from the right ear to the left hemisphere (Kimura 2011). Studies have shown a significant link between right-ear advantage and speech-in-noise recognition in young adults (Bidelman and Bhagat 2015) and in older populations (Tadros et al. 2005). Because this right-ear advantage was not observed in the controls and the two groups were age-matched, it is unlikely that the observed between-ear difference can be solely attributed to aging. The right-ear advantage increases with normal aging and age-related hearing loss (Jerger et al. 1994; Kam and Keith 2010; Roup 2011; Roup et al. 2006). The increased right-ear advantage reported in older adults is not entirely due to a change of hearing sensitivity but may be attributed to a worse performance in the left ear, indicating a deficit in speech recognition in that ear (Jerger et al. 1994; Roup 2011). As proposed by Jerger et al. (1994), a left-ear disadvantage can be caused by a decline in cognitive functions such as in memory, attention, and processing speed, or a loss in efficiency of interhemispheric transfer at the corpus callosum.
Using a dichotic listening task, Cuny et al. (2004a) confirmed that patients with bilateral tinnitus retained a significant right-ear advantage similar to that of the controls. Although the QuickSIN test has been used to demonstrate a right-ear advantage in young adults with normal hearing sensitivity and no audiological complaints (Bidelman and Howell 2016), our findings support the right-ear advantage (or more specifically, left-ear disadvantage) hypothesis only in the tinnitus group, likely due to the wide age range (23–63 years) of normal hearing participants in our study. The right-ear advantage found in our patients may imply that tinnitus degrades speech processing in a manner similar to aging or hearing impairment, and can possibly result in an inefficient tinnitus intervention with binaural fitting of amplification or noise generators.
Procedural Learning Effect and Heterogeneity of the QuickSIN Lists
Without using a practice list in the QuickSIN test to familiarize participants to the listening task, a procedural learning effect can result in a better performance in the ear being tested later. However, there were no indications of procedural learning effects in the current study, which is also in line with Yund and Woods (2010) that the QuickSIN test demonstrates small procedural learning effects. It is also likely that the between-ear difference was caused by the heterogeneity of the QuickSIN lists, as some studies reported the lack of interlist equivalency for the QuickSIN (McArdle and Wilson 2006; Walden and Walden 2004). The findings from McArdle and Wilson (2006) indicate that some lists were significantly different from others for listeners with hearing loss. Notwithstanding these findings, there is no evidence on how the heterogeneity of the QuickSIN lists affects the performance of listeners with normal hearing sensitivity (Killion et al. 2004; McArdle and Wilson 2006).
Impact of Tinnitus Severity and Loudness on Speech-in-Noise Recognition
Our findings showed a statistical trend of the correlation between the THI and the LME scores (r = 0.48, P = 0.081), which is in keeping with previous findings that self-rated tinnitus loudness can contribute to increased perceived tinnitus handicap (Figueiredo et al. 2009; Hiller and Goebel 2007; Huang et al. 2007; Kuk et al. 1990). Interestingly, the self-rated loudness perception measured by LME and the psychoacoustic loudness measures (LD500 and LDWN) were not significantly correlated. This finding, supported by the weak correlations between tinnitus loudness matching and THI, is in line with previous studies showing that loudness measured audiometrically does not relate to tinnitus severity (Hiller and Goebel 2007; Hoekstra et al. 2014; Probst et al. 2016). This mismatch between psychoacoustic estimates of loudness and self-reported loudness rating supports the novel cognitive-behavioral model of tinnitus by McKenna et al. (2014), implying that the distorted perception of tinnitus can maintain tinnitus distress and further affect behavioral responses.
Heterogeneity of the tinnitus population may explain the differences between our study and reports from extant literature; for example, higher mean THI scores in a mild to moderate severity range than the THI score in the current study were reported when similar age groups were studied (Hennig et al. 2011; Huang et al. 2007). Gilles et al. (2016) shared comparable tinnitus characteristics with the current study: the majority of their participants reported bilateral tinnitus with slight tinnitus severity measured by the tinnitus questionnaire (Goebel and Hiller 1994); however, only younger participants with recently developed tinnitus (mean tinnitus duration 2 years; SD 1.2 years) were recruited in their study. Therefore, it may be necessary to examine the effect of age, tinnitus duration (e.g., recent onset versus long term), and tinnitus severity with a range of scores to better understand the impact of tinnitus on speech-in-noise performance.
