Abstract
A 70-year-old man visited our emergency department, whose laboratory test results revealed leucocytosis, anaemia, thrombocytopenia and high levels of serum lactate dehydrogenase. In addition, the peripheral blood smear revealed neutrophilic granulocytes with nuclear hypolobation (pseudo-Pelger-Hüet anomaly), hypogranulation and no myeloperoxidase reactivity. Genetic testing of the peripheral blood sample was as follows: G-band, 46XY,t(9;22)(q34;q11.2) (20/20); fluorescence in situ hybridisation BCR/ABL fusion signal, 97%; and analysis of exons 5–9 of the p53 gene, mutation (Pro72Arg) in exon 4 protein. On the basis of these findings, the patient was diagnosed with chronic myelogenous leukaemia (CML) in chronic phase with a p53 mutation and treated with hydroxyurea, dasatinib and nilotinib. Neutrophilic granulocytes with the anomalies were no longer observed after achieving cytogenetic remission. To the best of our knowledge, this is the first report of CML case with the anomalies, in which a p53 mutation without chromosome 17 abnormalities was identified.
Keywords: Haematology (drugs and medicines), malignant and benign Haematology
Background
Nuclear hypolobation (pseudo-Pelger-Hüet anomaly (PPA)) and hypogranulation of neutrophilic granulocytes are anomalies, associated with myelodysplastic syndrome (MDS), acute myelogenous leukaemia (AML) and chronic myelogenous leukaemia (CML) in blast phase,1 and CML in accelerated phase.2 Here, we report a case of CML in chronic phase (WHO classification revised fourth edition) with a p53 mutation accompanied by these anomalies.
Case presentation
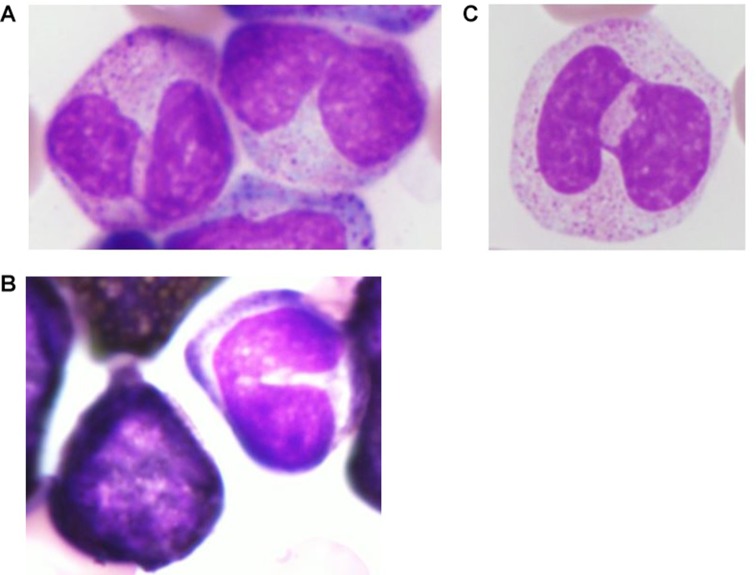
A 70-year-old man with a history of glaucoma, cataract and surgery for fracture of bilateral lower legs, visited our emergency department on the day of admission with a week-long complaint of nausea, vomiting and cough. His body temperature was 37.7°C, blood pressure was 119/67 mm Hg, pulse rate was 86 beats per min and oxygen saturation was 94% in ambient air. Chest auscultation revealed no rales or murmur. The abdomen was soft, without distention, rebound tenderness, guarding and palpable hepatomegaly. Splenomegaly was palpable 3 cm below the left costal margin, and lymphadenopathies (<1 cm in diameter) in the bilateral inguinal region were palpable. He had operation scars on his bilateral lower legs. His laboratory test results revealed leucocytosis (white cell count, 325.6×109 cells/L), anaemia (haemoglobin level, 5.8 g/dL), thrombocytopenia (platelet count, 40×109 cells/L) and high levels of serum lactate dehydrogenase (3653 IU/L). Peripheral blood smear revealed an increase in the number of granulocytes in different stages of maturation, leucoerythroblastosis and basophilia (myeloblasts, 1.0%; erythroblasts, 9.3 cells/100 white cell count; and basophils, 2.1%). In addition, neutrophilic granulocytes with PPA were observed (figure 1), which accounted for 11% of cells in the peripheral blood smear, with some presenting hypogranulation and no myeloperoxidase reactivity. A CT scan revealed hepatomegaly and splenomegaly (12.3 cm×6.2 cm×17.0 cm), generalised lymphadenopathies (<1 cm in diameter) and blebs in the lung. A bone marrow (BM) examination was conducted. The BM could not be aspirated (dry tap). A BM biopsy was performed that revealed hypercellular marrow and myelofibrosis (grade 3). The results of genetic