Abstract
The importance of medical imaging in the diagnosis and monitoring of cancer cannot be overstated. As personalized cancer treatments are gaining popularity, a need for more advanced imaging techniques has grown significantly. Nanoparticles are uniquely suited to fill this void, not only as imaging contrast agents but also as companion diagnostics. This review provides an overview of many ways nanoparticle imaging agents have contributed to cancer imaging, both preclinically and in the clinic, as well as charting future directions in companion diagnostics. We conclude that, while nanoparticle-based imaging agents are not without considerable scientific and developmental challenges, they enable enhanced imaging in nearly every modality, hold potential as in vivo companion diagnostics, and offer precise cancer treatment and maximize intervention efficacy.
Keywords: nanoparticles, cancer, imaging, contrast agent, theranostics, companion diagnostics, EPR effect, personalized medicine, molecular imaging
Graphical Abstract

Medical imaging is an integral component of cancer care and is routinely used to detect and assess tumor progression based on anatomical or functional evaluations. For example, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) is an accepted diagnostic and decision-making tool in oncology. TNM (tumor, nodes, metastases) staging and the RECIST (response evaluation criteria in solid tumors) criteria are routinely implemented in cancer patient management through magnetic resonance imaging (MRI) or computed tomography (CT) scans. Since the early 1990s, the FDA has begun approving imaging-based surrogate end points to expedite the passage of investigational drugs. Although these imaging capabilities have contributed significantly to cancer research and patient management, there is still a great need for targeted imaging techniques that can evaluate changes at the molecular level in vivo, as such information is needed within the context of precision medicine.
Small-molecule and antibody-based imaging probes have demonstrated potential for in vivo molecular imaging. Recent developments in nanotechnology have suggested that nanoparticle-based imaging probes may offer additional benefits over traditional imaging techniques. In general, nanoparticle-based delivery vehicles provide enhanced stability, tumor accumulation, and circulation times as compared to small molecules delivered alone.1 The strengths of a nanoparticle-based system are well-known and documented (i.e., large surface area to volume ratio, high tunability, and the possibility of a multimodal nature), such that their application in cancer medicine (as well as many other diseases)2–4 is only natural. To date, the clinical applications of nanoparticles (hereafter, NPs) in imaging settings have been limited, although a few NPs for drug delivery have been FDA-approved (for example, Doxil5 and Abraxane).6 In the past, NPs as imaging agents have suffered several setbacks in the FDA approval pipelines due to health and scientific challenges. Nevertheless, NPs offer significant advantages in the design of not just contrast agents but also companion diagnostic platforms, such that persistent continuation of their development is warranted. This review is not intended to provide a comprehensive review of all NP-based contrast agents; instead, it will focus on their unique applications in cancer detection and management. As most NP-based imaging contrast agents remain preclinical, interested readers should refer to specific reviews cited throughout this paper concerning individual NPs and their specific applications.
NANOPARTICLES AS IMAGING AGENTS
While NPs as imaging agents offer additional information on conventional imaging techniques, they also enable new capabilities such as in vivo Raman imaging,7 magnetic particle imaging (MPI), or multimodality imaging with the use of a single agent. NPs can be coated with a so-called “stealth” layer of polymer or carbohydrates8,9 to enhance their stability, biocompatibility, and safety, and they can also be customized for patient-specific imaging applications. Many preclinical studies were reported throughout the literature in the development of NP-based contrast agents for various imaging modalities: quantum dots for optical imaging, gold NPs and iodinated liposomes for CT, gold nanorods and dye-containing NPs for photoacoustic imaging, gold nanostars and silver nanoclusters for surface-enhanced Raman spectroscopy, iron oxide NPs for MRI, chelator-based or intrinsically radiolabeled NPs for PET/SPECT, and gas-filled nanobubbles for ultrasound imaging (among others). Within oncological studies, NPs may be functionalized with targeting moieties or coated to avoid immune recognition and improve circulation, such as through polymer or carbohydrate stealth layers. This section provides an overview of current NP-enabled imaging modalities and techniques, as well as their status in clinical translation.
Magnetic Resonance Imaging
Magnetic resonance imaging has leveraged NPs as contrast agents. These NPs can provide positive contrast in T1-weighted (spin–lattice relaxation) images or negative contrast in T2-weighted (transverse relaxation) images by changing the corresponding relaxivities of nearby water protons. Notably, superparamagnetic iron oxide NPs (SPIONs) have been used in animal studies since the 1990s; extensive studies also have been conducted in the past decade to make them suitable for biomedical applications in patients.10 SPIONs are used predominantly as T2 contrast agents for MRI, which create localized dark regions in MR images. Some SPIONs have undergone or are in the midst of clinical trials in cancer patients, including ferumoxytol,11 ferumoxtran (Figure 1B),12,13 and ferucarbotran NPs.14 Ferumoxytol, the only FDA-approved drug for anemia treatment, is used as an “off-label” MRI contrast agent in 20 ongoing clinical trials for different diseases and indications; at the same time, ferumoxtran-10 is currently undergoing clinical trials in Europe (NCT02751606, NCT03223064, NCT02549898, NCT02997046).15 Ferumoxytol has also been explored in cardiovascular MRI as an alternative contrast agent for magnetic resonance angiography to circumvent concerns of renal toxicity by conventional gadolinium-contrast MRI; however, the progress has been slow.16,17 Nevertheless, preclinical studies have used different forms of NPs for contrast-enhanced MRI, including molecularly targeted SPIONs to noninvasively detect elevated mRNA levels after chronic drug exposure18 or track gene expression after gene therapy in rodent brains,19 more complex magnetic iron oxides for theranostic applications in cancer,20 gadolinium-doped NPs for locating radiosensitizers (Figure 2B),21,22 manganese oxide NPs as alternatives to Gd-based agents,23 and other metals for cell tracking.24
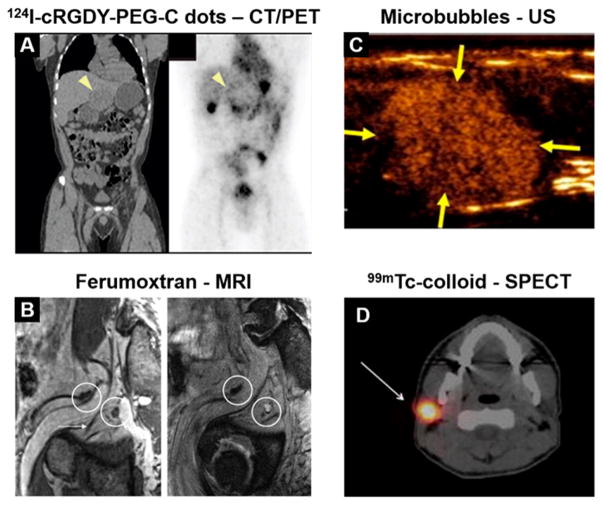
Figure 1.
Clinical examples of NP imaging agents. (A) Administration of 124I-cRGDY-PEG-C dots shows accumulation of the tracer near the periphery of a hepatic lesion. Reprinted with permission from ref 33. Copyright 2014 AAAS. (B) Ferumoxtran-enhanced MRI enables visualization of a metastatic lymph node, which appears hyperintense in a T2*-weighted image postcontrast (right) due to its lack of NP uptake. Reproduced with permission from ref 13. Copyright 2009 RSNA Publications. (C) Targeted microbubbles provide contrast of a breast lesion using ultrasound. Reprinted with permission from ref 50. Copyright 2017 American Society of Clinical Oncology. All rights reserved. (D) 99mTc-colloid preferentially accumulates in a sentinel lymph node. Reproduced with permission from ref 42. Copyright 2017 Springer.