Implication of Speech-in-Noise Recognition: from Peripheral Audibility to Cognitive Function
Speech-in-noise recognition has been shown to be related to several cognitive functions, which include but are not limited to working memory (Akeroyd 2008), processing speed, task switching, attention, and executive control (Ellis et al. 2016). Additionally, the cognitive spare capacity, which is closely related to working memory in terms of short-term storage and processing of information, tends to decrease for higher level processing of speech in adverse conditions (Ronnberg et al. 2011; Rudner and Lunner 2014). According to the information-degradation hypothesis, one possibility is that the effect of tinnitus (in the normal hearing condition) is another factor in degrading incoming information, and has an effect similar to the effect of hearing impairment on cognitive decline; tinnitus as a distracting sound makes it more effortful for speech-in-noise recognition, which results in the depletion of cognitive resources (Arlinger et al. 2009; CHABA 1988; Degeest et al. 2017; Pichora-Fuller 2003; Wayne and Johnsrude 2015). It is probable that our results cannot be solely interpreted by the structural difference in hemispheres, as studies have shown that the right-ear advantage can be influenced or modified by attention, or by other cognitive functions such as working memory (for a review, see Hiscock and Kinsbourne 2011). How this impacts cognitive abilities in the long term in chronic tinnitus is unknown. Because of the lack of neuropsychological assessments to evaluate cognitive functions in the present study, we are unable to posit the correlation between speech-in-noise performance and cognitive functions in our tinnitus group. Additionally, as tinnitus patients can react to their tinnitus differently depending on the context of event, studies that incorporate factors such as personality and implement more realistic listening environments for tinnitus evaluation are warranted (Searchfield 2014).
To conclude, we found a right-ear advantage of speech-in-noise ability in individuals with bilateral tinnitus and normal hearing sensitivity. The speech-in-noise performance showed an impact of loudness (as measured via psychoacoustic measures) but not of self-reported tinnitus severity, or self-rated loudness. The findings underline the importance of including speech-in-noise testing in tinnitus patients to evaluate the interaction between peripheral and cognitive factors and for tinnitus management when amplification or noise generators are recommended to tinnitus patients with normal hearing sensitivity. Future studies that include individuals with bothersome tinnitus are needed to better understand the impact of severity on speech recognition, which in turn can be useful for developing interventions ameliorating the effect of tinnitus and improving speech-in-noise performance.
Acknowledgements
The authors would like to thank Anthony Tsao for assisting with data collection and Dr. Sa Shen for providing advice on aspects of the statistical analysis. We are deeply grateful to Dr. Richard Tyler for his insightful comments on earlier versions of this manuscript.
Funding Information
The study was supported in part by the Department of Defense, award number W81XWH-15-2-0032 (PI: Husain) and a Center for Wounded Veterans in Higher Education (UIUC) seed grant (PI: Husain).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Yihsin Tai, Phone: (217) 333-2230, Email: ytai5@illinois.edu.
