Abstract
Key points
Activation of the shoulder abductor muscles in the arm opposite a unilateral brain injury causes involuntary increases in elbow, wrist and finger flexion in the same arm, a phenomenon referred to as the flexion synergy.
It has been proposed that flexion synergy expression is related to reduced output from ipsilesional motor cortex and corticospinal pathways.
In this human subjects study, we provide evidence that the magnitude of flexion synergy expression is instead related to a progressive, task‐dependent recruitment of contralesional cortex.
We also provide evidence that recruitment of contralesional cortex may induce excessive activation of ipsilateral reticulospinal descending motor pathways that cannot produce discrete movements, leading to flexion synergy expression.
We interpret these findings as an adaptive strategy that preserves low‐level motor control at the cost of fine motor control.
Abstract
A hallmark of hemiparetic stroke is the loss of fine motor control in the contralesional arm and hand and the constraint to a grouped movement pattern known as the flexion synergy. In the flexion synergy, increasing shoulder abductor activation drives progressive, involuntary increases in elbow, wrist and finger flexion. The neural mechanisms underlying this phenomenon remain unclear. Here, across 25 adults with moderate to severe hemiparesis following chronic stroke and 18 adults without neurological injury, we test the overall hypothesis that two inter‐related mechanisms are necessary for flexion synergy expression: increased task‐dependent activation of the intact, contralesional cortex and recruitment of contralesional motor pathways via ipsilateral reticulospinal projections. First, we imaged brain activation in real time during reaching motions progressively constrained by flexion synergy expression. Using this approach, we found that cortical activity indeed shifts towards the contralesional hemisphere in direct proportion to the degree of shoulder abduction loading in the contralesional arm. We then leveraged the post‐stroke reemergence of a developmental brainstem reflex to show that anatomically diffuse reticulospinal motor pathways are active during synergy expression. We interpret this progressive recruitment of contralesional cortico‐reticulospinal pathways as an adaptive strategy that preserves low‐level motor control at the cost of fine motor control.
Keywords: stroke, motor control, neural plasticity
Key points
Activation of the shoulder abductor muscles in the arm opposite a unilateral brain injury causes involuntary increases in elbow, wrist and finger flexion in the same arm, a phenomenon referred to as the flexion synergy.
It has been proposed that flexion synergy expression is related to reduced output from ipsilesional motor cortex and corticospinal pathways.
In this human subjects study, we provide evidence that the magnitude of flexion synergy expression is instead related to a progressive, task‐dependent recruitment of contralesional cortex.
We also provide evidence that recruitment of contralesional cortex may induce excessive activation of ipsilateral reticulospinal descending motor pathways that cannot produce discrete movements, leading to flexion synergy expression.
We interpret these findings as an adaptive strategy that preserves low‐level motor control at the cost of fine motor control.
Introduction
Fine motor control is both a defining feature of primates and an essential skill for successfully interacting with complex environments. However, the primary motor pathways responsible for this control, the lateral corticospinal tract (lCST), are often the most directly impacted following unilateral brain injury. The direct effects of reduced lCST fibre density, in addition to secondary adaptive changes in the brain and spinal cord, severely compromise precise, individuated control of single joints (Stinear et al. 2007; Schaechter et al. 2009; Puig et al. 2013). In particular, this ability is replaced by the constraint to highly stereotyped patterns of coarse, multi‐joint movements known as limb synergies (Brunnstrom, 1970). This transition is most apparent in the contralesional upper limb post stroke, where increased shoulder abductor activation drives simultaneous, involuntary activation of the elbow, wrist and finger flexors (Dewald et al. 1995; Sukal et al. 2007; Miller & Dewald, 2012). This phenomenon is termed the upper limb ‘flexion synergy’ (Foerster, 1936; Twitchell, 1951; Brunnstrom, 1970). The rapid emergence of the flexion synergy, even with minimal shoulder abduction activity, makes it one of the most ubiquitous and functionally limiting motor impairments; even mild expression can severely limit the elbow extension and hand opening necessary for object acquisition (Sukal et al. 2007; Miller & Dewald, 2012; Lan et al. 2017).
We hypothesize that two critical, inter‐related mechanisms are necessary for flexion synergy expression: increased activation of the intact, contralesional cortex and recruitment of contralesional motor pathways that have ipsilateral spinal projections. Options for such pathways include the non‐decussating ipsilateral ventral corticospinal tract (vCST), the vestibulospinal tract (VeST), and the reticulospinal tract (ReST). The ReST is particularly well suited to drive flexion synergy expression for the following reasons in particular: (1) across multiple species, including humans, the reticular formation has consistently been shown to receive rich cortical inputs via direct cortico‐reticular projections and collaterals from corticospinal fibres (Peterson et al. 1974; Ugolini & Kuypers, 1986; Keizer & Kuypers, 1989; Kably & Drew, 1998; Jankowska & Edgley, 2006; Yeo et al. 2012); (2) electrical stimulation of the origin of the ReST in non‐human primates produces coordinated multi‐joint flexion patterns in the ipsilateral upper limb (Holstege & Kuypers, 1987; Buford & Davidson, 2004; Davidson & Buford, 2006; Herbert et al. 2010; Zaaimi et al. 2012); (3) the ReST is a primary input to segmental propriospinal networks (Mitchell et al. 2016), which have also been implicated in mediating flexion synergy expression (McMorland et al. 2015); and (4) fractional anisotropy of the contralesional ReST is greater in individuals with stronger expression of paretic limb synergies (Owen et al. 2017). The ReST also has a strong monoaminergic neuromodulatory component, and evidence suggests that upregulation of this component may contribute to the development of hyperexcitable stretch‐sensitive reflexes post stroke (McPherson et al. 2008).
Here, we test two predictions that follow from the hypothesized role of contralesional cortico‐reticulospinal networks in flexion synergy expression. First, if contralesional cortical activity is a component of flexion synergy expression, then the magnitude of this activity will increase and decrease in direct proportion to the degree that movements of the paretic upper limb are constrained by flexion synergy expression. Second, if the ReST is upregulated post stroke (via increased contralesional cortical recruitment), then brainstem/spinal reflexes known to utilize ReST motor pathways will become exaggerated in the paretic musculature.
We tested these predictions in 25 adults with chronic, hemiparetic stroke and 18 adults without neurological injury using a combination of high resolution neuroimaging and advanced robotics. Across experiments, we show a clear cortical signature of flexion synergy expression and we demonstrate that brainstem reflexes known to use ReST motor pathways are upregulated during movements constrained by flexion synergy expression. Our findings strongly suggest that dynamic recruitment of contralesional cortico‐reticulospinal networks is associated with flexion synergy expression. Our findings also provide a framework for developing new, potentially more effective, therapies for motor rehabilitation following unilateral brain injury that facilitate use of remaining viable ipsilesional corticospinal projections.
Methods
Ethical approval
In total, 25 adults with a stroke‐induced unilateral brain injury resulting in chronic hemiparesis and 18 adults without known neurological injury participated in this investigation after providing informed, written consent to participate (Table 1). All protocols were approved by the Institutional Review Board of Northwestern University in accordance with the ethical standards stipulated by the 1964 Declaration of Helsinki for research involving human participants, except for registration in a database.
Table 1.
Participant demographics
| HD‐EEG experiments | ATNR experiments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant | Age | Sex | Years post stroke | Paretic arm | FMA | Participant | Age | Sex | Years post stroke | Paretic arm | FMA |
| P1 | 59 | F | 4 | R | 16 | P1 | 61 | F | 6 | R | 16 |
| P2 | 84 | M | 4 | L | 25 | P2 | 84 | M | 4 | L | 25 |
| P3 | 57 | M | 7 | L | 31 | P3 | 60 | M | 10 | L | 19 |
| P4 | 61 | M | 11 | R | 16 | P4 | 63 | M | 12 | R | 13 |
| P5 | 52 | M | 7 | R | 18 | P5 | 49 | M | 3 | L | 18 |
| P6 | 60 | F | 5 | R | 18 | P6 | 68 | M | 8 | L | 19 |
| P7 | 54 | F | 21 | R | 19 | P7 | 57 | F | 2 | R | 19 |
| P8 | 52 | M | 11 | L | 23 | P8 | 54 | M | 3 | L | 25 |
| P9 | 60 | M | 3 | R | 25 | P9 | 60 | M | 14 | L | 30 |
| P10 | 63 | M | 4 | L | 43 | P10 | 44 | M | 21 | R | 33 |
| P11 | 65 | M | 7 | L | 34 | ||||||
| P12 | 61 | M | 6 | L | 35 | ||||||
| P13 | 39 | M | 5 | L | 37 | ||||||
| P14 | 45 | M | 12 | L | 39 | ||||||
| P15 | 37 | M | 2 | R | 40 | ||||||
| Averages | 60 | 8 | 23 | 56 | 8 | 27 | |||||
| Std dev. | 9 | 5 | 8 | 12 | 5 | 9 | |||||
FMA: Fugl‐Meyer Motor assessment; participants 1–4 (in italics) are common to both experimental cohorts; Age, years post‐stroke and FMA scores are current at the time of experiment in cases where particpants completed both experimental protocols. Std dev., standard deviation.
Participants
Our investigation was split into two phases: (1) high‐density electroencephalography (HD‐EEG) and flexion synergy expression experiments, and (2) asymmetric tonic neck reflex (ATNR) and flexion synergy expression experiments. Exclusion criteria were common to both phases of the investigation and included the following: recent decline in health and/or hospitalization, any current painful condition, recent changes in the medical management of hypertension, an inability to sit for prolonged periods of time (>∼2–3 h), and an inability to follow a simple 3‐step command. Additionally, participants were excluded if they had bilateral motor or sensory impairments and/or severe wasting or contracture of the impaired upper limb. Overpressure at the end range of motion was used as a medical screening tool to rule out current inflammation/pain at the cervical spine, shoulder, elbow, wrist and fingers. All participants had passive range of motion to at least 90° of shoulder flexion and abduction, and the tested limb was able to be positioned in the horizontal plane without discomfort (i.e. in mid‐range or 0° of internal/external rotation).
In phase 1 of the investigation, we tested 10 adults with moderate to severe upper limb motor impairment following stroke‐induced unilateral brain injury (≥1 year prior to enrollment; Table 1) and 8 age‐matched adults without known neurological injury (7 male, 1 female, all right hand dominant). Motor function was evaluated using the upper‐extremity portion of the Fugl‐Meyer Motor Assessment (FMA), with scores ranging from 16 to 43 of a possible 66 (lower scores indicate greater impairment). In phase 2 of the investigation, we tested 15 adults with moderate to severe upper limb motor impairment following stroke‐induced unilateral brain injury (≥1 year prior to enrollment; Table 1), and 10 non‐age matched adults without known neurological injury. Upper limb FMA scores ranged from 13 to 40 out of 66.
ACT‐3D robotic system
For all experimental protocols, we quantified the impact of flexion synergy expression during upper limb reaching movements with a robotic system, the ACT‐3D (Sukal et al. 2007; Ellis et al. 2008, 2016) (Fig. 1 A). The ACT‐3D system consists of an admittance‐controlled HapticMaster robot (Moog‐FCS, The Netherlands) fitted with a 6 degrees‐of‐freedom loadcell on an instrumented gimbal, a Biodex experimental chair (Biodex Medical Systems, Shirley, NY, USA), and a custom graphical user interface. The ACT‐3D allows unrestrained motion of the upper limb in three dimensions, and can impose forces upon its user. Its graphical user interface displays an avatar of the participant's arm, which moves in real‐time with actual limb displacement.
Figure 1. Increased SABD loading drives increased flexion synergy expression.
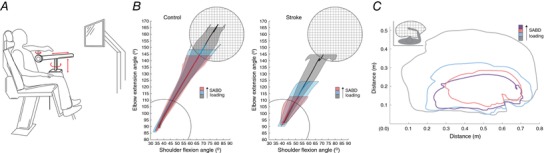
A, schematic representation of the ACT‐3D system, which allows free motion in three dimensions, can impose forces on its user, and enables dynamic quantification of flexion synergy expression. B, group‐averaged trajectories over the first 1 s of ballistic reaching for control (left) and stroke (right), depicted in joint angle space; control, N = 8; stroke, N = 10. This task was used for HD‐EEG and reaching experiments. Elbow and shoulder joint excursion were invariant to shoulder abduction loading in control participants, while excursion at both joints decreased monotonically as shoulder abduction loading increased post stroke. C, representative reaching work area data from a single stroke participant (upper limb FMA: 24/66). Across participants, work area (expressed as a percentage of on‐table area) averaged 69.5% in the Floating condition, 56.5% when lifting and producing 25% maximum voluntary shoulder abduction torque (25% SABD MVT), and 45.1% when lifting and producing 50% maximal voluntary shoulder abduction torque. B and C, decrease in elbow extension ability with increased shoulder abduction loading is the characteristic feature of flexion synergy expression. Grey: moving over a frictionless virtual table (Table); blue: limb fully supported by ACT‐3D, as if weightless (Floating); pink: generating 25% of maximum voluntary shoulder abduction torque; purple: generating 50% of maximum voluntary shoulder abduction torque. [Color figure can be viewed at http://wileyonlinelibrary.com]
Because activation of shoulder abductor muscles elicits the most vigorous expression of the flexion synergy, we configured the ACT‐3D to impose vertical forces that required participants to generate specific amounts of shoulder abduction torque to maintain a horizontal limb orientation during reaching. For HD‐EEG experiments, participants performed ballistic reaches (to maximize descending neural drive) while for ATNR experiments, participants made large, slow, circular reaches (to measure work area). Both protocols were performed at three shoulder abduction loading levels. In addition to providing a robust measure of flexion synergy expression, the work area metric has been previously cross‐validated with clinical measures across the World Health Organization ICF model (Ellis et al. 2008).
HD‐EEG and flexion synergy
Three distinct motor tasks were performed, all in the ACT‐3D: reaching while sliding on a frictionless virtual table (‘Table’), horizontal reaching in free space with the limb fully supported by the robot (‘Floating’), and reaching in free space while lifting a load equal to 25% of the subject's maximum voluntary shoulder abduction torque (‘25% SABD MVT’). Each reaching trial began with a 3–5 s wait in the start position (85° shoulder abduction, 40° shoulder flexion, and 90° elbow flexion) followed by a self‐initiated ballistic reaching motion to a single target requiring an additional 70° of elbow extension and 40° of shoulder flexion. A total of 120 trials were performed for each level of abduction loading.
Surface electromyographic (EMG) signals were recorded from 12 muscles of the arm and trunk on either the dominant limb side of control participants or the paretic limb side of participants with stroke. These muscles included the brachioradialis, biceps brachii, and the long and lateral heads of the triceps brachii at the elbow; the anterior, intermediate, and posterior deltoids at the shoulder; the pectoralis major vertical and horizontal fibres and latissimus dorsi of the trunk; and the flexor and extensor carpi radialis at the wrist. EMG activity from three muscles – brachioradialis, lateral head of the triceps, and the intermediate deltoid – was also recorded on the opposite arm to detect potential bilateral activation. All EMG data were filtered (high pass at 6 Hz and low pass at 500 Hz) and sampled at 1024 Hz. Amplitudes were normalized to maximum EMG values obtained during maximum voluntary contractions using a six degrees of freedom load cell.
During all motor tasks, an HD‐EEG system with 160 active Ag/AgCl electrodes (Active II, Biosemi, Inc., Amsterdam, The Netherlands) was placed over the head. Electro‐oculogram (EOG) electrodes were placed above and below the eye to detect eye movements. Electrode positions were recorded using a 3D magnetic digitizer (Polhemus, Colchester, VT, USA). The presence of eye and/or muscle movement artifacts in any of the HD‐EEG channels eliminated that channel from further analysis (for a given trial). All trials were aligned by arm EMG onset, which was detected offline using a statistical thresholding algorithm written in MATLAB (The MathWorks, Natick, MA, USA). HD‐EEG data were averaged and imported into the CURRY 5.0 software environment (Compumedics Neuroscan Ltd., Charlotte, NC, USA) for low pass filtering (cutoff frequency of 50 Hz) and baseline correction. A mean global field power (MGFP) trace was calculated for each subject as the root mean square of the re‐referenced HD‐EEG channels. This calculated value was used to assess the quality of the averaged data, but not for current density reconstruction.
A subject‐specific boundary element method (BEM) model was created in Curry from T1‐weighted magnetic resonance images (MRI) of each participant and used to reconstruct cortical activity. MRI data were collected on 3T Siemens MAGNETOM Trio scanner (Siemens AG, Erlangen, Germany), using approximately 176–192 contiguous images in the sagittal plane with voxel dimensions of 1.0 × 1.0 × 1.0 mm and a voxel matrix of 256 × 256). The BEM model represents the volume conductor between the cortex and the scalp, and is split into three compartments for the skin, skull and brain with 10 mm, 9 mm and 7 mm resolution, respectively. Coefficients of conductivity used for each compartment were 0.25 S m−1 for skin, 0.017 S m−1 for skull, and 1.79 S m−1 for brain (Geddes & Baker, 1967; Law, 1993; Baumann et al. 1997; Yao & Dewald, 2005). Co‐registration of the electrode positions to the skin was then performed by superimposing the locations of anatomical landmarks (nasion and two preauricular points) to the scalp.
We used low resolution electromagnetic tomography (LORETA) to reconstruct cortical activity by estimating the strength of sources for measured scalp HD‐EEG that were constrained on a subject‐specific cortical triangular mesh (we use ‘cortical activity’ as a general term to refer to the activity of HD‐EEG sources). LORETA computes a smooth current density distribution, with the assumption that neighbouring neurons are activated simultaneously and synchronously, and thus have similar strengths (Pascual‐Marqui et al. 1994, 2002). The LORETA Lp norm method with parameter P = 1 has been shown to provide better source localization ability than a variety of other inverse methods, including moving dipoles and minimum Lp norm (Yao & Dewald, 2005; Grova et al. 2006; Bai et al. 2007). The estimated spatial resolution is ∼3–5 mm using a 3 mm resolution cortex source distribution (Yao & Dewald, 2005). Current density strengths were measured in units of microamps per square millimeter (μA mm2), but were then normalized to the highest strength observed in the task to facilitate comparisons across subjects.
After reconstructing the cortical activity, we selected active sources residing in regions of interest (ROI) including the supplementary motor area (SMA), premotor (PM), primary motor (M1), and primary somatosensory (S1) cortices. To facilitate group spatial analysis, locations were transformed to the normalized Montreal Neurological Institute template coordinate system. Cortical activity was averaged over 100 ms immediately prior to movement onset. This window encompasses neural activity related to the release of a motor command, which occurs before reach‐related sensory feedback or muscle activity. Specifically for the characteristic Bereitschaftspotential (BP) seen in HD‐EEG activity of self‐initiated movements (Kornhuber & Deecke, 1965), this behaviour usually takes place during the late BP or early motor potential phases (Shibasaki & Hallett, 2006; Colebatch, 2007). Active sources were identified as those that exceeded a 95% confidence level above baseline mean strength for at least 10 ms (consecutively). We then used a laterality index (LI), similar to a weighted laterality index used in fMRI analysis (Cramer et al. 1997), to determine the relative contributions of each cerebral hemisphere to the source activity for each task. The LI a single measure across all regions of interest (SMA, M1, PM and S1). It equals 1 for a completely contralateral source distribution and −1 for a completely ipsilateral source distribution, and 0 for an equal bilateral source distribution.
ATNR and flexion synergy
In individuals with acquired brain injury, head rotation elicits a developmental brainstem reflex known as the asymmetric tonic neck reflex (ATNR) (Ellis et al. 2012). In normal expression of the ATNR, as observed in early motor development, rotation of the head away from one arm causes involuntary flexion of that arm coupled with simultaneous extension of the contralateral arm (i.e. the arm faced by the head) (Shevell, 2009). For testing the impact of ATNR on reaching ability, the ACT‐3D was positioned such that the participant's arm was in the transverse plane located at shoulder height (perpendicular to the line of gravity) and the participant's head was oriented in one of three directions: head forward, contralateral head rotation (face pointed away from the paretic/contralesional arm), or ipsilateral head rotation (face pointed at the paretic/contralesional arm). For rotated positions, the head was rotated until 5° from full cervical spine rotation.
Once the head was positioned, participants made large, slow circles exploring their maximum reaching range of motion (i.e. work area) in the horizontal plane. Work area was first evaluated with the arm supported on and sliding along a horizontal haptic surface (Table condition), allowing the participant to learn the task. Next, the participant performed the same motions actively lifting the arm off of the haptic surface during controlled abduction loading conditions normalized to the weight of their arm. In all, five 15‐s trials were performed for each of three abduction‐loading conditions: Table, 50%, and 100% of arm weight. The work area tasks were also completed for all three head orientations. Trials with unintended head rotation were omitted from analysis. Endpoint/hand position data were sampled from the ACT‐3D robotic device at 60 Hz during work area trials. Work area was calculated as the largest area contained within the superimposed circular motions obtained from individual trials at a given abduction load level. To account for the anthropometric differences between participants, each participant's work area values were expressed as a percentage of the work area performed in the Table condition. The percentage change in work area from head‐forward to both ipsilateral and contralateral head rotation was computed for each abduction loading condition.
Statistics
Prior to all statistical analyses, we first confirmed the normality of our datasets (and subgroups) with the Kolmogorov‐Smirnov test and the Shapiro‐Wilk test. As appropriate, sphericity was assessed with Mauchly's Test; the Greenhouse‐Geiser correction was used for comparisons that violated the assumption of sphericity. To account for family‐wise error rate in multiple comparisons, a Bonferroni correction was used. A P value of 0.05 or less was considered significant in all cases, and all statistical analyses were performed using IBM SPSS Statistics software (IBM Inc., Armonk, NY, USA).
For HD‐EEG and flexion synergy experiments, the primary outcome measure was the change in magnitude of cortical activity as a function of shoulder abduction load level. We used two‐way ANOVAs to assess this outcome, with the two independent factors being participant cohort (control or stroke) and shoulder abduction load level (Table, Floating and 25% SABD MVT) and a dependent variable of cortical activity magnitude. If there was not a significant interaction of cohort and load level, then one‐way ANOVAs were used to compare cortical measures between groups and across levels within each group. We then used simple linear regression analysis and two‐tailed Pearson's correlation coefficients to determine the strength of linear relationships between the three levels of shoulder abduction loading and joint kinematic, EMG, and cortical activity measures.
The primary outcome measure for ATNR experiments was the percentage change in reaching work area from the head‐forwards condition to the head‐contralateral position. We assessed this outcome using a 2‐way mixed model ANOVA, which tested the potential impact of independent factors shoulder abduction load (table, 50% and 100% limb weight), group (stroke and control), and the interaction of abduction load and group on percentage change in work area. The secondary outcome measure for ATNR experiments was the percentage change in reaching work area from the head‐forwards condition to the head‐ipsilateral position. This comparison also utilized a 2‐way mixed model ANOVA with independent factors of shoulder abduction load, group (stroke and control), and the interaction of load level and group.
Results
HD‐EEG and flexion synergy expression
First, we verified that increased shoulder abduction loading led to progressive reductions in concurrent elbow extension ability in the contralesional arm (Fig. 1 B and C ; Fig. 2). This is the defining feature of the flexion synergy. Expectedly, increased shoulder abduction loading did not cause significant reductions in straight line or circular reaching ability in adults without neurological impairment, regardless of shoulder abduction load.
Figure 2. Kinematic data for straight line ballistic reaches.
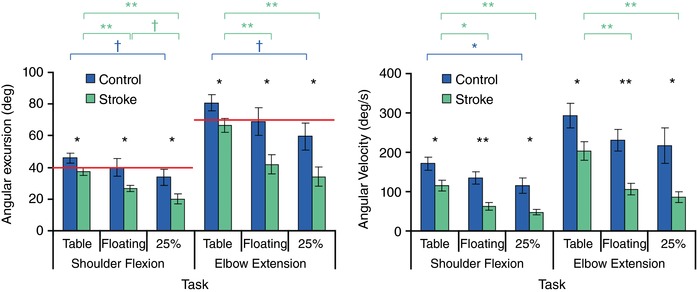
Left, group‐average maximum joint angular excursions for control (blue) and stroke (green) participants. Red line: angular excursion required to reach target. Right, group‐average maximum angular velocities for shoulder flexion and elbow extension for control and stroke participants. Statistical significance is indicated above each graph and is colour coded to indicate between‐group (black) or between‐task (blue or violet) one‐way ANOVAs († P < 0.1, * P < 0.05, ** P < 0.01). [Color figure can be viewed at http://wileyonlinelibrary.com]
Next, we acquired HD‐EEG data during planning and execution of ballistic, centre‐out reaching tasks in the dominant (control) or contralesional (stroke) upper limb (Fig. 3 A). In neurologically uninjured control participants, cortical activity was primarily observed in the hemisphere contralateral to the arm being tested, regardless of shoulder abduction load (Fig. 3 B). In adults with stroke, however, contralesional cortical activity rapidly and progressively increased as shoulder abduction loading, and thus synergy expression, increased (Fig. 3 C).
Figure 3. Exhaustion of ipsilesional motor circuits precipitates recruitment of contralesional cortex and flexion synergy expression.
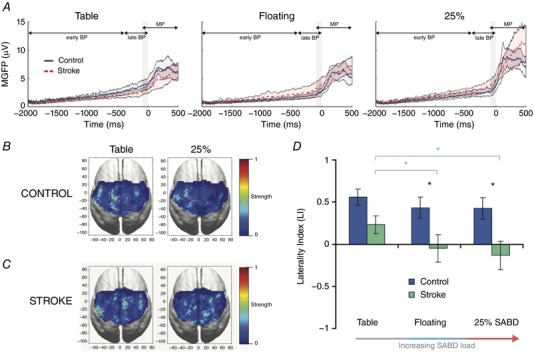
A, group‐average mean global field power over 100 ms immediately prior to movement onset (gray patch). Cortical activity is presented in reference to the Bereitschaftpotential (BP), including the early BP, late BP, and motor potential (MP). B, C, group‐average cortical current density reconstructions are shown for control (B) and stroke (C) over 100 ms immediately prior to movement onset. All brains are oriented such that the left side is contralateral to the tested arm. Thus, the left side corresponds to the lesioned hemisphere in stroke, and the right side is contralesional (ipsilateral to the paretic upper limb). Cortical activity was primarily localized in the contralateral hemisphere across all tasks for control participants. Stroke participants exhibited increased activity in the contralesional (R) hemisphere as SABD loading increased. D, cortical activation laterality. In control participants (blue), mean laterality index (LI) was indistinguishable across shoulder abduction (SABD) load levels. In stroke (green), mean LI decreased from 0.27 to −0.12 to −0.21 from Table to Floating to 25% shoulder abduction loading, with significant decreases from Table to Floating and from Table to 25% shoulder abduction. Stroke participants had significantly lower LI values than the control participants for Floating and 25% shoulder abduction (P = 0.01, 0.02, respectively), but only a strong trend towards lower mean LI for Table (P = 0.08). Mean global field power, MGFP. [Color figure can be viewed at http://wileyonlinelibrary.com]
We then employed a laterality index (LI), using a 100 ms window of the HD‐EEG data prior to the start of centre‐out reaching tasks, to estimate the overall contribution of each hemisphere to the source activity during different shoulder abduction loading conditions (Cramer et al. 1997). A value of 1 on this metric represents a cortical source distribution completely contralateral to the tested arm, while a value of −1 represents a source distribution completely ipsilateral to the tested arm. Control participants displayed statistically indistinguishable mean LI values of 0.56, 0.43 and 0.42 for Table, Floating and 25% maximal shoulder abduction torque (25% SABD MVT), respectively (Fig. 3 D). These values reflected predominantly contralateral cortical activity. In contrast, adults with stroke exhibited average LI values of 0.27, −0.12 and −0.21, respectively, across the same conditions (Fig. 3 D). This trend represented a rise in contralesional activity as synergy expression increased.
We found a non‐significant difference in LI between the control and stroke groups for the Table condition (P = 0.08) and statistically significant between‐group differences for Floating and 25% SABD MVT (Fig. 3 D; P = 0.01, 0.02, respectively). Increased contralesional cortical activity in stroke (i.e. a shift towards negative LI) was also strongly correlated with poorer performance on kinematic movement parameters, including reduced angular excursion for shoulder flexion and elbow extension (Pearson's r = 0.56 and r = 0.59, respectively; P < 0.01 for both) and reduced angular velocity for shoulder flexion (r = 0.32, P = 0.10) and elbow extension (r = 0.37, P = 0.06). These findings are consistent with the qualitative observation that differences in reaching ability between individuals post stroke and individuals without neurological injury are smallest when sliding over a hard yet frictionless surface (due to minimal synergy expression) and largest during production of shoulder abduction torque (due to increased synergy expression).
Within the unilateral brain injury cohort, we also conducted a subgroup analysis based on whether the paretic limb was the dominant or the non‐dominant arm. We found a significant difference in LI only for the Table condition (P = 0.02). Unexpectedly, the non‐dominant arm had more positive LI values, and hence more contralateral activity. This is a clear contradiction of previous postulates, which claim that non‐dominant arms introduce more bilateral activation. Therefore, it is reasonable to conclude that the shifts in activity localization that we found are not simply artifacts of using the non‐dominant limb.
To localize the source of altered cortical activity as a function of shoulder abduction load level, we also quantified cortical activation in primary motor cortex (M1), pre‐motor cortex (PM), supplemental motor area (SMA), and primary sensory cortex (S1) of both hemispheres (Fig. 4 A and B). We found that the increase in contralesional cortical activity seen during increasing flexion synergy expression was driven primarily by increased activation of the contralesional M1 and PM regions, which were significantly increased in adults with stroke compared to control participants at the 25% maximum shoulder abduction load level (Fig. 4 B). By comparison, adults without neurological injury tended to exhibit reductions in cortical activity in these regions as shoulder abduction loading increased. These opposing trends highlight the robustness of the increased contralesional activation with increasing synergy expression, and confirm that our changes in LI were not a mathematical byproduct of stable contralesional cortical activity with reduced ipsilesional activity. Finally, we found a significant positive correlation between greater clinically assessed motor impairment and the degree of contralesional bias in LI values at the Floating and 25% SABD MVT levels (Fig. 5).
Figure 4. Flexion synergy expression is associated with increased cortical activity in contralesional M1 and PM regions.
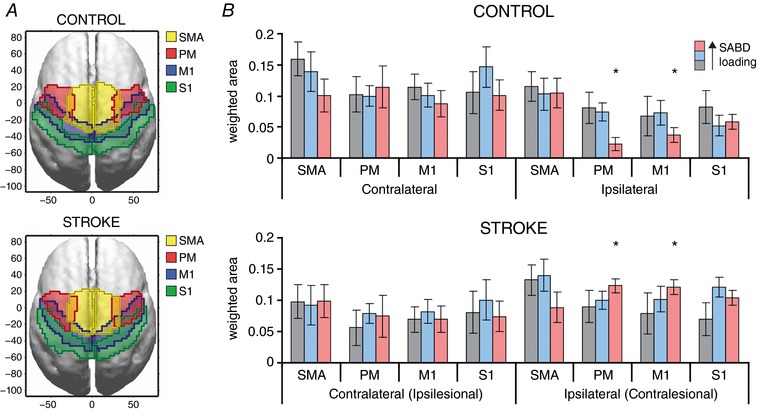
A, outermost boundaries for cortical regions of interest are shown for the control (top) and stroke (bottom) on a normalized brain in MNI coordinates. Boundaries overlapped due to anatomical variations in the locations of these areas across subjects. B, group‐average total strengths of sources within each subregion of the sensorimotor cortices (SMA, PM, M1 and S1) on either the contralateral (ipsilesional) or ipsilateral (contralesional) hemisphere. Grey: Table; Blue: Floating; Pink: 25% SABD MVT. Total strength was significantly different between control and stroke participants for 25% shoulder abduction in the ipsilateral (contralesional) PM and M1 subregions. Unit‐less amplitude reflects within‐subject normalization of activity to individual maximum. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5. Increased contralesional cortical activity is directly related to clinically assessed motor impairment.
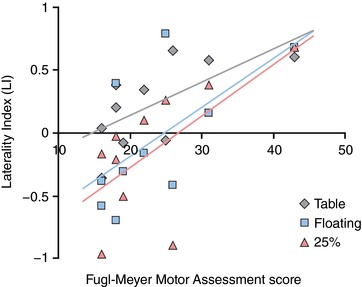
We used linear regression analysis to relate lateralization of cortical activity to FMA impairment score, stratified by the amount of shoulder abduction loading (Table: grey; Floating: blue; 25% SABD MVT: pink). Negative LI values correspond to increased contralesional activity, and lower FMA scores indicate more severe impairment. For the Table condition: R 2 = 0.59, P = 0.09; for Floating R 2 = 0.93, P < 0.001; for 25% SABD MVT: R 2 = 0.57, P = 0.07. We also performed one‐way ANOVAs to compare LI values between two stroke subgroups based on level of impairment: a moderately impaired group consisting of FMA scores between 25–43 and a severely impaired group consisted of FMA scores between 16–19. This analysis reported significantly lower LI values for the severely impaired group in the Table (P = 0.05) and Floating (P = 0.03) conditions but not the 25% SABD MVT condition. [Color figure can be viewed at http://wileyonlinelibrary.com]
ATNR and flexion synergy expression
We measured maximum‐possible circular reaching work area with the head facing forward, rotated towards, and rotated away from the dominant (control) or contralesional (stroke) upper limb. Contralesional arm work area was inversely proportional to shoulder abduction torque regardless of head orientation (due to flexion synergy expression post stroke; Fig. 6 A). However, we found that rotation of the head away from the contralesional arm led to significant additional reductions in reaching work area (P < 0.01) that were indistinguishable across load levels (P = 0.69; overall mean percentage decrease: 10 ± 10%) (Fig. 6 B). Conversely, work area was not a function of either shoulder abduction load or head orientation in the dominant upper limb of control participants (P > 0.05; overall mean percentage change: 0 ± 3%) (Fig. 6 B), nor did rotation of the head towards the contralesional upper limb post stroke lead to significant changes in work area.
Figure 6. Elicitation of the ATNR exacerbates flexion synergy expression post stroke.
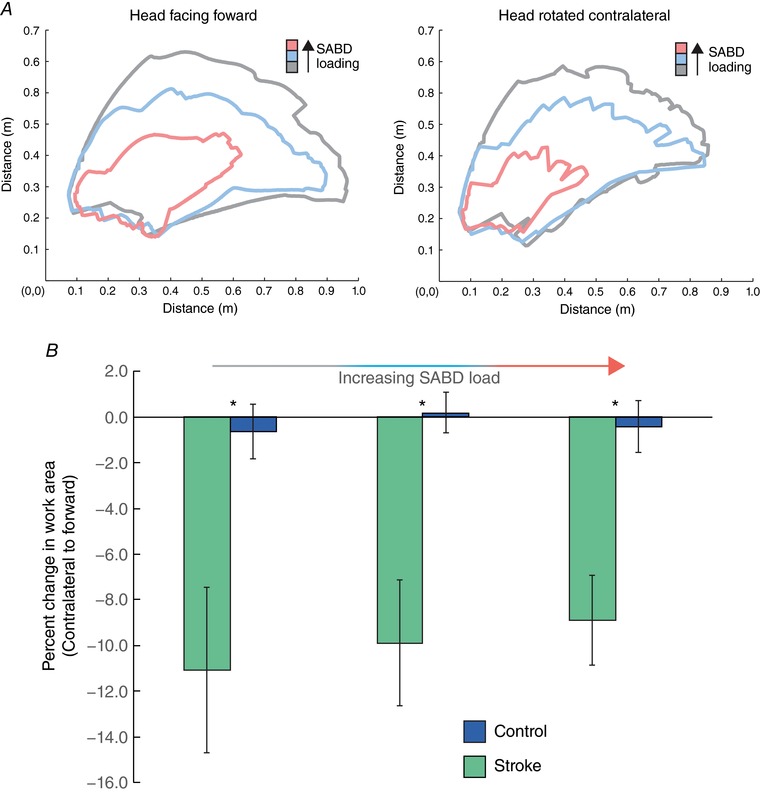
A, representative reaching work area data for an individual stroke participant with the head forward (left) and contralateral (right), for Table (grey), 50% (blue) and 100% (pink) of limb weight. A reduction in reaching work area is visually apparent with contralateral head rotation. B, group summary data illustrating the difference in reaching work area between the head‐forward and head‐rotated conditions, expressed as a percentage of the head‐forward area. Data are presented as mean and standard error of work area (m2) for participants with stroke (green; N = 15) and controls (blue; N = 10) for Table, 50% and 100% of limb weight with the head facing forward and head facing contralateral to the tested arm. A significant overall effect of group was present for contralateral head rotation (P = 0.003), and asterisks indicate a significant difference (P ≤ 0.017 with Bonferroni correction) between groups found by the post hoc pairwise comparisons. There was no effect of shoulder abduction (SABD) load level (P = 0.686) on percentage change in work area with contralateral rotation, nor a significant interaction of shoulder abduction load by group (P = 0.76). [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
Contralesional cortical activation and flexion synergy initiation
In early conceptions of the post‐stroke interhemispheric competition model, reduced ipsilesional motor cortical excitability was presumed to drive tonic disinhibition of contralesional cortex, while increased ipsilesional cortical excitability was associated with decreased contralesional excitability (Netz et al. 1997; Traversa et al. 1998; Fujii & Nakada, 2003; McMorland et al. 2015). Decreased motor‐evoked potentials in the contralesional (i.e. paretic) arm following ipsilesional transcranial magnetic stimulation (TMS) and increased motor‐evoked potentials in the contralesional arm following contralesional TMS (compared with adults without neurological injury) are consistent with this framework (Turton et al. 1996; Schwerin et al. 2008, 2011). Related, suppression of contralesional M1 excitability has been shown to improve motor control in people with mild impairment post stroke during isometric biceps contractions (Bradnam et al. 2012). However, the post‐stroke interhemispheric competition model has recently been challenged by a meta‐analysis of the available literature (McDonnell & Stinear, 2017), which found no consistent evidence of increased contralesional cortical excitability or altered interhemispheric inhibition post stroke. Complicating matters further, considerably less is known about contralesional cortical activity during volitional movements of the paretic limb. Thus, the role of contralesional cortex in flexion synergy expression, if any, has remained unclear.
We tested the prediction that if contralesional cortical activity is associated with flexion synergy expression, then the magnitude of contralesional cortical activity should increase and decrease in direct proportion to the degree that movements of the paretic upper limb are constrained by flexion synergy expression. Our primary finding was a progressive rise in contralesional cortical activity in PM and M1 and a progressive reduction in reaching performance as shoulder abduction loading increased in the paretic arm (evidenced by shifts in LI). We found no such shifts in cortical laterality in participants without neurological injury, where reaching performance was not compromised by increased shoulder abduction loading. In fact, in these participants, cortical activity in PM and M1 ipsilateral to the tested arm tended to decrease as shoulder abduction loading increased. This finding suggests that flexible activation of contralesional cortical regions is a key component of the characteristic graded nature of flexion synergy expression.
ReST motor pathways and flexion synergy expression
For contralesional cortical activity to be involved with initiation of paretic limb movements, it must have a conduit for transmitting motor commands to spinal motor pools. Descending motor pathways of the ReST are one possibility. These pathways originate in nuclei of the ponto‐medullary reticular formation (PMRF), where they receive inputs directly from ipsilateral PM and M1 (Keizer & Kuypers, 1984, 1989; Ugolini & Kuypers, 1986; Matsuyama & Drew, 1997; Kably & Drew, 1998). Motor pathways of the ReST also have substantial projections to ipsilateral spinal motor pools (Nyberg‐Hansen, 1966; Holstege et al. 1979; Matsuyama et al. 1999, 2004), where they convey specific motor commands via direct and indirect connections with spinal motoneurons (Lawrence & Kuypers, 1968; Riddle et al. 2009; Baker, 2011; Zaaimi et al. 2012; McMorland et al. 2015; Mitchell et al. 2016). ReST motor fibres have characteristically diffuse vertical projections within the cervical enlargement (Kuypers, 1964; Sjölund & Björklund, 1982), and they also form the primary input to segmental propriospinal networks (compared to the CST) (Mitchell et al. 2016). Through both of these means, ReST motor fibres drive simultaneous activation of muscle groups spanning multiple joints – a critical component of flexion synergy expression.
We tested the prediction that if the ReST is recruited by contralesional cortex during flexion synergy expression, then brainstem/spinal reflexes known to utilize contralesional ReST motor pathways should become preferentially exaggerated in the paretic musculature due to increased ReST excitability. The ATNR recruits ReST motor pathways via feedback from cervical spine joint receptors and muscle spindle afferents from perivertebral muscles onto the nucleus gigantocellularis of the PMRF (McCough et al. 1950; Bakker & Richmond, 1982; Chan et al. 1987). Thus, we used the ATNR as the basis for this investigation.
We found that when participants with moderate to severe post‐stroke hemiparesis rotated their head away from the paretic arm, there was a statistically significant reduction in reaching range of motion in the paretic arm (in addition to the effects of flexion synergy expression). Because ATNR elicitation hinges upon activation of the PMRF opposite the direction of head rotation (Srivastava et al. 1983), unmasking of the ATNR with ipsilesional head rotation indicates that ReST motor pathways originating on the same side as the paretic limb are upregulated/active during flexion synergy expression. Conversely, we found that rotation of the head towards the paretic arm, or rotation in any direction for participants without neurological injury, resulted in no significant changes in work area. Although the classical ATNR pattern would predict increased extension ability in the paretic arm with contralesional head rotation, this would require activation of the ipsilesional PMRF. Given the direct effects of stroke‐induced damage to the internal capsule, it is likely that the lack of increased extension ability during ipsilateral head rotation in the paretic limb resulted from a decreased ipsilesional cortico‐reticular drive.
Potential role of other motor pathways in flexion synergy expression
Because our study was not specifically designed to investigate potential contributions of the vCST, the rubrospinal tract (RuST), or the VeST to flexion synergy expression, their role, if any, remains unclear. However, the documented structure–function of these pathways appears to be less suitable for driving flexion synergy expression than that of the contralesional ReST. For example, although the vCST can account for up to 30% of all CST fibres in humans (Nathan et al. 1990), only ∼10% of these fibres terminate in spinal lamina IX, with still fewer synapsing directly with motoneurons (Jankowska & Edgley, 2006). Indeed, the majority of vCST fibres terminate on commissural interneurons in lamina VIII, where they subsequently impact contralateral motor pools (Jankowska & Edgley, 2006). And, because the ipsilesional vCST is highly susceptible to stroke‐induced damage, its crossed actions on contralesional motor pools are likely to be diminished, not enhanced. The RuST likewise mediates predominantly contralateral effects on spinal motoneurons, decussating in the ventral tegmentum before terminating contralaterally. Thus, the contralesional RuST is also an unlikely contributor. Interestingly, however, the ipsilesional RuST may play an important role in motor recovery post stroke, particularly for the hand, and warrants continued investigation (Ruber et al. 2012; Takenobu et al. 2014; Owen et al. 2017). Regarding the VeST, the majority of direct cortico‐vestibular inputs appear to arise in primary somatosensory cortex (Brodmann areas 2, 3a) and PM cortex, not primary motor cortex (Licata et al. 1987; Akbarian et al. 1993, 1994; Wilson et al. 1999). Nevertheless, our finding of increased contralesional PM activity during flexion synergy expression, combined with VeST–ReST inter‐connectivity, suggests that VeST pathways cannot be excluded from consideration. Finally, the direct effects of ReST collaterals on contralateral motoneurons are both weak and seen in only ∼10% of cells (Jankowska et al. 2003). This implies that the ipsilesional ReST is less critical for flexion synergy expression than the contralesional ReST, which is consistent with our ATNR results.
Limitations
Because our assays of ReST activity in humans were necessarily indirect, we cannot unequivocally state that flexion synergy expression requires progressive recruitment of contralesional cortico‐reticulospinal pathways. However, when considered with the known structure–function of ReST motor pathways in non‐human primates (Holstege & Kuypers, 1987; Buford & Davidson, 2004; Davidson & Buford, 2006; Baker, 2011), recent work in humans showing increased ReST white matter integrity in individuals with severe synergy expression post stroke (Owen et al. 2017), and the presence of long‐latency ipsilateral MEPs in the paretic arm (Schwerin et al. 2008), our findings strongly suggest that contralesional cortico‐reticulospinal pathways are active during flexion synergy expression. Future work using targeted neuropharmacological probes that modify the neuromodulatory component of the ReST are warranted to further increase our understanding of the potential contribution of this system to flexion synergy expression.
Clinical implications and conclusions
We have previously shown that neurorehabilitation approaches challenging people to work against the flexion synergy pattern while progressively increasing shoulder abduction loading can lead to significant improvements in reaching ability (Ellis et al. 2009a, b), potentially by promoting use of the remaining ipsilesional CST. Suppression of contralesional cortical excitability via non‐invasive brain stimulation has also shown promise for improving control of paretic limb muscles, ostensibly by the same mechanism (Bradnam et al. 2012). Given these early successes, combinatorial strategies that simultaneously leverage the benefits of these approaches hold considerable promise for driving further functional gains, and may have the additional benefit of reducing other post‐stroke motor abnormalities.
In conclusion, we interpret the increased contralesional cortical activation and putative recruitment of ReST pathways post stroke as an attempt to increase descending neural drive to the paretic limb as spared ipsilesional motor resources become exhausted. Although this appears to be an adaptive strategy intended to preserve shoulder abduction torque production, it comes at the cost of fine, fractionated motor control. Indeed, movement is produced, but it becomes progressively constrained to the stereotypical multi‐joint pattern characteristic of the flexion synergy (highlighted by the correlation between LI and clinically assessed motor impairment; Fig. 5). Nevertheless, the benefits of preserving some movement capability – even if coarse – would seem to outweigh the potential alternative of paralysis.
Additional information
Competing interests
The authors have no financial or other conflicts of interest to disclose.
Author contributions
Experiments were conducted at Northwestern University. Authors contributions are as follows: J.G.M., A.C., M.D.E. and J.Y. designed and conducted experiments and analysed and interpreted data; C.J.H. and J.P.A.D. designed and supervised all experiments, and interpreted data; all authors contributed to preparation of the manuscript. All authors approve the final version of the manuscript, agree to be accountable for its contents, agree that all persons designated as authors qualify for authorship, and agree that all who qualify for authorship are listed.
Funding
National Institutes of Health grants: R01NS054269, R01HD047569, and R01HD039343.
Translational perspective
Precise, dexterous movements are a central component of primate motor control. However, following unilateral brain injury resulting from a stroke, cerebral palsy, or focal brain trauma, this ability is often severely compromised opposite the lesion. Complicating the matter further, the loss of fine motor control distally – for example, at the hand – is exacerbated as proximal muscles are driven at a greater proportion of their maximum. Our results provide the first systematic, multi‐modal evidence in humans that the constraint to grouped movement patterns reflects both a progressive recruitment of contralesional cortical regions and an increased reliance on brainstem motor pathways that are incapable of producing discrete movements. These findings depart from the standard notion that utilization of contralesional motor resources is statically increased or decreased post injury, and have important translational implications. Namely, we found that the degree of contralesional cortical activity during activation of shoulder muscles was directly correlated with clinically assessed motor impairment post stroke. Thus, our results motivate therapeutic strategies promoting continued use of viable regions of the ipsilesional hemisphere (i.e. restorative interventions more so than compensatory approaches), because the adaptive strategy of recruiting contralesional motor resources appears to preserve low‐level function at the cost of fine control. In summary, rehabilitative strategies that rationally incorporate physical therapy, targeted neuropharmacology, and emerging technologies to aid spared ipsilesional resources are likely to best address the underlying mechanisms of impairment and enhance recovery.
Acknowledgements
The authors thank Carolina Carmona, PT, DPT, and Justin Drogos, PT, DPT for participant recruitment, assistance with data collection and coordination of study scheduling. The authors also thank Natasha Panzarella, PT, DPT, Victoria Jones, PT, DPT, Aaron Buchler, PT, DPT, and Kathleen Burke, PT, DPT for assistance with data collection.
Biographies
Jacob McPherson holds a BS in Biomedical Engineering from the University of North Carolina and MS and PhD degrees in Biomedical Engineering from Northwestern University. He completed post‐doctoral fellowships in Physiology and Biophysics at the University of Washington, where he was a Sackler Foundation Scholar of Integrative Biophysics, and in Physical Therapy at Northwestern University. Currently, he is an Assistant Professor of Biomedical Engineering at Florida International University.

Albert Chen holds BS and MS degrees in Electrical Engineering from Stanford University, and a PhD in Biomedical Engineering from Northwestern University. He is currently Senior Manager of Engineering at athenahealth, Inc.
J. G. McPherson and A. Chen contributed equally to this work.
Edited by: Janet Taylor & Richard Carson
References
- Akbarian S, Grusser OJ & Guldin WO (1993). Corticofugal projections to the vestibular nuclei in squirrel monkeys: further evidence of multiple cortical vestibular fields. J Comp Neurol 332, 89–104. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Grusser OJ & Guldin WO (1994). Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey. J Comp Neurol 339, 421–437. [DOI] [PubMed] [Google Scholar]
- Bai X, Towle VL, He EJ & He B (2007). Evaluation of cortical current density imaging methods using intracranial electrocorticograms and functional MRI. Neuroimage 35, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN (2011). The primate reticulospinal tract, hand function and functional recovery. J Physiol 589, 5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker DA & Richmond FJ (1982). Muscle spindle complexes in muscles around upper cervical vertebrae in the cat. J Neurophysiol 48, 62–74. [DOI] [PubMed] [Google Scholar]
- Baumann SB, Wozny DR, Kelly SK & Meno FM (1997). The electrical conductivity of human cerebrospinal fluid at body temperature. IEEE Trans Biomed Eng 44, 220–223. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Barber PA & Byblow WD (2012). Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex 22, 2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnstrom S ( 1970). Movement Therapy in Hemiplegia: A Neurophysiological Approach. Medical Dept., New York. [Google Scholar]
- Buford JA & Davidson AG (2004). Movement‐related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159, 284–300. [DOI] [PubMed] [Google Scholar]
- Chan YS, Kasper J & Wilson VJ (1987). Dynamics and directional sensitivity of neck muscle spindle responses to head rotation. J Neurophysiol 57, 1716–1729. [DOI] [PubMed] [Google Scholar]
- Colebatch JG ( 2007). Bereitschaftspotential and movement‐related potentials: origin, significance, and application in disorders of human movement. Mov Disord 22, 601–610. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP & Rosen BR (1997). A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 28, 2518–2527. [DOI] [PubMed] [Google Scholar]
- Davidson AG & Buford JA (2006). Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173, 25–39. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS & Rymer WZ (1995). Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 118, 495–510. [DOI] [PubMed] [Google Scholar]
- Ellis MD, Drogos J, Carmona C, Keller T & Dewald JP (2012). Neck rotation modulates flexion synergy torques, indicating an ipsilateral reticulospinal source for impairment in stroke. J Neurophysiol 108, 3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MD, Lan Y, Yao J & Dewald JP (2016). Robotic quantification of upper extremity loss of independent joint control or flexion synergy in individuals with hemiparetic stroke: a review of paradigms addressing the effects of shoulder abduction loading. J Neuroeng Rehabil 13, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MD, Sukal T, DeMott T & Dewald JP (2008). Augmenting clinical evaluation of hemiparetic arm movement with a laboratory‐based quantitative measurement of kinematics as a function of limb loading. Neurorehabil Neural Repair 22, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MD, Sukal‐Moulton T & Dewald JP (2009a). Progressive shoulder abduction loading is a crucial element of arm rehabilitation in chronic stroke. Neurorehabil Neural Repair 23, 862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MD, Sukal‐Moulton TM & Dewald JP (2009b). Impairment‐based 3‐D robotic intervention improves upper extremity work area in chronic stroke: targeting abnormal joint torque coupling with progressive shoulder abduction loading. IEEE Trans Robot 25, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster O (1936). Motorische Felder und Bahnen In Handbuch der Neurologie, eds. Bumke O. & Foerster O, pp. 1–357. Springer‐Verlag, Berlin. [Google Scholar]
- Fujii Y & Nakada T (2003). Cortical reorganization in patients with subcortical hemiparesis: neural mechanisms of functional recovery and prognostic implication. J Neurosurg 98, 64–73. [DOI] [PubMed] [Google Scholar]
- Geddes LA & Baker LE (1967). The specific resistance of biological material–a compendium of data for the biomedical engineer and physiologist. Med Biol Eng 5, 271–293. [DOI] [PubMed] [Google Scholar]
- Grova C, Daunizeau J, Lina JM, Benar CG, Benali H & Gotman J (2006). Evaluation of EEG localization methods using realistic simulations of interictal spikes. Neuroimage 29, 734–753. [DOI] [PubMed] [Google Scholar]
- Herbert WJ, Davidson AG & Buford JA (2010). Measuring the motor output of the pontomedullary reticular formation in the monkey: do stimulus‐triggered averaging and stimulus trains produce comparable results in the upper limbs? Exp Brain Res 203, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG & Boer RC (1979). Anatomical evidence for direct brain stem projections to the somatic motoneuronal cell groups and autonomic preganglionic cell groups in cat spinal cord. Brain Res 171, 329–333. [DOI] [PubMed] [Google Scholar]
- Holstege JC & Kuypers HG (1987). Brainstem projections to spinal motoneurons: an update. Neuroscience 23, 809–821. [DOI] [PubMed] [Google Scholar]
- Jankowska E & Edgley SA (2006). How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 12, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K & Edgley SA (2003). Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci 23, 1867–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kably B & Drew T (1998). Corticoreticular pathways in the cat. I. Projection patterns and collaterization. J Neurophysiol 80, 389–405. [DOI] [PubMed] [Google Scholar]
- Keizer K & Kuypers HG (1984). Distribution of corticospinal neurons with collaterals to lower brain stem reticular formation in cat. Exp Brain Res 54, 107–120. [DOI] [PubMed] [Google Scholar]
- Keizer K & Kuypers HG (1989). Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res 74, 311–318. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH & Deecke L (1965). [Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials.]. Pflugers Arch Gesamte Physiol Menschen Tiere 284, 1–17. [PubMed] [Google Scholar]
- Kuypers HG ( 1964). The descending pathways to the spinal cord, their anatomy and function. Prog Brain Res 11, 178–202. [DOI] [PubMed] [Google Scholar]
- Lan Y, Yao J & Dewald JPA (2017). The impact of shoulder abduction loading on volitional hand opening and grasping in chronic hemiparetic stroke. Neurorehabil Neural Repair 31, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SK (1993). Thickness and resistivity variations over the upper surface of the human skull. Brain Topogr 6, 99–109. [DOI] [PubMed] [Google Scholar]
- Lawrence DG & Kuypers HG (1968). The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain‐stem pathways. Brain 91, 15–36. [DOI] [PubMed] [Google Scholar]
- Licata F, Li Volsi G, Maugeri G & Santangelo F (1987). Integration of cortical and peripheral information in the lateral vestibular nucleus in the cat. Neurosci Lett 77, 293–297. [DOI] [PubMed] [Google Scholar]
- McCough CG, Ling TH & Deering ID (1950). Location of receptors for tonic neck reflexes. Am J Med Sci 219, 347. [PubMed] [Google Scholar]
- McDonnell MN & Stinear CM (2017). TMS measures of motor cortex function after stroke: a meta‐analysis. Brain Stimul 10, 721–734. [DOI] [PubMed] [Google Scholar]
- McMorland AJ, Runnalls KD & Byblow WD (2015). A neuroanatomical framework for upper limb synergies after stroke. Front Hum Neurosci 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson JG, Ellis MD, Heckman CJ & Dewald JP (2008). Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol 100, 3236–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K & Drew T (1997). Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracer Phaseolus vulgaris‐leucoagglutinin. J Comp Neurol 389, 617–641. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B & Mori S (1999). Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA‐L. J Comp Neurol 410, 413–430. [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M & Mori S (2004). Locomotor role of the corticoreticular‐reticulospinal‐spinal interneuronal system. Prog Brain Res 143, 239–249. [DOI] [PubMed] [Google Scholar]
- Miller LC & Dewald JP (2012). Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol 123, 1216–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EJ, McCallum S, Dewar D & Maxwell DJ (2016). Corticospinal and reticulospinal contacts on cervical commissural and long descending propriospinal neurons in the adult rat spinal cord; evidence for powerful reticulospinal connections. PLoS One 11, e0152094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW, Smith MC & Deacon P (1990). The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain 113, 303–324. [DOI] [PubMed] [Google Scholar]
- Netz J, Lammers T & Homberg V (1997). Reorganization of motor output in the non‐affected hemisphere after stroke. Brain 120, 1579–1586. [DOI] [PubMed] [Google Scholar]
- Nyberg‐Hansen R (1966). Functional organization of descending supraspinal fibre systems to the spinalcord. Anatomical observations and physiological correlations. Ergeb Anat Entwicklungsgesch 39, 3–48. [PubMed] [Google Scholar]
- Owen M, Ingo C & Dewald JPA (2017). Upper Extremity motor impairments and microstructural changes in bulbospinal pathways in chronic hemiparetic stroke. Front Neurol 8, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual‐Marqui RD, Esslen M, Kochi K & Lehmann D (2002). Functional imaging with low‐resolution brain electromagnetic tomography (LORETA): a review. Methods Find Exp Clin Pharmacol 24 (Suppl. C), 91–95. [PubMed] [Google Scholar]
- Pascual‐Marqui RD, Michel CM & Lehmann D (1994). Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18, 49–65. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Anderson ME & Filion M (1974). Responses of ponto‐medullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res 21, 19–44. [DOI] [PubMed] [Google Scholar]
- Puig J, Blasco G, Daunis IEJ, Thomalla G, Castellanos M, Figueras J, Remollo S, van Eendenburg C, Sanchez‐Gonzalez J, Serena J & Pedraza S (2013). Decreased corticospinal tract fractional anisotropy predicts long‐term motor outcome after stroke. Stroke 44, 2016–2018. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA & Baker SN (2009). Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29, 4993–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruber T, Schlaug G & Lindenberg R (2012). Compensatory role of the cortico‐rubro‐spinal tract in motor recovery after stroke. Neurology 79, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN & Makris N (2009). Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 30, 3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M & MacKinnon C (2008). Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res 185, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerin SC, Yao J & Dewald JP (2011). Using paired pulse TMS to facilitate contralateral and ipsilateral MEPs in upper extremity muscles of chronic hemiparetic stroke patients. J Neurosci Methods 195, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell M (2009). The tripartite origins of the tonic neck reflex: Gesell, Gerstmann, and Magnus. Neurology 72, 850–853. [DOI] [PubMed] [Google Scholar]
- Shibasaki H & Hallett M (2006). What is the Bereitschaftspotential? Clin Neurophysiol 117, 2341–2356. [DOI] [PubMed] [Google Scholar]
- Sjölund BH & Björklund A (1982). Brain Stem Control of Spinal Mechanisms: Proceedings of the 1st Eric K. Fernström Symposium, held in Lund (Sweden) on 10–13 November 1981. Elsevier Biomedical Press, New York. [Google Scholar]
- Srivastava UC, Manzoni D, Pompeiano O & Stampacchia G (1983). Frequency response of medullary reticulospinal neurons to sinusoidal rotation of the neck. Adv Otorhinolaryngol 30, 302–305. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK & Byblow WD (2007). Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 130, 170–180. [DOI] [PubMed] [Google Scholar]
- Sukal TM, Ellis MD & Dewald JP (2007). Shoulder abduction‐induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res 183, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenobu Y, Hayashi T, Moriwaki H, Nagatsuka K, Naritomi H & Fukuyama H (2014). Motor recovery and microstructural change in rubro‐spinal tract in subcortical stroke. Neuroimage Clin 4, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M & Rossini PM (1998). Follow‐up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Res 803, 1–8. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C & Lemon RN (1996). Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol 101, 316–328. [DOI] [PubMed] [Google Scholar]
- Twitchell TE ( 1951). The restoration of motor function following hemiplegia in man. Brain 74, 443–480. [DOI] [PubMed] [Google Scholar]
- Ugolini G & Kuypers HG (1986). Collaterals of corticospinal and pyramidal fibres to the pontine grey demonstrated by a new application of the fluorescent fibre labelling technique. Brain Res 365, 211–227. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Zarzecki P, Schor RH, Isu N, Rose PK, Sato H, Thomson DB & Umezaki T (1999). Cortical influences on the vestibular nuclei of the cat. Exp Brain Res 125, 1–13. [DOI] [PubMed] [Google Scholar]
- Yao J & Dewald JP (2005). Evaluation of different cortical source localization methods using simulated and experimental EEG data. Neuroimage 25, 369–382. [DOI] [PubMed] [Google Scholar]
- Yeo SS, Chang MC, Kwon YH, Jung YJ & Jang SH (2012). Corticoreticular pathway in the human brain: diffusion tensor tractography study. Neurosci Lett 508, 9–12. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS & Baker SN (2012). Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135, 2277–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]


