Abstract
Methaemoglobin is a form of haemoglobin in which the ferrous (Fe2+) ion contained in the iron–porphyrin complex of haem is oxidised to its ferric (Fe3+) state. Methaemoglobinaemia, the presence of methaemoglobin in the blood, is most commonly treated with methylene blue. However, methylene blue cannot be used in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency as it is ineffective in such patients and it can worsen G6PD deficiency haemolysis. We report the case of a 30-year-old man who presented with clinical features of G6PD deficiency-associated haemolysis and was found to have severe methaemoglobinaemia (35%). He was administered blood transfusions and intravenous ascorbic acid. His methaemoglobinaemia resolved within 24 hours. This case demonstrates the successful management of a patient with severe methaemoglobinaemia in the setting of G6PD deficiency haemolysis. Emergency physicians should be aware of the possible co-occurrence of severe methaemoglobinaemia in a patient with G6PD deficiency haemolysis.
Keywords: haematology (drugs and medicines), emergency medicine, general practice / family medicine, haematology (incl blood transfusion)
Background
Methaemoglobin is a form of haemoglobin in which the ferrous (Fe2+) ion contained in the iron–porphyrin complex of haem is oxidised to its ferric (Fe3+) state. Methaemoglobinaemia, the presence of methaemoglobin in the blood, is a haemoglobinopathy that is usually acquired, although it may rarely be congenital.1 Methaemoglobinaemia impairs the ability of haemoglobin to transport oxygen molecules to tissue capillary beds, thereby impairing oxygenation of the body. This results in a hypoxic state that may manifest as headache, nausea, fatigue and confusion; if left untreated, it can progress to lactic acidosis, seizures, coma and even death.2 Methylene blue is the most commonly used treatment for methaemoglobinaemia. However, methylene blue cannot be used in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency as it is ineffective and can worsen haemolysis by increasing oxidative stress.3 Here, we report the case of a 30-year-old man who presented with clinical features of G6PD deficiency-associated haemolysis and was found to have methaemoglobinaemia (35%). He was treated with blood transfusions and intravenous ascorbic acid, and improved subsequently. This case demonstrates the successful management of a patient with severe methaemoglobinaemia in the setting of G6PD deficiency haemolysis.
Case presentation
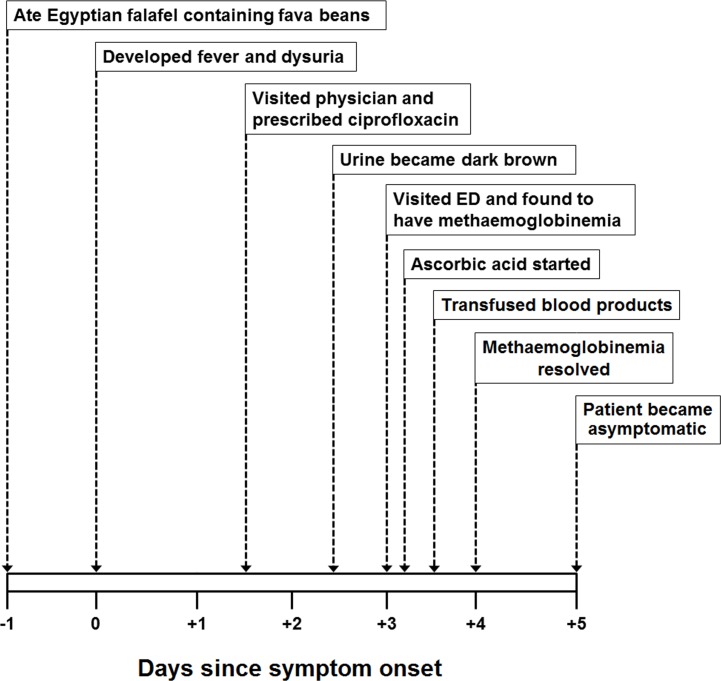
A 30-year-old Nepalese man presented to the emergency department of our institution with fever, burning micturition and cola-coloured urine for the past 3 days. He first developed fever with rigors and chills and burning micturition. One day prior to the onset of these symptoms, he ate an Egyptian falafel containing fava beans. He went to a general physician on the second day of symptoms, who prescribed him ciprofloxacin (500 mg two times a day) and acetaminophen (1000 mg three times a day). After taking these medications for 1 day, he noticed that his urine became dark brown in colour (on day 3) and he also developed back pain. This compelled him to visit the emergency department of our institution (see figure 1). On systemic inquiry, he reported having generalised weakness, headache and mild shortness of breath on exertion. On examination, he was febrile (oral temperature of 38.5°C) and tachycardiac with a regular pulse rate of 110/min. Pulse oximetry showed oxygen saturation of 85% on room air and his respiratory rate was 24/min. General examination was notable for conjunctival pallor and scleral icterus. Systemic examination was remarkable for an ejection systolic murmur (grade II/VI on the Levine scale) at the left lower sternal edge.