Fatima T. Husain, Phone: (217) 333-2230, Email: husainf@illinois.edu
References
- Akeroyd MA. Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol. 2008;47(sup2):S53–S71. doi: 10.1080/14992020802301142. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute (1999) Maximum permissible ambient noise levels for audiometric test rooms [DOI] [PubMed]
- American National Standards Institute (2010) Specification for audiometers
- Anderson S, Kraus N. Objective neural indices of speech-in-noise perception. Trends Amplif. 2010;14(2):73–83. doi: 10.1177/1084713810380227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G, Westin V. Understanding tinnitus distress: introducing the concepts of moderators and mediators. Int J Audiol. 2008;47(sup2):S106–S111. doi: 10.1080/14992020802301670. [DOI] [PubMed] [Google Scholar]
- Arlinger S, Lunner T, Lyxell B, Pichora-Fuller MK. The emergence of cognitive hearing science. Scand J Psychol. 2009;50(5):371–384. doi: 10.1111/j.1467-9450.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- Basile CE, Fournier P, Hutchins S, Hebert S. Psychoacoustic assessment to improve tinnitus diagnosis. PLoS One. 2013;8:28–41. doi: 10.1371/journal.pone.0082995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Bhagat SP. Right-ear advantage drives the link between olivocochlear efferent “antimasking” and speech-in-noise listening benefits. Neuroreport. 2015;26(8):483–487. doi: 10.1097/WNR.0000000000000376. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Howell M. Functional changes in inter- and intra-hemispheric cortical processing underlying degraded speech perception. NeuroImage. 2016;124(Pt A):581–590. doi: 10.1016/j.neuroimage.2015.09.020. [DOI] [PubMed] [Google Scholar]
- CHABA Speech understanding and aging. J Acoust Soc of Am. 1988;83(3):859–895. doi: 10.1121/1.395965. [DOI] [PubMed] [Google Scholar]
- Cuny C, Chéry-Croze S, Bougeant JC, Koenig O. Investigation of functional hemispheric asymmetry of language in tinnitus sufferers. Neuropsychology. 2004;18(2):384–392. doi: 10.1037/0894-4105.18.2.384. [DOI] [PubMed] [Google Scholar]
- Cuny C, Norena A, El Massioui F, Chery-Croze S (2004b) Reduced attention shift in response to auditory changes in subjects with tinnitus. Audiol Neurotol 9:294–302, 5, doi: 10.1159/000080267 [DOI] [PubMed]
- Davis A, El Rafaie A. Epidemiology of tinnitus. In: Tyler RS, editor. Tinnitus handbook. San Diego: Singular; 2000. pp. 1–24. [Google Scholar]
- Degeest S, Keppler H, Corthals P. The effect of tinnitus on listening effort in normal-hearing young adults: a preliminary study. J Speech Lang Hear Res. 2017;60(4):1036–1045. doi: 10.1044/2016_JSLHR-H-16-0090. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Molander P, Ronnberg J, Lyxell B, Andersson G, Lunner T. Predicting speech-in-noise recognition from performance on the trail making test: results from a large-scale internet study. Ear Hear. 2016;37(1):73–79. doi: 10.1097/AUD.0000000000000218. [DOI] [PubMed] [Google Scholar]
- Figueiredo RR, Azevedo AAD, Oliveira PDM. Correlation analysis of the visual-analogue scale and the Tinnitus Handicap Inventory in tinnitus patients. Braz J Otorhinolaryngol. 2009;75(1):76–79. doi: 10.1016/S1808-8694(15)30835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles A, Schlee W, Rabau S, Wouters K, Fransen E, Van de Heyning P. Decreased speech-in-noise understanding in young adults with tinnitus. Front Neurosci. 2016;10:1–14. doi: 10.3389/fnins.2016.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel G, Hiller W. The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO. 1994;42(3):166–172. [PubMed] [Google Scholar]
- Hennig TR, Costa MJ, Urnau D, Becker KT, Schuster LC. Recognition of speech of normal-hearing individuals with tinnitus and hyperacusis. Int Arch Otorhinolaryngol. 2011;15:21–28. [Google Scholar]
- Henry JA, Zaugg TL, Myers PJ, Kendall CJ, Turbin MB. Principles and application of educational counseling used in progressive audiologic tinnitus management. Noise Health. 2009;11(42):33–48. doi: 10.4103/1463-1741.45311. [DOI] [PubMed] [Google Scholar]
