Abstract
A 47-year-old woman with a medical history of Raynaud’s phenomenon presented with fever, cough and shortness of breath. She was found to have left lower lobe consolidation and pleural effusion and was treated as a case of pneumonia. During the hospital course, her respiratory status worsened, and she was intubated on the third hospital day. To investigate the high A–a gradient, a Computerized Tomographic Pulmonary Embolism (CTPE) study was done which identified a large left lower pulmonary artery embolism. She was also found to have a new murmur, and an echocardiogram demonstrated a large lesion on tricuspid valve. However, multiple sets of her blood cultures came back consistently negative. Alternative diagnoses for culture-negative endocarditis were considered, and a full set of rheumatological workup was done. Laboratory tests were suggestive of antiphospholipid syndrome, hence the diagnosis of tricuspid valve Libman-Sacks endocarditis was made.
Keywords: systemic lupus erythematosus, pulmonary embolism
Background
Non-bacterial thrombotic endocarditis (NBTE) accounts for an important cardiac manifestation of collagen vascular diseases (CVDs). The sterile vegetations in such cases are commonly seen in the aortic and mitral valves and are commonly incidentally discovered. However, these vegetations are not always benign and can embolise and cause life-threatening systemic or pulmonary embolism. Proinflammatory state rendered by the circulating cytokines and the activated platelets are believed to be the cause of valvular abnormalities in CVDs. The same also makes the patient prone to spontaneously form clots on the damaged valves and embolise to systemic and pulmonary circulations. Clinical manifestations in such cases, especially in patients with no diagnosed CVDs, can be difficult to decipher and diagnostically challenging. A detailed history and physical examination emphasising on subtle points such as Raynaud’s phenomenon, previous episodes of clots or emboli, recurrent febrile illnesses can shed light on the final diagnosis in such cases.
Case presentation
A 47-year-old woman with a medical history of Raynaud’s phenomenon and autoamputation of left great toe presented to the emergency department with low-grade fevers, cough and shortness of breath. Her vital signs on admission were stable. On physical examination, there was reduced breath sounds in the left lung base. Laboratory tests on admission are listed in table 1, included a complete blood count, comprehensive metabolic panel, one set of blood culture and lactic acid. Laboratory tests were pertinent for a white cell count (WCC) of 22.6×109/L, alanine aminotransferase of 78 U/L, aspartate aminotransferase of 46 U/L and alkaline phosphatase of 187 U/L. Chest X-ray was performed which demonstrated left lower lobe infiltrates and pleural effusion. A clinical diagnosis of community-acquired pneumonia was thus made. She was started on oral levofloxacin 750 mg daily and was admitted to regular medical floor.
Table 1.
First encounter laboratory data (Abnormal values shown in bold characters)
| Test | Result | Reference range |
| WCC | 22.6 109 /L | 4.5–11 109/L |
| Haemoglobin | 11.3 g/dL | 12–16 g/dL |
| Sodium | 141 mmol/L | 135–145 mmol/L |
| Potassium | 4.4 mmol/L | 2.5–5.2 mmol/L |
| Creatinine | 0.5 mg/dL | 0.4–1.1 mg/dL |
| ALT | 78 IU/L | 10–43 IU/L |
| AST | 46 IU/L | 13–41 IU/L |
| ALP | 178 IU/L | 25–100 (IU/L) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
After getting admitted to the hospital, patient’s WCC trended down. However, she started having left-sided chest pain on day 1 of her hospital stay. Pain was pleuritic in nature and was exacerbated by taking deep breaths and relieved only with opiate pain medications. On day 2, she also had one episode of blood-stained sputum, and her oxygen saturation was 98% on 4 L nasal cannula at rest, however on ambulation, it dropped down to 85%. Towards the evening that day, she started having shortness of breath at rest and was started on bi-level positive airway pressure (BPAP) at an fractional inspired oxygen (FiO2) of 50%. As clinically the patient was worsening, antibiotics were changed to intravenous vancomycin 1.250 g every 12 hours and intravenous cefepime 2 g every 12 hours.