Figure 1.
A figure depicting the timeline and sequence of events that occurred in the present case. ED, emergency department.
Investigations and diagnosis
His laboratory investigations were notable for a haemoglobin of 8.4 g/dL (reference range: 13.0–17.0 g/dL) and total leucocyte count of 15.0×109 cells/L (reference range: 4–10×109 cells/L). Peripheral smear showed marked polychromasia, anisocytosis, many nucleated red blood cells (RBC), few bite cells and many blister cells. His aspartate aminotransferase was elevated (126 U/L; reference range: 5–34 U/L), while alanine aminotransferase was normal (25 U/L; reference range: 0–45 U/L). Total bilirubin was also elevated at 222 μmol/L (reference range: 3.4–20.5 μmol/L) with a direct bilirubin of 10.8 μmol/L (reference range: 0–8.8 μmol/L) suggestive of indirect hyperbilirubinaemia. Serum creatinine, urea, electrolytes and coagulation profile were within normal limits. He was initially kept on oxygen 8 L/min by face mask, but pulse oximetry showed persistently low oxygen saturation (86%). His arterial blood gas was done, which revealed oxygen saturation of 99% (suggestive of a ‘saturation gap’), PaO2 of oxygen of 95 mm Hg (reference range: 83–108 mm Hg), lactic acid of 0.9 mmol/L (reference range: 0.5–1.6 mmol/L) and methaemoglobin of 35% (reference range: 0.0%–1.5%). Oxygen therapy was reduced to 1 L/min by nasal cannula and he was started on intravenous hydration. Urinalysis was ordered, which was notable for +3 blood and +1 leucocyte. Urine microscopy showed only 2 RBCs and 23 pus cells. His blood and urine cultures were sent and he was empirically started on ceftriaxone (1000 mg one time a day) for a possible urinary tract infection. A plain chest radiograph was ordered, which was unremarkable. Ultrasonography of abdomen was also performed, which was unremarkable. Subsequently, results of haemolysis workup showed a reticulocyte count of 15.3% (Reticulocyte Production Index: 3.5%), lactate dehydrogenase of 2894 U/L (reference range: 125–220 U/L), haptoglobin level of <5 mg/dL (reference range: 14–258 mg/dL), G6PD level of <35 units per trillion RBCs (reference range: 245–500 units per trillion RBCs) and negative direct Coomb’s test.
Treatment
Haematology and toxicology teams were consulted. Haematology team advised to avoid methylene blue as it can worsen G6PD deficiency-associated haemolysis. Toxicology team advised to start intravenous ascorbic acid (1000 mg four times a day) and continue supportive care. Patient was admitted to the medical floor for further evaluation and management.