- Hiller W, Goebel G. When tinnitus loudness and annoyance are discrepant: audiological characteristics and psychological profile. Audiol Neurotol. 2007;12(6):391–400. doi: 10.1159/000106482. [DOI] [PubMed] [Google Scholar]
- Hiscock M, Kinsbourne M. Attention and the right-ear advantage: what is the connection? Brain Cogn. 2011;76(2):263–275. doi: 10.1016/j.bandc.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Hoekstra CEL, Wesdorp FM, van Zanten GA. Socio-demographic, health, and tinnitus related variables affecting tinnitus severity. Ear Hear. 2014;35(5):544–554. doi: 10.1097/AUD.0000000000000045. [DOI] [PubMed] [Google Scholar]
- Huang CY, Lee HH, Chung KC, Chen HC, Shen YJ, Wu JL. Relationships among speech perception, self-rated tinnitus loudness and disability in tinnitus patients with normal pure-tone thresholds of hearing. ORL. 2007;69(1):25–29. doi: 10.1159/000096713. [DOI] [PubMed] [Google Scholar]
- Jain C, Sahoo JP. The effect of tinnitus on some psychoacoustical abilities in individuals with normal hearing sensitivity. Int Tinnitus J. 2014;19(1):28–35. doi: 10.5935/0946-5448.20140004. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8(4):221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Jerger J, Chmiel R, Allen J, Wilson A. Effects of age and gender on dichotic sentence identification. Ear Hear. 1994;15(4):274–286. doi: 10.1097/00003446-199408000-00002. [DOI] [PubMed] [Google Scholar]
- Kam ACS, Keith RW. Aging effect on dichotic listening of Cantonese. Int J Audiol. 2010;49(9):651–656. doi: 10.3109/14992021003793942. [DOI] [PubMed] [Google Scholar]
- Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, Banerjee S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2004;116(4):2395–2405. doi: 10.1121/1.1784440. [DOI] [PubMed] [Google Scholar]
- Kimura D. From ear to brain. Brain Cogn. 2011;76(2):214–217. doi: 10.1016/j.bandc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of the Tinnitus Handicap Questionnaire. Ear Hear. 1990;11(6):434–445. doi: 10.1097/00003446-199012000-00005. [DOI] [PubMed] [Google Scholar]
- McArdle RA, Wilson RH. Homogeneity of the 18 QuickSIN lists. J Am Acad Audiol. 2006;17(3):157–167. doi: 10.3766/jaaa.17.3.2. [DOI] [PubMed] [Google Scholar]
- McArdle RA, Wilson RH, Burks CA. Speech recognition in multitalker babble using digits, words, and sentences. J Am Acad Audiol. 2005;16(9):726–739. doi: 10.3766/jaaa.16.9.9. [DOI] [PubMed] [Google Scholar]
- McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70–79. doi: 10.1016/j.heares.2016.05.009. [DOI] [PubMed] [Google Scholar]
- McKenna L, Handscomb L, Hoare DJ, Hall DA. A scientific cognitive-behavioral model of tinnitus: novel conceptualizations of tinnitus distress. Front Neurol. 2014;5:196. doi: 10.3389/fneur.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, Myers PJ, Newman CW, Sandridge S, Turk DC, Folmer RL, Frederick EJ, House JW, Jacobson GP, Kinney SE, Martin WH, Nagler SM, Reich GE, Searchfield G, Sweetow R, Vernon JA. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33(2):153–176. doi: 10.1097/AUD.0b013e31822f67c0. [DOI] [PubMed] [Google Scholar]
- Moller AR. Tinnitus: presence and future. Prog Brain Res. 2007;166:3–16. doi: 10.1016/S0079-6123(07)66001-4. [DOI] [PubMed] [Google Scholar]
- Moon IJ, Won JH, Kang HW, Kim DH, An YH, Shim HJ. Influence of tinnitus on auditory spectral and temporal resolution and speech perception in tinnitus patients. J Neurosci. 2015;35(42):14260–14269. doi: 10.1523/JNEUROSCI.5091-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman CW, Jacobson GP, Spitzer JB. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Soli SD, Sullivan JA. Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95(2):1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Norena AJ. Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. Audiol Neurotol. 2015;20(1):53–59. doi: 10.1159/000380749. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK. Cognitive aging and auditory information processing. Int J Audiol. 2003;42:S26–S32. doi: 10.3109/14992020309074641. [DOI] [PubMed] [Google Scholar]
