Abstract
Endovascular treatment has been the mainstay of therapy for repair of both ruptured and unruptured cerebral aneurysms. Flow diverter devices offer a new option for the treatment of complex aneurysms that were previously not amenable to coiling. Procedural adverse effects include intracranial haemorrhage and ischaemic stroke, which usually occur on the same day. Delayed complications are rare. We report a case of a patient who underwent placement of a pipeline embolisation device and developed delayed neurological deficits, which were thought to be an inflammatory reaction to the hydrophilic coating used in guidewires and microcatheters. Our patient was treated with a course of steroids, with improvement of her neurological deficits and resolution of MRI findings. As the use of flow diverter devices has increased, variable and delayed complications of such therapy are increasingly being reported in the literature.
Keywords: neurology (drugs and medicines), neuroimaging, interventional radiology
Background
Prior to the development of the Guglielmi detachable coil in 1990, aneurysms were primarily repaired by surgical clipping; since then endovascular treatment has become the first-line treatment for intracranial aneurysms of anterior circulation. Furthermore, flow diverter stents have become an important and effective means of treating giant, wide-neck complex aneurysms.1 Common complications from endovascular therapy are usually immediate and are either haemorrhagic or thromboembolic. With the increasing use of flow diverter devices, there have been emerging reports in the literature that describe delayed complications with this procedure. Hydrophilic polymers such as polyvinylpyrrolidone (PVP) are widely used to coat endovascular devices to reduce endothelial damage, for greater manoeuvrability during the procedure and to decrease thrombogenicity.2 Foreign body reaction due to polymer embolisation is emerging as a new potential cause of postprocedural ischaemic stroke and intraparenchymal haemorrhage (IPH).3 We hereby add to the literature and describe the case of a patient who underwent placement of a pipeline embolisation device (PED) and had delayed neurological deficits that were not due to haemorrhage or ischaemia.
Case presentation
A middle-aged woman with a medical history of hypothyroidism, rheumatoid arthritis (RA) on maintenance methotrexate and history of breast cancer treated with a mastectomy presented to the office with a complaint of chronic headaches associated with mild visual changes that had been worsening recently. On CT scan, she was found to have a 7 mm wide-neck, supraclinoid right internal carotid artery (ICA) aneurysm. She was scheduled for elective treatment of the aneurysm with placement of a PED.
On the day of the procedure, the right femoral artery was accessed using a 5 French (Fr) sheath, through which a 5 Fr Davis catheter (Cook Medical, Bloomington, Indiana) was introduced over 0.035 Terumo guidewire (Terumo Medical, Somerset, New Jersey) for the diagnostic portion of the procedure. Once the aneurysm was identified and the anatomy delineated on fluoroscopy, the diagnostic Davis catheter (Cook Medical) and sheath were removed over an Amplatz wire (Boston Scientific, Marlborough, Massachusetts). Then a Neuron MAX 0.088 inch guide sheath (Penumbra, Alameda, California) was advanced over the Amplatz wire to the right ICA. A Navien 0.054’ (Covidien, Irvine, California) intermediary support catheter was advanced into the supraclinoid right ICA, through which a Marksman microcatheter (Medtronic, Minneapolis, Minnesota) was deployed. However, the procedure operators had difficulty getting the Marksman microcatheter past the aneurysm neck. An attempt was made with the help of Synchro 0.014 inch guidewire (Stryker, Fremont, California) without success. An Asahi 0.010 inch hydrophilic wire (Asahi Intecc, Japan) was then tried with the same result. A combination of both wires was then successfully used to advance the Marksman microcatheter through the supraclinoid ICA into the distal middle cerebral artery.
A 3.75×18 mm pipeline flow diverter device (Covidien) was then successfully deployed in the vessel with good positioning on fluoroscopy. The right ICA remained patent without any evidence of stenosis. A follow-up CT scan of the head showed no haemorrhage or ischaemic changes, and she had an uncomplicated hospital stay postprocedurally. She was discharged on dual antiplatelet therapy.
