Abstract
Patient: Female, 79
Final Diagnosis: Splenic rupture due to babesia microti infection
Symptoms: Abdominal discomfort • chest pain • fever • tachycardia
Medication: —
Clinical Procedure: Splenectomy
Specialty: Infectious Diseases
Objective:
Rare disease
Background:
Babesiosis is an emerging, tick-borne zoonosis caused by intraerythrocytic protozoa of the genus Babesia. Babesia microti is the main pathogen causing human disease and is endemic in the northeastern and upper midwestern parts of the USA. Severity of infection ranges from mild, self-limited, febrile viral-like illness accompanied by nonspecific symptoms to life-threatening infection complicated by severe hemolytic anemia, disseminated intravascular coagulation (DIC), acute respiratory distress syndrome (ARDS), and renal or/and hepatic failure. Splenic rupture (SR) is a very rare but life-threatening complication of severe B. microti infection.
Case Report:
A 79-year-old female farmer from Wisconsin, USA was admitted during summer with hemorrhagic shock secondary to spontaneous splenic rupture. She was transfused with 3 units of packed red blood cells (PRBC) and underwent emergent splenectomy. Postoperatively, she recovered well and was discharged on postoperative day 4. However, she was re-admitted on postoperative day 10 for febrile hemolytic anemia. Further exposure history was obtained and was significant for multiple tick bites 8 weeks preceding the index hospitalization. She was promptly diagnosed with babesiosis and Lyme disease co-infection. She responded favorably to 10 days of azithromycin and atovaquone and 21 days of oral doxycycline.
Conclusions:
Despite its rare occurrence, SR due to B. microti infection is a dreaded complication that can rapidly progress to hemorrhagic shock and death. In contrast to other complications of babesiosis, SR is not correlated with parasite burden or immune status of the affected host. Babesiosis should be considered as part of the differential diagnosis in patients from endemic areas presenting with atraumatic splenic rupture.
MeSH Keywords: Babesia microti, Lyme Disease, Splenic Infarction, Splenic Rupture
Background
Babesiosis is a worldwide, vector-borne disease caused by intraerythrocytic protozoa of the genus Babesia. It is endemic in the northeast, upper midwest and pacific northwest regions of the United States (U.S.) and in western Europe, occurring mostly during the spring and summer months [1,2]. The white-footed mouse is the primary reservoir host for Babesia microti, and Ixodes scapularis ticks are the primary vector for transmission of disease to humans [1]. Geographic distribution of babesiosis in the United States closely follows that of Lyme disease, as they share the same vector, I. scapularis [1,2]. The majority of cases in the U.S. are caused by B. microti, while in Europe, B. divergens, which is associated with more severe illness, is the most common pathogen. A smaller number of cases caused by B. duncani have been reported in northern California and Washington State and a few sporadic cases of infection caused by B. divergens-like organisms have been described in patients from Kentucky and Missouri [2,3]. The disease is named after Victor Babes, a Hungarian microbiologist who was the first to identify the organism in 1888 [2]. The first human case, caused by B. divergens, was described in a splenectomized Yugoslavian farmer in 1956, [4] and the first case in the U.S. was described in an immunocompetent man from Massachusetts’s Nantucket Island in 1969 (hence the name, “Nantucket fever”) [1,5].
While the bite of an infected tick remains the primary route of transmission of babesiosis, vertical transmission [6] and transmission by blood transfusion have been reported in the literature [3]. Recently, donor-derived babesiosis was reported in 2 renal transplant recipients who received allografts from the same deceased donor who was multiply transfused on the day of his death [8]. The incidence of tick-borne infections in the U.S. is on the rise due to multiple factors including larger deer populations, increasing tick populations, and increased proximity between humans and ticks due to rural development, as well as increased awareness of disease and availability of better diagnostic methods [9]. As of January 2011, babesiosis is a mandatory reportable disease in the U.S. [10]. While up to 20% of adults and 50% of children have asymptomatic disease [11,12], clinical manifestations of symptomatic patients range from mild febrile illness with nonspecific, viral-like symptoms to life-threatening multiorgan failure with a fatality rate of 6–21%. Infection in the elderly, asplenic, and immunocompromised patients has been associated with higher mortality [13–15]. Splenic rupture is a rarely reported complication of B. microti infection. Here, we report a case of splenic rupture leading to hemorrhagic shock in a patient with B. microti and Borrelia burgdorferi co-infection and review the existing literature on splenic complications associated with B. microti infection.