- Probst T, Pryss R, Langguth B, Schlee W (2016) Emotional states as mediators between tinnitus loudness and tinnitus distress in daily life: results from the “TrackYourTinnitus” application. Sci Rep 6(1). 10.1038/srep20382 [DOI] [PMC free article] [PubMed]
- Ronnberg N, Stenfelt S, Rudner M (2011) Testing listening effort for speech comprehension using the individuals’ cognitive spare capacity. Audiol Res 1(1S). 10.4081/audiores.2011.e22 [DOI] [PMC free article] [PubMed]
- Roup CM. Dichotic word recognition in noise and the right-ear advantage. J Speech Lang Hear Res. 2011;54(1):292–297. doi: 10.1044/1092-4388(2010/09-0230). [DOI] [PubMed] [Google Scholar]
- Roup CM, Wiley TL, Wilson RH. Dichotic word recognition in young and older adults. J Am Acad Audiol. 2006;17(4):230–240. doi: 10.3766/jaaa.17.4.2. [DOI] [PubMed] [Google Scholar]
- Rudner M, Lunner T. Cognitive spare capacity and speech communication: a narrative overview. Biomed Res Int. 2014;2014:1–10. doi: 10.1155/2014/869726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu IS, Ahn JH, Lim HW, Joo KY, Chung JW. Evaluation of masking effects on speech perception in patients with unilateral chronic tinnitus using the hearing in noise test. Otol Neurotol. 2012;33(9):1472–1476. doi: 10.1097/MAO.0b013e31826dbcc4. [DOI] [PubMed] [Google Scholar]
- Searchfield GD. Tinnitus what and where: an ecological framework. Front Neurol. 2014;5:271. doi: 10.3389/fneur.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, Frisina ST, Mapes F, Kim SH, Frisina DR, Frisina RD. Loss of peripheral right-ear advantage in age-related hearing loss. Audiol Neurootol. 2005;10(1):44–52. doi: 10.1159/000082307. [DOI] [PubMed] [Google Scholar]
- Theodoroff SM, Folmer RL. Repetitive transcranial magnetic stimulation as a treatment for chronic tinnitus: a critical review. Otol Neurotol. 2013;34(2):199–208. doi: 10.1097/MAO.0b013e31827b4d46. [DOI] [PubMed] [Google Scholar]
- Tillman TW, Carhart R (1966) An expanded test for speech discrimination utilizing CNC monosyllabic words: Northwestern University Auditory Test No. 6. Northwestern University Auditory Research Lab, Evanston, IL [DOI] [PubMed]
- Tyler RS. Psychological management of tinnitus. In: Tyler RS, editor. Tinnitus handbook. San Diego: Singular; 2000. pp. 263–279. [Google Scholar]
- Tyler RS, Baker LJ. Difficulties experienced by tinnitus sufferers. J Speech Hear Disord. 1983;48(2):150–154. doi: 10.1044/jshd.4802.150. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Ji H, Perreau A, Witt S, Noble W, Coelho C. Development and validation of the tinnitus primary function questionnaire. Am J Audiol. 2014;23(3):260–272. doi: 10.1044/2014_AJA-13-0014. [DOI] [PubMed] [Google Scholar]
- Vielsmeier V, Kreuzer PM, Haubner F, Steffens T, Semmler PRO, Kleinjung T, Schlee W, Langguth B, Schecklmann M. Speech comprehension difficulties in chronic tinnitus and its relation to hyperacusis. Front Aging Neurosci. 2016;8:1–8. doi: 10.3389/fnagi.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden TC, Walden BE. Predicting success with hearing aids in everyday living. J Am Acad Audiol. 2004;15(5):342–352. doi: 10.3766/jaaa.15.5.2. [DOI] [PubMed] [Google Scholar]
- Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(Pt B):154–166. doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Wilson RH. Development of a speech-in-multitalker-babble paradigm to assess word-recognition performance. J Am Acad Audiol. 2003;14(9):453–470. [PubMed] [Google Scholar]
- Wilson RH, McArdle RA, Smith SL. An evaluation of the BKB-SIN, HINT, QuickSIN, and WIN materials on listeners with normal hearing and listeners with hearing loss. J Speech Lang Hear Res. 2007;50(4):844–856. doi: 10.1044/1092-4388(2007/059). [DOI] [PubMed] [Google Scholar]
- Wong PC, Ettlinger M, Sheppard JP, Gunasekera GM, Dhar S. Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear. 2010;31(4):471–479. doi: 10.1097/AUD.0b013e3181d709c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yund EW, Woods DL. Content and procedural learning in repeated sentence tests of speech perception. Ear Hear. 2010;31(6):769–778. doi: 10.1097/AUD.0b013e3181e68e4a. [DOI] [PubMed] [Google Scholar]