At her 2-week follow-up appointment, the patient complained of severe right-sided headaches that had been going on for 3 days. She described her headache as being persistent, sharp and a 10 out of 10 in severity. Her headaches were associated with nausea, photophobia, phonophobia, left hand and leg weakness with numbness, and a tingling sensation of her left lower extremities. Her neurological exam during the office visit was unremarkable, with stable vital signs. She was referred to the emergency room for an urgent non-contrast CT head, which showed a non-specific, faint, low-density area in the right frontal lobe. There was concern that she may have been experiencing ischaemic symptoms from stenosis of the PED. A CT angiogram of the head and neck was done, which showed a patent PED device, with good flow and no ischaemic or haemorrhagic changes. The patient was given Zofran, Reglan and Fioricet in the emergency department with resolution of symptoms and was discharged with a diagnosis of migraine, along with orders for an outpatient MRI.
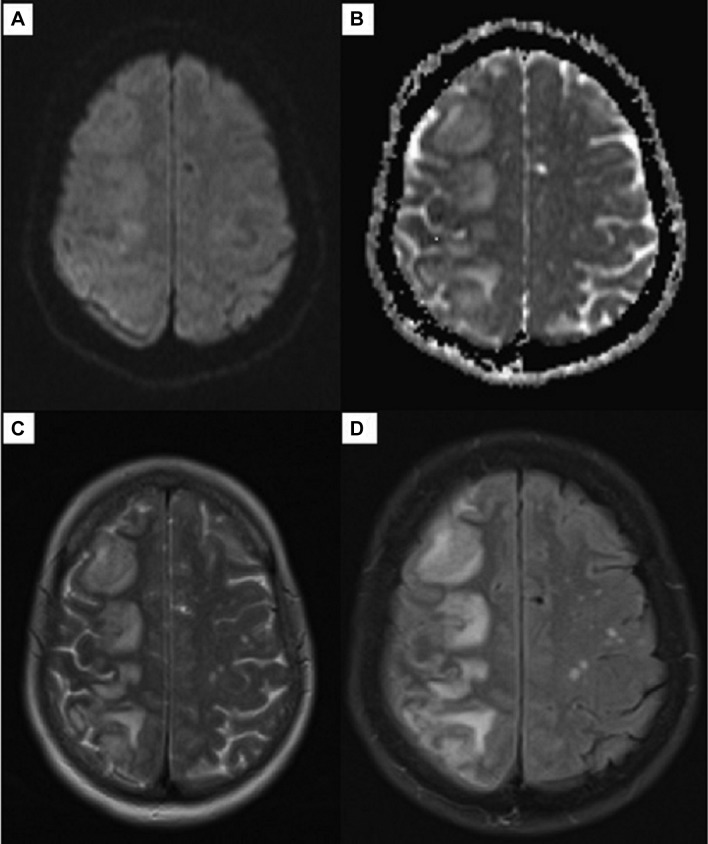
Her outpatient brain MRI without contrast showed multiple areas of abnormal signal within the subcortical white matter at the right frontal, parietal, occipital and temporal lobes, with vasogenic oedema and negative diffusion restriction (figure 1). Given the MRI findings she was asked to return to the hospital the same day for admission and further work-up. She also reported on the phone that her symptoms were progressing. Her vital signs during the admission were stable. Basic laboratory values and rheumatological markers were unremarkable. Her neurological exam was significant for left upper and lower extremity weakness, with 4 out of 5 on strength testing, as compared with 5 out of 5 strength in her right upper and lower extremities. She also exhibited decreased sensory perception on her left side in comparison with her right. Cranial nerves were intact and there were no meningeal signs. An MRI with contrast was performed in the hospital; the T1 postcontrast sequences showed multiple, rounded, enhancing foci at the right frontal, parietal, temporal and occipital regions (figure 2).
Figure 1.
MRI of the brain without contrast. Diffusion-weighted sequence (A), apparent diffusion coefficient map (B), T2-weighted (C) and T2-weighted fluid-attenuated inversion recovery (D).
Figure 2.