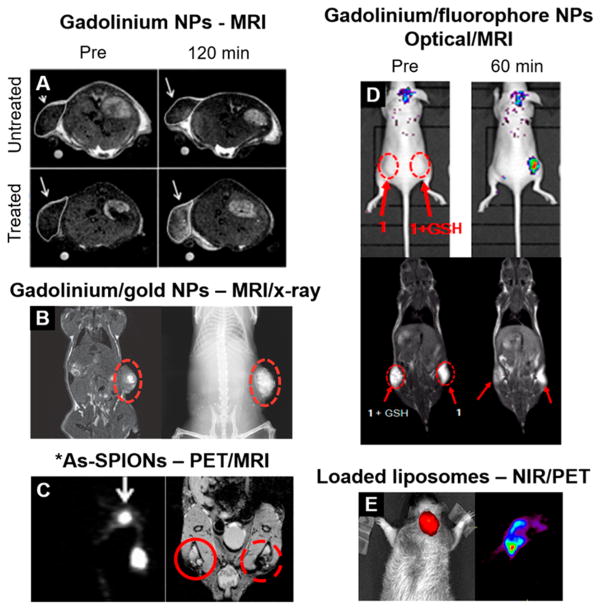
Figure 2.
Multimodality and “smart” NP imaging platforms. (A) Gadolinium NPs self-assemble in the presence of caspase 3/7 enzymes (“treated” group), increasing MRI contrast. Reproduced with permission from ref 79. Copyright 2014 The Royal Society of Chemistry. (B) Gadolinium–gold nanoshells provide contrast in both MRI (left) and X-ray (right) imaging. Reproduced with permission from ref 22. Copyright 2014 John Wiley & Sons. (C) SPIONs labeled with *As allow PET and MRI-based sentinel lymph node mapping. Reproduced with permission from ref 69. Copyright 2013 John Wiley & Sons. (D) NPs disassemble in the presence of GSH in the tumor, activating fluorescence. MRI also shows the presence of the NPs. Reprinted from ref 77. Copyright 2016 American Chemical Society. (E) Liposomes loaded with NIR dyes and 64Cu enable fluorescent and PET imaging of an orthotopic brain tumor. Reprinted from ref 70. Copyright 2012 American Chemical Society.
Optical Imaging
Optical imaging techniques display high spatial resolution but have limited detection depth in tissue due to the inherent attenuation of signals in this wavelength range, limiting them to superficial lesion detection. Many NPs are inherently fluorescent and are ideally suited for optical or near-infrared (NIR) imaging.25 Photoluminescent NPs have versatile surface functionalization and optical properties and are popular in preclinical imaging studies. Several lanthanide-doped upconverting NPs (UCNPs) have also been designed to improve depth penetration with minimal tissue damage, zero autofluorescence, and multicolor capabilities for in vivo imaging and photothermal therapy (PTT)26,27 in preclinical settings. Engineered photoluminescent NPs such as quantum dots (QDs) have been used for fluorescent imaging of cells and tissues for the last 20 years because of their high quantum yields and versatile spectra. Unfortunately, traditional QDs contain cadmium, which is toxic28,29 and limits their clinical usage. Current developments of cadmium-free QDs have gained some attention with their improved biocompatibility, but they still face significant challenges. When depth penetration is not a limiting factor, fluorescent dye-loaded NPs can be used for image-guided tumor surgery.30–32 For example, the first-inhuman trial of fluorescent core–shell silica NPs (Cornell dots or C dots) received FDA Investigational New Drug (IND) approval as a drug for targeted molecular imaging.33 NP-enabled optical imaging detection techniques are expected to continue to be an important field in which NPs can contribute to cancer management.
Nuclear Medicine Techniques
Nuclear medicine techniques, such as PET and single photon emission computed tomography (SPECT), provide unparalleled detection sensitivity when compared to other imaging approaches.34 NPs can be radiolabeled with or without the use of a chelator or intrinsically radiolabeled with any number of radionuclides for in vivo biodistribution and tumor targeting studies.35 With all these possible design variations, the field of radiolabeled NP contrast agents has made significant progress. Early applications of radiolabeled NPs in oncology focused on the use of biomolecule-like NPs, such as RNAs,36,37 or cyclodextrin polymer-based NPs containing camptothecin.38 Radiolabeled drug-delivering NPs have been used to establish drug uptake profiles and to predict treatment responses.39 Radiolabeled NPs have also been used for molecular imaging of atherosclerosis40 and many other diseases. There are several 99mTc-labeled colloid-based SPECT imaging agents for cancer diagnosis that are currently clinically approved, either in the U.S. or EU, including Techneoll, Nanocoll, Hepatate, Nanocis, and Senti-Scint, primarily for lymphoscintigraphy of different cancer indications.41 Figure 1D shows the potential advantage of SPECT/CT with the injection of 99mTc-labeled sulfur colloid in providing surgeons with three-dimensional anatomic landmarks.42 In more recent years, nearly every type of NP has been radiolabeled and tested with a wide range of radionuclides and disease models.
Computed Tomography and X-ray Imaging
Computed tomography is one of the most widely used imaging techniques for cancer screening due to its wide availability, relatively low cost, and high-throughput capabilities. The contrast of bone and lung tissues is readily visible on CT; however, imaging of soft tissues requires the use of high-Z contrast agents. Clinical CT contrast agents are often iodine-containing small molecules, but these usually suffer from short circulation half-lives and notable toxicity. The use of NPs alleviates these concerns, incorporating iodine or gold (or other large atoms) into a NP structure that is more stable and circulates for a longer time, often with active targeting to a tumor site to enhance specificity.43 Nanostructures explored for oncological imaging with CT include gold nanorods, shells, and dendrimers,44 iodine-containing liposomes,45 and lanthanide oxide NPs.46 Also, micelle-based CT contrast agents can be made to target highly expressed molecular signatures during disease progression (e.g., atherosclerosis, inflammation, and cancer).47 Although the field has seen rapid advancement in recent years, NP-based CT imaging techniques are still in the preclinical space.
Ultrasound Imaging
Ultrasound imaging (US), with its accessibility, low cost, and lack of ionizing radiation, is a very common modality for detecting potential malignancies. However, up until recently, US was limited in cancer applications due to its inability to distinguish tissue boundaries that often do not exhibit the required differential scattering properties. The only contrast agents that can be used in US are microbubbles and their variations. Due to their large size (1–8 μm), microbubbles are restricted to vasculature imaging and hence had limited usefulness in oncology.48 While most US contrast agents are applied in cardiac applications, the advent of smaller-sized particles has enabled US to play an increasing role in cancer management. While not necessarily on the nanoscale, Sonazoid US contrast agents have been demonstrated to enhance the detection of malignant prostate lesions using transrectal US.49 Similar clinical-grade, kinase insert domain receptor-targeted contrast microbubbles were evaluated by another group and shown to be clinically feasible and safe based on results from the first-in-human study in patients with breast and ovarian lesions (Figure 1C).50 Within the nanoscale, generation and utilization of nanobubbles targeting cancer antigen 125 (CA-125) have also been demonstrated to offer an improved diagnosis of epithelial ovarian cancer.48
Photoacoustic Imaging
Like US, photoacoustic (PA) imaging utilizes sound waves to produce an image. Unlike US, however, PA imaging is enabled by irradiation of tissue with NIR (“light-in”) and detection of the resulting pressure waves (“sound-out”) with an US transducer.51 In addition to measurements from general US such as anatomy and flow rates, PA imaging can provide additional information such as the optical, physiological, and mechanical properties of tissues. Many tissues show inherent contrast in PA imaging, but the differences in this contrast between healthy and diseased tissue are often not sufficient to be adequately detected. To this end, contrast agents for PA imaging have been developed, many of which are NPs.52 Both organic and inorganic NPs have been used in this still relatively new imaging technique. Inorganic NP frameworks of metals (in particular, gold),53 QDs,54 other semiconductors,55 and UCNPs56 have been successfully used to visualize cancer lesions preclinically, taking advantage of the inherent NIR absorption of these structures. Carbon NPs, particularly single-walled carbon nanotubes, have been used for molecular PA imaging studies, although their biocompatibility is still a significant issue.57 Another strategy for developing PA contrast agents involves the incorporation of small molecules or dyes that have high NIR absorbance into organic or inorganic NPs, such as loading indocyanine green into perfluorocarbon (PFC) nanodroplets.57,58 While this technique does rely on small molecules, encapsulating them within an NP structure improves quantum yield, circulation time, and stability, and allows the possibility of targeted delivery to cancer sites. Currently, NP-enabled PA imaging techniques are still in the early stages of exploration.