In the ward, patient was noted to have a progressive drop in his haemoglobin reaching a nadir of 5.9 g/dL within 24 hours. He was transfused 2 units of packed RBCs with regular monitoring of his haemoglobin and supportive care. His methaemoglobin level was also monitored regularly, which normalised after 24 hours.
Outcome and follow-up
Final reports of blood and urine cultures revealed no growth. Blood smears for malarial parasite (sent thrice) were negative. Ceftriaxone and ascorbic acid were stopped and he was planned for discharge. He was educated about G6PD deficiency and the types of food and medications to avoid in the future. Patient continued to follow-up in haematology outpatient clinic and remained healthy. His repeat haemoglobin improved to 13.6 g/dL at a 3 month follow-up visit. Haemoglobin electrophoresis performed on outpatient basis was also normal.
Discussion
Methaemoglobin is an abnormal form of haemoglobin that impairs the ability of haemoglobin to unload oxygen molecules into tissue capillary beds, thereby impairing oxygenation of the body.1 Consequently, this results in a hypoxic state that impairs the generation of ATP in all cells of the body. Over time, this can lead to dysfunction of virtually every organ of the body, although metabolically active cells of the body (such as neurons) are at most risk.2 Clinically, patients with methaemoglobinaemia report non-specific symptoms (such as anxiety, lightheadedness and headache) which are related to the degree of methaemoglobinaemia.4 At concentrations of 30%–50%, patients may develop confusion, tachycardia and tachypnoea. At higher concentrations (50%–70%), coma, seizures and arrhythmias can occur, which may lead to death if left untreated.5 In our patient, methaemoglobin level was 35% and he reported symptoms of fatigue and headache. He also had tachycardia and tachypnoea, although fever and anaemia contributed to this as well. More importantly, our patient had a significant degree of anaemia, which can result in more severe manifestations for a given degree of methaemoglobinaemia.
Methaemoglobinaemia is most commonly an acquired condition, although it can rarely be congenital. Congenital methaemoglobinaemia often presents within the first few hours or days of life and is caused by cytochrome-b5 or cytochrome-b5 reductase deficiency.6 Cytochrome-b5 and cytochrome-b5 reductase are two enzymes that reduce methaemoglobin to haemoglobin by transferring electrons from reduced nicotinamide adenine dinucleotide to methaemoglobin. Acquired methaemoglobinaemia can be caused by toxins, chemicals or drugs that have strong oxidative potential.7 Nitrates and nitrites are classically described to cause methaemoglobinaemia. Long-term use of dapsone in patients with dermatitis herpetiformis has also been described to cause methaemoglobinaemia.8 Local anaesthetics (such as benzocaine and prilocaine) have been frequently implicated in causing methaemoglobinaemia, especially in infants and young children.9 Cases of methaemoglobinaemia secondary to illicit drug use have also been reported in the literature.10 Some recent reports described cases of methaemoglobinaemia in young infants secondary to ingestion of well water containing a high concentration of nitrates.11 In our patient, methaemoglobinaemia was likely a combined consequence of severe G6PD deficiency and ingestion of ciprofloxacin—a drug rarely implicated in methaemoglobinaemia.12 However, in most cases of methaemoglobinaemia associated with G6PD deficiency, the degree of methaemoglobinaemia is mild (<5%).2 4 In the present case, our patient developed severe methaemoglobinaemia (35%) in conjunction with G6PD deficiency-associated haemolysis, which might have been secondary to an undetected concomitant enzyme deficiency.