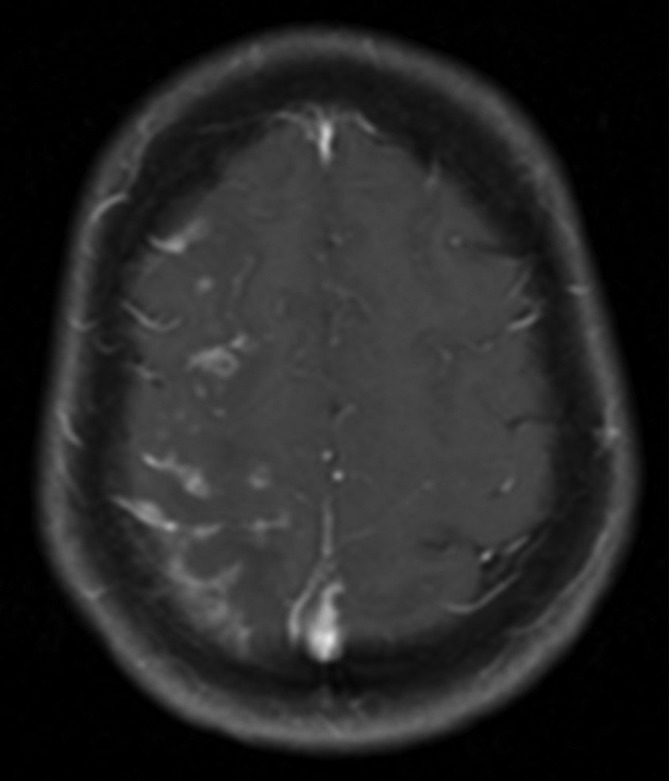
MRI of the brain with contrast. Postgadolinium-T1 image 4 weeks post placement of a pipeline embolisation device at the supraclinoid portion of the right internal carotid artery shows cortical and subcortical enhancement in the right frontal, parietal and occipital lobes.
Differential diagnosis
A shower of foreign body emboli was among the top differentials being considered given the patient’s clinical presentation and brain MRI with contrast, which showed significant enhancing lesions in the distribution of the vascular territory of the catheterised arteries with perilesional oedema. There was no diffusion restriction suggestive of ischaemic events. She was afebrile with unremarkable labs and no meningeal signs. Her indolent presentation made an infectious aetiology unlikely, and as such a lumbar puncture was not deemed necessary; however, cerebritis was considered a differential diagnosis. A diagnosis of complex migraines was also considered, although was less likely given neurological deficits on physical exam during this hospitalisation.
Treatment
She was treated with a high dose of methylprednisolone 250 mg intravenously every 6 hours for 1 day, with marked symptomatic improvement in her headaches and left-sided weakness and paresthesia, and then was switched to prednisone 30 mg daily, which was slowly tapered over a course of 2 months.
Outcome and follow-up
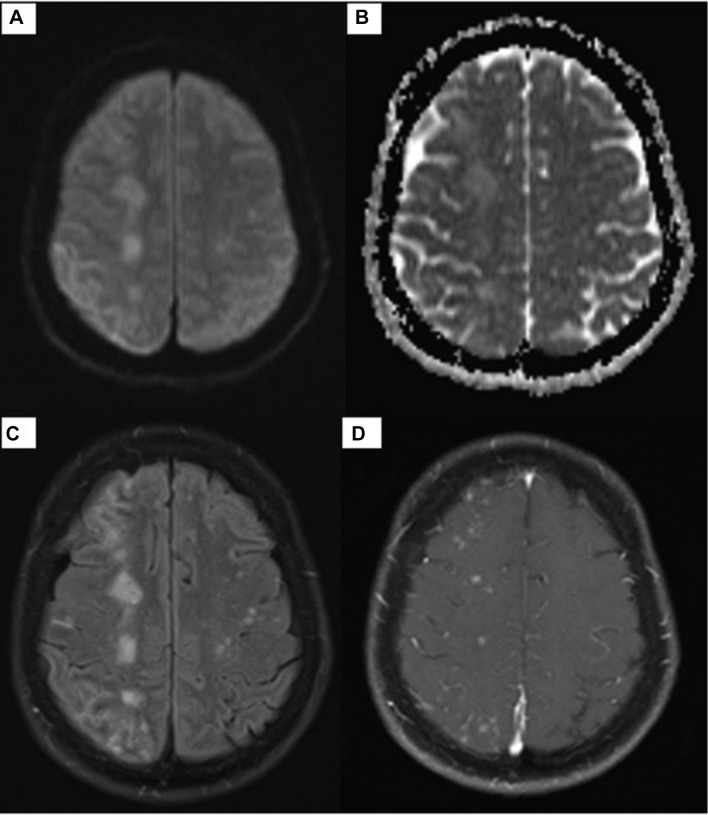
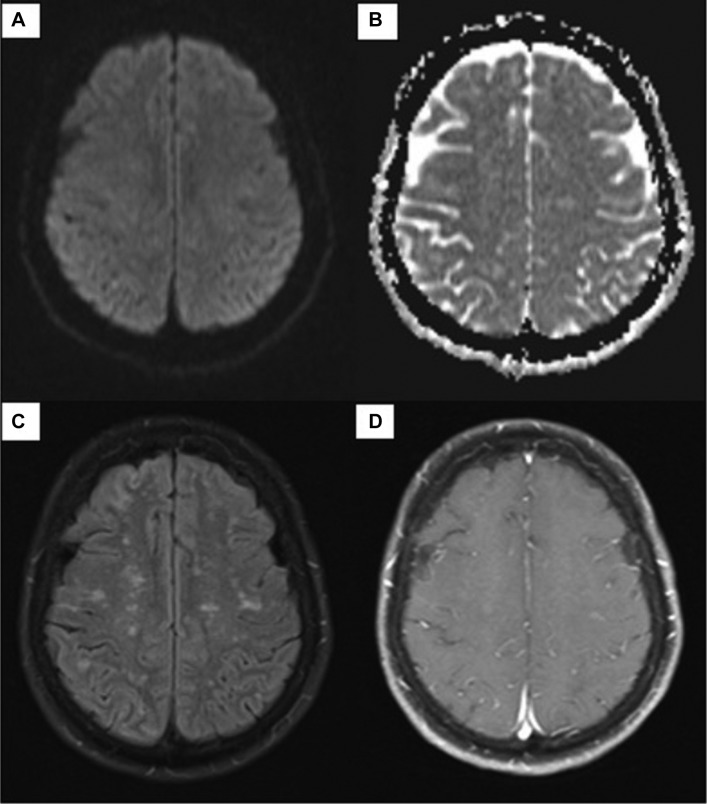