Surface-Enhanced Raman Spectroscopy
In addition to providing imaging contrast, NPs enable novel imaging techniques due to the unique properties of nanoscale materials. One such emerging technology is surface-enhanced Raman spectroscopy. While Raman spectroscopy has been utilized as an in vitro technique for quite some time,59 recent developments in NP construction have demonstrated its utility for in vivo detection.7,60 Raman spectroscopy takes advantage of inelastic scattering of photons (known as Raman scattering) and compares the energy of incident and emitted photons to generate molecular-level data. Very few incident photons are naturally inelastically scattered, limiting the technique’s sensitivity. The addition of a noble metal surface with high curvature can increase this percentage, enabling detection of measurable signals. This phenomenon is known as surface-enhanced Raman scattering (SERS). Several SERS contrast agents have recently been developed, primarily based on gold and silver NPs.61 For example, gold nanostar structures were recently demonstrated by Kircher’s group to allow microscopic detection of cancer lesions following administration of low amounts of the NPs.62 Likewise, silver NPs have been incorporated into nanocluster structures and demonstrated effective targeting and imaging of cancer cells.63 Without a NP-based contrast agent, SERS imaging would not be a viable in vivo technique.
Advanced NP-Enabled Imaging Techniques
NPs can be designed to offer unique imaging capabilities and applications which have the potential for clinical translation. This section outlines the advances and benefits of these NP designs in oncological imaging and treatment.
Multimodal Imaging
A primary benefit of utilizing a NP platform for imaging is the relative ease of incorporating contrast agents for multiple imaging modalities (Figure 2).64,65 Some NPs inherently provide multimodal contrast, such as QDs for optical/PA,66 gold NPs for CT/PA/SERS,67 and SPIONs for MRI/PA.68 NPs can also offer a simple platform for surface modifications to incorporate additional contrast agents for multiple modalities, through the addition of chelators for radiolabeling or encapsulation of small imaging molecules. Figure 2 demonstrates several such platforms. For example, Gd-conjugated gold–silica nanoshells allowed multimodal image-guided PTT using MRI/X-ray (Figure 2B)22 and chelator-free radio-arsenic-labeled SPIONs (*As-SPIONs, * = 71, 72, 74, 76) allowed quantification of *As-SPION uptake in lymph nodes using PET/MRI (Figure 2C).69 Additionally, liposomes loaded with both near-infrared dyes and 64Cu-enabled dual-modality (PET/fluorescence) imaging of orthotopic brain tumor models (Figure 2E).70 Clinically, C-dots incorporate Cy5 within the structure for fluorescent imaging, cRGD peptides on the surface for tumor targeting of integrin αvβ3, and 124I (half-life: 4.2 days) for PET imaging.33 Preliminary clinical safety studies indicated no toxicity to late-stage melanoma patients, renal clearance of the small NPs, and preferential accumulation of the tracer in cancerous lesions, visualized by serial PET scans (Figure 1A). Tumors were observed in organs ranging from the liver to the brain, indicating that the tracer may hold promise in many disease sites. The successful initial translation of this multimodal NP has undoubtedly provided hope that other NPs may be soon to follow.
Magnetic Particle Imaging
Magnetic particle imaging is a relatively new imaging technique that uses SPIONs to construct three-dimensional images under both static and dynamic magnetic fields like MRI.71 Different from the detection of SPIONs using MRI via indirect measurement of proton signal changes, MPI directly detects signals from SPIONs. Because there are no susceptibility artifacts from these SPIONs as in MRI, MPI is well suited for vascular imaging and intervention. MPI was first investigated as an alternative to subtraction angiography with CT and iodine-containing agents.72 More recently, MPI has been used to track iron oxide-labeled stem cells or macrophages implicated in injuries or disease progression.73 Design and development of tailored SPIONs, as well as the MPI scanner systems, are both active areas of research.
“Smart” NPs for Improved Signal Specificity
“Smart” NPs can be activated by external triggers—they change their properties in some way in response to surrounding environment and thus minimize background noise. Activatable (or switchable) NP sensors have shown success in amplifying the sensitivity of clinical in vitro assays below their standard detection limits, reaching the femto- and picomolar levels.74 When applied in vivo, these activatable NPs are expected to exhibit higher tumor-to-background imaging ratios as their signal would only be turned on in response to a cancer-specific marker. Triggers to activate these NPs could include pH changes,75,76 glutathione reduction (Figure 2D),77,78 or enzymatic cleavage (Figure 2A).79 The NPs themselves may provide contrast in any number of modalities, mostly in fluorescent,80,81 photoacoustic,75,82 and MR imaging,79,83 or combinations thereof.77,84,85 For example, using PA imaging, in vivo assessment of the tumor microenvironment was achieved by pH-responsive albumin-NIR self-assembled NPs75 or ligand-targeted mesoporous silica NPs.76 Using MRI, Mn2+-doped bioinspired calcium phosphate NPs could be “turned on” in the tumor microenvironment, brightening the tumor and identifying the hypoxic regions within tumors.86 Enhanced MRI signal was achieved in response to elevated levels of caspase 3/7 in HeLa tumors with drug-induced apoptosis by Ye and coworkers (Figure 2A).79 When tumors were treated with doxorubicin to induce apoptosis, the caspase-responsive NPs accumulated over twice as much when compared to treatment-naïve tumors, evidenced through both nearly doubled MRI signals and ex vivo analysis.
NPs for Image-Guided Surgery
Accurate tumor margin delineation is a significant concern for surgical cancer interventions, since untreated residual disease can lead to tumor recurrence. The use of image-guided surgery has enabled the delineation of tumor margins, thereby resulting in more positive outcomes for patients.87 These techniques employ optical, NIR, or radioactive probes that ideally are specific for cancerous tissues, so that surgeons can remove all tissues that are marked by the probe. NPs have been demonstrated preclinically to have numerous advantages over their counterpart small-molecule dyes for this application, such as enhanced accumulation and retention in the tumor, and the ability to be actively targeted to malignant tissues.88 Carbon NPs were evaluated in clinical trials to detect small central neck metastatic lymph nodes and subsequently resect them (NCT02724176).89 Previously mentioned C-dots also allowed for mapping of cancerous sentinel lymph nodes, tested in a clinical trial (NCT02106598).33,90 Image-guided surgery stands to be one of the areas in which NPs can find their way into everyday clinical practice.
Theranostic NPs for Monitoring and Controlling Localized Treatment
The use of a NP-based platform enables the use of novel imaging techniques, as well as several imaging modalities simultaneously. However, imaging is not the only area to benefit from incorporation of NP technologies. Many therapeutic options become available thanks to NPs and the phenomena that can only occur at the nanoscale. Such options include PTT,91 photodynamic therapy (PDT),92 targeted drug delivery,93 radiosensitization,94 immunotherapies,95 and other novel treatment techniques.96,97 NP-enabled drug delivery is by far the most widely used application of NPs: there are more than 40 ongoing clinical trials involving NP-based chemo-therapeutics or combination therapies in addition to photothermal therapies and radiosensitization in cancer treatment. The use of NPs as sensitization agents has the benefit that these treatments (e.g., lasers and radiation treatments) can be physically targeted to diseased tissue, and normal tissue toxicity can be limited.