Case Report
A 79-year-old female farmer from Wisconsin was admitted in early August for left-sided chest pain that started one hour prior to admission. The pain was located in the left lower chest, was constant and sharp, and was exacerbated with movement and deep breathing. She complained of dizziness and profound fatigue and denied fevers, chills, or cough. Her past medical history was significant for hypertension, coronary artery disease, and atrial fibrillation for which she was anticoagulated with warfarin. She was a nonsmoker and did not use alcohol or abuse illicit drugs. She denied any recent trauma. On presentation, she was lethargic with unstable vital signs with a heart rate of 110 beats/min, blood pressure of 80/50 mmHg, and respiratory rate of 24–30 breaths per min. She was afebrile and had an oxygenation saturation of 88% on ambient air. She appeared to be in moderate to severe distress. Cardiothoracic examination was normal with the exception of tachycardia. Abdominal examination revealed diffuse abdominal tenderness with guarding and absent bowel sounds. Laboratory data were significant for hemoglobin of 6.5 g/dL, white blood cell count (WBC) of 14.9×103, platelets of 91×103/L, aspartate aminotransferase (AST) of 60 U/L, ala-nine aminotransferase (ALT) of 48 U/L, international normalized ratio (INR) of 1.1, and normal renal function and serum electrolytes. Computed tomography (CT) of the chest was negative for pulmonary embolism; however, abdominal CT showed splenic rupture with associated hematoperitoneum (Figure 1). The patient underwent emergent splenectomy and received 3 units of PRBC. Histologic examination of the removed spleen revealed mild red pulp hyperplasia, but white pulp was unremarkable. There was no evidence of malignancy or intracellular inclusions. The postoperative course was unremarkable and she was discharged home on postoperative day 4. She received post-splenectomy pneumococcal and meningococcal vaccinations.
Figure 1.
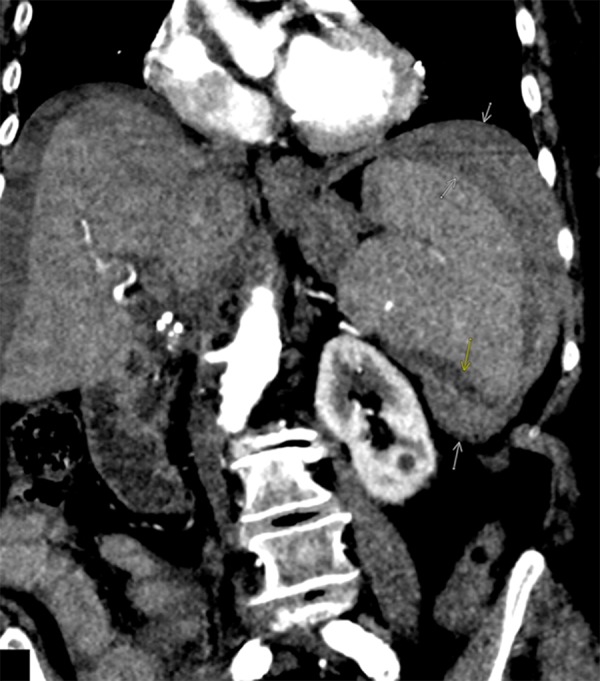
A coronal image from contrast-enhanced CT demonstrates a perisplenic hematoma and hemoperitoneum (white arrows) related to splenic rupture (yellow arrow).