After a day of methylprednisolone, our patient had marked symptomatic improvement in her headaches, left-sided weakness and paresthesia. Two follow-up MRIs with and without contrast showed near-complete resolution of the enhancing lesions (figures 3 and 4).
Figure 3.
MRI of the brain with and without contrast. Diffusion-weighted sequence (A), apparent diffusion coefficient map (B), T2-weighted fluid-attenuated inversion recovery (C) and postgadolinium-T1 (D) after 2 weeks of steroids show improvement in the enhancement previously noted in the right frontal, parietal and occipital lobes.
Figure 4.
Two-month follow-up MRI of the brain with and without contrast shows marked improvement of enhancement. Diffusion-weighted sequence (A), apparent diffusion coefficient map (B), T2-weighted fluid-attenuated inversion recovery (C) and postgadolinium-T1 (D).
Discussion
A few case reports have described delayed complications of endovascular therapy of intracranial aneurysms using a PED. Hu et al described three cases of delayed ipsilateral fatal IPH, which were observed 3 days, 6 days and 2 weeks after uneventful treatment of aneurysm with a PED. All three patients underwent postmortem evaluation, and the vasculature of the haemorrhagic regions of all three patients was found to contain PVP.3 The three devices that were common to the three procedures were 0.035 Terumo guidewire, the Synchro 0.014 microguidewire and the Cook Shuttle guiding sheath. Although the Cook Shuttle was not used in our patient, the Terumo and Synchro microguidewires were used.