The combination of therapeutic and diagnostic entities into a single unit has been termed “theranostics”,98 and due to their versatile nature, NPs can be designed and customized for theranostic applications. By incorporating an imaging moiety into the same structure as a therapeutic entity, these theranostic NPs can help visualize drug distribution and monitor treatment response in real-time. Recent years have seen a high number of reports on theranostic NPs, which were summarized in several reviews.98–103 These theranostic NPs may serve a similar purpose to companion diagnostics–patients can be stratified based upon the uptake of the NPs (as evidenced through imaging), which may correlate to therapeutic success. The main difference here, however, is that the imaging and therapeutic entities are coupled to a single agent, rather than being administered sequentially.
To date, the application of theranostic NPs in the clinic is limited, likely due to the increased complexity that comes along with combining both imaging and therapy moieties,99 which makes the regulatory review of these agents more challenging. Each component of these theranostic NPs, including the core materials, targeting or therapeutic moieties, surface coatings, as well as the imaging labels, needs to be evaluated and deemed safe for in vivo applications. However, in preclinical studies, nearly every imaging modality has been combined with a therapy mechanism and demonstrated significant promise for cancer diagnosis and treatment.
MATERIAL DESIGNS OF DIFFERENT PARTICLE FAMILIES USED IN IMAGING AND THERANOSTICS
Several materials or their combinations have been used in the design of NPs to support the applications described above. Below, we present a brief discussion of these different material groups and show their best matches when it comes to potential applications in cancer interventions.
Liposomes
Capable of encapsulating nearly any small molecule, liposomes hold enormous potential for drug delivery and theranostics.101,104 Since liposomal formulations of small-molecule drugs have already received FDA approval, it has been suggested that these biocompatible particles may experience expedited clinical translation.105 A wide range of theranostic liposomal NPs has been reported. For example, ultrasound-active liposomes were loaded with cisplatin, and, upon administration of high-frequency ultrasound pulses, the cisplatin was released, and tumor growth was slowed.106 Similarly, heating of liposomes containing both Gd-DTPA chelates and doxorubicin enabled visualization of MRI contrast enhancement, which was found to correlate to the drug concentration in the tumor environment as the liposomal contents were released.107 Additionally, the T1 relaxation enhancement within tumors was predictive of response to heat-induced doxorubicin therapy, with a change in R1 of greater than 3 × 10−5 ms−1 corresponding to complete tumor remission. Highly complex theranostic liposome systems have also been developed, with one such platform incorporating IR-dyes, Gd-DOTA chelates, and 64Cu-DOTA chelates for NIR, MR, and PET imaging, respectively, along with loading of doxorubicin for therapy (Figure 2E).70
Carbon-Based NPs
Many NP platforms have been designed using carbon for medical applications, including carbon nanotubes (CNTs), dots, and graphene oxides.108 Clinically, carbon NPs have been utilized for identifying sentinel lymph nodes for surgical removal in several cancers, including thyroid,109 breast,110 and gastric111 cancers. When carbon NPs were injected around the primary tumor site 6–12 h before surgery in gastric cancer patients, the sensitivity, specificity, and accuracy of positive-stained lymph nodes as determined during laparoscopy were 90, 100, and 98.9%, respectively.111 Similar results were found in other cancers, wherein the use of preoperative carbon NP sentinel lymph node mapping increased the number of metastatic lymph nodes detected and minimized unnecessary surgery.
Many carbon NPs can load large amounts of cargo such as drugs or smaller NPs for theranostic applications, and intrinsically possess theranostic characteristics, as well. For instance, CNTs are both NIR and Raman–active, and this strong NIR absorbance allows them to be effective photothermal therapy agents.112 Zhao et al. developed polydopamine and PEG-coated CNTs, which were then labeled with 131I and Mn2+ ions.111 These CNTs were visualized in vivo using both MRI (using Mn2+) and gamma (using 131I) imaging and could treat tumors through radionuclide therapy and photothermal therapy, demonstrating the versatility of this platform. Graphene oxide NPs have also been utilized as photoacoustic and photothermal therapy agents, the effects of which were amplified through the loading of indocyanine green.113 In this study, 4T1 tumor growth was completely halted after photothermal therapy, without systemic toxicity. The versatility and biocompatibility of carbon NPs indicates that they have yet to reach their full potential in cancer theranostics.
Silica NPs
Silica-based NPs are used as biocompatible, inorganic platforms for cancer theranostics. In particular, mesoporous silica has been demonstrated to be easily modified and loaded with any number of imaging or therapeutic entities for many theranostic options, in a comparable manner to liposomes.114 While the silica NP itself does not possess theranostic capabilities, the use of this NP enables successful administration of other, possibly less biocompatible, entities in vivo. These mesoporous silica NPs (MSNs) can incorporate small metal NPs, such as gold, for enhanced PTT and NIR imaging.115 Likewise, tungsten sulfide and iron oxide NPs were coated with silica, then loaded with doxorubicin, for CT/MRI/NIR/PTT/chemo theranostics of tumor models.116 Easy surface modification of the MSNs allows for directing of the NPs to any number of targets, including neovasculature,117,118 or antigens on the cancer cell surface,76,119 to increase the specific uptake of the NPs. When targeted to neovasculature, mesoporous silica–coated copper sulfide NPs accumulated in preclinical breast cancer models at about 6%ID/g at 24 h postinjection, evidenced through PET images using attached 64Cu (Figure 3A).117 While this is only a small portion of the intravenously injected NP dose, effective photothermal therapy was still achieved through almost complete tumor destruction after a single (15 min, 980 nm) laser irradiation. In this study, the silica did not contribute to the PTT; rather, it enabled targeted administration of CuS, optimization of the NP biodistribution, and facile surface modifications.
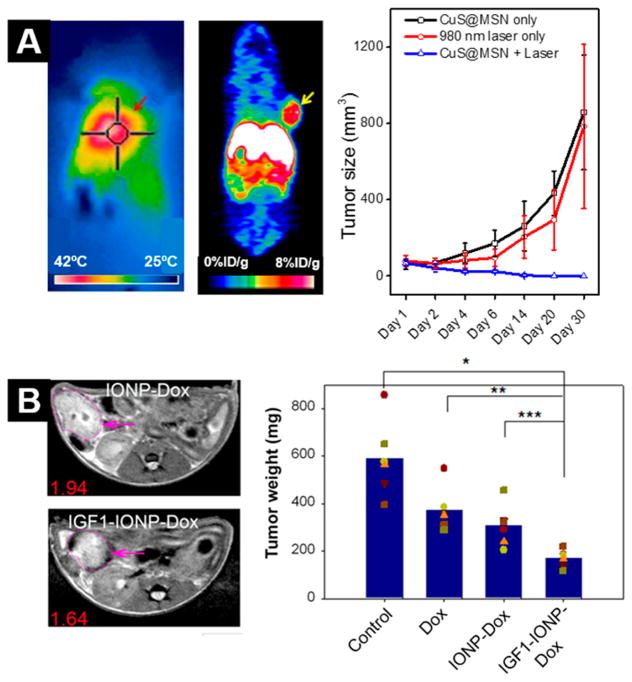
Figure 3.
Preclinical examples of theranostic NPs. (A) Copper sulfide NPs embedded in mesoporous silica and radiolabeled with 64Cu enable successful photothermal therapy (left) and PET imaging (right) of tumor xenografts. Reproduced from ref 117. Copyright 2015 American Chemical Society. (B) Iron oxide NPs (IONP) can be loaded with drugs, such as doxorubicin (Dox), and targeted to cancerous tissue using IGF1 to facilitate targeted chemotherapy and MRI. Reproduced from ref 137. Copyright 2015 American Chemical Society.