G6PD is a rate-limiting enzyme of the pentose phosphate pathway, which reduces nicotinamide adenine dinucleotide phosphate (NADPH) from its oxidised state (NADP) to its reduced state (NADPH). NADPH is required in RBCs to maintain adequate intracellular levels of glutathione—a free radical scavenger. G6PD deficiency can lead to depletion of glutathione within erythrocytes and render them vulnerable to damage from oxidative compounds. Oxidative stress in various forms can lead to haemolysis in patients with G6PD deficiency.13 A variety of drugs can precipitate G6PD deficiency-associated haemolysis including dapsone, nitrofurantoin, primaquine, methylene blue, dimercaprol and rasburicase.14 The only food that can trigger haemolysis in patients with G6PD deficiency is fava beans.15 16 Certain dietary and herbal supplements may also cause low level of haemolysis in G6PD deficiency, such as Acalypha indica, Coptis chinensis, Salix caprea and Lawsonia inermis.17–19 Severe illness, especially an acute infection, can precipitate G6PD deficiency-associated haemolysis.20 In our patient, acute haemolysis was likely a combined consequence of severe acute infection, exposure to ciprofloxacin and possibly, ingestion of fava beans (contained in the falafel).
The antidote of choice for reversal of methaemoglobinaemia is methylene blue. Methylene blue is typically administered in a dose of 1–2 mg/kg as an intravenous infusion over 5 min. In most cases, methylene blue is effective and reverses methaemoglobinaemia rapidly (usually in less than 60 min).21 The mechanism of action involves the conversion of methylene blue to leucomethylene blue by the enzyme NADPH methaemoglobin reductase, which in turn reduces methaemoglobin to haemoglobin. The enzyme NADPH methaemoglobin reductase requires NADPH, which it oxidises to NADP. As NADPH is primarily produced by the enzyme G6PD, patients deficient in G6PD cannot convert methylene blue to leucomethylene blue (as NADPH methaemoglobin reductase cannot operate effectively because NADPH is in short supply). On the contrary, methylene blue can worsen G6PD deficiency-associated haemolysis as it can lead to generation of free radicals and oxidative damage to RBC membrane.22 In patients with G6PD deficiency who develop methaemoglobinaemia, methylene blue cannot be used and alternative treatment options need to be used.
Different alternative treatments have been described in the literature for managing patients with methaemoglobinaemia and G6PD deficiency. In patients with mild-to-moderate G6PD deficiency and no evidence of haemolysis, it may be prudent to administer methylene blue at a lower dose with close monitoring.23 Another antidote that has been described in in vitro studies is N-acetyl cysteine (NAC). In a study by Wright and colleagues, NAC successfully reduced methaemoglobin levels in an in vitro model of G6PD deficiency.24 However, in a randomised controlled trial published in 2000, intravenous NAC failed to reverse sodium nitrite-induced methaemoglobinaemia in healthy volunteers.25 Due to this reason, we avoided the use of NAC in the present case. Another antidote that has been described in previously published literature is ascorbic acid. Numerous in vitro studies have shown that ascorbic acid (vitamin C) can treat methaemoglobinaemia by removing key precursors of oxidative damage and reducing methaemoglobin.26 In a case report from Korea, Park and Lee described the use of high-dose intravenous ascorbic acid (10 g four times a day) for the treatment of methaemoglobinaemia (due to non-availability of methylene blue).27 However, in the case reported by Park and Lee, the patient did not have G6PD deficiency. In a series of five cases reported by Rino et al, intravenous ascorbic acid was successful for the treatment of methaemoglobinaemia.28 The dose range reported in this case series ranged from 1 g three times a day to 2 g four times a day. The degree of methaemoglobinaemia ranged from 6.4% to 43%. In our patient, we used 1 g intravenous ascorbic acid every 6 hours in consultation with toxicology team and clinical pharmacists. Additionally, the patient received transfusion of 2 units of packed RBCs. Subsequently, he recovered successfully and his methaemoglobin level normalised within 24 hours. Apart from ascorbic acid, a few other treatment options have also been described in the literature, such as riboflavin,29 cimetidine30 and ketoconazole.31 In cases where methaemoglobinaemia persists despite all measures, exchange transfusion has been used as a last resort with favourable results.32
