Abstract
Neurogenic tumours of the mediastinum in adults occur most often at the posterior mediastinum, majority of which are benign of nerve sheath in origin. A 72-year-old woman, known asthmatic, presented with chronic symptoms of hoarseness, dysphagia, chest heaviness, easy fatigability, cough, epigastric pain, feeling of abdominal fullness and choking with food intake and at a supine position. Treated for other disorders, routine chest X-ray incidentally found a homogenous convex radiodensity at the right paratracheal area; mass which was also observed with CT and 18F-fludeoxyglucose-positron emission tomography/CT scan studies. Mediastinoscopy with biopsy showed spindle to plump cells with strong S100 positivity. Thoracoscopic surgery done to completely excise the mass found it to be benign schwannoma.
Keywords: neural sheath tumour, neurilemmoma, schwannoma
Background
Previous medical history in a patient is important in history taking. However, in patients who are presenting with common symptoms attributable to the medical history, refractory to usual treatment and additional atypical presentations should prompt the search of another possible diagnosis.
In this case report, we describe the importance of increased suspicion for another possible diagnosis. In this case, a mass was undetected early on in a patient known to have asthma and gastro-oesophageal reflux disease with multiple chronic progressive and refractory symptoms.
Case presentation
A 72-year-old woman, known asthmatic, was incidentally found to have a homogeneous convex radiodensity at the right paratracheal area during routine chest X-ray. She had been chronically complaining of hoarseness, dysphagia, easy fatigability, chest heaviness, cough, epigastric pain with sour taste in the mouth, feeling of abdominal fullness and choking with food intake and at a supine position. She had medical history of asthma, gastrointestinal gastro-oesophageal reflux disease, hepatitis B carrier and goitre. Her mother died of hepatocellular carcinoma and her father died of thyroid cancer and tuberculosis. She is a non-smoker, non-alcoholic beverage drinker and denied illicit drug use.
Investigations
Prior to the chest X-ray with positive finding, upper gastrointestinal series, chest X-ray, two-dimensional echocardiography, dobutamine stress test and CT angiogram done were essentially normal. She was treated with salbutamol and proton pump inhibitor, both of which provided only temporary relief of the cough and choking sensation.
With the latest chest X-ray finding, CT scan with contrast was done which showed 3.4×3.3×5.0 cm (AP x W x H) non-enhancing ovoid density with central hypodense area along the right paratracheal region (figure 1). 18F-fludeoxyglucose-positron emission tomography (FDG-PET)/CT scan was then done which showed mildly enhancing soft tissue density with central area of necrosis in the right paratracheal region measuring 4.1×3.8×4.9 cm. The said density was noted to mildly efface the brachiocephalic veins, brachiocephalic artery, left common carotid and left subclavian artery. No invasion or significant compression of vessels was noted. The trachea and mainstem bronchi are patent with no endobronchial lesion seen. The lesion was noted to have increased FDG uptake except centrally. Also, thyroid function tests done were normal.
Figure 1.
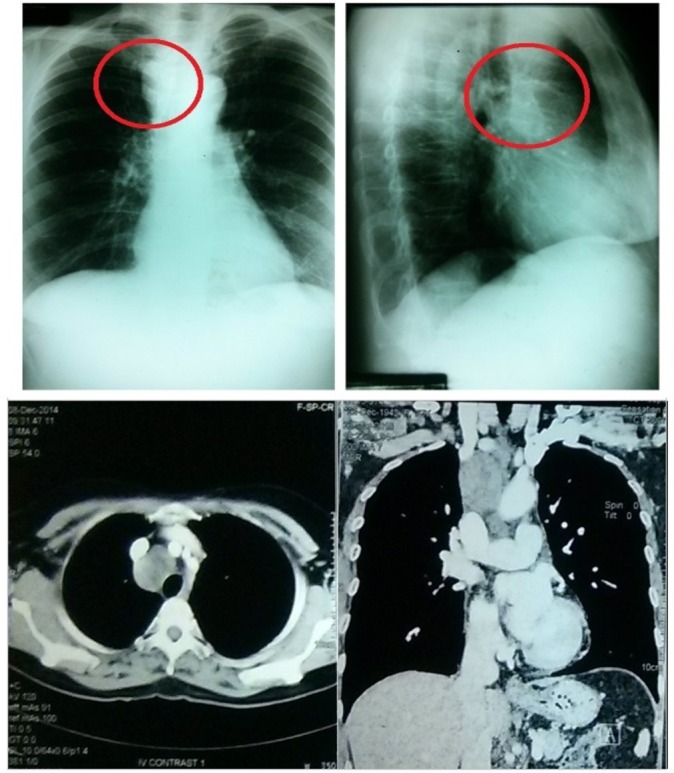
Imaging studies. In the chest X-ray, encircled is the right paratracheal mass. CT chest with contrast also showed this non-enhancing ovoid density with central hypodensity.
Differential diagnosis
Given the age, the previous medical history of tuberculosis and goitre, the family history of her father’s thyroid cancer and the presence of central necrosis with above studies, the differential diagnoses were lymphoma, ectopic thyroid mass, tuberculoma and sarcoidosis.
Treatment
Biopsy was then the next approach. Options presented to the patient were endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), mediastinoscopy and video-assisted thoracoscopic surgery (VATS) with possible thoracotomy.
Despite reported use of EBUS-TBNA, this option was excluded because of the possible hypoxia as a complication in this patient with known asthmatic condition. VATS with possible thoracotomy was also excluded due to the patient’s fear of thoracotomy procedure invasiveness.
Tan-cream to brown, irregular, soft to firm tissues were sampled via mediastinoscopy. Histologically, the specimen showed mildly cellular neoplasm composed of spindle to plump cells that are strongly S100 positive and smooth muscle actin negative. Hence, neural tumour with degenerative changes was reported.
Immediately after the procedure, the patient noted decrease in chest heaviness with ability to inhale deeply without coughing. Because of this, complete resection was opted.
Prior to complete resection, upper endoscopy was done which showed oesophagitis, chronic atrophic gastritis, gastric polyps and acute gastric mucosal erosions at the antrum. She was then treated with proton pump inhibitor, prokinetic and cytoprotective.
During VATS, a firm encapsulated oval mass that measured 4.5×3.0×2.0 cm was seen on the right anterior aspect of the trachea. The mass was adherent but easily dissected from the superior vena cava and azygous vein. The mass was complex with both solid and cystic components (figure 2).
Figure 2.
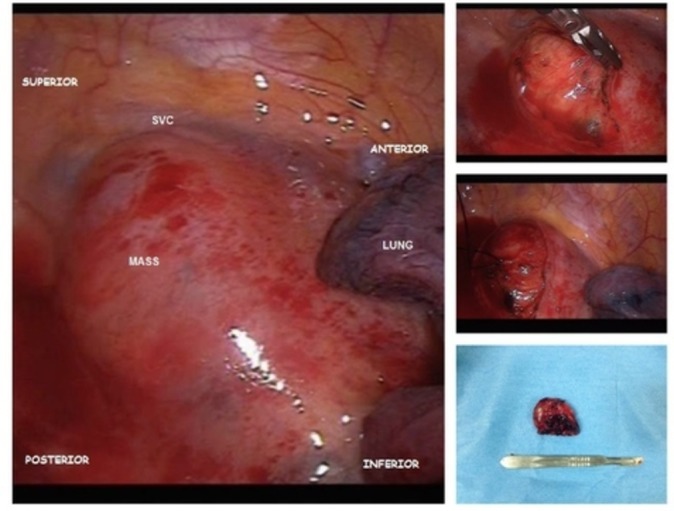
Mediastinal mass intraoperatively and postoperatively. SVC, superior vena cava.
Excised mass submitted to pathology was described to be encapsulated light to dark brown fairly ovoid rubbery tissue that measured 4.2×3.8×1.7 cm. Microscopically, the mass showed biphasic pattern with predominant cellular compact areas and loose spongy areas. The cellular compact area showed interlacing fascicles of elongated spindle cells with areas of nuclear palisading arranged in linear stocks. Also seen were focal areas of haemorrhage with numerous haemosiderophages and foamy histiocytes (figure 3).
Figure 3.
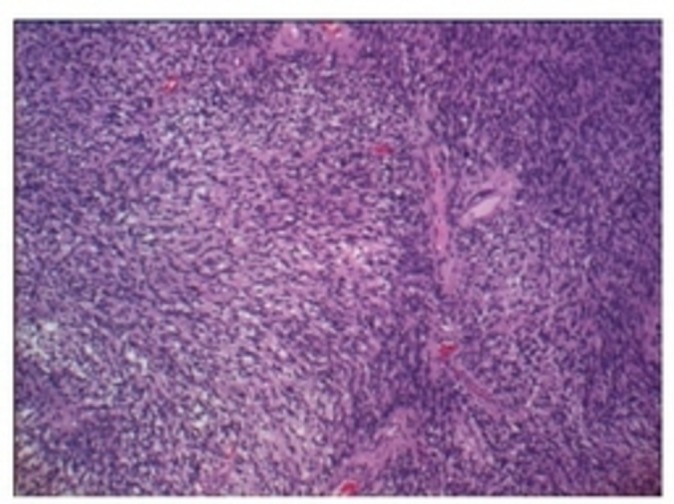
Microscopic findings of the mass with biphasic pattern.
Outcome and follow-up
Postoperatively, patient reported increased vocal amplitude, resolution of choking sensation, decreased cough frequency and ability to inhale deeply and resolution of chest heaviness. Two years postoperatively, repeat CT chest imaging showed no recurrence of the mediastinal mass.
Discussion
Mediastinal tumours encompass a wide spectrum of diseases that can only be definitively diagnosed histologically. With the advancement of imaging, several studies were made to assist in diagnosing these masses anatomically (table 1) and radiographically (table 2).
Table 1.
Differential diagnosis of a mediastinal tumour by anatomic location22
| Anterior | Middle | Posterior |
| Thymoma | Lymphoma | Neurogenic tumour |
| Teratoma, seminoma | Pericardial cyst | Bronchogenic cyst |
| Lymphoma | Bronchogenic cyst | Enteric cyst |
| Carcinoma | Metastatic cyst | Xanthogranuloma |
| Parathyroid adenoma | Systemic granuloma | Diaphragmatic hernia |
| Intrathoracic goitre | Meningocele | |
| Lipoma | Paravertebral abscess | |
| Lymphangioma | ||
| Aortic aneurysm |
Table 2.
Radiographic differential diagnosis of a mediastinal tumour23
| Fatty | Cystic | Solid | Uncommon |
| Lipoma | Bronchogenic cyst | Mediastinal goitre | Parathyroid adenoma |
| Thymolipoma | Duplication cyst | Thymic hyperplasia | Haematoma |
| Mediastinal neuroenteric cyst | Thymoma | Haemangioma | |
| Pericardial cyst | Thymic carcinoma | Sarcoma | |
| Thymic cyst | Thymic carcinoids | Extramedullary haematopoiesis | |
| Lymphangioma | Lymphoproliferative disorders |
Fibrosing mediastinitis | |
| Pancreatic pseudocyst | Germ cell tumours | ||
| Neurogenic tumours |
Most often, these mediastinal tumours are incidentally found. Patients are often asymptomatic and, when present, are non-specific. Usually, symptoms presented themselves secondary to the enlarging mass in relation to its neighbouring structures. Hence, symptoms range from none to life-threatening respiratory compromise.
Mediastinal tumours may either be benign or malignant; more than two-thirds of which are benign,1 but patients with symptoms at presentation are more likely to be malignant.2
For adults, the most common mediastinal tumours are primary thymic neoplasms, thyroid masses and lymphoma.3 Although definite consensus on mediastinal neurogenic tumours as to its age and gender predilections are conflicting, studies are in agreement that neurogenic tumours are most commonly found in the posterior mediastinum.
Neurogenic tumours constitute 21% of all primary mediastinal tumours in adults,4 and are grouped into nerve sheath, ganglion cell and paraganglion cell tumours.5 Of the three, nerve sheath tumours are the most common.
Mediastinal nerve sheath tumours commonly originate from intercostal nerves and sympathetic chain situated at the posterior mediastinum. But recent studies documented nerve sheath tumours to arise, though less frequently, from the vagus nerve,6 phrenic nerve,7 recurrent nerve8 and pulmonary artery,9 areas outside of the posterior mediastinum.
The majority of studies report that neural sheath tumours affect both genders in the 20–50 age groups.10 11 However, these tumours are more common in women.12
Nerve sheath tumours can either be benign or malignant, with the malignant variant to be less common. The benign variant has several subtypes, of which schwannoma and neurofibroma are the most common.13
Schwannomas or neurilemmomas are tumours that originate from Schwann cells derived from neural crest.14 They are slow-growing mass with low potential for malignant transformation.9 Described to be encapsulated and well-circumscribed mass that are often solitary, when it exists as multiple masses, they occur with neurofibromatosis.
Similar to other mediastinal masses, mediastinal schwannomas are clinically asymptomatic unless enlarged that causes compression of nearby structures. With the combined natures of being slow growing and asymptomatic, these tumours are often incidentally found radiographically for other purposes.
When large enough, chest X-ray shows mediastinal schwannomas as homogenous opacity. However, CT and MRI are better tools in assisting in the diagnosis of these mediastinal masses and for preoperative planning through the characteristics of the mass and its relationship to neighbouring structures.
On CT, schwannomas are well-defined homogenous masses that appear heterogeneous with intravenous contrast administration,15 with low attenuation areas representing hypocellularity or cystic degeneration.16 On the other hand, schwannomas on MRI show heterogeneous signal intensities on T1-weighted and T2-weighted sequences, with hyperintensity in T2 images indicating cystic degeneration.15
Another imaging study that has additional advantage of knowing the metabolic activity of masses is the FDG-PET. There have been recent interests in the application of FDG-PET in mediastinal mass.17 Though increase uptake in FDG-PET may lean towards malignancy, other more benign conditions such as infection and inflammatory processes are also possible. And in schwannomas specifically, FDG uptake is variable.18
However, imaging studies does not give a definitive diagnosis. Because mediastinal schwannomas are less common especially outside that of the posterior mediastinum, options are usually sought first to determine the histology of the mass. Biopsy can be done via mediastinoscopy, EBUS-TBNA or endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Another option is to do intraoperative frozen section of the mass during VATS.
Among the four above-mentioned options, mediastinoscopy and VATS are more invasive than EBUS-TBNA and EUS-FNA, thus making the use of the latter two more attractive.
EBUS-TBNA and EUS-FNA are two different techniques that compliment one another. EUS-FNA allows better access to the posterior mediastinum that cannot be accessed by EBUS-TBNA, while EBUS-TBNA is well established in sampling subcarinal and right paratracheal areas.19 However, combination of both still limits the access of the anterior mediastinum.20 And despite their less invasiveness, they are not without any complications such as hypoxia and bleeding.21
The definitive treatment for benign schwannomas is surgical resection with rare recurrence rate.22 However, its benign nature causes some to advice a more conservative approach.14 There are several surgical approaches that can be used, such as VATS, sternotomy and thoracotomy. But what dictates one approach over the other depends on both the anatomic location of the mass and surgical expertise.
Histologically, schwannomas show mixture of two distinct patterns, Antoni A and B. Antoni A areas are highly cellular consisting of spindle cells, while Antoni B areas are hypocellular in connective tissue stroma. In addition, Antoni B areas are noted to be prone to degeneration. Immunohistochemically, schwannomas show strong S100 positivity.9
Learning points.
Schwannoma is rare in the location of the middle mediastinum. It is a benign and indolent type of mass that is easy to miss.
For patients with asthma and gastro-oesophageal reflux disease (GORD) that are refractory to treatment, workup should be considered to rule out another diagnosis.
Symptoms not typical of asthma and GORD such as chest pain and choking episodes, respectively, can be indicators of other underlying conditions.
In working up mediastinal mass, there are several alternatives available with its own advantages and disadvantages.
Mediastinal mass that is found to be benign schwannoma, has a good prognosis with improvement of symptomatologies.
Footnotes
Contributors: PD, the main author, wrote the paper. CL, the surgery resident, contributed to the images, involved in the care of the patient and provided information of the workup and management. JS, the thoracic surgeon, operated on the patient.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893–909. 10.1378/chest.128.4.2893 [DOI] [PubMed] [Google Scholar]
- 2.Davis RD, Oldham HN, Sabiston DC. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 1987;44:229–37. 10.1016/S0003-4975(10)62059-0 [DOI] [PubMed] [Google Scholar]
- 3.Laurent F, Latrabe V, Lecesne R, et al. Mediastinal masses: diagnostic approach. Eur Radiol 1998;8:1148–59. 10.1007/s003300050525 [DOI] [PubMed] [Google Scholar]
- 4.Nason KS, Maddaus MA, Luketich JD, et al. Chest wall, lung, mediastinum, and pleura : Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG. Schwartz’s principles of surgery, 9th edition. New York: McGraw-Hill, 2010. [Google Scholar]
- 5.Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest 1997;112:1344–57. [DOI] [PubMed] [Google Scholar]
- 6.Rammos KS, Rammos SK, Foroulis CN, et al. Schwannoma of the vagus nerve, a rare middle mediastinal neurogenic tumor: case report. J Cardiothorac Surg 2009;4:68 10.1186/1749-8090-4-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza B, Lowe A, Stewart B, et al. A rare case of Schwannoma of the intrathoracic phrenic nerve. ANZ J Surg 2010;80:841–2. 10.1111/j.1445-2197.2010.05504.x [DOI] [PubMed] [Google Scholar]
- 8.Sasaki K, Kohno T, Mun M, et al. Thoracoscopic removal of middle mediastinal schwannoma originating from recurrent nerve. Thorac Cardiovasc Surg 2008;56:375–7. 10.1055/s-2008-1038471 [DOI] [PubMed] [Google Scholar]
- 9.Elstner K, Granger E, Wilson S, et al. Schwannoma of the pulmonary artery. Heart Lung Circ 2013;22:231–3. 10.1016/j.hlc.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 10.Baranović M, Macan D, Begović EA, et al. Schwannoma with secondary erosion of mandible: case report with a review of the literature. Dentomaxillofac Radiol 2006;35:456–60. 10.1259/dmfr/32200965 [DOI] [PubMed] [Google Scholar]
- 11.Chrysomali E, Papanicolaou SI, Dekker NP, et al. Benign neural tumors of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:381–90. 10.1016/S1079-2104(97)90036-6 [DOI] [PubMed] [Google Scholar]
- 12.Ribet ME, Cardot GR. Neurogenic tumors of the thorax. Ann Thorac Surg 1994;58:1091–5. 10.1016/0003-4975(94)90464-2 [DOI] [PubMed] [Google Scholar]
- 13.Gabhane SK, Kotwal MN, Bobhate SK. Morphological spectrum of peripheral nerve sheath tumors: a series of 126 cases. Indian J Pathol Microbiol 2009;52:29–33. 10.4103/0377-4929.44958 [DOI] [PubMed] [Google Scholar]
- 14.Dhull AK, Kaushal V, Atri R, et al. Giant neurilemmoma of the vagus nerve: a case report and review of literature. J Indian Med Assoc 2012;110:926–8. [PubMed] [Google Scholar]
- 15.Tahir MZ, Fatimi SH, Enam SA. Ancient schwannoma presenting as a thoracic mass. Surg Neurol 2007;68:534–6. 10.1016/j.surneu.2006.12.058 [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Lee KS, Yoo JH, et al. Middle mediastinal lesions: imaging findings and pathologic correlation. Eur J Radiol 2000;35:30–8. 10.1016/S0720-048X(99)00156-4 [DOI] [PubMed] [Google Scholar]
- 17.Kaira K, Abe M, Nakagawa K, et al. 18F-FDG uptake on PET in primary mediastinal non-thymic neoplasm: a clinicopathological study. Eur J Radiol 2012;81:2423–9. 10.1016/j.ejrad.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 18.De Waele M, Carp L, Lauwers P, et al. Paravertebral schwannoma with high uptake of fluorodeoxyglucose on positron emission tomography. Acta Chir Belg 2005;105:537–8. 10.1080/00015458.2005.11679777 [DOI] [PubMed] [Google Scholar]
- 19.Choi YR, An JY, Kim MK, et al. The diagnostic efficacy and safety of endobronchial ultrasound-guided transbronchial needle aspiration as an initial diagnostic tool. Korean J Intern Med 2013;28:660–7. 10.3904/kjim.2013.28.6.660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasufuku K, Nakajima T, Fujiwara T, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal masses of unknown etiology. Ann Thorac Surg 2011;91:831–6. 10.1016/j.athoracsur.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 21.Boland JM, Colby TV, Folpe AL. Intrathoracic peripheral nerve sheath tumors-a clinicopathological study of 75 cases. Hum Pathol 2015;46:419–25. 10.1016/j.humpath.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 22.Crapo JD, Glassroth J, Karlinsky J, et al. ; Baum’s textbook of pulmonary diseases. Philadelphia, PA: Lippincott Williams & Wilkins, 2004:883–912. [Google Scholar]
- 23.Juanpere S, Cañete N, Ortuño P, et al. A diagnostic approach to the mediastinal masses. Insights Imaging 2013;4:29–52. 10.1007/s13244-012-0201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