The patient was re-admitted to the hospitalist service on postoperative day 10 with fever, abdominal discomfort, and fatigue that started shortly after the previous discharge from hospital. She reported mild frontal headache without photophobia or neck stiffness. She denied vomiting, diarrhea, chest pain, cough, urinary urgency, dysuria, joint pains, or skin rash. She lived on a farm without exposure to livestock but had a pet cat. She denied cat bites or scratches. She denied traveling outside the U.S. However, she remembered removing several ticks from her arms approximately 8 weeks prior to her first admission. She did not receive blood product transfusion prior to her previous admission. Physical exam revealed a well-developed woman in no distress. She was febrile with a temperature of 39°C. Her blood pressure was 100/40 mmHg and heart rate was 110 beats/min with irregularly irregular rhythm and no murmurs. Respirations were 18 breaths per min. The lungs were clear on auscultation bilaterally without wheezing, rhonchi, or crackles. An examination of the abdomen revealed a well-healing surgical scar without wound dehiscence, erythema, or drainage and was soft and non-tender on palpation. There were no skin rashes and joints showed no evidence of synovitis. Her extremities were warm and well-perfused without edema. Laboratory testing showed hemoglobin 6.3 g/dL, hematocrit 18.2%, normal WBC with differential, platelets 132×10/L, INR 1.1, lactate dehydrogenase (LDH) 1144 U/L, haptoglobin <10 ng/dL, AST 78U/L, ALT 20 U/L, and total bilirubin 3.4 mg/dL. Procalcitonin was 0.78 ng/ml. Blood cultures remained without any growth. Microscopic examination of the peripheral smear revealed numerous red cells with parasitic inclusions characteristic of Babesia spp. with an estimated parasitemia of 1.3% (Figures 2, 3). Additionally, there were also features of hyposplenism/asplenism, including increased poikilocytosis and Howell-Jolly bodies. Nucleated red blood cells and reticulocytes were also seen, which can be attributed to the patient’s asplenism or a compensatory bone marrow response to anemia (Figures 2, 3). A tick-borne panel was ordered and it was positive for Babesia microti IgM, IgG, and PCR, as well as Borrelia burgdorferi IgM and IgG in serum. Serology and PCR for Anaplasma phagocytophilum and Ehrlichia spp. were negative. Other workups included serologies for cytomegalovirus (CMV) and Epstein-Barr virus (EBV), which were reflective of previous exposure (IgM-negative and IgG-positive) and negative Bartonella henselae serology. The patient showed clinical improvement after a 14-day course of atovaquone and azithromycin for babesiosis and a 21-day course of oral doxycycline for early localized Lyme disease. In retrospect, we believe that the splenic rupture occurred as a consequence of underlying babesiosis that went unrecognized during the initial admission due to paucity of other symptoms that are commonly associated with babesiosis. The occurrence of febrile illness associated with hemolytic anemia after splenectomy led to the prompt workup and diagnosis of babesiosis and Lyme co-infection in this patient.
Figure 2.
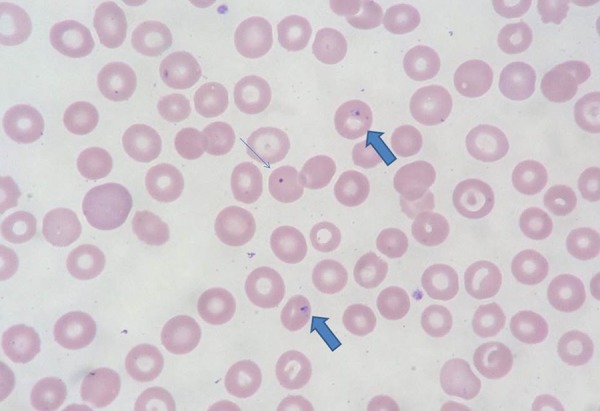
The peripheral smear with 1000× magnification. Thin arrow denotes Howell-Jolly body (nuclear remnant, indicative of hyposplenism/asplenism). Thick arrows show parasitic inclusions (Modified Wright Giemsa stain).
Figure 3.
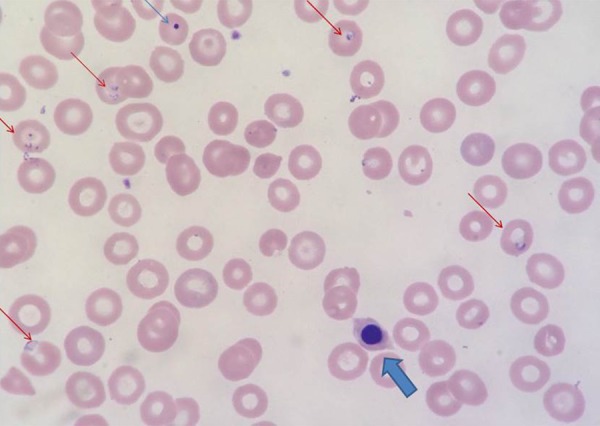
This peripheral smear under 1000× magnification demonstrates: nucleated red cell (thick arrow); Howell-Jolly body (thin arrow) and numerous cells with parasitic inclusions are seen (red arrows) (Modified Wright Giemsa stain).