There are several caveats in the interpretation of this case report which deserve attention. First of all, it must be recognised that the pathogenesis of methaemoglobinaemia differs depending on its aetiology and, therefore, efficacy of various treatment options may differ for patients with the same degree of methaemoglobinaemia depending on their aetiopathogenesis.28 Second, in the present case, patient received transfusion of 2 units of packed RBCs. Although exchange transfusion was not used in this case, simple transfusion in patients with significant anaemia can offset methaemoglobinaemia by increasing the proportion of oxyhaemoglobin in the blood. This precludes an accurate assessment of the effect of ascorbic acid on methaemoglobinaemia in this case. Third, in the present case, patient had G6PD deficiency, which precluded the use of methylene blue. In cases where methylene blue is not contraindicated, it remains the treatment choice as its efficacy in clinical settings is well established and it reverses methaemoglobinaemia within minutes.33 This is in contrast to the use of ascorbic acid which typically requires 12–24 hours to have any significant effect and, therefore, it is less favourable for patients with severe manifestations of methaemoglobinaemia.26–28 Lastly, data from in vitro studies shows that ascorbic acid can only significantly reduce methaemoglobin at concentrations of ≥10 mM.26 27 Such high plasma concentrations of ascorbic acid can only be achieved with intravenous administration as gastrointestinal absorption of ascorbic acid is regulated and precludes the attainment of such plasma concentrations.32 It is also worth noting that innate mechanisms and metabolic reactions can slowly reduce methaemoglobin to haemoglobin.34 However, the rate of physiological reduction of methaemoglobin is estimated to be approximately 3% per day,35 which is insufficient for patients with symptomatic methaemoglobinaemia.
In a nutshell, we presented the case of a 30-year-old man who presented with clinical features of a urinary tract infection complicated by G6PD deficiency-associated haemolysis and methaemoglobinaemia. G6PD deficiency haemolysis was likely a combined consequence of severe infection, ingestion of fava beans and exposure to ciprofloxacin, and manifested clinically as haemoglobinuria and back pain. Methaemoglobinaemia resulted from severe G6PD deficiency and exposure to ciprofloxacin, although an undetected, concomitant enzyme deficiency might also have contributed (given the degree of methaemoglobinaemia). Patient’s shortness of breath and presence of a ‘saturation gap’ were accounted for by methaemoglobinaemia. Successful management of this rare clinical presentation entailed the use of blood products, intravenous ascorbic acid and antibiotics (to treat underlying infection).
Learning points.
Methaemoglobinaemia should be suspected in a patient who has a persistently low oxygen saturation (typically 85%) on pulse oximetry with a normal oxygen saturation on arterial blood gas analysis (the so-called ‘saturation gap’).
Diagnosis of methaemoglobinaemia can be readily made by co-oximetry.
Methaemoglobinaemia can occur concomitantly with glucose-6-phosphate dehydrogenase (G6PD) deficiency and methylene blue should be avoided in such cases.
Blood transfusions (simple or exchange) and intravenous ascorbic acid may be feasible treatment options for patients with symptomatic methaemoglobinaemia and concomitant G6PD deficiency-associated haemolysis.
Footnotes
Contributors: All authors were involved in the direct care of the patient and conceived the idea of writing a case report. They collected the data and interpreted it. AR performed a literature review and wrote down the discussion section of the manuscript. MS and DK wrote down the case presentation section of the manuscript. AJ substantially reviewed the whole manuscript for important intellectual content. All authors read the manuscript and approved it for submission in its current form.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine 2004;83:265–73. [DOI] [PubMed] [Google Scholar]
- 2. Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011;104:757–61. 10.1097/SMJ.0b013e318232139f [DOI] [PubMed] [Google Scholar]
- 3. Liao YP, Hung DZ, Yang DY. Hemolytic anemia after methylene blue therapy for aniline-induced methemoglobinemia. Vet Hum Toxicol 2002;44:19–21. [PubMed] [Google Scholar]
- 4. Ashurst J, Wasson M. Methemoglobinemia: a systematic review of the pathophysiology, detection, and treatment. Del Med J 2011;83:203–8. [PubMed] [Google Scholar]
- 5. Da-Silva SS, Sajan IS, Underwood JP. Congenital methemoglobinemia: a rare cause of cyanosis in the newborn-a case report. Pediatrics 2003;112:e158–e161. 10.1542/peds.112.2.e158 [DOI] [PubMed] [Google Scholar]
- 6. Kinoshita A, Nakayama Y, Kitayama T, et al. Simulation study of methemoglobin reduction in erythrocytes. Differential contributions of two pathways to tolerance to oxidative stress. Febs J 2007;274:1449–58. [DOI] [PubMed] [Google Scholar]
- 7. Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–56. 10.1016/S0196-0644(99)70167-8 [DOI] [PubMed] [Google Scholar]
- 8. Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011;45:1103–15. 10.1345/aph.1Q139 [DOI] [PubMed] [Google Scholar]
- 9. Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg 2009;108:837–45. 10.1213/ane.0b013e318187c4b1 [DOI] [PubMed] [Google Scholar]
- 10. Hunter L, Gordge L, Dargan PI, et al. Methaemoglobinaemia associated with the use of cocaine and volatile nitrites as recreational drugs: a review. Br J Clin Pharmacol 2011;72:18–26. 10.1111/j.1365-2125.2011.03950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greer FR, Shannon M; American Academy of Pediatrics Committee on Nutrition American Academy of Pediatrics Committee on Environmental Health. Infant methemoglobinemia: the role of dietary nitrate in food and water. Pediatrics 2005;116:784–6. 10.1542/peds.2005-1497 [DOI] [PubMed] [Google Scholar]
- 12. Wilburn-Goo D, Lloyd LM. When patients become cyanotic: acquired methemoglobinemia. J Am Dent Assoc 1999;130:826–31. 10.14219/jada.archive.1999.0306 [DOI] [PubMed] [Google Scholar]
- 13. Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008;371:64–74. 10.1016/S0140-6736(08)60073-2 [DOI] [PubMed] [Google Scholar]
- 14. Youngster I, Arcavi L, Schechmaster R, et al. Medications and glucose-6-phosphate dehydrogenase deficiency. Drug Saf 2010;33:713–26. 10.2165/11536520-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 15. Mehta A, Mason PJ, Vulliamy TJ. Glucose-6-phosphate dehydrogenase deficiency. Best Pract Res Clin Haematol 2000;13:21–38. 10.1053/beha.1999.0055 [DOI] [PubMed] [Google Scholar]
- 16. Luzzatto L, Arese P. Favism and glucose-6-phosphate dehydrogenase deficiency. N Engl J Med 2018;378:60–71. 10.1056/NEJMra1708111 [DOI] [PubMed] [Google Scholar]
- 17. Raupp P, Hassan JA, Varughese M, et al. Henna causes life threatening haemolysis in glucose-6-phosphate dehydrogenase deficiency. Arch Dis Child 2001;85:411–2. 10.1136/adc.85.5.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senanayake N, Sanmuganathan PS. Acute intravascular haemolysis in glucose-6-phosphate dehydrogenase deficient patients following ingestion of herbal broth containing Acalypha indica . Trop Doct 1996;26:32 10.1177/004947559602600113 [DOI] [PubMed] [Google Scholar]