Noble Metal NPs
Gold, silver, and platinum NPs provide inherent contrast in CT, fluorescence, PA, and SERS imaging and have PTT capabilities, making them a natural fit for cancer theranostics.44,120–123 Just as in imaging applications, they may be surface-functionalized to target any number of cancer antigens (with peptides or antibodies), making precise imaging and therapy viable. The gold NP platform has also been combined with other moieties to enable further multimodality theranostics, such as coating with Gd-chelates for CT/MRI/PTT124 or encapsulation of gold nanoshells with iron oxide and indocyanine green for CT/MRI/NIR/PTT.125 In vivo studies of the latter NP showed successful visualization of pancreatic tumor burden using both NIR and T2-weighted MRI, while photothermal treatments were extremely effective in vitro.
Likewise, silver NPs have been widely used as antibacterial agents in the clinic;126 however, their applications in cancer theranostics are just beginning to be explored preclinically. Silver NPs have demonstrated inherent anticancer effects, as well as contrast in many imaging modalities.127,128 Most of these studies with silver NPs to date have been performed in vitro; however, a few early stage preclinical in vivo studies have been conducted. One such investigation by Tan et al. utilized silver NPs coated with polyaniline, PEG, and indocyanine green (ICG) to enable PA and fluorescence imaging, as well as photothermal and photodynamic therapies.129 Tumors were clearly visualized by fluorescence imaging as soon as 8 h postinjection and the contrast remained until the end of the imaging study (48 h). While the use of the silver NPs and NIR irradiation for PTT inhibited tumor growth in comparison to controls, the addition of ICG and subsequent PDT resulted in almost complete tumor eradication.
Platinum NPs are the least explored of these three for imaging; however, elemental Pt inherently demonstrates anticancer effects which are currently used for treating many cancers (e.g., carboplatin).130 Many platinum prodrugs can be loaded into other NP platforms for delivery of the cargo,131 or platinum itself can be directly incorporated into the NP structure. One such study by Hariri et al. utilized FePt NPs for targeting of irradiated tumors using a peptide specific for radiation-induced vascular damage.132 In vivo fluorescence imaging demonstrated the irradiated tumors to be the organs with highest uptake of the NPs, verified by ex vivo platinum content analysis of tissues. FePt NPs have great potential as stable contrast agents for both MRI and CT.133,134
Magnetic NPs
Iron oxide NPs, especially SPIONs, inherently provide theranostic capabilities due to their magnetic nature.135 While they provide contrast in MRI, their interaction with an alternating magnetic field can also enable magnetic hyperthermia of tumor tissues.96 One such study employed SPIONs loaded with curcumin, and the combination of the anticancer effects of the drug, along with magnetic hyperthermia enabled by the SPIONs, resulted in an increased therapeutic effect in preclinical models.136 While these NPs may be functionalized with targeting ligands to monitor doxorubicin delivery (Figure 3B),137 magnetic NPs can also be guided to a superficial site of interest in vivo through the application of a localized external magnetic field138 leading to increased tumor accumulation of the NPs. Limited early stage clinical trials have explored this technique, including the use of intraoperative monitoring of magnetically active, doxorubicin-containing iron NPs.139 In this study, a 5-kG magnet was placed on the skin of patients near liver tumor sites while the NPs were injected intra-arterially, essentially pulling the NP and attached drugs out of the blood pool and into the diseased tissue. After up to three doses of the NPs, at least 60% of the tumor volume was covered by the NPs, compared to a maximum of 30% of the normal liver. SPIONs also find application in lymph node mapping to determine the extent of metastatic disease through direct injection into tumor sites and imaging of the NP movement from that area (Figure 2C).69
Semiconducting NPs
QDs and UCNPs also demonstrate inherent theranostic capabilities.140–142 Their NIR range absorbance makes them an ideal candidate for not only fluorescent imaging but also for PDT. While the use of longer-wavelength light enables deeper tissue penetration, it also limits background autofluorescence. Indeed, this area has experienced increased research efforts in recent years, with several new UCNP-143 or QD-based144,145 agents in preclinical trials. Of course, in addition to the intrinsic theranostics achieved with these NPs, they have been loaded with other agents to enable further multimodality imaging and therapy, such as through radiolabeling for PET or therapy, doping with metal ions for MRI, and loading with high-Z atoms for CT contrast.142,146 For example, tungsten sulfide QDs were used by Yong et al. for multifunctional theranostics of tumors, with imaging contrast in both CT and PA imaging allowing localization of tumors, and enhanced therapeutic efficacy achieved in both radiotherapy and photothermal therapy.144 Following injection of the NPs and localized combination treatment (radiotherapy + photothermal), tumors were completely eradicated; at the same time, single treatments with either PTT or radiotherapy also shrunk tumors, but to a lesser extent. QDs are thus certainly “all-in-one” platforms for nanotheranostics.
NANOPARTICLES AS COMPANION DIAGNOSTICS
The use of companion and complementary diagnostic tests along with current and future cancer treatments has the potential to improve the outcomes of cancer treatment significantly. Such diagnostic tools give physicians the ability to accurately predict which patients may or may not respond to a given therapy, or are susceptible to toxicities from that therapy. The current state of companion diagnostics, which are unique to an individual treatment option, has been thoroughly reviewed by Agarwal.147
Companion diagnostics have gained momentum in recent years, demonstrating clear benefits not only for patients through smarter treatment decisions, but for drug companies, as well. The use of a companion diagnostic during clinical trials has been indicated to provide an expedited process in addition to reduced costs through a guided selection of eligible patients.148 Most of these tests have traditionally been performed in vitro through testing of genetic or protein markers from biopsy samples, such as Her2/Neu expression in breast cancer,149 ALK rearrangements in nonsmall cell lung cancer,150 and KRAS mutations in colorectal cancer.151 The information gained from these tests has proven its utility, as patients who undergo a treatment that has a companion diagnostic have been demonstrated to have fewer grade 3 or 4 adverse reactions and a decreased rate of treatment discontinuation.152
Imaging-Based Companion Diagnostics
When compared to the increasing number of in vitro companion diagnostics, the list of imaging-based companion diagnostics is short. As the only FDA-approved imaging companion diagnostic, Ferriscan is an MRI measurement to evaluate liver iron concentrations in patients receiving deferasirox to prevent chronic iron overload.153 This test uses a proprietary algorithm to analyze R2 images of liver tissue, producing color maps of iron concentration. Based on this information, physicians can monitor the thalassemia status of patients and make further treatment decisions. One other imaging test received the initial European Medicines Agency (EMA) approval as a companion diagnostic–the use of 99mTc-etarfolatide as a SPECT evaluation of folate receptor presence in ovarian cancer patients who are candidates for Vintafolide therapy.81 The final approval of this diagnostic-drug combination hinged on the results of the phase III Vintafolide PROCEED trial, which, unfortunately, yielded negative results (NCT01170650).
While the preclinical examples of immunoPET for determining tumors’ biomarker status are numerous, only a handful of imaging-based companion diagnostics have gotten into early stages of clinical development, mostly through the use of radiolabeled antibodies.154,155 Patients are being imaged with radiolabeled therapeutics to determine their HER2 status for predicting treatment response in patients with metastatic breast cancer. For example, PET/CT of 89Zr-labeled trastuzumab or 64Cu-DOTA-trastuzumab were included as techniques to identify patients who were unlikely to benefit from an expensive anti-HER2 agent (NCT01565200, NCT02827877).156 Similarly, 99mTc-EC0652 is in a phase I trial for determining PSMA levels in prostate cancer patients during the simultaneous evaluation of the therapeutic efficacy of EC1169 in patients with recurrent metastatic, castration-resistant prostate cancer (NCT02202447). Although all in Phase I, these clinical trials demonstrate the continuing interest in developing imaging-based companion diagnostics for patient stratification and improved outcomes.
It is not expected that these imaging-based diagnostics would replace traditional biopsy-based analysis; instead, they could serve as a natural complement, providing whole-body information rather than localized detailed information. Imaging-based companion diagnostics may also provide a more realistic representation of in vivo biomarker status, as ex vivo tissue staining may overestimate bioavailability of many markers. However, just as with biopsy-based methods, standardized analysis protocols need to be established for imaging companion diagnostics, to differentiate specific and background uptake.