tests on peripheral blood sample were as follows: G-band, 46XY,t(9;22)(q34;q11.2) (20/20); fluorescence in situ hybridisation (FISH) BCR/ABL; fusion signal, 97%; analysis of exons 5–9 of the p53 gene, mutation (Pro72Arg) in exon 4 protein. On the basis of these findings, a diagnosis of CML in chronic phase (WHO classification revised fourth edition) with a p53 mutation was made. On the day of admission, daily administration of 1000 mg hydroxyurea was initiated for leucocytosis. The dose of hydroxyurea was gradually increased to 4000 mg daily. Two weeks later, hydroxyurea was discontinued, because of a reduction in the white cell count. One day later, the use of a tyrosine kinase inhibitor (TKI), 40 mg dasatinib daily, was initiated, which was subsequently discontinued 4 days later because of upper gastrointestinal bleeding. Thirteen days later, daily administration of 300 mg nilotinib was initiated; dyslipidaemia and hyperglycaemia developed as adverse effects. Three months later, genetic tests of the peripheral blood sample revealed the following: FISH BCR/ABL, fusion signal 0% and PCR major BCR/ABL mRNA, 3.0×103 copies/µgRNA. The patient achieved cytogenetic remission. One month later, neutrophilic granulocytes with the anomalies were no longer observed in the peripheral blood smear.
figure 1.
Microscopic findings of the peripheral blood smear (on the day of admission) (A): neutrophilic granulocyte with nuclear hypolobation (PPA) (×1000, May-Giemsa stain); (B): neutrophilic granulocyte with nuclear hypolobation (PPA) and hypogranulation (×1000, May-Giemsa stain); (C): left, myeloid cell was positive; right, neutrophilic granulocyte with nuclear hypolobation was negative (×1000, myeloperoxidase stain). PPA, pseudo-Pelger-Hüet anomaly.
Discussion
Nuclear hypolobation (PPA), hypogranulation of neutrophilic granulocytes and myeloperoxidase deficiency are anomalies, which are associated with MDS, AML, CML in blast phase,1 and CML in accelerated phase.2
Lai et al3 reported that MDS and AML with chromosome 17 abnormalities are associated with PPA. They identified 11 patients with a 17p deletion resulting from translocations involving 17p: t(5;17)(p11;p11) in four cases, t(7;17)(p11;p11) in five cases, complex (5;17)(q23;p12) translocation with dicentric chromosome in one case and t(17;?)(p11–12;?) in one case. All these patients but one case had PPA observed in bone marrow mature granulocytes.
p53 is a tumor-suppressor gene, which is localised on chromosome 17p. Soenen et al4 investigated a correlation of anomalies (PPA and hypogranulation) with p53 mutation and overexpression. They analysed 17 cases of AML and MDS with 17p deletion by whole chromosome painting. The FISH analysis with probes spanning the 17p arm, including a p53 gene probe, was performed in 16 cases of AML and MDS with chromosome 17 abnormalities. In 14 cases, the FISH analysis revealed a 17p deletion of variable extent, but it always included deletion of the p53 gene. All 14 patients had PPA and hypogranulation, and all but one had a p53 mutation and/or overexpression. The other two patients had no 17p deletion by the FISH analysis; they neither had PPA nor hypogranulation. The p53 mutation analysis was conducted in only one of the two patients, and the result was negative. Based on these results, the correlation of the anomalies with p53 mutation and overexpression was indicated.
In CML, PPA has been reported in blastic phase.1 In 11 of 83 CML cases in blast phase, PPA was observed in the peripheral blood smear. Chromosomal analysis of the 11 patients revealed chromosome 17 abnormalities in addition to t(9;22)(q34;q11). The chromosome 17 abnormalities were as follows: i(17q), eight cases; unbalanced translocation involving chromosome 17, two cases; and monosomy 17, one case. The morphological and chromosomal study of the remaining 72 patients demonstrated neither PPA nor 17p involvement. Based on these results, the correlation between chromosome 17 abnormalities with PPA has also been suggested in blast phase of CML.5
Furthermore, Xu et al2 studied this correlation also. They diagnosed CML in accelerated phase by WHO classification third edition and reported that patients with CML in accelerated phase with PPA and hypogranulation demonstrated chromosome 17p abnormalities in addition to t(9;22)(q34;q11.2), whereas those without the anomalies did not exhibit chromosome 17p abnormalities. However, in one case with anomalies, the 17p deletion was not identified by G-banding or FISH using a DNA probe to the p53 locus at 17p13.
Our case was of CML which demonstrated anomalies (PPA and hypogranulation) and p53 mutation. AML and MDS cases with the anomalies were accompanied by p53 mutations in all 14 cases but one.4 This report also suggests the correlation between the anomalies and p53 mutation. A plausible assumption is that the anomalies in our case might have been caused by the p53 mutation. TKI reduces clones with both t(9;22)(q34;q11.2) and the p53 mutation, resulting in the disappearance of the anomalies. This case displayed two characteristic points. To the best of our knowledge, our case was the first CML case in chronic phase (WHO classification revised fourth edition) with anomalies, in which a p53 mutation without chromosome 17 abnormalities was identified. Second, the anomalies disappeared after the patient achieved cytogenetic remission.
Learning points.
Nuclear hypolobation (pseudo-Pelger-Huet anomaly (PPA)) and hypogranulation of neutrophilic granulocytes are anomalies, which are associated with MDS, AML and CML in accelerated phase and blast phase.
In MDS and AML, a 17p deletion and p53 mutations are associated with PPA and hypogranulation.
Footnotes
Contributors: MS and JT treated the patient. MKo diagnosed the myelofibrosis. MS wrote the manuscript. MKa organised all process.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kuriyama K, Tomonaga M, Matsuo T, et al. . Diagnostic significance of detecting pseudo-Pelger-Huët anomalies and micro-megakaryocytes in myelodysplastic syndrome. Br J Haematol 1986;63:665–9. 10.1111/j.1365-2141.1986.tb07550.x [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Dolan MM, Nguyen PL. Diagnostic significance of detecting dysgranulopoiesis in chronic myeloid leukemia. Am J Clin Pathol 2003;120:778–84. 10.1309/P2QUGBB9NVWV4JUD [DOI] [PubMed] [Google Scholar]
- 3.Laï JL, Zandecki M, Fenaux P, et al. . Translocations (5;17) and (7;17) in patients with de novo or therapy-related myelodysplastic syndromes or acute nonlymphocytic leukemia. A possible association with acquired pseudo-Pelger-Huët anomaly and small vacuolated granulocytes. Cancer Genet Cytogenet 1990;46:173–83. [DOI] [PubMed] [Google Scholar]
- 4.Soenen V, Preudhomme C, Roumier C, et al. . 17p Deletion in acute myeloid leukemia and myelodysplastic syndrome. Analysis of breakpoints and deleted segments by fluorescence in situ. Blood 1998;91:1008–15. [PubMed] [Google Scholar]
- 5.Sessarego M, Ajmar F. Correlation between acquired pseudo-Pelger-Huet anomaly and involvement of chromosome 17 in chronic myeloid leukemia. Cancer Genet Cytogenet 1987;25:265–70. 10.1016/0165-4608(87)90187-7 [DOI] [PubMed] [Google Scholar]