Cruz et al4 described the development of subacute non-lethal MRI enhancing lesions with and without neurological symptoms in seven patients (two were treated with conventional aneurysm coiling, two with flow diverter stents, two with balloon-assisted coiling and one with stent-assisted coiling) and concluded that foreign body emboli were likely responsible. Shapiro et al reported five cases of MRI enhancing lesions associated with neurological symptoms noted after placement of a PED in four of the five cases and after one case of stent-supported coiling. While no biopsy was pursued in our patient, Shapiro et al did biopsies on two of their cases, which found granulomatous angiitis encasing a foreign material that stained similarly to the PVP coating. They proposed that PVP embolisation may be related to intercatheter friction created by the sophisticated catheter support systems.5 In our patient, several attempts were made before the final catheter was successfully positioned for deployment of the PED, which involved twisting and sliding manoeuvres. This could have potentially caused the hydrophilic coating to dislodge and embolise to the cortical-subcortical areas distally in the vascular territory of the catheterised arteries. Our patient did not have an acute infarct or haemorrhage but developed a diffuse inflammatory reaction that involved the white matter ipsilateral to the PED deployment site. Both our case and the Shapiro et al cases highlighted that this presentation developed over time with improvement in patients’ symptoms and near-resolution of enhancing lesions on MRI (figures 3 and 4) after treatment with steroids.
Our patient had a history of RA and hypothyroidism, and therefore may be predisposed to autoimmune phenomena, which may also respond to a course of steroids. Lorentzen et al6 described a similar case in a patient with a history of psoriasis who developed a diffuse inflammatory reaction in the white matter ipsilateral to the stent and distal to the stent site after an elective stenting of a wide-neck paraophthalmic aneurysm of the left ICA using a PED.
We believe that our patient gradually developed a delayed inflammatory reaction, likely to PVP, which responded well to a course of steroids. We suggest that as emerging complications of endovascular treatment of aneurysms are identified, catheter manufacturers should be vigilant on the potential adverse effects. Given the tight-fitting catheter system, neurointerventionists should also be more careful while manoeuvring these devices to minimise potential embolic complications that may arise from intercatheter friction, or consider higher size sheaths and catheters for access.
Learning points.
Complications from endovascular treatment of aneurysms generally tend to be immediate. Delayed complications are rare.
Given the increasing use of flow diverter devices for the treatment of aneurysms, we have seen clinically variable and delayed complications from the placement of such devices.
Delayed reactions to stent and microcatheter coatings, which may be thromboembolic and inflammatory in nature, are increasingly being recognised. Treatment with steroids should be considered.
Footnotes
Contributors: NS: primarily involved in creating the initial draft, design and reviewing the literature. MMH: predominantly helped in looking up the EMR for relevant information about the patient, acquisition of data and planning. AS: contributed mainly towards revision, adding relevant images, proper organisation and finishing the paper. JF: reviewed the paper, gave feedback, and helped analyse the data and understand the concept. All authors contributed substantially and approved the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Benaissa A, Tomas C, Clarençon F, et al. Retrospective analysis of delayed intraparenchymal hemorrhage after flow-diverter treatment: presentation of a retrospective multicenter trial. AJNR Am J Neuroradiol 2016;37:475–80. 10.3174/ajnr.A4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RI, Mehta RI, Fishbein MC, et al. Intravascular polymer material after coil embolization of a giant cerebral aneurysm. Hum Pathol 2009;40:1803–7. 10.1016/j.humpath.2009.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu YC, Deshmukh VR, Albuquerque FC, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the Pipeline Embolization Device. J Neurosurg 2014;120:365–74. 10.3171/2013.11.JNS131599 [DOI] [PubMed] [Google Scholar]
- 4.Cruz JP, Marotta T, O’Kelly C, et al. Enhancing brain lesions after endovascular treatment of aneurysms. AJNR Am J Neuroradiol 2014;35:1954–8. ajnr.A3976v1-0 10.3174/ajnr.A3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro M, Ollenschleger MD, Baccin C, et al. Foreign body emboli following cerebrovascular interventions: clinical, radiographic, and histopathologic features. AJNR Am J Neuroradiol 2015;36:2121–6. 10.3174/ajnr.A4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorentzen AO, Nome T, Bakke SJ, et al. Cerebral foreign body reaction after carotid aneurysm stenting. Interv Neuroradiol 2016;22:53–7. 10.1177/1591019915609171 [DOI] [PMC free article] [PubMed] [Google Scholar]