NP Imaging To Determine Strength of the EPR Effect
One area in which NP-based companion diagnostics are a natural fit is the exploration of the enhanced permeability and retention (EPR) effect which plays a significant role in the release of NPs from blood circulation to tumor tissue and as such is a determinant of the effectiveness of NP-based therapeutics. While this property of cancers’ vasculature has been recognized for over 30 years, there are still many questions surrounding it. Most importantly, the strength of the EPR effect varies considerably across tumor types, across patient cohorts within the same type, and even within different tumor sites for a single patient.157 Indeed, an increased understanding of this phenomenon would enable the more rational design of nanotherapeutics and allow nanotechnology to reach its full potential in oncology. Most NP-formulated drugs that have been clinically translated to date utilize the EPR effect for passive targeting of tumors (or accumulation of NPs within the tumor), making this understanding critically important.158 Preclinically, most initial studies utilize subcutaneous xenograft models which often display a high level of EPR and may lead to an overestimation of the effectiveness of a drug. Thus, both clinically and preclinically, a greater understanding of this phenomenon is warranted.
Traditional imaging techniques may be able to predict the biodistribution of NP therapeutics by evaluating the strength of the EPR effect. Such approaches have been employed using dynamic contrast-enhanced CT tumor perfusion imaging to predict liposomal distributions,159 or diffusion-weighted MRI to estimate diffusibility of NPs into a tumor.160 A significant correlation was found between measures of tumor perfusion such as Ktrans and AUCiox and the uptake of CT-active liposomes in tumors (AUCliposome and Cpeak), as evidenced through serial CT studies by Stapleton et al.159 Another study utilized both dynamic contrast-enhanced MRI and vessel size index MRI to evaluate the EPR status of tumors.161 Within the eight preclinical tumor models studied, both subcutaneous and orthotopic, it was determined that a combination of the permeability and blood volume fraction as measured by MRI could aid in classifying tumors as either EPR-positive or negative. These studies indicate that well-established imaging techniques hold great potential for application to novel NP imaging and therapeutic entities.
Another natural means of exploring the EPR effect is through using a companion NP capable of noninvasive visualization. One preclinical study thoroughly compared the biodistribution of magnetic NPs and therapeutic NPs, finding that they matched quite well (Figure 4A).162 Not only did the localization of magnetic NPs mirror that of their therapeutic counterparts, but MRI-based assessment of the diagnostic NPs could predict treatment outcomes based on the uptake of ferumoxytol. Similar strategies have been used clinically as well, using dextran-coated iron oxide NPs for the elucidation of EPR effects.163
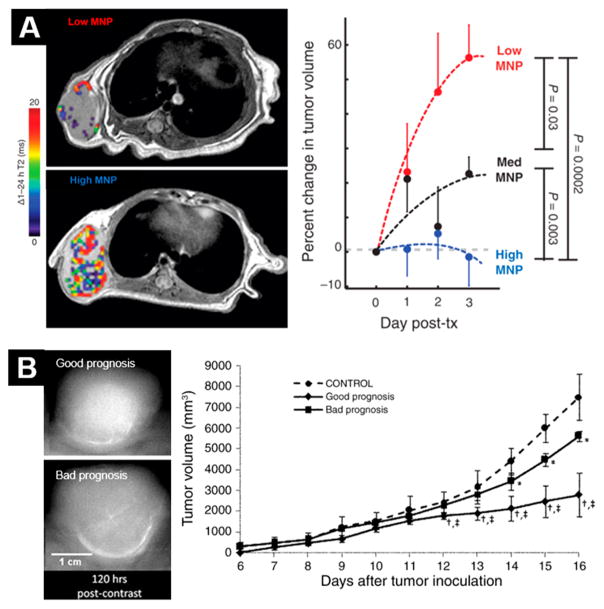
Figure 4.
Examples of NPs as companion diagnostics. (A) Magnetic NP accumulation predicts therapeutic response to treatment with a corresponding therapeutic NP. Reprinted with permission from ref 162. Copyright 2015 AAAS. (B) Uptake of iodinated liposomes predicts response to liposomes carrying therapeutic payloads, with higher accumulation (upper tumor) indicating a good prognosis. Reproduced with permission from ref 168. Copyright 2009 RSNA Publications.
PET has also been used preclinically to evaluate differences in the EPR effect in large animal models, using 64Cu-labeled liposomes in spontaneous cancers in dogs.164 This study showed that the EPR effect varies widely across cancer types and patients–seven of eight carcinomas displayed high tracer uptake (indicating strong EPR), while only one of four sarcomas displayed the same. The metal-chelating ability of certain drugs (i.e., bisphosphonates and anthracyclines) was also exploited to efficiently label and track drug-loaded liposomes with positron-emitting radionuclides in a study by Edmonds et al., providing a means to track the exact therapeutic entities in vivo using PET.165 The authors demonstrated efficient (80–100%) radiolabeling of several types of drug-loaded liposomes with 52Mn, 64Cu, and 89Zr, with >80% stability over the course of several days. In vivo studies with 89Zr-PLA liposomes provided clear tumor contrast, with total tumor uptake greater than 8%ID/g a week after injection.
The EPR state of cancer before therapy may have a significant impact on the efficacy of NP-based treatments, and imaging with NP companion diagnostics may be the best way to evaluate this variable. For example, 64Cu-labeled HER2-targeted doxorubicin NPs (64Cu-MM-302) and PET/CT were included as a part of a clinical trial to identify breast cancer patients who were suited for treatment with therapeutic NPs based on their tumor EPR level (NCT01304797).166 Tumor uptake of the radiolabeled NPs varied from 0.52 to 18.5%ID/g at 24 to 48 h across patients, as well as across primary and metastatic sites, allowing the researchers to retrospectively define a level of uptake at which successful therapy could be predicted (hazard ratio = 0.42).
Liposomes containing high concentrations of iodine for CT contrast have been used to study tumor uptake preclinically as well as in the clinic167 and may serve to predict uptake of liposomal formulations of many drugs, such as in the preclinical mammography study by Karathanasis et al. (Figure 4B).168 Mice were stratified in this study based upon uptake of the iodinated NP, as uptake of the contrast agent predicted response to liposomes loaded with chemotherapy drugs.
As the level of EPR may change during cancer treatment, longitudinal evaluation of the EPR effect is warranted. Interventions such as photothermal therapy,169 induction of high blood pressure, and administration of TGF-β inhibitors158 have been employed to artificially increase the EPR-mediated accumulation of NPs, with varying levels of success. In-depth exploration of these treatments and their impact on the EPR status of a tumor may be facilitated through imaging with NP contrast agents. One such study evaluated the distribution of radiolabeled, PEGylated, doxorubicin-loaded gold NPs in rabbit liver tumors following several treatment options.170 While NP accumulation in tumors was observed for all the treatment groups when combined with nanoembolization (radiofrequency ablation, irreversible electroporation, and laser-induced thermal therapy), the timing of this accumulation varied and the subtissue localization of the NPs was also different across groups. Intracellular localization was only observed for the embolization+electroporation group, drug release was only observed following thermal therapy, and a faster uptake of NPs in the tumor was observed after radiofrequency ablation. Thus, depending on the purpose of a NP therapeutic, different combinations of therapy should be applied for maximum effects and optimization of the EPR effect.
Targeted NPs for Companion Diagnostics
As more targeted NP treatments beyond leveraging of the EPR effect become available, the need for companion diagnostics for these treatments will also increase.171 For this reason, targeted NPs for companion diagnostics are beginning to be explored preclinically, through the use of many modalities and platforms. Nearly every type of previously described NP has been conjugated with a targeting ligand to determine a tumor’s biomarker expression through imaging, but not yet necessarily correlated with a therapeutic outcome.172 For example, HER2 targeting has been employed for gold NPs that preferentially accumulated in antigen-positive breast cancer models, evidenced through CT imaging,173 or iron oxide NPs through PA imaging.174 Iron oxide NPs provide very strong PA contrast; however, they are often not strongly retained in tumors. Thus, the addition of a HER2-targeting antibody fragment was found to increase the uptake of 20 nm iron oxide NPs, providing clear contrast in HER2-expressing xenografts.174 Similarly, the use of tumor vasculature targeting enhanced the uptake of silica NPs in murine breast cancer models, visualized by PET,175 and also of iron oxide NPs in MRI scans.176 Compared to nontargeted silica NPs, targeting to CD105 (a marker of angiogenesis) allowed the same NPs to accumulate at twice as high a level (6%ID/g vs 3%ID/g) in 4T1 tumors.130
The logical next step for these platforms is to determine whether the response to therapeutic versions of the same NPs corresponds to the uptake of the imaging NP. One consideration for these targeted NPs is whether they can accumulate above background levels, which are dictated by the inherent EPR effect; thus, in-depth studies are necessary to determine whether the extra complication of targeting ligands provides a clear benefit, both for diagnostic and therapeutic NPs.171
The ability to longitudinally image NPs in vivo provides cancer researchers with an invaluable tool to gain insight into the dynamic interaction of NPs in a biological system. Such potential is already being realized, as the knowledge gained from the noninvasive imaging of NP biodistribution can inform the rational design of next-generation NP-based platforms. It is expected that NP-enabled, imaging-based companion diagnostics will also be moving to clinical space in the near future as NP therapeutic formulations also continue to make an impact.
CONCLUDING REMARKS
NPs have been providing a growing level of utility in cancer imaging and improved therapeutic strategies. However, while their small size is what endows them with a great deal of this promise, the nanoscale properties of these materials lead to many scientific challenges for clinical translation.
NP Manufacturing, Characterization, and Large-Scale Production
A critical component of successful translation of NPs is the ability to manufacture them consistently for clinical use177 and maintain their structural uniformity (size and shape), surface charge and consistent loading of any drug or imaging payloads. The issue of reproducibility, among other reasons, has come into play with the revoking of approval of some NP-based agents such as iron oxides.178 Additionally, while the theranostic capabilities of NPs have shown promise, it should be noted that, with the addition of every new moiety, the NP’s complexity increases.
Throughout the literature, a wide variety of methods have been used to characterize NPs.179 For example, particle size and size distributions of the NPs have been reported using X-ray diffraction, small-angle X-ray scattering, dynamic light scattering, scanning electron microscopy, or transmission electron microscopy, which each offer diverse information. Another example is zeta-potential which provides information about the surface charge and stabilities of NPs. Because such measurements are sensitive to the pH or ionic strength of the solution, reports on the zeta-potential of NPs without information about the method used and condition of the solution are not sufficient. The lack of standardized physicochemical characterizations and reporting of NP parameters can lead to a lack of reproducibility and difficulty in comparing across synthesis methodologies. To overcome this challenge, the Nanotechnology Characterization Laboratory, a part of the NCI’s Alliance for Nanotechnology in Cancer Program, provides standardized protocols and performs physicochemical characterization of NPs to support the translation of NP development.180,181 Standardized characterization methods and reporting that are agreed upon by the nanotechnology field are needed to further mature the NP biomedical field.
There have been some large-scale, reproducible manufacturing methods for NPs used for producing NPs in early clinical trials.182–184 While most large-scale NP productions are based on chemical and physical approaches, biological-based methods are gaining interest in recent years due to their relatively low cost and eco-friendly natures.185
Biodistribution and Toxicity Considerations of NPs
Among the barriers to the widespread application of NPs in medicine are their potential toxicity and unique biodistribution patterns.186 Indeed, it is well-known that the behaviors of materials in bulk cannot be generalized to their nanoscale properties. Therefore, the complete evaluation of NPs’ biocompatibility is critical for successful application in vivo. This should take the form of in vitro testing and multiple preclinical studies. For in vitro assessment, tests such as MTT187 and Ames188 assays, while simple, provide basic biocompatibility information. Advanced cell culture models, such as 3D culture and flow models, better mimic the in vivo situation and should also be employed.189 When moving into in vivo experiments, clinically relevant situations need to be evaluated. This would include considerations such as dosing and injection regimens, excretion and accumulation profiles, metabolic byproducts, and both acute and long-term effects of the administered agents, all of which require longitudinal monitoring strategies. Importantly, patients will ideally be living many years after the administration of an imaging NP; thus, preclinical testing needs to take place on a time scale that is proportionally appropriate. As discussed below, the preclinical models also matter a great deal for fully understanding a NP’s biodistribution. Several strategies have been developed to increase the biocompatibility of NPs, outlined elsewhere.190
Choice of Proper Preclinical Models
Proper preclinical models need to be chosen and validated for both imaging and therapeutic NPs, including the use of positive and negative cell lines, clinically relevant tumor models, and representative treatment strategies. Often, as previously mentioned, the xenograft models in preclinical studies may demonstrate unrealistic levels of the EPR effect, providing false hope. Thus, many different types of tumors (with different growth rates, vasculature properties, etc.) need to be utilized. Simply using the tumor which provides the best NP uptake will certainly bias the results; thus, a critical evaluation of all NP agents (and imaging agents in general) is essential. Additionally, more advanced tumor models, such as orthotopic xenografts,191 may provide clinically relevant information that will provide insight into potential translational application. It is noteworthy that the choice of preclinical model matters greatly when determining the biodistribution of a NP. It has been shown that the clearance of NPs varies across different animal species. Like humans, mice, rats, monkeys, chickens, and rabbits show primary uptake of larger NPs in the liver; at the same time, sheep, pigs, goats, and cats have high levels of the same NPs in the lungs, trapped in pulmonary vasculature.192 Proper preclinical models should thus be carefully chosen.
Selection of Imaging Biomarkers, Imaging Protocols, and Correlation of Image Signals to Concentration of NPs
Recognizing the importance of quantitative imaging, the Radiological Society of North America (RSNA) established the Quantitative Imaging Biomarkers Alliance (QIBA) in 2007 to advance quantitative imaging tools and interpretation in clinical trials and clinical practice. However, NP-based quantitative imaging tools and methods for clinical applications currently do not exist. Attempts have been made to correlate in vivo NP signals with NP concentration in preclinical studies through establishing calibration curves; however, these calibrations may not necessarily represent the observations in larger animals and humans. Although several molecular markers are robust for detecting cancer cells in vitro, in vivo imaging NPs targeting these molecular markers have not been sufficiently validated for clinical decisions. There is a need to validate NP-based imaging biomarkers and establish consensus in imaging protocols and calibration curves for human applications.
Commercial Incentive for Developing Imaging NPs as Companion Diagnostics
A few unique challenges are present for the development and approval of NP-based companion diagnostics and theranostics. The most prominent barrier to commercialization of NP imaging agents is their development costs. Specifically, NPs for the sole use as imaging agents have a much lower return on investment than drug delivery systems.193 Hence, pharmaceutical companies are reluctant to invest in the development of NP imaging agents. Further preclinical development of these agents and demonstration of their increasing utility could change that situation.
The FDA issued “Guidance for Industry: In Vitro Companion Diagnostic Devices” in 2014 to recommend the codevelopment of drug and companion diagnostic tests at an earlier stage of the drug development process. Pharmaceutical companies were encouraged to develop the companion diagnostic in-house or partner with outside research facilities. In vivo imaging-based companion diagnostics bear an additional requirement of a full, rigorous, premarket regulatory approval, as they are injected into the body and may directly or indirectly affect treatment decisions and outcomes. Also, the profit margins (and therefore potentially the incentive for development) on NP-based imaging companion diagnostics are not as high as those for their counterpart drugs since the companion diagnostic agent is only administered a few times, for patient stratification or efficacy monitoring, compared to the longitudinal need for the drug. Last, concerns like intellectual property may be at the heart of why few imaging-based companion diagnostics are on the market today.147 Because most developments of NP-based imaging agents, associated detection methods, and protocols are from academic institutions, research partnerships between academic and industrial investigators with a priori intellectual property agreements could accelerate the translation of promising NP imaging and theranostic tools and technologies.