- 19. Wong HB. Singapore kernicterus. Singapore Med J 1980;21:556–67. [PubMed] [Google Scholar]
- 20. Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am 2016;30:373–93. 10.1016/j.hoc.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 21. Clifton J, Leikin JB. Methylene blue. Am J Ther 2003;10:289–91. 10.1097/00045391-200307000-00009 [DOI] [PubMed] [Google Scholar]
- 22. Sills MR, Zinkham WH. Methylene blue-induced Heinz body hemolytic anemia. Arch Pediatr Adolesc Med 1994;148:306–10. 10.1001/archpedi.1994.02170030076017 [DOI] [PubMed] [Google Scholar]
- 23. Metz EN, Balcerzak P, Sagone AL. Mechanisms of methylene blue stimulation of the hexose monophosphate shunt in erythrocytes. J Clin Invest 1976;58:797–802. 10.1172/JCI108531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright RO, Woolf AD, Shannon MW, et al. N-acetylcysteine reduces methemoglobin in an in-vitro model of glucose-6-phosphate dehydrogenase deficiency. Acad Emerg Med 1998;5:225–9. 10.1111/j.1553-2712.1998.tb02617.x [DOI] [PubMed] [Google Scholar]
- 25. Tanen DA, LoVecchio F, Curry SC. Failure of intravenous N-acetylcysteine to reduce methemoglobin produced by sodium nitrite in human volunteers: A randomized controlled trial. Ann Emerg Med 2000;35:369–73. 10.1016/S0196-0644(00)70056-4 [DOI] [PubMed] [Google Scholar]
- 26. Dunne J, Caron A, Menu P, et al. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J 2006;399:513–24. 10.1042/BJ20060341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park S-Y, Lee K-W, Kang T-S. High-dose vitamin C management in dapsone-induced methemoglobinemia. Am J Emerg Med 2014;32:684.e1–684.e3. 10.1016/j.ajem.2013.11.036 [DOI] [PubMed] [Google Scholar]
- 28. Rino PB, Scolnik D, Fustiñana A, et al. Ascorbic acid for the treatment of methemoglobinemia: the experience of a large tertiary care pediatric hospital. Am J Ther 2014;21:240–3. 10.1097/MJT.0000000000000028 [DOI] [PubMed] [Google Scholar]
- 29. Dötsch J, Demirakça S, Kratz M, et al. Comparison of methylene blue, riboflavin, and N-acetylcysteine for the reduction of nitric oxide-induced methemoglobinemia. Crit Care Med 2000;28:958–61. 10.1097/00003246-200004000-00008 [DOI] [PubMed] [Google Scholar]
- 30. Coleman MD, Rhodes LE, Scott AK, et al. The use of cimetidine to reduce dapsone-dependent methaemoglobinaemia in dermatitis herpetiformis patients. Br J Clin Pharmacol 1992;34:244–9. 10.1111/j.1365-2125.1992.tb04131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tingle MD, Coleman MD, Park BK. An investigation of the role of metabolism in dapsone-induced methaemoglobinaemia using a two compartment in vitro test system. Br J Clin Pharmacol 1990;30:829–38. 10.1111/j.1365-2125.1990.tb05448.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhat P, Sisler I, Collier AB. Exchange transfusion as treatment for rasburicase induced methemoglobinemia in a glucose-6-phosphate dehydrogenase deficient patient. Pediatr Blood Cancer 2008;51:568 10.1002/pbc.21582 [DOI] [PubMed] [Google Scholar]
- 33. Dötsch J, Demirakça S, Cryer A, et al. Reduction of NO-induced methemoglobinemia requires extremely high doses of ascorbic acid in vitro. Intensive Care Med 1998;24:612–5. 10.1007/s001340050623 [DOI] [PubMed] [Google Scholar]
- 34. Dorman SC, Kenny CF, Miller L, et al. Role of redox potential of hemoglobin-based oxygen carriers on methemoglobin reduction by plasma components. Artif Cells Blood Substit Immobil Biotechnol 2002;30:39–51. 10.1081/BIO-120002726 [DOI] [PubMed] [Google Scholar]
- 35. Power GG, Bragg SL, Oshiro BT, et al. A novel method of measuring reduction of nitrite-induced methemoglobin applied to fetal and adult blood of humans and sheep. J Appl Physiol 2007;103:1359–65. 10.1152/japplphysiol.00443.2007 [DOI] [PubMed] [Google Scholar]