Discussion
The most common cause of splenic rupture is blunt abdominal trauma. Spontaneous splenic rupture is a rare condition that can be classified according to Renzulli et al. [16] as either atraumatic-idiopathic or atraumatic-pathological splenic rupture, with the latter being more common (7% and 93%, respectively). The causes of atraumatic-pathologic splenic rupture are neoplasm (30.3%), infections (27.3%), and inflammatory, non-infectious conditions (20%). Several bacterial, viral, and protozoan pathogens have been reported to cause splenic rupture, with Plasmodium spp., EBV, and CMV being the most frequently implicated agents. Splenic rupture associated with bacterial infection such as Streptococcus spp., Staphylococcus spp., Coxiella burnetii, Bartonella henselae, and Rickettsia species have been described [17]. Babesia species have been infrequently associated with atraumatic splenic rupture [18,20–28].
A PubMed database search for articles published in English using the key words “Babesia and splenic rupture”, “Babesiosis and splenic rupture”, “Babesia and hemorrhagic shock” and “Babesia and splenic infarct”, yielded 9 cases of babesiosis associated with splenic rupture and 3 cases associated with splenic infarct. Interestingly, all cases were reported in the U.S and among these, only 1 case [27] was identified in a patient who recently immigrated to the U.S. from Ecuador. All the cases of splenic infarct or splenic rupture were due to B. microti infection. The patients’ median age was 57.3 years (range, 23 to 72). The male-to-female ratio was 5: 1. Of the 12 reported cases, 1 patient died due to multiorgan failure, which corresponds to a mortality rate of 8.3%. The parasitemia level was documented in 8 of 12 cases and varied from 0.5% to 30%, with a mean parasitemia level of 6.3%. Splenomegaly was reported in 10 of 12 patients. Eight patients (80%) had mild splenomegaly, 10% had moderate splenomegaly, and 10% had massive splenomegaly. In 2 patients, spleen size was not reported.
Unlike the majority of reported cases, our patient was a woman. The level of parasitemia in our patient was mild (1.3%), significantly lower than the median level in cases reported thus far. Similar to previously described cases, our patient had mild splenomegaly (14 cm in diameter and 300 g weight) and the red pulp showed hyperplasia but the white pulp was unremarkable. It is important to recognize that splenic rupture as a complication usually occurs in otherwise healthy and nonimmunocompromised patients, unlike other severe manifestation of babesiosis that are most commonly seen in the elderly and immunocompromised [28]. In addition, the level of parasitemia is not correlated with the risk of splenic rupture, since the majority of patients who developed splenic rupture only had mild parasitemia. This is in contrast with other severe manifestations of babesiosis. Reviewing the literature, of the 12 cases described, none were immunocompromised and the majority (83.3%) were male patients, which is similar to a previous observation of patients with splenic rupture associated with CMV infection [29]. It is interesting to note that Plasmodium vivax, which usually causes less severe infection when compared to P. falciparum, may be more commonly associated with splenic rupture in patients with malaria [30–32].
The exact mechanism by which Babesia and malaria lead to splenic rupture is unknown, but several theories have been proposed. Kuwayama and Briones, who were the first to describe splenic rupture associated with babesiosis [17] in 2008, theorized that degradation of parenchymal integrity, rather than elevated intracapsular pressure, contributed to splenic rupture in their patient, based on the observation that the ruptured spleen in their patient was not enlarged, but, interestingly, had abnormal, friable parenchyma. Their theory is further supported by the fact that a majority of reported cases (80%) of babesiosis with splenic rupture had mild splenomegaly (Table 1). Another theory favors the development of small subcapsular hematomas and/or splenic infarcts during acute parasitemia, which can subsequently lead to focal necrosis and splenic rupture. It has been proposed that splenic infarct and splenic rupture are different stages along a single continuum rather than 2 different processes [18,29–31]. Similar to malaria, infection with B. microti is associated with an increase in cytokines TNF-α, IFN-γ, and IL-10, as well as increase in endothelial activation markers and hypercoagulability due to decreased levels of antithrombin III, protein C, and protein S, [18,33–35]. These changes, in turn, can increase cytoadhesion of infected RBCs to endothelial cells, causing endothelial injury and microthrombus formation, further leading to splenic infarction and rupture [35–38].
Table 1.