The use of NP-based imaging agents for companion diagnostics holds potential to improve cancer management significantly. With the rapid development of new NPs, targeting options, and “smart” NPs, this potential is only expected to grow. While NPs do present a unique set of challenges and concerns, their possible benefits to cancer patients indeed seem to outweigh the downsides. Interdisciplinary collaboration of scientists across disciplines including materials science, biology, physics, chemistry, and pharmacy has been enabling NP-based cancer interventions to move forward. NP-based imaging agents have already demonstrated clear advantages for the noninvasive visualization of cancer as evidenced by successful early stage clinical trials. We hope this review brings additional awareness to the challenges and opportunities for the further development and clinical implementation of NP-based imaging agents for companion diagnostics.
Acknowledgments
Funding
NIH Grants T32GM008505, T32CA009206, and P30CA014520, and the NIH Summer Internship Program.
VOCABULARY
- companion diagnostic
an in vitro diagnostic device or an imaging tool that provides information that is essential for the safe and effective use of a corresponding therapeutic product (FDA definition)
- theranostics
the combination of therapeutic and diagnostic components into a single agent
- enhanced permeability and retention effect (EPR effect)
a property of cancers that describes their permeable and defective vasculature; EPR effects allow nanoparticles to extravasate from blood circulation leading to accumulation in tumors
- contrast agent
a substance used to enhance the contrast of a structure in the body while performing medical imaging
- molecular imaging
the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems (Society of Nuclear Medicine and Molecular Imaging definition)
- biomarker
a biological molecule found in blood, other bodily fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease (NCI definition)
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Kim TH, Lee S, Chen X. Nanotheranostics for Personalized Medicine. Expert Rev Mol Diagn. 2013;13:257–269. doi: 10.1586/erm.13.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschbaum K, Sonner JK, Zeller MW, Deumelandt K, Bode J, Sharma R, Krüwel T, Fischer M, Hoffmann A, Costa da Silva M, Muckenthaler MU, Wick W, Tews B, Chen JW, Heiland S, Bendszus M, Platten M, Breckwoldt MO. In Vivo Nanoparticle Imaging of Innate Immune Cells can Serve as a Marker of Disease Severity in a Model of Multiple Sclerosis. Proc Natl Acad Sci U S A. 2016;113:13227–13232. doi: 10.1073/pnas.1609397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Wan X, Zheng X, Shao X, Liu Q, Zhang Q, Qian Y. Dual-Functional Nanoparticles Targeting Amyloid Plaques in the Brains of Alzheimer’s Disease Mice. Biomaterials. 2014;35:456–465. doi: 10.1016/j.biomaterials.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 4.Stendahl JC, Sinusas AJ. Nanoparticles for Cardiovascular Imaging and Therapeutic Delivery, Part 1: Compositions and Features. J Nucl Med. 2015;56:1469–1475. doi: 10.2967/jnumed.115.160994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barenholz Y. Doxil(R)–The First FDA-Approved Nano-Drug: Lessons Learned. J Controlled Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Kundranda MN, Niu J. Albumin-Bound Paclitaxel in Solid Tumors: Clinical Development and Future Directions. Drug Des, Dev Ther. 2015;9:3767–3777. doi: 10.2147/DDDT.S88023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreou C, Kishore SA, Kircher MF. Surface-Enhanced Raman Spectroscopy: A New Modality for Cancer Imaging. J Nucl Med. 2015;56:1295–1299. doi: 10.2967/jnumed.115.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karakoti AS, Das S, Thevuthasan S, Seal S. PEGylated Inorganic Nanoparticles. Angew Chem, Int Ed. 2011;50:1980–1994. doi: 10.1002/anie.201002969. [DOI] [PubMed] [Google Scholar]
- 9.García KP, Zarschler K, Barbaro L, Barreto JA, O’Malley W, Spiccia L, Stephan H, Graham B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small. 2014;10:2516–2529. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Zhou Z, Mao H, Yang L. Magnetic Nanoparticles for Precision Oncology: Theranostic Magnetic Iron Oxide Nanoparticles for Image-Guided and Targeted Cancer Therapy. Nanomedicine. 2017;12:73–87. doi: 10.2217/nnm-2016-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging Applications for Ferumoxytol as a Contrast Agent in MRI. J Magn Reson Imaging. 2015;41:884–898. doi: 10.1002/jmri.24691. [DOI] [PubMed] [Google Scholar]
- 12.Bernd H, De Kerviler E, Gaillard S, Bonnemain B. Safety and Tolerability of Ultrasmall Superparamagnetic Iron Oxide Contrast Agent: Comprehensive Analysis of a Clinical Development Program. Invest Radiol. 2009;44:336. doi: 10.1097/RLI.0b013e3181a0068b. [DOI] [PubMed] [Google Scholar]
- 13.Heesakkers RA, Jager GJ, Hovels AM, de Hoop B, van den Bosch HC, Raat F, Witjes JA, Mulders PF, van der Kaa CH, Barentsz JO. Prostate Cancer: Detection of Lymph Node Metastases Outside the Routine Surgical Area with Ferumoxtran-10-Enhanced MR Imaging. Radiology. 2009;251:408–414. doi: 10.1148/radiol.2512071018. [DOI] [PubMed] [Google Scholar]
- 14.Rief M, Wagner M, Franiel T, Bresan V, Taupitz M, Klessen C, Hamm B, Asbach P. Detection of Focal Liver Lesions in Unenhanced and Ferucarbotran-Enhanced Magnetic Resonance Imaging: A Comparison of T2-Weighted Breath-Hold and Respiratory-Triggered Sequences. Magn Reson Imaging. 2009;27:1223–1229. doi: 10.1016/j.mri.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Daldrup-Link HE. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology. 2017;284:616–629. doi: 10.1148/radiol.2017162759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn JP, Nguyen KL, Han F, Zhou Z, Salusky I, Ayad I, Hu P. Cardiovascular MRI with Ferumoxytol. Clin Radiol. 2016;71:796–806. doi: 10.1016/j.crad.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE, Dosa E, Finn JP, Gahramanov S, Harisinghani M, Macdougall I, Neuwelt A, Vasanawala SS, Ambady P, Barajas R, Cetas JS, Ciporen J, DeLoughery TJ, Doolittle ND, Fu R, et al. Current and Potential Imaging Applications of Ferumoxytol for Magnetic Resonance Imaging. Kidney Int. 2017;92:47–66. doi: 10.1016/j.kint.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu CH, Ren JQ, Yang J, Liu CM, Mandeville JB, Rosen BR, Bhide PG, Yanagawa Y, Liu PK. DNA-Based MRI Probes for Specific Detection of Chronic Exposure to Amphetamine in Living Brains. J Neurosci. 2009;29:10663–10670. doi: 10.1523/JNEUROSCI.2167-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J, Chen YI, Liu CH, Chen PC, Prentice H, Wu JY, Liu PK. Noninvasive Tracking of Gene Transcript and Neuroprotection after Gene Therapy. Gene Ther. 2016;23:1–9. doi: 10.1038/gt.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas R, Park IK, Jeong YY. Magnetic Iron Oxide Nanoparticles for Multimodal Imaging and Therapy of Cancer. Int J Mol Sci. 2013;14:15910–15930. doi: 10.3390/ijms140815910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lux F, Sancey L, Bianchi A, Cremillieux Y, