Abstract
The amplitude modulations (AMs) in speech signals are useful cues for speech recognition. Several adaptation mechanisms may make the detection of AM in noisy backgrounds easier when the AM carrier is presented later rather than earlier in the noise. The aim of the present study was to characterize temporal adaptation to noise in AM detection. AM detection thresholds were measured for monaural (50 ms, 1.5 kHz) pure-tone carriers presented at the onset (‘early’ condition) and 300 ms after the onset (‘late’ condition) of ipsilateral, contralateral, and bilateral (diotic) broadband noise, as well as in quiet. Thresholds were 2–4 dB better in the late than in the early condition for the three noise lateralities. The temporal effect held for carriers at equal sensation levels, confirming that it was not due to overshoot on carrier audibility. The temporal effect was larger for broadband than for low-band contralateral noises. Many aspects in the results were consistent with the noise activating the medial olivocochlear reflex (MOCR) and enhancing AM depth in the peripheral auditory response. Other aspects, however, indicate that central masking and adaptation unrelated to the MOCR also affect both carrier-tone and AM detection and are involved in the temporal effects.
Keywords: olivocochlear reflex, overshoot, sound envelope, central masking, dynamic range adaptation
INTRODUCTION
The amplitude modulations (AMs) in speech signals are useful cues for speech recognition (e.g., Lorenzi et al. 2006; Shannon et al. 1995; Zeng et al. 2005). Several mechanisms may make the detection of AM in noisy backgrounds easier when the AM carrier is presented later rather than earlier in the noise. The aim of the present study was to characterize temporal effects on monaural AM detection in simultaneous ipsilateral, contralateral, and bilateral (diotic) noise.
One of the mechanisms that could facilitate AM detection in noise is the linearization of basilar membrane (BM) responses by activation of the medial olivocochlear (MOC) efferents. MOC efferents originate in the right and left medial superior olivary complexes and innervate outer hair cells (OHCs) at both cochleae (Guinan 2006). Ipsilateral and contralateral sounds can activate ipsilateral and contralateral MOC efferents reflexively. The activation of MOC efferents hyperpolarizes OHCs (Blanchet et al. 1996) and reduces the motion of the BM for low- and mid-level sounds but not for high-level sounds, thus linearizing BM input/output curves (dashed line in Fig. 1a) (Cooper and Guinan 2003, 2006; Murugasu and Russell 1996). In noisy backgrounds, the leading noise could linearize BM responses by activation of the ipsilateral and/or the contralateral MOC reflex (MOCR). Such linearization could enhance the effective AM depth as represented at the output of the BM (compare the blue and black AM representations in the ordinate of Fig. 1a) and thus facilitate AM detection.
Fig. 1.
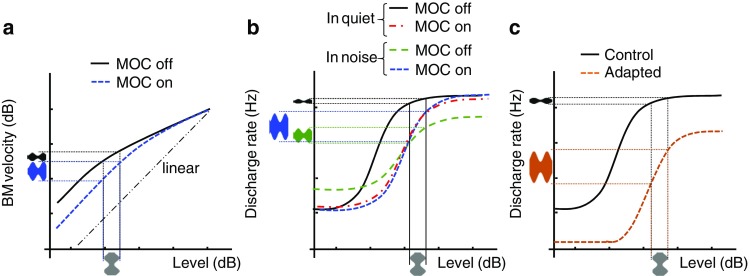
Schematic representation of three mechanisms that could facilitate AM-depth coding and detection. a Basilar membrane velocity versus sound pressure level with and without MOC efferent activation (adapted from Cooper and Guinan 2006). b Auditory nerve rate-level functions in quiet and in background noise with and without MOC efferent activation (adapted from Guinan 2006). c Dynamic range adaptation of auditory neurons to the most frequently occurring level (adapted from Wen et al. 2009). Each panel illustrates the internal representation (ordinate) of the acoustic AM depth (abscissa) associated with each mechanism. See main text for further details
The MOCR could also facilitate the detection of AM in noise by enhancing the representation of AM depth in the discharge rate of auditory nerve (AN) fibers. Winslow and Sachs (1988) measured AN fiber discharge rates to tone bursts in background noise with and without electrical stimulation of the olivocochlear bundle, as well as in quiet. They found that the background noise increased and decreased the discharge rate at low and high levels, respectively, thus reducing the dynamic range of AN fibers (compare the green dashed line with the continuous line in Fig. 1b). The electrical stimulation of the olivocochlear bundle shifted the rate-level curve to higher levels but restored the dynamic range of AN fibers to values observed in quiet (blue dashed line in Fig. 1b). The dynamic range restoration can improve the representation of AM depth in the discharge rate of AN fibers, at least for some carrier levels (compare the blue and green AM representations in the ordinate of Fig. 1b), and thus facilitate AM detection. Furthermore, Winslow and Sachs (1988) calculated the just-noticeable difference in intensity in the absence and presence of MOC efferent stimulation with a statistical model, and observed that the AN dynamic range restoration improved the ability of single fibers to code signal intensity changes in the presence of broadband noise (their Fig. 12). This supports the idea that the MOCR could facilitate the perception of changes in intensity present in the fluctuations of an AM probe.
A third mechanism that could facilitate AM detection in noise is the adaptation of the dynamic range of central auditory neurons. Wen et al. (2009) reported that for a continuous sound of varying level, the dynamic range of AN fibers shifted to the most frequently occurring level in the sound. When the most frequent sound level was within the region of saturated responses, the level-varying stimuli reduced the firing rate and shifted the rate-level functions of AN fibers to higher levels, as depicted in Fig. 1c (see also Figs. 2a and 4a in Wen et al. 2009). Similar results were reported by Dean et al. (2005, 2008) for neurons in the inferior colliculus, and by Watkins and Barbour (2008) for neurons in the auditory cortex. In noise backgrounds, the rate-level curves of auditory neurons could shift horizontally toward the noise level, and thus facilitate AM coding and detection when the carrier level is within the dynamic range of the adapted rate-level curve, as illustrated in Fig. 1c.
The present study was motivated by the hypothesis that the MOCR may facilitate the detection of AM in simultaneous noise. To our knowledge, only Almishaal et al. (2017) have investigated a related hypothesis. They measured AM detection thresholds for a narrow-band noise AM carrier centered at 5 kHz as a function of carrier level in the presence and in the absence of a 200 ms, 40 dB sound pressure level (SPL) ipsilateral notched noise precursor. Without the precursor, AM detection thresholds were worse at mid than at lower or higher levels. They argued that this was because BM responses are more compressive at mid-levels than at lower or higher levels. Compression would reduce the magnitude of the representation of envelope fluctuations in the mechanical BM response and thus hinder AM detection. Most importantly, however, they reported that AM detection thresholds improved, particularly at mid-to-high levels, when the noise preceded the probe. They reasoned that this improvement was consistent with the noise precursor activating the ipsilateral MOCR and linearizing BM responses (Fig. 1a). They admitted, however, that their findings were equally consistent with the dynamic range adaptation mechanisms described above (Fig. 1c) enhancing AM depth cues, and that those mechanisms could not be ruled out as an explanation for (or a contributor to) their findings.
To our knowledge, studies are lacking about temporal effects in AM detection for tonal carriers presented in simultaneous noise. Many studies, however, have investigated temporal effects on pure tone detection. Those studies have shown that the detectability of a short probe tone in noise improves when the tone onset is delayed in the noise compared to when it is presented at the noise onset, an effect known as ‘overshoot’ (Zwicker 1965). Overshoot was first explained in terms of short-term adaptation in the AN. The discharge rate of AN fibers would adapt in response to the long noise masker and much less so (or nothing at all) in response to the shorter probe tone (Smith and Zwislocki 1975). The progressive adaptation to the masker would cause a progressive increase in the neural signal-to-masker ratio that could facilitate the detection of the probe when it is delayed from the masker onset. However, short-term neural adaptation alone cannot explain the greater amount of overshoot at mid than at lower or higher levels (Bacon 1990; von Klitzing and Kohlrausch 1994) or the reduction in overshoot when contralateral precursors are used (Bacon and Liu 2000). Many studies have suggested that the MOCR could be the major contributor to overshoot (Jennings et al. 2011, 2016; McFadden et al. 2010; Schmidt and Zwicker 1991; Strickland 2001, 2004; von Klitzing and Kohlrausch 1994; Walsh et al. 2010). Other studies, however, have suggested that overshoot is unlikely related to the MOCR and is instead mediated by central auditory processes (Fletcher et al. 2013, 2015; Keefe et al. 2009; Verschooten et al. 2017).
Here, we use the overshoot paradigm to investigate potential temporal effects on AM detection in simultaneous noise and the possible involvement of the MOCR in those effects. For this reason, our parameters and experimental conditions were selected based on the characteristics of the MOCR. We measured AM detection thresholds for 50 ms probes presented at the onset (‘early’ condition) and 300 ms after the onset (‘late’ condition) of a 60 dB SPL simultaneous noise. Because the overall onset time constant of the MOCR is about 280 ms (Backus and Guinan 2006), we assumed that the MOCR would be active in the late but not in the early condition. If the MOCR facilitates the representation of a sound’s envelope in the mechanical BM response (by linearization of BM responses, Fig. 1a) and/or in the AN response (by dynamic-range restoration, Fig. 1b), then AM detection thresholds should be lower in the late than in the early condition.
Two experiments were conducted. Experiment 1 was aimed at comparing the magnitude of the temporal effect on AM detection thresholds for ipsilateral, contralateral and bilateral (diotic) noises. Lilaonitkul and Guinan (2009a) reported greater suppression of stimulus frequency otoacoustic emissions (OAEs) levels with bilateral than with ipsilateral or contralateral broadband noise (BBN) MOCR elicitors (their Fig. 3c). This indicates a greater linearization of BM responses for bilateral than for unilateral MOCR elicitors. Based on this, we hypothesized that the temporal effect on AM detection thresholds would be larger for bilateral than for ipsilateral or contralateral noises. Experiment 2 was aimed at investigating the effect of contralateral noise bandwidth on AM detection thresholds. Contralateral BBN elicitors suppress OAE levels more than narrow-band noise (NBN) or tone MOCR elicitors (Lilaonitkul and Guinan 2009a; Lisowska et al. 2002; Maison et al. 2000). Based on this, we hypothesized that a BBN would produce greater temporal effects on AM-detection thresholds than a low-band noise (LBN).
GENERAL METHODS
Participants
Experimental procedures were approved by the Human Experimentation Ethics Committee of the University of Salamanca (Spain). A different number of normal-hearing subjects participated in each experiment (details are given below). Participants were volunteers and were not paid for their services.
Stimuli
Amplitude modulation detection thresholds were measured for a monaural probe presented in the left ear. The mathematical expression describing the AM probe was:
| 1 |
where m is the modulation depth (0 ≤ m ≤ 1), fm is the modulation rate (40 Hz), ϕ is the starting phase of the modulation (−π/2), fc is the carrier frequency (1.5 kHz), and t denotes time in seconds. The probe duration was 50 ms, including 4-ms raised-cosine rise/fall ramps. To match the levels of the modulated and unmodulated probes, the amplitude of the AM probe was adjusted after applying the amplitude modulation.
The characteristics of the probe were chosen considering three factors:
The overall onset time constant of the MOCR is around 280 ms (Backus and Guinan 2006), and the MOCR has a 20–30 ms period of “hesitation” over which it does not change behavioral (McFadden et al. 2010) or physiological responses (Backus and Guinan 2006). Therefore, ideally the probe duration would need to be shorter than 30 ms to prevent the probe from activating the MOCR by itself. For low modulation rates, however, AM detection thresholds are higher for short than for long probes (Viemeister 1979), presumably because the greater the number of modulation cycles in the probe, the more opportunities there are to sample the envelope fluctuations. A 30-ms probe would have made the AM task very difficult. As a compromise, we used a probe duration of 50 ms. This probe would have activated the MOCR minimally but at the chosen modulation rate (40 Hz), it had only two modulation cycles and so the AM-detection task was still hard.
When BBN is used to activate the MOCR, MOCR suppression of OAEs is greater for a 1 kHz probe than for 0.5 or 4 kHz probes (Fig. 3 in Lilaonitkul and Guinan 2009a). Therefore, the carrier frequency was chosen to be equal to 1.5 kHz to stay close to the frequency region where MOCR effects on OAEs are known to be stronger (the suitability of this choice is discussed in the Discussion).
The spectrum of an AM sinusoid contains the carrier frequency (fc) and two sideband frequencies (fc ± fm). The equivalent rectangular bandwidth of the auditory filter at the carrier frequency (1.5 kHz) is about 186 Hz (Glasberg and Moore 1990). Based on this, the modulation rate (40 Hz) was chosen so that the carrier frequency and the two sidebands (1460 and 1540 Hz) would be detected with a single auditory filter. In other words, we assumed that listeners could not detect the sidebands as separate frequency components and that their responses during the task would be based on detecting the amplitude fluctuations in the carrier envelope only. Our assumption is supported by the fact that normal-hearing listeners have constant AM detection thresholds for 1 and 2 kHz carriers modulated at 10, 20, 40, and 50 Hz, but better thresholds for higher modulation rates, presumably because they can detect the sidebands at the higher modulation rates (Kohlrausch et al. 2000; Moore and Glasberg 2001).
Fig. 3.
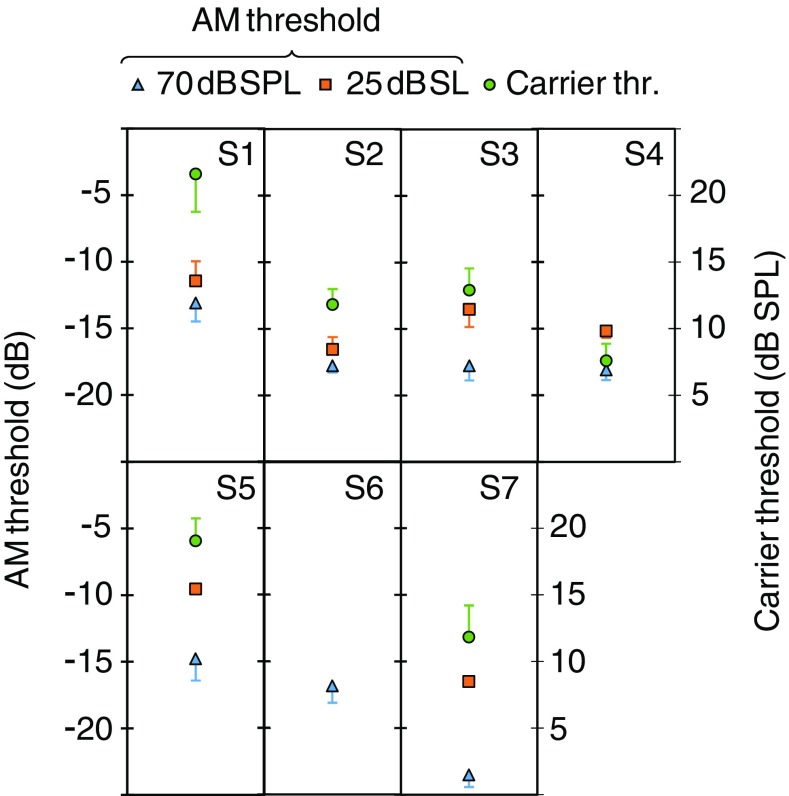
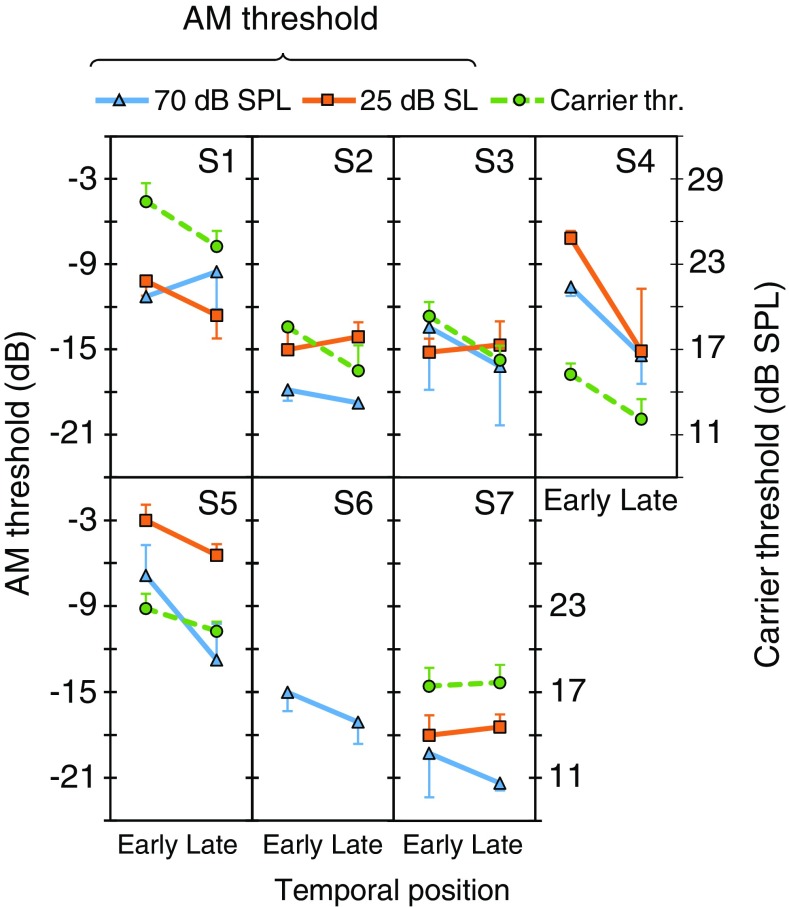
AM detection thresholds for 70 dB SPL (triangles) and 25 dB SL (squares) probes, and carrier detection thresholds (circles) in quiet for each subject. Each data point represents the mean of three estimates; error bars illustrate one standard deviation
AM detection thresholds were measured in quiet and in noise. The noise was either BBN (0.1–10 kHz) (Experiments 1 and 2) or LBN (0.1–1 kHz) (Experiment 2). The noise level was fixed at 60 dB SPL because it has been shown elsewhere that this type of noise is capable of activating the MOCR without activating the middle-ear muscle reflex (e.g., Aguilar et al. 2013). The noise had a duration of 400 ms in all conditions and was gated with onset and offset raised-cosine ramps of 4-ms of duration. As explained in the Introduction, AM detection thresholds were measured for AM probes at the noise onset and 300 ms after the noise onset.
Procedure
AM detection thresholds were measured using a three-alternative forced-choice (3AFC) adaptive procedure. Three intervals were presented to the participants accompanied by lights in a computer monitor. Unmodulated carriers were presented in two of the intervals, and an amplitude modulated carrier (the ‘target’) was presented in another interval. The interval containing the target was chosen at random in each trial and participants were instructed to identify the target interval by pressing a key on the computer keyboard. Feedback was given to the participants on the correctness of their responses. Except in the quiet condition, the noise was presented in the three intervals. The time period between the intervals was 500 ms, and it was defined as the silence period between the offset and the onset of the noise, or as the silent period between the offset and the onset of the AM probe in the quiet condition.1
The modulation depth of the AM probe (m) decreased after two successive correct responses and increased after an incorrect response (two-down, one-up adaptive rule). AM threshold was thus defined as the modulation depth giving 70.7 % correct responses in the psychometric function (Levitt 1971). The modulation depth varied using a logarithmic procedure as 20⋅log10(m). m was equal to 0 (−∞ dB) for unmodulated stimuli, and it was equal to 1 (0 dB) for 100 % modulation. The initial modulation depth was −6 dB, and changed by 4 dB until the second reversal in AM depth occurred and by 2 dB thereafter. The procedure continued until 12 reversals in modulation depth were obtained, and the AM detection threshold was calculated as the mean of the modulation depths at the last eight reversals. At least three AM thresholds were obtained for each condition and their mean was taken as the AM threshold. The standard deviations within and across measures for all participants were always less than 6 dB.
Carrier Detection Thresholds
In Experiment 1, AM detection thresholds were measured for probes at a fixed sound pressure level and at a fixed sensation level (SL) (see below) across conditions. To set the latter, absolute thresholds for the 1.5 kHz carrier were measured in the same conditions used to measure AM detection thresholds. That is, the carrier was presented monaurally in the left ear and its detection threshold was measured in quiet, in the presence of ipsilateral, contralateral, and bilateral (diotic) noise, and early and late in the noise (seven thresholds in total).
Absolute detection thresholds for the AM carrier tone were measured using a 3AFC, two-down, one-up adaptive procedure. Three intervals were presented accompanied by lights in a computer monitor, and the carrier tone was present in one of the three intervals chosen at random. In the conditions with noise, the noise was presented in the three intervals and the carrier tone was presented in only one of them. In the quiet condition, the carrier tone was presented in one of the intervals only. As in the AM experiment, the lights were presented gated with each interval, thus their duration was 400 ms in noise and 50 ms in quiet. The inter-stimulus time interval was 500 ms. Participants were instructed to identify the interval containing the tone by pressing a key on the computer keyboard, and feedback was given. The initial tone level was typically 60 dB SPL for ipsilateral and bilateral noises and 40 dB SPL for the contralateral noise or in quiet. The tone level decreased after two successive correct responses and increased after an incorrect response. The level change was 4 dB until the second reversal in level occurred, and 2 dB thereafter. The procedure continued until 12 level reversals occurred and the detection threshold was obtained as the mean of the carrier tone levels at the last eight reversals. The resulting threshold was discarded when the corresponding standard deviation exceeded 4 dB. Three valid threshold estimates were obtained for each condition and their mean was taken as the carrier detection threshold. The standard deviation of the three valid threshold estimates was less than 4 dB for all participants.
Apparatus
Stimuli were generated with custom-made Matlab software and played via an RME Fireface 400 soundcard at a sampling rate of 44.1 kHz, and with 24-bit resolution. In Experiment 1, stimuli were presented to the listeners using circumaural Sennheiser HD580 headphones; in Experiment 2 stimuli were presented using Sennheiser HD580 headphones for five listeners and Sennheiser HD265 headphones for the other seven listeners. Listeners sat in a double-wall sound attenuating booth during all measurements. Sound pressure levels were calibrated by placing the headphones on a KEMAR equipped with a Zwislocki DB-100 artificial ear connected to a sound level meter. Calibration was performed at 1 kHz only and the obtained sensitivity was used at all other frequencies.
Statistical Analyses
Repeated-measures analyses of the variance (RMANOVA) or paired Student’s t test were used as appropriate to test for the statistical significance of temporal position, carrier level, noise laterality, and/or noise bandwidth on across-subject mean carrier detection or AM detection thresholds (see below). As explained in the Introduction, we hypothesized that carrier detection thresholds or AM detection thresholds would be better (lower) in the late than in the early conditions. Accordingly, we applied one-tailed tests when testing for differences in temporal position (see below). We applied two-tailed tests for all other comparisons. For tests involving multiple groups or variables, post hoc pairwise comparisons were conducted using Bonferroni corrections for multiple comparisons. An effect was regarded as statistically significant when the null hypotheses could be rejected with 95 % confidence (p < 0.05).
EXPERIMENT 1: EFFECT OF NOISE LATERALITY ON AM DETECTION
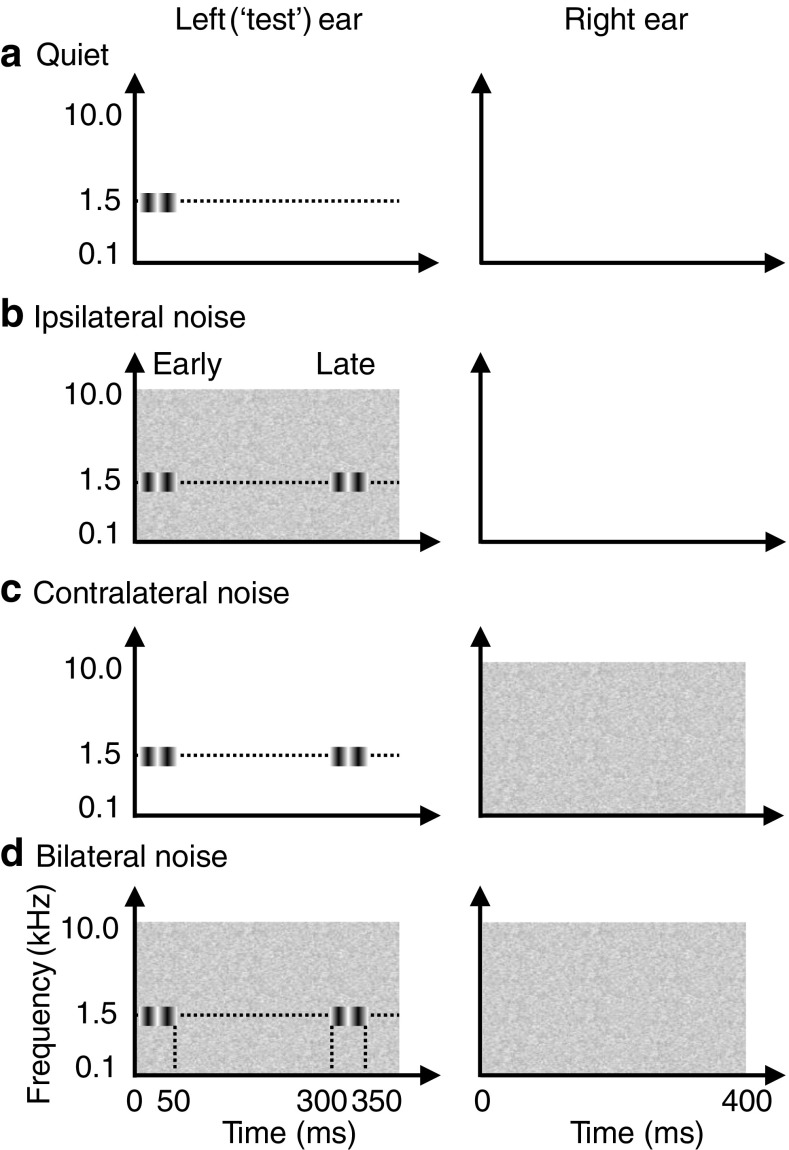
The aim was to investigate the effects of noise laterality and probe level on monaural AM detection. AM detection thresholds were measured in quiet (Fig. 2a), with BBN presented to the same ear as the probe (ipsilateral noise; Fig. 2b), with BBN presented to the opposite ear from the probe (contralateral noise; Fig. 2c), and with bilateral (diotic) BBN (bilateral noise; Fig. 2d). For each of the three noise lateralities, AM detection thresholds were measured in the early and late conditions. This amounted to seven AM detection thresholds per listener: one in quiet plus six in noise (3 noise lateralities × 2 temporal positions). The seven conditions were administered in random order for each participant.
Fig. 2.
Schematic representation of the different conditions in which AM detection thresholds were measured. The AM probe always was presented to the left ear (the ‘test’ ear), and AM thresholds were measured in quiet (a) and with ipsilateral (b), contralateral (c) and bilateral (d) noises
AM detection thresholds were first measured using AM carriers at 70 dB SPL in all conditions. We, however, expected overshoot to make the fixed-SPL carriers more audible in the late than in the early condition. The differences in audibility might have affected AM detection to some uncertain extent. For this reason, as a control, we measured carrier detection thresholds in the seven conditions and AM detection thresholds for carrier levels at 25 dB SL; that is, 25 dB above the individual detection threshold for the 1.5 kHz carrier tone in each condition. The corresponding SPL values are given in Table 1.
Table 1.
Individual and mean sound pressure levels (in dB) corresponding to 25 dB SL. Values are missing for subject S6 because AM detection thresholds were not measured for the 25 dB SL carrier for that subject
| Noise laterality | |||||||
|---|---|---|---|---|---|---|---|
| Bilateral | Ipsilateral | Contralateral | |||||
| Subject ID | Quiet | Early | Late | Early | Late | Early | Late |
| S1 | 46.6 | 72.2 | 69.4 | 72.7 | 70.5 | 52.4 | 49.3 |
| S2 | 36.8 | 69.8 | 65.8 | 69.6 | 68.8 | 43.6 | 40.5 |
| S3 | 37.9 | 72.0 | 68.6 | 74.8 | 70.4 | 44.3 | 41.3 |
| S4 | 32.6 | 66.2 | 64.8 | 71.1 | 71.9 | 40.3 | 37.1 |
| S5 | 44.1 | 70.0 | 69.3 | 72.9 | 71.3 | 47.8 | 46.3 |
| S7 | 36.8 | 66.8 | 65.4 | 70.5 | 69.5 | 42.4 | 42.7 |
| Mean | 39.1 | 69.5 | 67.2 | 71.9 | 70.4 | 45.1 | 42.8 |
| SD | 5.2 | 2.5 | 2.1 | 1.9 | 1.2 | 4.3 | 4.3 |
Seven volunteers (five women) with normal clinical audiograms (thresholds ≤ 20 dB HL; ANSI 1996) and no self-reported history of hearing impairment participated in this experiment. Their mean age was 35.1 years (SD = 12.5 years). Except S4, all other participants were experienced in AM detection tasks. For participant S4, four AM detection thresholds were measured in each condition for the 70 dB SPL carrier level, and the first threshold estimate in each condition was regarded as training and discarded from further analysis. Subject S6 was available for a limited amount of time, enough to obtain her AM detection thresholds with the 70 dB SPL carriers but not the other thresholds.
Carrier Detection Thresholds in Quiet
Individual thresholds for the 1.5 kHz carrier tone in quiet are depicted by circles in Fig. 3 (units in the right ordinate). Individual carrier thresholds measured in the presence of bilateral, ipsilateral and contralateral noises are illustrated by circles in Figs. 4, 5 and 6, respectively, with units in the right ordinates. Group mean thresholds are shown in Fig. 7. Individual and mean thresholds were generally higher in noise than in quiet regardless of noise laterality. For the early condition, the mean increase was 30.3, 32.8 and 6.0 dB for bilateral, ipsilateral and contralateral noises, respectively; for the late condition, the mean increase was 28.1, 31.3 and 3.7 dB, respectively. All increases were statistically significant and were comparatively larger for ipsilateral or bilateral noises than for the contralateral noise.
Fig. 4.
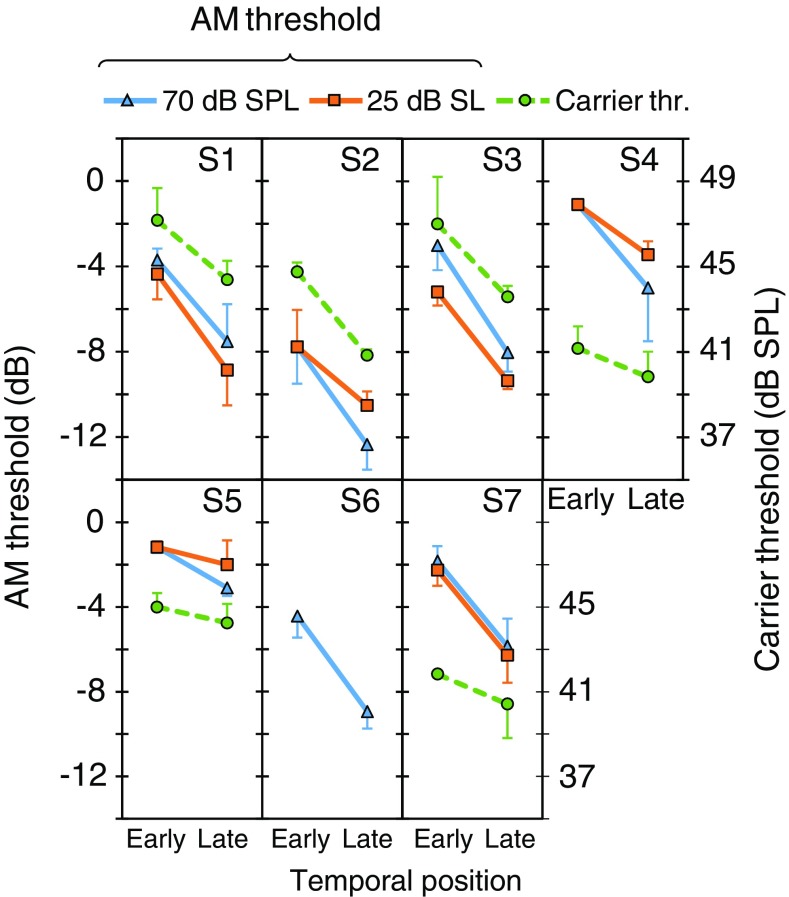
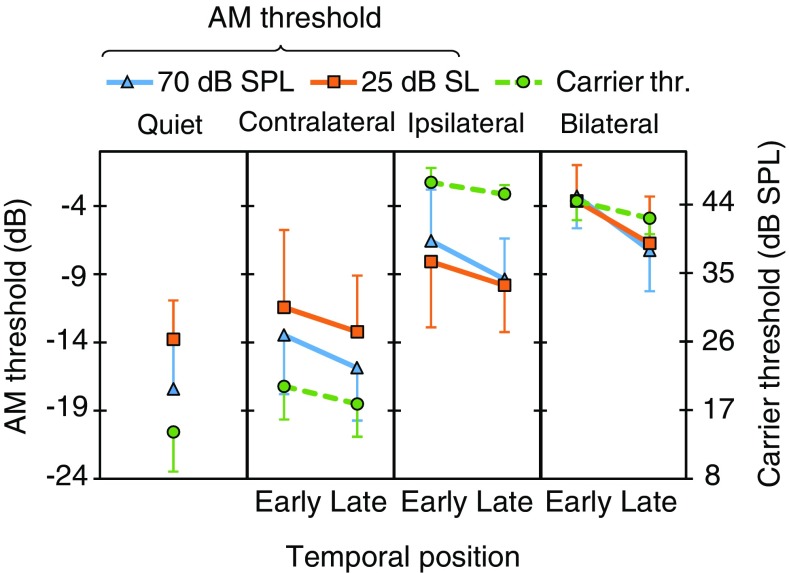
The effect of probe temporal position in bilateral BBN on carrier detection thresholds (circles, dashed traces) and AM detection thresholds (continuous traces). Each panel shows data for an individual listener. Two AM detection thresholds are shown per listener: one for 70 dB SPL (triangles) and one for 25 dB SL (squares) carriers, as indicated by the inset at the top of the figure. Each data point is the mean of three measures; error bars illustrate one standard deviation. Note that the left ordinate is for AM detection thresholds while the right ordinate is for carrier detection thresholds. See main text for details
Fig. 5.
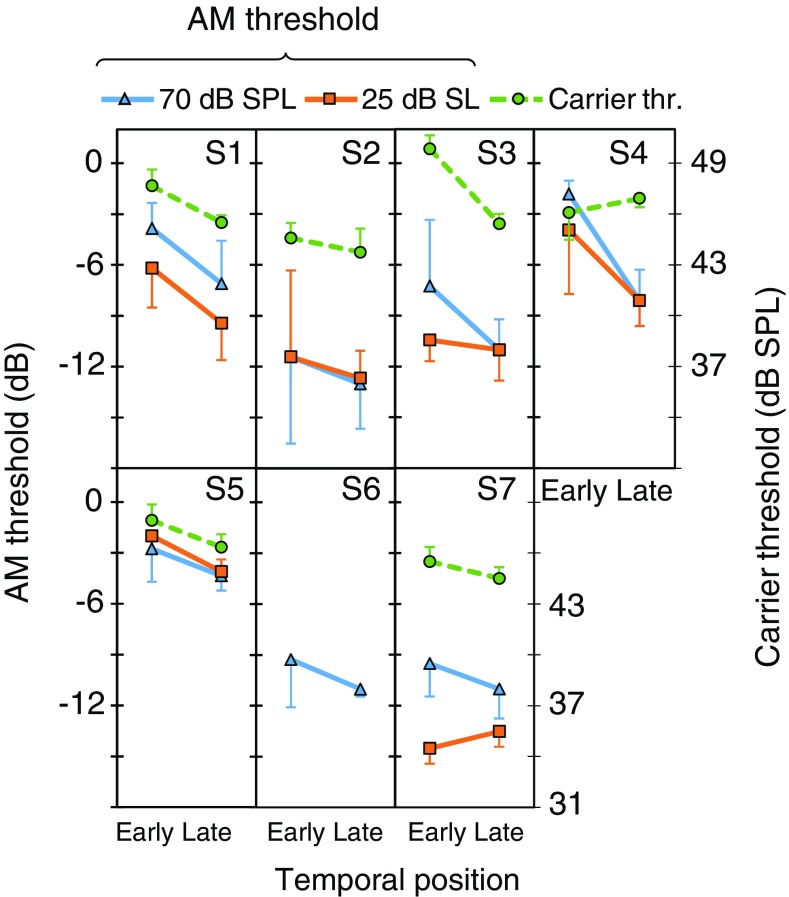
As Fig. 4 but for ipsilateral BBN
Fig. 6.
As Fig. 4 but for contralateral BBN
Fig. 7.
Across-subject mean carrier detection thresholds and AM detection thresholds. From left to right, each panel shows the mean data in quiet, and in the presence of contralateral, ipsilateral, and bilateral broadband noises, as indicated at the top of the panels. Otherwise, the layout is as for Figs. 3–6
Temporal Effect on Carrier Detection Thresholds
Figures 4 to 6 show the effect of temporal position on carrier detection threshold for each participant and noise laterality. For 16 of the 18 measures (6 subjects × 3 noise lateralities), thresholds were lower in the late than in the early conditions; the exceptions were S4 for an ipsilateral noise (Fig. 5) and S7 for a contralateral noise (Fig. 6). This shows that overshoot occurred for virtually all of the present participants and noise lateralities.
Figure 7 illustrates across-subject mean carrier detection thresholds in the early and late conditions. A two-way RMANOVA was conducted to test for the effect of temporal position, noise laterality or their interaction on carrier detection thresholds. Thresholds were significantly lower in the late than in the early condition [F(1,5) = 19.8, p = 0.004]. Figure 8a shows the mean temporal effect on carrier threshold for the different noise lateralities; that is, the across-subject mean of early minus late threshold differences. The magnitude of the temporal effect was 2.3 dB (SD = 1.3) for bilateral, 1.5 dB (SD = 1.7) for ipsilateral, and 2.3 dB (SD = 1.4) for contralateral noises. Post hoc analyses showed that the temporal effect was statistically significant for bilateral (p = 0.004), ipsilateral (p = 0.042) and contralateral (p = 0.005) noises. The RMANOVA also revealed a significant effect of noise laterality [F(2,10) = 279.7, p < 0.001]. Carrier detection thresholds were higher for ipsilateral than for bilateral noises (p = 0.043), consistent with the existence of a binaural masking level difference (BMLD).2 The interaction between temporal effect and noise laterality was not significant [F(2,10) = 0.81, p = 0.470], indicating that there were no statistically significant differences in the magnitude of the temporal effect across the three noise lateralities.
Fig. 8.
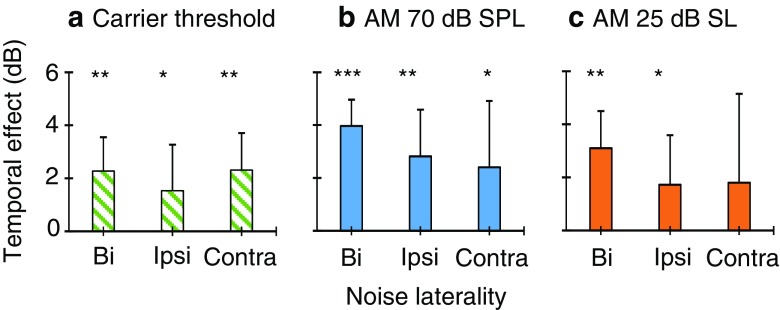
Magnitude of the temporal effect on carrier detection thresholds (a) and AM detection thresholds for 70 dB SPL (b) and 25 dB SL carriers (c). The temporal effect represents the threshold in the early condition minus the threshold in the late condition. Panel b shows the mean of seven subjects, while panels a and c show the mean of six subjects. Error bars illustrate one standard deviation of the mean. Asterisks indicate statistical significant differences at p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***)
In summary, carrier detection thresholds were higher in the presence than in the absence of ipsilateral, contralateral, or bilateral noises. Thresholds were higher in the early than in the late condition, which demonstrated overshoot for the present participants. The magnitude of overshoot (or temporal effect) was not statistically different across noise lateralities.
AM Detection Thresholds in Quiet
Individual and mean AM detection thresholds in quiet are shown in Figs. 3 and 7, respectively. Thresholds for the 70 dB SPL and 25 dB SL carriers are illustrated by triangles and square symbols, respectively, with units in the left ordinates. Mean AM detection thresholds were lower for the 70 dB SPL (mean = − 17.5 dB, SD = 3.6 dB) than for 25 dB SL carriers (mean = − 13.8 dB, SD = 2.9 dB) and the difference was statistically significant (N = 6, paired 2-tailed Student’s t test, p = 0.009). These values and the effect of level were consistent with those reported elsewhere for similarly short carriers and low modulation rates (e.g., Viemeister 1979).
Figure 7 shows that the AM detection thresholds were best (lowest) in quiet and got gradually worse (increased) in the presence of contralateral, ipsilateral or bilateral noise in the early condition. This pattern occurred both for 70 dB SPL and 25 dB SL carriers. A RMANOVA revealed a significant effect of noise laterality (none, contralateral, ipsilateral, or bilateral) on AM detection thresholds in the early condition for both the 70 dB SPL [F(3,18) = 51.6, p < 0.001] and 25 dB SL [F(3,15) = 17.9, p < 0.001] carriers. Post hoc pairwise comparisons revealed that AM detection thresholds in quiet were not statistically different from thresholds measured with contralateral noise (p = 0.079 at 70 dB SPL; or p = 1.0 at 25 dB SL), but they were lower than those measured with ipsilateral (p = 0.001 at 70 dB SPL; or p = 0.051 at 25 dB SL) or bilateral (p = 0.001 at 70 dB SPL, p = 0.003 at 25 dB SL) noises.
Temporal Effect on AM Detection Thresholds
Participants S4 and S5 could not detect the AM probe with bilateral BBN noise in the early condition, even when the modulation depth was 100 % (m = 1, 0 dB). In those cases, the AM threshold was arbitrarily set to − 1 dB (i.e., half the final step size in modulation depth), which was deemed more conservative than setting it to 0 dB for revealing any temporal effect on AM detection threshold.
Equal Sound-Pressure-Level Carriers
Individual AM detection thresholds for 70 dB SPL carriers in the early and late conditions and for bilateral, ipsilateral and contralateral noises are represented by triangles in Figs. 4 to 6, with units in the left ordinate. Thresholds were lower (better) in the late than in the early conditions for all participants and noise lateralities, except for S1 with a contralateral noise for whom thresholds were 1.8 dB better in the early than in the late condition. This indicates that presenting the probe 300 ms after the noise onset facilitated AM detection. Figure 8b shows that the average (mean ± SD) AM-detection threshold improvement was 4.0 ± 1.0, 2.8 ± 1.8 and 2.4 ± 2.5 dB for bilateral, ipsilateral, and contralateral noises, respectively.
Figure 7 shows mean AM detection thresholds for the two temporal positions and for the three noise lateralities. For either temporal position, AM detection thresholds worsen gradually when going from the left-most to the right-most panel in the figure. That is, AM detection thresholds were smallest (best) with contralateral noise, followed by thresholds with ipsilateral noise, and were highest (worst) with bilateral noise.
A two-way RMANOVA was conducted to test for the effects of temporal position (early versus late), noise laterality (bilateral, ipsilateral, or contralateral) or their interaction on AM detection thresholds. AM detection thresholds were worse in the early than in the late condition [F(1,6) = 57.3, p < 0.001]. Post hoc analyses showed significantly worse AM detection thresholds in the early than in the late condition for bilateral (p < 0.001), ipsilateral (p = 0.003) and contralateral (p = 0.023) noises. The RMANOVA also showed a significant effect of noise laterality on AM detection thresholds [F(2,12) = 49.2, p < 0.001]: AM thresholds for the bilateral noise were worse than for the ipsilateral (p = 0.047) or the contralateral (p = 0.001) noises; thresholds were also worse for the ipsilateral than for the contralateral noise (p < 0.001). The interaction between temporal effect and noise laterality was not significant [F(2,12) = 1.3, p = 0.305]; that is, the magnitude of the temporal effect was not statistically different across the three noise lateralities.
Equal Sensation-Level Carriers
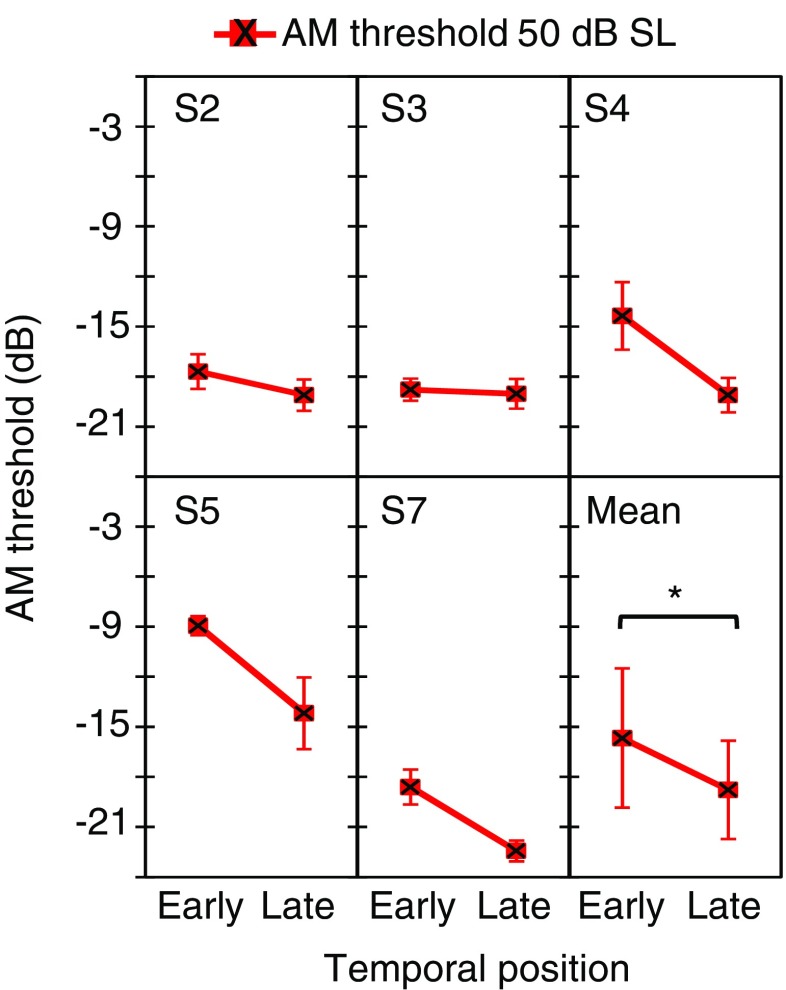
Individual AM detection thresholds for the 25 dB SL probes are represented by square symbols in Figs. 4 to 6. For bilateral noises, the pattern of results was consistent with that for the 70 dB SPL probes. For ipsilateral noises, the pattern of results was consistent for the two carrier levels except for S3 and S7, who went from having a clear temporal effect at 70 dB SPL to not having one at 25 dB SL. This is curious for S7, because the carrier SPLs were barely different between the two conditions (Table 1). For contralateral noises, only three of the six participants (S1, S4, and S5) showed better thresholds in the late than in the early conditions; the other three participants (S2, S3 and S7) had very similar AM detection thresholds in the two temporal positions. This may be due to the low sensation level of the probe, and will be discussed later. Figure 8c shows that the average (mean ± SD) temporal effect on AM detection thresholds was 3.1 ± 1.4, 1.7 ± 1.9, and 1.8 ± 3.4 dB for bilateral, ipsilateral and contralateral noises, respectively.
As for the 70 dB SPL probes, AM detection thresholds worsened gradually when going from the left-most to the right-most panels in Fig. 7, indicating that AM detection was easiest with the contralateral noise, followed by the ipsilateral noise, and became most difficult with the bilateral noise.
A two-way RMANOVA indicated worse AM thresholds in the early than in the late condition [F(1,5) = 11.9, p = 0.009]. The temporal effect was significant for the bilateral (p = 0.002) and ipsilateral (p = 0.036) noises, but not for the contralateral noise (p = 0.123). The RMANOVA also revealed a significant effect of noise laterality on AM detection thresholds [F(2,10) = 17.7, p = 0.001]. AM detection thresholds were worse for bilateral than for contralateral noises (p = 0.017). They were also worse for ipsilateral than for contralateral noises (p = 0.006). However, thresholds for bilateral and ipsilateral noises were not statistically different (p = 0.095). The interaction between the temporal effect and noise laterality was not significant [F(2,10) = 0.77, p = 0.486].
Figure 8b, c allows a direct comparison of the magnitude of the temporal effect for equal sound pressure level (70 dB SPL) or equal sensation level (25 dB SL) carriers. A two-way RMANOVA indicated that the magnitude of the temporal effect was not statistically different across the three noise lateralities [F(2,10) = 1.0, p = 0.413] or across carrier levels [F(1,5) = 2.39, p = 0.183]. The interaction between noise laterality and carrier level was not significant [F(2,10) = 0.15, p = 0.864]. Altogether, these results indicate that the temporal effect on AM detection threshold was unlikely due to improved audibility of the AM carrier in the late compared to the early condition.
In summary, AM detection thresholds were best (lowest) in quiet and worst (highest) in the presence of a bilateral noise. Bilateral and ipsilateral noises produced a significant temporal effect on AM detection thresholds for carriers with both equal SPLs and equal SLs. Contralateral noises, by contrast, produced a significant temporal effect on AM detection thresholds for carriers at fixed SPL only. Although the mean temporal effect was slightly greater for bilateral than for ipsilateral or contralateral noises, the effect of noise laterality was not statistically significant. The size of the temporal effect was not statistically different for 70 dB SPL and 25 dB SL probes.
EXPERIMENT 2: EFFECT OF CONTRALATERAL NOISE BANDWIDTH ON AM DETECTION
This experiment aimed at testing the effect of noise bandwidth on AM-detection thresholds. AM detection thresholds were measured in quiet, and in the early and late conditions for BBN and LBN. Only contralateral noises were used and the carrier level was fixed at 70 dB SPL. Because contralateral BBN elicitors suppress OAE levels more than narrow-band noise MOCR elicitors (Lilaonitkul and Guinan 2009a; Lisowska et al. 2002; Maison et al. 2000), we expected the BBN to produce greater temporal effects on AM-detection thresholds than the LBN. We note that the bandwidth of the LBN was lower in frequency than the frequency of the AM carrier tone. This choice was made because contralateral narrow-band noise is more effective in activating the MOCR when its spectrum is centered half to one octave below the probe frequency than when it is centered at the probe frequency (see Lilaonitkul and Guinan 2009b).
Twelve volunteers (eight women) participated in this experiment (mean age = 32.7 years, SD = 12.0 years). Five of them also participated in Experiment 1 and had audiometric thresholds ≤ 20 dB HL; the other seven participants had self-reported normal hearing and no history of hearing disorders.
Results
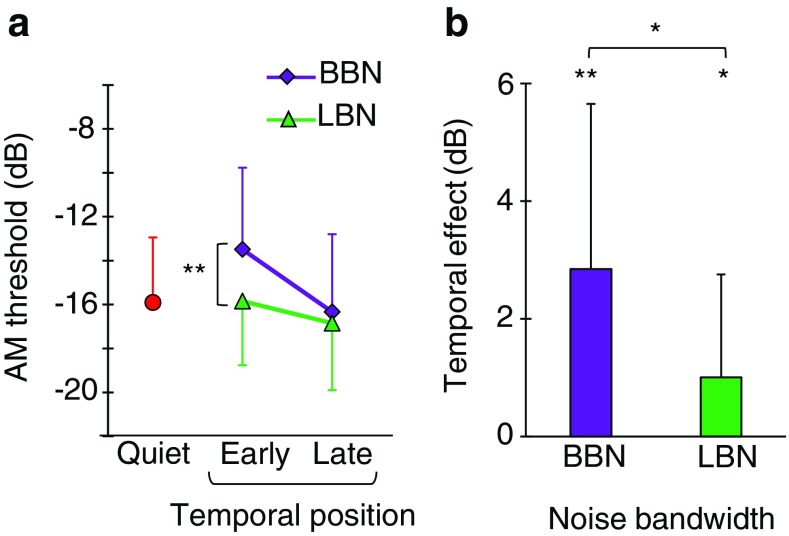
Figure 9a shows mean AM detection thresholds in quiet (circles) and in the presence of contralateral BBN (diamonds) and LBN (triangles). In noise, thresholds are shown for probes presented early and late in the noise. Pairwise comparisons showed that the mean threshold in quiet was not statistically different from the mean threshold in any of the four noise conditions.
Fig. 9.
a Mean AM detection thresholds in quiet (circles), and for contralateral BBN (diamonds) and LBN (triangles). The probe level was 70 dB SPL. b Mean temporal effect magnitude as a function of noise bandwidth. Data are the mean for 12 participants. Error bars illustrate one standard deviation
With contralateral BBN and LBN, eight of the twelve subjects showed better AM detection thresholds in the late than in the early condition (although the subjects in question were different for the two noise bandwidths). As a result, mean AM detection thresholds were lower in the late than in the early condition (Fig. 9a). The temporal effect, however, was more pronounced for BBN than for LBN (Fig. 9b).
A two-way RMANOVA was conducted to test for the effect of probe temporal position and noise bandwidth on AM detection threshold. The test indicated that AM detection thresholds were better (lower) in the late than in the early condition [F(1,11) = 10.4, p = 0.004]. Post hoc analyses showed that the temporal effect was significant for the two noise bandwidths (BBN, p = 0.003; and LBN, p = 0.037). The RMANOVA also showed a significant effect of noise bandwidth; that is, AM detection thresholds were better (lower) for LBN than for BBN [F(1,11) = 12.4, p = 0.005]. The interaction between probe temporal position and noise bandwidth was significant [F(1,11) = 8.33, p = 0.015]. AM detection thresholds in the early condition were better for LBN than for BBN (p = 0.001), and were not statistically different in the late condition (p = 0.300). As a result, the temporal effect was significantly greater for BBN than for LBN (Fig. 9b).
DISCUSSION
We have found a statistically significant temporal effect on AM detection for 70 dB SPL tonal carriers and for bilateral, ipsilateral and contralateral BBN. The magnitude of the effect was slightly larger for bilateral noises than for ipsilateral or contralateral noises, although the differences across noise lateralities was not statistically significant (Fig. 8b). The effect also occurred for carriers at 25 dB SL for ipsilateral and bilateral BBN (Fig. 8c), and its magnitude was not statistically different from that for 70 dB SPL carriers. We have also found a significant temporal effect on AM detection for contralateral LBN, though smaller in magnitude than the effect for contralateral BBN (Fig. 9b).
Comparison with Earlier Studies
To our knowledge, no other study has measured temporal effects on AM detection using the stimulus design and conditions employed here. Nonetheless, insofar as is possible, a comparison between the present data and data reported in earlier related studies may be useful. Sheft and Yost (1990) measured the sensitivity for detecting AM segments in a broadband noise carrier presented monaurally. They reported that AM detection was easier (i.e., AM depth thresholds were smaller) when the modulated segment was preceded by 500-ms unmodulated carrier than when it was not. A careful analysis of their results reveals, however, that for an AM segment duration of 400 ms, this temporal effect occurred for modulation rates from 2 to 10 Hz but not for 40-Hz modulation rate (their Fig. 8), the modulation rate employed here. It also reveals that for 50-ms long AM segments, the temporal effect did not occur for 40-Hz modulation rate (compare the data in their Figs. 4 and 6). In other words, no temporal effect was present in the data of Sheft and Yost (1990) for the stimuli and conditions most similar to those employed here.
Almishaal et al. (2017) measured AM detection thresholds for a narrow-band noise carrier centered at 5 kHz in the presence and in the absence of an ipsilateral noise precursor with a spectral notch at the AM carrier frequency. They reported AM detection thresholds for 50-ms carriers to be 3–5 dB better with than without the precursor over the 65–85 dB SPL carrier level range (their Fig. 3). Here, monaural AM detection thresholds were measured for probes at two temporal positions in simultaneous ipsilateral, contralateral and bilateral noise. The present stimulus design is thus different from that of Almishaal et al. (2017). Nonetheless, the present AM detection thresholds for 70 dB SPL carriers and ipsilateral BBN (the stimuli and condition most similar to any of those explored by Almishaal et al. 2017) were 2.8 dB better in the late than in the early condition. Therefore, insofar as a comparison between the two studies is possible, the results from the two studies are broadly consistent with each other.
On the Suitability of Using a 1.5 kHz Carrier Frequency
We hypothesized that the MOCR may contribute a temporal effect on AM detection, hence the present stimuli were chosen to maximize MOCR effects. Specifically, we chose the AM carrier frequency to be 1.5 kHz based on the fact that, for BBN MOCR elicitors, the suppression of OAEs by the MOCR is greater for probes at 1 kHz than at 0.5 kHz or 4 kHz (Lilaonitkul and Guinan 2009a). However, technical limitations prevented Lilaonitkul and Guinan from measuring MOCR suppression of OAEs at frequencies higher than 4 kHz and so it is uncertain if MOCR effects could be larger at higher frequencies. Based on psychoacoustical observations, some authors have argued that the MOCR is possibly stronger at higher frequencies. For example, the magnitude of overshoot on pure-tone detection is greater at frecuencies ≥ 4 kHz than at lower frequencies (Bacon and Takahashi 1992; Carlyon and White 1992) and contralateral BBN raises pure-tone detection thresholds more at 4 kHz than at 0.5 kHz (Aguilar et al. 2015). Other authors, however, have reported larger thresholds increases in the presence of contralateral BBN at 2 kHz than at lower or higher frequencies (Kawase et al. 2003). Therefore, though uncertain, it is possible that the temporal effects on AM detection may be greater for higher frequency carriers.
Possible Mechanisms Involved in the Temporal Effects on Carrier Detection
Several studies have shown that pure tone detection thresholds are higher in the presence of contralateral noise than in quiet when the tones are sufficiently delayed from the noise onset, an effect typically attributed to the noise activating the MOCR and thus reducing the BM gain for levels near threshold (e.g., Aguilar et al. 2015; Kawase et al. 2003). Smith et al. (2000) supported that interpretation. They observed that, in macaque, tonal thresholds increased with contralateral noise and that the increase became smaller after sectioning of the MOC efferents. Consistent with the cited studies, the present carrier thresholds were significantly higher with contralateral noise in the late condition than in quiet (circles in Fig. 7).
However, the MOCR alone cannot explain the found increase in carrier thresholds with contralateral noise. Carriers were short (50 ms) and thus insufficiently long to activate the MOCR by themselves. In addition, they were presented to the contralateral ear and thus could not have been masked by interaction with the noise in the auditory periphery. If a reduction in BM gain by the MOCR were the only mechanism responsible for the threshold increase, one would expect carrier thresholds to be similar in quiet and in the early condition, and to worsen (increase) in the late condition. Instead, thresholds were higher in the early condition than in quiet (Fig. 7). Smith et al. (2000) argued that, although the elevation in tone thresholds by contralateral noise is mediated to some extent by the MOCR, the elevations for noise-target onset delays shorter than 100 ms reflect a “purely central process” because that delay is too short for the MOCR to be active. In other words, Smith et al. (2000) supported ‘central masking’ for short noise-target onset delays (Zwislocki et al. 1967). Our elevated carrier thresholds with contralateral noise in the early condition also demonstrate ‘central masking’.
Furthermore, we found thresholds with contralateral noise to be higher in the early than in the late condition; that is, we found overshoot with contralateral noise (Zwislocki et al. 1967). Overshoot for ipsilateral noise has been traditionally explained by a reduction in BM gain by the MOCR (e.g., Jennings et al. 2016; Strickland 2001, 2004; von Klitzing and Kohlrausch 1994). However, because masking in the early condition is likely central in origin, it would be hard to explain how the MOCR could improve thresholds over time. Instead, it is conceivable that adaptation occurs in binaural, central auditory neurons. In other words, the improvement in carrier threshold from the early to the late conditions for contralateral noise suggests that adaptation unrelated to the MOCR occurs in binaural, central auditory neurons. This is in line with the conclusions from psychoacoustical (Fletcher et al. 2013, 2015) and physiological studies (Keefe et al. 2009; Verschooten et al. 2017) that suggest that overshoot is a product of central auditory processing. The nature of this processing is uncertain. Alternatively, the threshold increase in the early condition (relative to quiet) might reflect transient masking and the temporal effect a release from transient masking (e.g., Bacon and Moore, 1987).
Possible Mechanisms Involved in the Temporal Effects on AM Detection
The existence of a temporal effect on AM detection for carriers with equal SLs minimizes the possibility that the effect was due to the carrier being more audible when presented later rather than earlier in the BBN. Instead, it seems reasonable to assume that the effect reflects an enhancement of AM depth in the late versus the early condition. The question is: what are the mechanisms responsible for this enhancement?
Several aspects in the present data suggest that the MOCR may be responsible for the enhancement. First, all participants had normal hearing and the noise level employed here is sufficient to activate the MOCR without activating the middle-ear muscle reflex (e.g., Aguilar et al. 2013; Lilaonitkul and Guinan 2009a). Therefore, the MOCR must have been active during the experiment to some extent.
Second, for ipsilateral or bilateral noises, the temporal effect was consistent with the leading noise activating the MOCR. This activation would linearize BM input/output curves and/or restore of the dynamic range of AN fibers. Either or both these mechanisms could enhance the representation of the AM envelope in the late condition (as shown in Fig. 1a, b) and thus facilitate AM detection. For contralateral noise, the temporal effect was consistent with the MOCR linearizing BM responses. This linearization would be consistent with us finding a significant temporal effect for the 70 dB SPL carrier but not for the 25 dB SL carrier (Fig. 8). That is, a temporal effect did not occur for the 25 dB-SL carrier possibly because the carrier SPL (44 dB SPL, Table 1) fell within the low-level linear region of human BM responses (Lopez-Poveda et al. 2003; Johannesen and Lopez-Poveda 2008), but did occur for the 70 dB SPL carrier because its level fell within the compressive region of human BM responses at 1.5 kHz (Fig. 10 in Johannesen and Lopez-Poveda 2008). Admittedly, the BM linearization explanation would predict AM thresholds in the early condition to be better for the 25 dB SL than for 70 dB SPL, which was not the case perhaps because of the low sensation level of the 25 dB SL carriers. In an attempt to corroborate the linearization interpretation, we measured the temporal effect for contralateral noise using a higher carrier level at 50 dB SL, whose corresponding SPL (mean = 68 dB SPL) presumably fell within the compressive region of BM responses. In this case, the mean temporal effect was 3.1 dB and was statistically significant (Fig. 10), thus supporting the idea that for contralateral noises, the temporal effect occurs by linearization of BM responses mostly when the carrier level falls within the compressive region of BM responses.
Fig. 10.
The effect of probe temporal position on AM detection thresholds in contralateral BBN and for 50 dB SL carriers. Each panel shows data for an individual listener and for the mean. Each data point is the mean of three measures and error bars illustrate one standard deviation. The asterisk indicates a statistical significant difference at p < 0.05
A third aspect in support of the MOCR interpretation is that previous studies have shown that contralateral NBN causes smaller reductions in OAE levels than BBN, regardless of whether the NBN spectrum is centered at or below the OAE test frequency (Maison et al. 2000; Lilaonitkul and Guinan 2009a). The spectrum of the contralateral LBN used in the present Experiment 2 was below the carrier frequency while the BBN overlapped with it, and the magnitude of the temporal effect on AM detection was smaller for the LBN than for the BBN (Fig. 9b), in qualitative agreement with the OAE data.
Lastly, we found a temporal effect on AM detection for both contralateral BBN and LBN (Fig. 9b) even though AM thresholds in the two noises and in the two temporal positions were not statistically different from the thresholds in quiet (Fig. 9a). In other words, for contralateral noise, the temporal effect occurred even in the absence of AM masking. The two AN rate-level functions for the quiet condition in Fig. 1b provide a possible explanation for this finding. In this example, the contralateral noise would not mask the AM probe because the noise is contralateral to the test ear but would activate the contralateral MOCR. The activation of the MOCR would inhibit BM responses and thus shift the AN rate-level function rightward. The horizontal shift would enhance the representation of AM depth in the AN discharge rate, and thus AM sensitivity.
By contrast, other aspects in the present data dispute the idea that the MOCR is responsible for the temporal effects in AM detection. First, previous studies have shown that ipsilateral and contralateral BBNs cause comparable reductions in OAE levels (Guinan et al. 2003; Lilaonitkul and Guinan 2009a) while bilateral BBN induces greater reductions than ipsilateral or contralateral BBN (Berlin et al. 1995; Guinan et al. 2003; Lilaonitkul and Guinan 2009a). If the reductions in OAE levels reflect a linearization of BM motion caused by activation of the MOCR, and if the present temporal effect on AM detection were mediated by that same mechanism, one would expect the magnitude of the temporal effect to be comparable for ipsilateral and contralateral BBN, but larger for bilateral BBN. In contrast with this expectation, the magnitude of the temporal effect on AM detection was not statistically different across noise lateralities, either for fixed-SPL (Fig. 8b) or fixed-SL carriers (Fig. 8c).
Second, the present data provide evidence for central masking in AM detection. For example: (1) AM detection thresholds for the 70 dB SPL carrier were better for ipsilateral than for bilateral BBN, in both the early and late conditions (Fig. 7). This is difficult to understand considering that carrier detection thresholds were higher for ipsilateral than for bilateral noise and, consequently, that carrier audibility was slightly better in the condition with the bilateral noise. (2) The effects of contralateral BBN on AM thresholds were similar to its effects on carrier thresholds (Fig. 7); namely, mean AM thresholds tended to be higher (worse) in the early condition than in quiet (albeit not statistically significantly) and improved in the late condition to values in quiet. As for carrier thresholds, it would be difficult to explain this pattern based on MOCR effects alone. Altogether, this suggests that the detection of monaural AM cues is likely a central process that integrates inputs from the two ears. Therefore, it is conceivable that the central adaptation and/or transient masking mechanisms described in the preceding section (to explain overshoot with contralateral BBN in carrier thresholds) also contribute to the temporal effects in AM detection.
Third, as illustrated in Fig. 1c, dynamic range adaptation could also enhance the neural representation of AM depth in noise, and its time course [100–400 ms in the AN (Wen et al. 2012) and 160 ms in the inferior colliculus (Dean et al. 2008)] is compatible with the temporal effect in AM detection reported here. Therefore, it is conceivable that dynamic range adaptation also contributes to the temporal effect in AM detection.
In summary, some aspects in the present data suggest that the MOCR may be responsible for the found temporal effects in AM detection while others point to more central processes.
Implications
It is generally agreed that the slow fluctuations in the envelope of speech in different spectral channels carry critical information for intelligibility (e.g., Shannon et al. 1995). Indeed, most cochlear implant users understand speech by using solely amplitude modulated electrical pulses (Wilson et al. 1991). It is generally accepted that the MOCR facilitates the reception of speech in noise by facilitating the representation of transient speech features in the AN response (reviewed by Guinan 2006). Assuming that the MOCR is involved to some extent in the present temporal effects on AM detection, the present results indicate that the MOCR may also help by enhancing AM cues. This idea is supported by recent evidence that cochlear implant users show better speech-in-noise intelligibility with a binaural sound coding strategies inspired by the MOCR than with standard strategies (Lopez-Poveda 2015; Lopez-Poveda et al. 2016, 2017). It remains uncertain, however, to what extent this intelligibility improvement is actually due to the MOCR-inspired strategy enhancing AM cues per se or to other concomitant benefits (see the Discussion in Lopez-Poveda et al. 2016).
CONCLUSIONS
There is a temporal effect (or ‘overshoot’) on AM-depth sensitivity in noise: AM detection thresholds are 2 to 4 dB better when the AM carrier is presented later than earlier in broad-band noise.
The temporal effect occurs for bilateral, ipsilateral, and contralateral noises, and the magnitude is not statistically different across noise lateralities.
For contralateral noises, the temporal effect was larger for broadband than for low-band noises.
The temporal effect on AM-depth detection is not due to ‘overshoot’ on carrier audibility. Instead, some of its characteristics appear consistent with the MOCR enhancing envelope cues in the peripheral auditory response, while others suggest that the enhancement is due to more central processes.
Incidentally, the present data suggest some central effects on carrier detection and AM detection.
Acknowledgements
We thank the three anonymous reviewers and the editor for their excellent comments on earlier versions of this manuscript. Work supported by a doctoral contract of the University of Salamanca and Banco Santander to MMP, and by FEDER and MINECO (BFU2015-65376-P) to EALP.
Footnotes
We note that the total duration of presentation for the three intervals was longer in the noise conditions (2200 ms = 400 ms per interval plus 500-ms for each of the two inter-interval time periods) than in quiet (1150 ms = 50 ms per interval plus 500-ms for each of the two inter-interval time periods). We have assumed that this difference did not affect the main results. Our assumption seems reasonable considering that (1) humans are able to store 7 ± 2 elements in short-term memory (Miller 1994), and this experiment required remembering only three elements to choose the odd one out; and (2) the main aim was to compare AM detection thresholds for the early and late conditions, for which the total duration of presentation was the same.
We expected to see this BMLD because the detection threshold for a monaural pure tone embedded in noise improves when a contralateral correlated noise is added (e.g., Hirsh 1948; Blodgett et al. 1962). The mean BMLD in the present data was 2.8 dB, thus comparable with the BMLD at the same signal frequency (1.5 kHz) reported elsewhere for similar, albeit not identical, stimuli (Webster 1951).
References
- Aguilar E, Eustaquio-Martin A, Lopez-Poveda EA. Contralateral efferent reflex effects on threshold and suprathreshold psychoacoustical tuning curves at low and high frequencies. J Assoc Res Otolaryngol. 2013;14:341–357. doi: 10.1007/s10162-013-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar E, Johannesen PT, Lopez-Poveda EA. Contralateral efferent suppression of human hearing sensitivity. Front Syst Neurosci. 2015;8:251. doi: 10.3389/fnsys.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almishaal A, Bidelman GM, Jennings SG. Notched-noise precursors improve detection of low-frequency amplitude modulation. J Acoust Soc Am. 2017;141:324–333. doi: 10.1121/1.4973912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSI . S3.6 specification for audiometers. New York: American National Standards Institute; 1996. [Google Scholar]
- Backus BC, Guinan JJ. Time-course of the human medial olivocochlear reflex. J Acoust Soc Am. 2006;119:2889–2904. doi: 10.1121/1.2169918. [DOI] [PubMed] [Google Scholar]
- Bacon SP. Effect of masker level on overshoot. J Acoust Soc Am. 1990;88:698–702. doi: 10.1121/1.399773. [DOI] [PubMed] [Google Scholar]
- Bacon SP, Liu L. Effects of ipsilateral and contralateral precursors on overshoot. J Acoust Soc Am. 2000;108:1811–1818. doi: 10.1121/1.1290246. [DOI] [PubMed] [Google Scholar]
- Bacon SP, Moore BCJ (1987) Transient masking and the temporal course of simultaneous tone-on-tone masking. J Acoust Soc Am 81:1073–1077 [DOI] [PubMed]
- Bacon SP, Takahashi GA. Overshoot in normal-hearing and hearing-impaired subjects. J Acoust Soc Am. 1992;91:2865–2871. doi: 10.1121/1.402967. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Hurley AE, Wen H, Kemp DT. Binaural noise suppresses linear click-evoked otoacoustic emissions more than ipsilateral or contralateral noise. Hear Res. 1995;87:96–103. doi: 10.1016/0378-5955(95)00082-F. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett HC, Jeffress LA, Whitworth RH. Effect of noise at one ear on the masked threshold for tone at the other. J Acoust Soc Am. 1962;34:979–981. doi: 10.1121/1.1918233. [DOI] [Google Scholar]
- Carlyon RP, White LJ. Effect of signal frequency and masker level on the frequency regions responsible for the overshoot effect. J Acoust Soc Am. 1992;91:1034–1041. doi: 10.1121/1.402629. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ. Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J Physiol. 2003;548:307–312. doi: 10.1113/jphysiol.2003.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ. Efferent-mediated control of basilar membrane motion. J Physiol. 2006;576:49–54. doi: 10.1113/jphysiol.2006.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci. 2005;8:1684–1689. doi: 10.1038/nn1541. [DOI] [PubMed] [Google Scholar]
- Dean I, Robinson BL, Harper NS, McAlpine D. Rapid neural adaptation to sound level statistics. J Neurosci. 2008;28:6430–6438. doi: 10.1523/JNEUROSCI.0470-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M, de Boer J, Krumbholz K. Is overshoot caused by an efferent reduction in cochlear gain? Adv Exp Med Biol. 2013;787:65–72. doi: 10.1007/978-1-4614-1590-9_8. [DOI] [PubMed] [Google Scholar]
- Fletcher M, de Boer J, Krumbholz K. Is off-frequency overshoot caused by adaptation of suppression? J Assoc Res Otolaryngol. 2015;16:241–253. doi: 10.1007/s10162-014-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC. Derivation of auditory filter shapes from notched-noise data. Hear Res. 1990;47:103–138. doi: 10.1016/0378-5955(90)90170-T. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4:521–540. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh IJ. The influence of interaural phase on interaural summation and inhibition. J Acoust Soc Am. 1948;20:536–544. doi: 10.1121/1.1906407. [DOI] [Google Scholar]
- Jennings SG, Ahlstrom JB, Dubno JR. Effects of age and hearing loss on overshoot. J Acoust Soc Am. 2016;140:2481–2493. doi: 10.1121/1.4964267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SG, Heinz MG, Strickland EA. Evaluating adaptation and olivocochlear efferent feedback as potential explanations of psychophysical overshoot. J Assoc Res Otolaryngol. 2011;12:345–360. doi: 10.1007/s10162-011-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen PT, Lopez-Poveda EA. Cochlear nonlinearity in normal-hearing subjects as inferred psychophysically and from distortion-product otoacoustic emissions input/output functions. J Acoust Soc Am. 2008;124:2149–2163. doi: 10.1121/1.2968692. [DOI] [PubMed] [Google Scholar]
- Kawase T, Ogura M, Sato T, Kobayashi T, Suzuki Y. Effects of contralateral noise on the measurement of auditory threshold. Tohoku J Exp Med. 2003;200:129–135. doi: 10.1620/tjem.200.129. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Schairer KS, Ellison JC, Fitzpatrick DF, Jesteadt W. Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot. J Acoust Soc Am. 2009;125:1595–1604. doi: 10.1121/1.3068443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlrausch A, Fassel R, Dau T. The influence of carrier level and frequency on modulation and beat-detection thresholds for sinusoidal carriers. J Acoust Soc Am. 2000;108:723–734. doi: 10.1121/1.429605. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–677. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ. Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol. 2009;10:459–470. doi: 10.1007/s10162-009-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ. Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol. 2009;101:1394–1406. doi: 10.1152/jn.90925.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska G, Smurzynski J, Morawski K, Namyslowski G, Probst R. Influence of contralateral stimulation by two-tone complexes, narrow-band and broad-band noise signals on the 2f1-f2 distortion product otoacoustic emission levels in humans. Acta Otolaryngol. 2002;122:613–619. doi: 10.1080/000164802320396286. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda E. A. (2015). Sound enhancement for cochlear implants. EU Patent WO 2015/169649 A1
- Lopez-Poveda EA, Eustaquio-Martin A, Stohl JS, Wolford RD, Schatzer R, Gorospe JM, Ruiz SS, Benito F, Wilson BS. Intelligibility in speech maskers with a binaural cochlear implant sound coding strategy inspired by the contralateral medial olivocochlear reflex. Hear Res. 2017;348:134–137. doi: 10.1016/j.heares.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Eustaquio-Martin A, Stohl JS, Wolford RD, Schatzer R, Wilson BS. A binaural cochlear implant sound coding strategy inspired by the contralateral medial olivocochlear reflex. Ear Hear. 2016;37:138–148. doi: 10.1097/AUD.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Plack CJ, Meddis R. Cochlear nonlinearity between 500 and 8000 Hz in listeners with normal hearing. J Acoust Soc Am. 2003;113:951–960. doi: 10.1121/1.1534838. [DOI] [PubMed] [Google Scholar]
- Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BC. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proc Natl Acad Sci U S A. 2006;103:18866–18869. doi: 10.1073/pnas.0607364103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Andeol G, Gallego S, Collet L. Activation of medial olivocochlear efferent system in humans: influence of stimulus bandwidth. Hear Res. 2000;140:111–125. doi: 10.1016/S0378-5955(99)00196-3. [DOI] [PubMed] [Google Scholar]
- McFadden D, Walsh KP, Pasanen EG, Grenwelge EM. Overshoot using very short signal delays. J Acoust Soc Am. 2010;128:1915–1921. doi: 10.1121/1.3480568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1994;101:343–352. doi: 10.1037/0033-295X.101.2.343. [DOI] [PubMed] [Google Scholar]
- Moore BC, Glasberg BR. Temporal modulation transfer functions obtained using sinusoidal carriers with normally hearing and hearing-impaired listeners. J Acoust Soc Am. 2001;110:1067–1073. doi: 10.1121/1.1385177. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Zwicker E. The effect of masker spectral asymmetry on overshoot in simultaneous masking. J Acoust Soc Am. 1991;89:1324–1330. doi: 10.1121/1.400656. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Sheft S, Yost WA. Temporal integration in amplitude modulation detection. J Acoust Soc Am. 1990;88:796–805. doi: 10.1121/1.399729. [DOI] [PubMed] [Google Scholar]
- Smith DW, Turner DA, Henson MM. Psychophysical correlates of contralateral efferent suppression. I. The role of the medial olivocochlear system in “central masking” in nonhuman primates. J Acoust Soc Am. 2000;107:933–941. doi: 10.1121/1.428274. [DOI] [PubMed] [Google Scholar]
- Smith RL, Zwislocki JJ. Short-term adaptation and incremental responses of single auditory-nerve fibers. Biol Cybern. 1975;17:169–182. doi: 10.1007/BF00364166. [DOI] [PubMed] [Google Scholar]
- Strickland EA. The relationship between frequency selectivity and overshoot. J Acoust Soc Am. 2001;109:2062–2073. doi: 10.1121/1.1357811. [DOI] [PubMed] [Google Scholar]
- Strickland EA. The temporal effect with notched-noise maskers: analysis in terms of input-output functions. J Acoust Soc Am. 2004;115:2234–2245. doi: 10.1121/1.1691036. [DOI] [PubMed] [Google Scholar]
- Verschooten E, Strickland EA, Verhaert N, Joris PX. Assessment of ipsilateral efferent effects in human via ECochG. Front Neurosci. 2017;11:331. doi: 10.3389/fnins.2017.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemeister NF. Temporal modulation transfer functions based upon modulation thresholds. J Acoust Soc Am. 1979;66:1364–1380. doi: 10.1121/1.383531. [DOI] [PubMed] [Google Scholar]
- von Klitzing R, Kohlrausch A. Effect of masker level on overshoot in running- and frozen-noise maskers. J Acoust Soc Am. 1994;95:2192–2201. doi: 10.1121/1.408679. [DOI] [PubMed] [Google Scholar]
- Walsh KP, Pasanen EG, McFadden D. Overshoot measured physiologically and psychophysically in the same human ears. Hear Res. 2010;268:22–37. doi: 10.1016/j.heares.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL. Specialized neuronal adaptation for preserving input sensitivity. Nat Neurosci. 2008;11:1259–1261. doi: 10.1038/nn.2201. [DOI] [PubMed] [Google Scholar]
- Webster FA. The influence of interaural phase on masked thresholds. I. The role of interaural time-deviation. J Acoust Soc Am. 1951;23:452–462. doi: 10.1121/1.1906787. [DOI] [Google Scholar]
- Wen B, Wang GI, Dean I, Delgutte B. Dynamic range adaptation to sound level statistics in the auditory nerve. J Neurosci. 2009;29:13797–13808. doi: 10.1523/JNEUROSCI.5610-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B, Wang GI, Dean I, Delgutte B. Time course of dynamic range adaptation in the auditory nerve. J Neurophysiol. 2012;108:69–82. doi: 10.1152/jn.00055.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of the crossed olivocochlear bundle. Hear Res. 1988;35:165–189. doi: 10.1016/0378-5955(88)90116-5. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Stickney GS, Kong YY, Vongphoe M, Bhargave A, Wei C, Cao K. Speech recognition with amplitude and frequency modulations. Proc Nat Acad Sci USA. 2005;102:2293–2298. doi: 10.1073/pnas.0406460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker E. Temporal effects in simultaneous masking and loudness. J Acoust Soc Am. 1965;38:132–141. doi: 10.1121/1.1909588. [DOI] [PubMed] [Google Scholar]
- Zwislocki JJ, Damianopoulos EN, Buining E, Glantz J. Central masking: some steady-state and transient effects. Percept Psychophys. 1967;2:59–64. doi: 10.3758/BF03212462. [DOI] [Google Scholar]