Published reports of patients with splenic complications resulting from Babesia microti infection.
| Case [ref] | Year published | Age | Sex | Country | Co infection | Parasitemia | Splenomegaly | Rupture or infarct | ICS | Treatment (days) | Splenectomy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [17] | 2008 | 61 | M | NJ, USA | No | 5% | Mild | R | No | AZ+ATQ (7) | Yes |
| 2 [20] | 2008 | 56 | M | MN, USA | No | N/A | Moderate | R | No | N/A | Yes |
| 3 [21] | 2008 | 58 | M | NJ, USA | APG | 0.5% | Mild | I | No | AZ+ATQ | No |
| 4 [21] | 2008 | 75 | F | NY USA | No | N/A | Massive | I | No | AZ+ATQ QI+CLIN | No, expired |
| 5 [22] | 2011 | 54 | M | MA, USA | No | 3% | N/A | R | No | AZ+ATQ | No |
| 6 [23] | 2011 | 23 | M | CT, USA | No | 30% | N/A | R | No | CLIN+QI | No |
| 7 [24] | 2011 | 70 | M | NY, USA | No | NA | Mild | R | No | AZ+ATQ (14) | No, SA embolization |
| 8 [25] | 2011 | 70 | M | NY, USA | No | 8% | Mild | R | No | CLIN+QI AZ+ATQ (16) | No, SA embolization |
| 9 [25] | 2011 | 36 | M | NY, USA | No | 3–4% | Mild | R | No | AZ+ATQ (21) | No |
| 10 [26] | 2014 | 54 | M | CT, USA | No | N/A | Mild | R | No | AZ+ATQ (10) | No |
| 11 [28] | 2015 | 59 | F | CT, USA | No | 0.9% | Mild | R | No | CLIN+ AZ +ATQ (14) | Yes |
| 12 [27] | 2016 | 72 | M | Ecuador | No | 0.5% | Mild | I | No | ATQ+PRG N/A | No |
Ref – reference; ICS – immunocompromised state; M – male; F – female; R – rupture; I – infarct; AZ – azithromycin; QI – quinine; CLIN – clindamycin; ATQ – atovaquone; PRG – Proguanil; APG – Anaplasma phagocytophilum; SA – splenic artery; NJ – New Jersey; MN – Minnesota; MA – Massachusetts; CT – Connecticut; NY – New York; N/A – not applicable/reported.
Apart from B. microti, I. scapularis can transmit several other pathogens including B. burgdorferi, B. miyamotoi, A. phagocytophilum, and Powassan virus. Unlike the other reported cases, our patient was co-infected with B. burgdorferi. Given the high incidence of co-infection with multiple pathogens among I. scapularis ticks in Wisconsin and Minnesota [40,41], it is not unusual that our patient was infected with 2 tick-borne pathogens simultaneously. We do not believe that B. burgdorferi contributed to splenic rupture in our patient, as early-stage Lyme disease is not known to be associated with any splenic complications.
Transfusion-transmitted babesiosis (TTB) has increased in incidence in recent years and is the most common transfusion-transmitted infection in the U.S. [7,42]. TTB tends to have a longer incubation period, is more severe, and has a reported mortality of up to 20% [42,43]. The higher observed mortality in TTB is most likely due to the fact that blood transfusion recipients are usually immunocompromised and have a plethora of other medical comorbidities, making them more susceptible to severe infection [2,43]. We considered the possibility of TTB in our patient, as she was transfused with 3 units of PRBC for hemorrhagic shock during her first admission. However, given her history of tick bites, presence of co-infection with B. burgdorferi, and residence in a highly endemic area, we are confident that our patient had subclinical illness, and splenic rupture was, in fact, the first manifestation of the infection. Following splenectomy, the disease manifested itself in a more typical fashion, with febrile hemolytic anemia. Also, our patient developed febrile hemolytic anemia approximately 2 weeks following PRBC transfusion and, while not impossible, it is highly unlikely to be TTB due to its longer incubation period (up to 6 months) when compared to tick-transmitted infection [42,43]. Finally, our patient had both IgM and IgG antibodies against B. microti, which argues in favor of acquisition of the infection much earlier, since it takes more than 2 weeks for antibodies to develop, especially in immunocompromised patients.
Definitive diagnosis of babesiosis is made by microscopic examination of Giemsa-stained thick and thin blood smears, which can reveal intraerythrocytic merozoites [2]. The ring forms of Babesia are pleomorphic and delicate and can closely resemble those of P. falciparum. Unlike malaria, the brown pigmentation of hemozoin and advanced forms such as schizonts or gametocytes are not seen in thin smears of patients with babesiosis. Other findings in favor of babesiosis include occasional extra-cellular forms and the classic tetrad or Maltese cross forms [2]. In cases where organism morphology is inconclusive and travel history is not helpful, the use of molecular diagnostics such as polymerase chain reaction (PCR) can prove particularly valuable [2]. Molecular diagnostics such as Babesia PCR assays offer a rapid and sensitive alternative to blood film examination and can help differentiate between the various species that cause babesiosis [2,44,45]. Finally, serology may be useful among patients with very low level of parasitemia or those who have cleared infection following therapy. IgM antibodies usually develop 2 weeks after initial infection, followed by IgG in subsequent weeks [2,45].
Treatment of babesiosis depends on the severity of infection and the patient’s immune status. Immunocompetent patients with mild to moderate infection can be treated with a combination of oral azithromycin and atovaquone for 7 to 10 days [46]. Patients with severe infection may benefit from parenteral clindamycin and oral or intravenous quinine. Immunocompromised patients are more likely to have severe infection with persistent and relapsing parasitemia and may require a prolonged duration of treatment. While the development of resistance to azithromycin and atovaquone in patients on prolonged treatment has been described, this remains a rare phenomenon [47]. Patients with severe organ dysfunction and high level of parasitemia (>10%) may also require urgent partial or complete exchange transfusion (ET), in addition to antimicrobial therapy [2,46,48]. There have been reports of successful treatment of babesiosis with ET, even with parasitemia levels as high as 50% [48]. Our patient was successfully treated with 14 days of atovaquone and azithromycin and 21 days of oral doxycycline. She continued to do well 3 months following completion of treatment, without evidence of recurrence of infection.
Treatment of splenic rupture depends on the hemodynamic stability of the patient. In patients who are hemodynamically stable, a conservative approach, including close monitoring in intensive care and as-needed transfusion, is recommended, due to multiple benefits of spleen preservation [49]. In patients who are hemodynamically stable but with either significant or ongoing bleeding, trans-catheter splenic artery (SA) embolization has been successful in several cases [24,25]. Splenectomy remains the gold standard in patients similar to ours, who present with acute abdomen and hemorrhagic shock [15]. In their systematic review, Renzulli et al. [15] found that 660 out of 774 patients (85.3%) with atraumatic splenic rupture were managed surgically with splenectomy. However, in our review of the literature on atraumatic-pathologic splenic rupture secondary to B. microti infection, out of the 12 reported cases, only 3 underwent splenectomy (25%), 1 patient died (8.3%), and 8 (66.6%) were managed conservatively with either SA embolization or with transfusion, bedrest, and antibiotics.
Mortality in patients with splenic rupture associated with B. microti infection is 8.3%, which is significantly lower than the mortality rate reported by Imbert et al. (22%) in their analysis of 55 cases of spontaneous splenic rupture secondary to malaria [19]. The most plausible explanation for this observation is that babesiosis tends to usually cause milder disease and is endemic in countries with advanced diagnostic and medical amenities. Despite its lower mortality in comparison to malaria, it is vital to be aware that babesiosis can cause life-threatening complications. Increased awareness of splenic rupture as one of these complications will lead to early recognition and treatment and, in turn, may save lives.
Conclusions
B. microti infection leading to splenic rupture and hemorrhagic shock has been rarely reported. We report a case of B. microti and B. burgdorferi co-infection in a female farmer from an endemic region, with a history of tick bites, whose first manifestation of babesiosis was splenic rupture. Due to paucity of other, better-recognized symptoms of babesiosis, infection was undetected on initial admission and was recognized on readmission, when disease manifested itself in a more typical fashion, in the absence of a spleen. We additionally reviewed the previously reported cases of splenic complication of babesiosis. Babesiosis should be considered in the differential diagnosis of atraumatic splenic rupture among patients with epidemiological risk factors such as residence in or travel to endemic areas or blood transfusion within the preceding 6 months. Interestingly, unlike the other complications of severe babesiosis, splenic rupture does not seem to be correlated with parasite load or host immune status.
Acknowledgments
The authors thank Hemant Hegde MD who took part in clinical care of this patient.
References:
- 1.Spielman A. Human babesiosis on Nantucket Island: Transmission by nymphal Ixodes ticks. Am J Trop Med Hyg. 1976;25(6):784–87. doi: 10.4269/ajtmh.1976.25.784. [DOI] [PubMed] [Google Scholar]
- 2.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366(25):2397–407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 3.Burgess MJ, Rosenbaum ER, Pritt BS, et al. Possible transfusion-transmitted Babesia divergens-like/MO-1 infection in an Arkansas patient. Clin Infect Dis. 2017;64(11):1622–25. doi: 10.1093/cid/cix216. [DOI] [PubMed] [Google Scholar]
- 4.Skrabalo Z, Deanovic Z. Piroplasmosis in man; Report of a case. Doc Med Geogr Trop. 1957;9(1):11–16. [PubMed] [Google Scholar]
- 5.Western KA, Benson GD, Gleason NN, et al. Babesiosis in a Massachusetts resident. N Engl J Med. 1970;283:854–56. doi: 10.1056/NEJM197010152831607. [DOI] [PubMed] [Google Scholar]
- 6.Joseph JT, Purtill K, Wong SJ, et al. Vertical transmission of Babesia microti, United States. Emerg Infect Dis. 2012;18(8):1318–21. doi: 10.3201/eid1808.110988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leiby DA. Transfusion-transmitted Babesia spp.: Bull’s-eye on Babesia microti. Clin Microbiol Rev. 2011;24(1):14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan MB, Herwaldt BL, Kazmierczak JJ, et al. Transmission of Babesia microti parasites by solid organ transplantation. Emerg Infect Dis. 2016;22(11) doi: 10.3201/eid2211.151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res. 2015;2015:587131. doi: 10.1155/2015/587131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee on Lyme disease and Other Tick-Borne diseases. The state of the science- critical needs and gaps in understanding prevention, amelioration, and resolution of Lyme and other tick-borne diseases: The short-term and long-term outcomes: Workshop report. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 11.Krause PJ, McKay K, Gadbaw J, et al. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg. 2003;68(4):431–36. [PubMed] [Google Scholar]
- 12.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29(2):357–70. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White DJ, Talarico J, Chang HG, et al. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158(19):2149–54. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 14.Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: Review of 34 cases and their complications. Clin Infect Dis. 2001;32(8):1117–25. doi: 10.1086/319742. [DOI] [PubMed] [Google Scholar]
- 15.Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46(3):370–76. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 16.Renzulli P, Hostettler A, Schoepfer AM, et al. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;96(10):1114–21. doi: 10.1002/bjs.6737. [DOI] [PubMed] [Google Scholar]
- 17.Kuwayama DP, Briones RJ. Spontaneous splenic rupture caused by Babesia microti infection. Clin Infect Dis. 2008;46(9):e92–95. doi: 10.1086/587175. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JH, Lee CS. Malaria-induced splenic infarction. Am J Trop Med Hyg. 2014;91(6):1094–100. doi: 10.4269/ajtmh.14-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imbert P, Rapp C, Buffet PA. Pathological rupture of the spleen in malaria: Analysis of 55 cases (1958–2008) Travel Med Infect Dis. 2009;7(3):147–59. doi: 10.1016/j.tmaid.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Froberg MK, Dannen D, Bernier N, et al. Case report: Spontaneous splenic rupture during acute parasitemia of Babesia microti. Ann Clin Lab Sci. 2008;38(4):390–92. [PubMed] [Google Scholar]
- 21.Florescu D, Sordillo PP, Glyptis A, et al. Splenic infarction in human babesiosis: Two cases and discussion. Clin Infect Dis. 2008;46(1):e8–11. doi: 10.1086/524081. [DOI] [PubMed] [Google Scholar]
- 22.Tobler WD, Jr, Cotton D, Lepore T, et al. Case report: Successful non-operative management of spontaneous splenic rupture in a patient with babesiosis. World J Emerg Surg. 2011;6:4. doi: 10.1186/1749-7922-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas HM, Brenes RA, Ajemian MS, et al. Successful conservative treatment of spontaneous splenic rupture secondary to Babesiosis: A case report and literature review. Conn Med. 2011;75(3):143–46. [PubMed] [Google Scholar]
- 24.Reis SP, Maddineni S, Rozenblit G, et al. Spontaneous splenic rupture secondary to Babesia microti infection: Treatment with splenic artery embolization. J Vasc Interv Radiol. 2011;22(5):732–34. doi: 10.1016/j.jvir.2011.01.439. [DOI] [PubMed] [Google Scholar]
- 25.El Khoury MY, Gandhi R, Dandache P, et al. Non-surgical management of spontaneous splenic rupture due to Babesia microti infection. Ticks Tick Borne Dis. 2011;2(4):235–38. doi: 10.1016/j.ttbdis.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Usatii N, Khachatrian A, Stratidis J. Spontaneous splenic rupture due to Babesia microti infection: Case report and review of the literature. IDCases. 2014;1(4):63–65. doi: 10.1016/j.idcr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Zoubi M, Kwak T, Patel J. Atypical challenging and first case report of babesiosis in Ecuador. IDCases. 2016;4:15–17. doi: 10.1016/j.idcr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farber FR, Muehlenbachs A, Robey TE. Atraumatic splenic rupture from Babesia: A disease of the otherwise healthy patient. Ticks Tick Borne Dis. 2015;6(5):649–52. doi: 10.1016/j.ttbdis.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Farber FR, Alliot C, Beets C, Besson M, et al. Spontaneous splenic rupture associated with CMV infection: Report of a case and review. Scand J Infect Dis. 2001;33(11):875–77. doi: 10.1080/00365540110027114. [DOI] [PubMed] [Google Scholar]
- 30.Zingman BS, Viner BL. Splenic complications in malaria: case report and review. Clin Infect Dis. 1993;16(2):223–32. doi: 10.1093/clind/16.2.223. [DOI] [PubMed] [Google Scholar]
- 31.Hamel CT, Blum J, Harder F, et al. Nonoperative treatment of splenic rupture in malaria tropica: Review of literature and case report. Acta Trop. 2002;82(1):1–5. doi: 10.1016/s0001-706x(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 32.Kim KK, Bae BK, Lee SB. Spontaneous splenic rupture in Plasmodium vivax malaria. Ann Surg Treat Res. 2014;87(1):44–46. doi: 10.4174/astr.2014.87.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohanty D, Ghosh K, Nandwani SK, et al. Fibrinolysis, inhibitors of blood coagulation, and monocyte derived coagulant activity in acute malaria. Am J Hematol. 1997;54(1):23–29. doi: 10.1002/(sici)1096-8652(199701)54:1<23::aid-ajh4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Vogetseder A, Ospelt C, Reindl M, et al. Time course of coagulation parameters, cytokines and adhesion molecules in Plasmodium falciparum malaria. Trop Med Int Health. 2004;9(7):767–73. doi: 10.1111/j.1365-3156.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- 35.Francischetti IM, Seydel KB, Monteiro RQ, et al. Plasmodium falciparum-infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes. J Thromb Haemost. 2007;5(1):155–65. doi: 10.1111/j.1538-7836.2006.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner GD, Ly VC, Nguyen TH, et al. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152(6):1477–87. [PMC free article] [PubMed] [Google Scholar]
- 37.Andrade BB, Reis-Filho A, Souza-Neto SM. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010;9:13. doi: 10.1186/1475-2875-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohnishi K. Serum levels of thrombomodulin, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin in the acute phase of Plasmodium vivax malaria. Am J Trop Med Hyg. 1999;60(2):248–50. doi: 10.4269/ajtmh.1999.60.248. [DOI] [PubMed] [Google Scholar]
- 39.Krause PJ, Daily J, Telford SR, et al. Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol. 2007;23(12):605–10. doi: 10.1016/j.pt.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Krause PJ, Telford SR, III, Spielman A. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996;275(21):1657–60. [PubMed] [Google Scholar]
- 41.Mitchell PD, Reed KD, Hofkes JM. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34(3):724–27. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: A description of cases. Ann Intern Med. 2011;155(8):509–19. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 43.Levin AE, Krause PJ. Transfusion-transmitted babesiosis: Is it time to screen the blood supply? Curr Opin Hematol. 2016;23(6):573–80. doi: 10.1097/MOH.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krause PJ, Telford S, III, Spielman A, et al. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J Clin Microbiol. 1996;34(11):2791–94. doi: 10.1128/jcm.34.11.2791-2794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krause PJ, Ryan R, Telford S, III, et al. Efficacy of immunoglobulin M serodiagnostic test for rapid diagnosis of acute babesiosis. J Clin Microbiol. 1996;34(8):2014–16. doi: 10.1128/jcm.34.8.2014-2016.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343(20):1454–58. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 47.Wormser GP, Prasad A, Neuhaus E, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis. 2010;50(3):381–86. doi: 10.1086/649859. [DOI] [PubMed] [Google Scholar]
- 48.Tanyel E, Guler N, Hokelek M, et al. A case of severe babesiosis treated successfully with exchange transfusion. Int J Infect Dis. 2015;38:83–85. doi: 10.1016/j.ijid.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 49.Rapp C, Debord T, Imbert P, et al. Splenic rupture in infectious disease: Splenectomy or conservative treatment? Report of three cases. Rev Med Interne. 2002;23(1):85–91. doi: 10.1016/s0248-8663(01)00518-5. [DOI] [PubMed] [Google Scholar]