On day 3, her oxygen requirements kept increasing, and she was on BPAP with FiO2 of 100 saturating at 95%. She was noted to have developed a new grade 2/6 systolic murmur heard best in the left lower sternal border. An ECG was performed which showed new right bundle branch block and T wave changes in leads V1 to V3 suggestive of right ventricular strain (figure 1). A transthoracic echocardiogram was performed which revealed severe tricuspid regurgitation, mobile mass on the tricuspid valve and severe pulmonary hypertension. The working diagnosis at this point was changed to a case of possible tricuspid infective endocarditis. However, she met only one major in the Dukes criteria for endocarditis, other possibilities for valve lesions were also entertained. Patient’s high A–a gradient, pulmonary hypertension and valve vegetation raised suspicion for pulmonary embolism, and Well’s score was calculated and was high probability. A CTPE study was also performed which showed a large thrombus in the pulmonary artery to the left lower lobe with a wedge-shaped area of hypoperfusion within the posterior and lateral basal segments of the left lower lobe suggestive of pulmonary infarction (figure 2). Intravenous unfractionated heparin was immediately started, and the patient was transferred to intensive care unit. Due to increased work of breathing and acute hypoxic respiratory failure, she was electively intubated and pressure-regulated volume-controlled mechanical ventilation was begun. Blood cultures both from the admission and on day 2 were persistently negative. Transoesophageal echocardiogram was performed which showed a 1.3×2.1 cm mass on tricuspid valve with severe tricuspid regurgitation while the right ventricle was within normal limits (figures 3 and 4). At this point, we sent laboratory tests for hypercoagulability and other rheumatology workup which are listed in table 2. It revealed antinuclear antibody (ANA) titters of 1:2560, positive anticentromere antibodies, anti-beta2 glycoprotein, antiphospholipid and anticardiolipin antibodies. Direct antiglobulin test was negative. With these results, a diagnosis of Libman-Sacks endocarditis was made.
Figure 1.
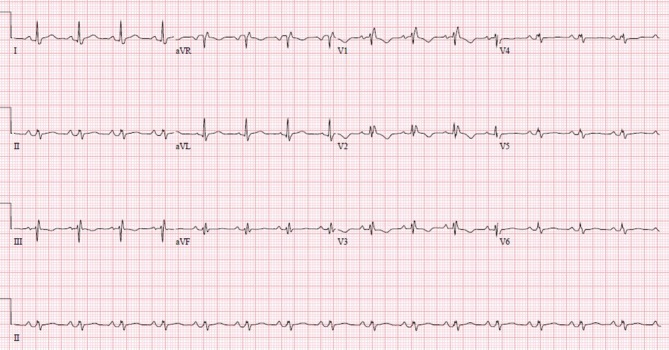
A 12-lead ECG shows new right bundle branch block pattern and T wave inversions in leads V1 to V3 which are new compared with the previous ECG done three days ago. This is suggestive of right ventricular strain.
Figure 2.
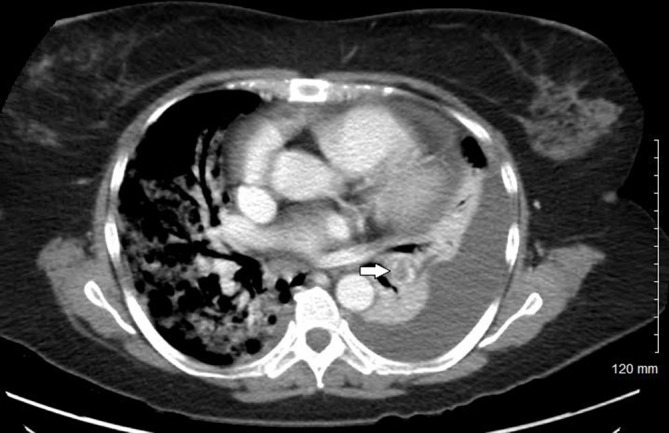
Perfusion defect due to a pulmonary embolus in the lobar artery to the left lower lobe (white arrow). Left pleural effusion with compression atelectasis can also be seen.
Figure 3.
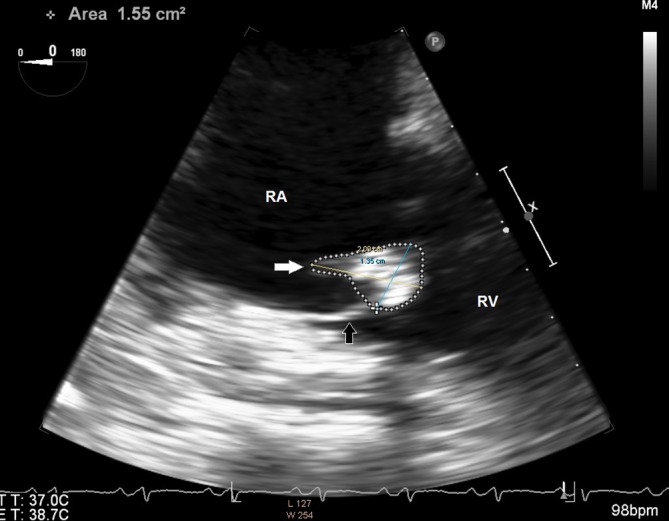
Transoesophageal echocardiogram image of the right heart. Right atrium (RA) and right ventricle (RV) are labelled. A 2.09x1.35 cm mass (white arrow) is seen in the tricuspid valve (black arrow).
Figure 4.
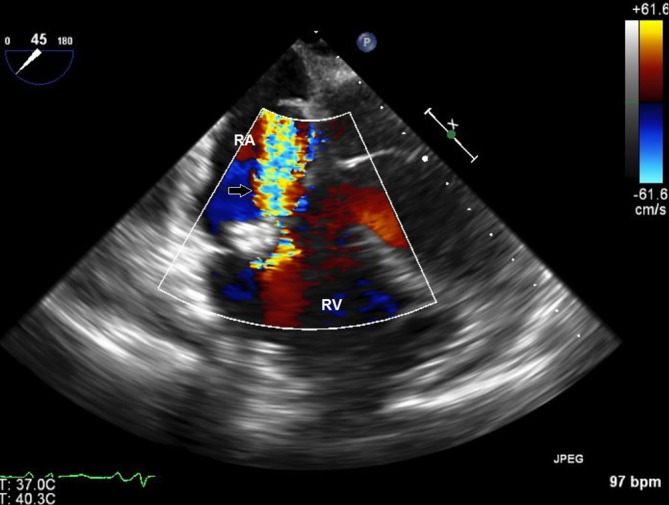
Colour Doppler image of the right heart in transoesophageal echocardiogram shows regurgitant jet (black arrow) from the right ventricle (RV) to the right atrium (RA).
Table 2.
Rheumatology laboratory data
| Test | Result | Reference range |
| ANA | 1:1280(centromere pattern) | <1:40 |
| Anti-Smith | >1 | <1.0 |
| C3 | 90 | 88–206 |
| C4 | 24 | 13–75 |
| Anti-SSA | <1 | <1.0 |
| Anti-SSB | <1 | <1.0 |
| Cardiolipin IgG | 94 | <15.0 |
| Cardiolipin IgM | 102 | <13.0 |
| Scl 70 AB | <1.0 | <1.0 |
| dsDNA AB | 6 | <5.0 |
| RF | <14.0 | <15.0 |
| B2 GPI IgG | 77 | <15.0 |
| B2 GPI IgM | >150 | <15.0 |
| Centromere antibodies | >8.0 | <1.0 |
| Anti-RNP | <20 | <20 |
ANA, antinuclear antibody; Scl, topoisomerase-1; RF, rheumatoid factor; dsDNA, double-stranded DNA; B2 GPI, beta 2 glycoprotein.
Treatment
The size of tricuspid vegetation warranted cardiothoracic surgery evaluation for possible valve replacement. However, since the patient showed significant improvement on a ventilator and a repeat transoesophageal echocardiogram showed size of the vegetation less than 2 cm, she was rendered not a candidate for replacement by the cardiothoracic team. She underwent an ultrasound duplex of her bilateral lower extremities and CT abdomen and pelvis to look for any alternate sources for her pulmonary embolism, both of which were negative. Hence, her pulmonary embolism was presumed to be from the tricuspid vegetations. Patient’s condition gradually improved, and she was weaned off the ventilator. She was put on Coumadin and was discharged home on lifelong anticoagulation. Blood cultures were consistently negative.
Discussion
Sterile valvular lesions that arise secondary to systemic malignancy or inflammatory conditions are termed NBTE. The subset of these lesions seen in Systemic Lupus Erythematosus (SLE) and less commonly in APLA syndromes are called Libman-Sacks endocarditis.1 The prevalence of these valvular lesions in patients with SLE ranges from 6% to 43% depending on the sensitivity of the diagnostic test. However, only a minority of these cases (8%) are clinically significant.2
Libman-Sacks endocarditis originates due to aggregation of activated platelets and fibrin spontaneously on the heart valves. The initiating event for this phenomenon is unknown, but is thought to be secondary to endothelial injury from the circulating autoantibodies, immune complexes and cytokines. This hypothesis is substantiated by the presence of immune complexes, mononuclear cells, platelets and fibrin on microscopy of these lesions. A high degree of correlation has been noted between the NBTE and presence of the three Antiphospholipid antibodies (anti-beta2 Glycoprotein, lupus anticoagulant and anticardiolipin antibodies).3
In the above case, our patient presented with symptoms suggestive of left lower lobe pneumonia. On detection of a new heart murmur, an echocardiogram was performed which showed large lesions on the tricuspid valve. The working diagnosis was changed to a case of possible tricuspid infective endocarditis. As she met only one major in the Dukes criteria for endocarditis, other possibilities for valve lesions were entertained. Prior antibiotic use, which is the most common cause for culture-negative Infective Endocarditis was ruled out by reaching out to the patient’s family. Fungal IE, IE secondary to fastidious HACEK bacteria, CVD causing Libman-Sacks endocarditis were high among our differentials. History of Raynaud’s phenomenon causing autoamputation in the past and the abnormal PT/INR studies were indicative of an underlying autoimmune process. The positive ANA, APLA and anticentromere antibodies led us to the final diagnosis of APLA disease with coexistent limited cutaneous systemic sclerosis syndrome. As there were no indications for surgery, we were not able to microscopically confirm our diagnosis. APLA is a condition which predisposes patients to both arterial and venous thrombi leading to pulmonary and systemic embolism. Patient’s hypoxic respiratory failure with high A–a gradient and haemoptysis during hospital stay was explained with a pulmonary embolism. Although this embolism could have originated from anywhere in the venous system, absence of clots in the deep leg veins in a Doppler study of bilateral lower extremity or in the pelvic veins, Inferior Vena Cava in a CT abdomen with intravenous contrast makes the abnormal tricuspid valve and its vegetation as the likely source of the emboli.
Management of LSE endocarditis can be medical or surgical. Medical management include anticoagulation to prevent formation of valvular or systemic thrombi from the underlying procoagulant condition. Steroids have been advocated in several reports.4 Although there is reduce inflammation aiding in quick healing with steroids, it has been shown that increasing fibrosis results in worse valvular damage.5 Steroids were avoided in our case because of the same. Indications for valve replacement in LSE is the same as those in IE namely severe valve dysfunction resulting in heart failure, large vegetations with significant embolic risk and recurrent emboli. Our patient did not meet these criteria either.
Although LSE is relatively common among SLE, APLA patients, these are mostly in the mitral or aortic valve. LSE resulting in isolated involvement of tricuspid valve is extremely rare. Culture-negative valve vegetations, even in the right-sided heart valves should raise the suspicion for NBTE among clinicians. Further questioning the patient in such cases can help unravel new clues towards diagnosis. Symptoms such as weight loss, night sweats (malignancy), Raynaud’s phenomenon and joint aches (CVD) are often overlooked by both patients and physicians. Revisiting and elaborating the history and physicals can provide invaluable information to aid the diagnosis in such cases.
Learning points.
Libman-Sacks endocarditis (LSE) is a condition associated with collagen vascular disease, most commonly SLE and less commonly APLA. The condition, although incidental, can in minority of the cases (8%) be clinically significant causing valve regurgitations, systemic or pulmonary emboli.
In cases where LSE becomes symptomatic, medical or surgical therapy can be adopted. Medical therapy includes anticoagulation with or without administration of steroids. Steroids, although noted to reduce valve inflammation and promote quick healing, can also worsen valve damage by aiding fibrosis. Indications for surgery in symptomatic LSE are severe valve dysfunction, congestive heart failure or recurrent pulmonary or systemic emboli while on anticoagulation.
Cases of cardiac valve vegetations with negative blood cultures are diagnostically challenging. While prior incomplete antibiotic therapy in a case of bacterial infective endocarditis is the most common cause, it is important to keep rarer causes such as fungal, HACEK, malignant or LSE in our list of differentials to avoid diagnostic errors.
Footnotes
Contributors: I, Dileep Unnikrishnan, certify that neither this manuscript nor one with substantially similar content under my authorship has been published or is being considered for publication elsewhere I have access to any data upon which the manuscript is based and will provide such data upon request to the editors or their assignees. I agree to allow the corresponding author to correspond with the editorial office, to review the uncorrected proof copy of the manuscript; and to make decisions regarding release of information in the manuscript. I have given final approval of the submitted manuscript for which I take public responsibility for whole content. According to the definition given by the International Committee of Medical Journal Editors (ICMJE), me and all the authors listed above qualify for authorship based on making one or more of the substantial contributions to the intellectual content. We also certify that me, Dileep Unnikrishnan, Nasreen Shaikh, Ahmad Sharayah and Chandler Patton have each contributed in both the care of the patient and drafting the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Libman E, Sacks B. A hitherto undescribed form of valvular and mural endocarditis. Arch Intern Med 1924;33:701–37. 10.1001/archinte.1924.00110300044002 [DOI] [Google Scholar]
- 2.Roldan CA, Qualls CR, Sopko KS, et al. Transthoracic versus transesophageal echocardiography for detection of Libman-Sacks endocarditis: a randomized controlled study. J Rheumatol 2008;35:224–9. [PubMed] [Google Scholar]
- 3.Ford PM, Ford SE, Lillicrap DP. Association of lupus anticoagulant with severe valvular heart disease in systemic lupus erythematosus. J Rheumatol 1988;15:597–600. [PubMed] [Google Scholar]
- 4.Hojnik M, George J, Ziporen L, et al. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation 1996;93:1579–87. 10.1161/01.CIR.93.8.1579 [DOI] [PubMed] [Google Scholar]
- 5.Hoffman R, Lethen H, Zunker U, et al. Rapid appearance of severe mitral regurgitation under high-dosage corticosteroid therapy in a patient with systemic lupus erythematosus. Eur Heart J 1994;15:138–9. 10.1093/oxfordjournals.eurheartj.a060367 [DOI] [PubMed] [Google Scholar]


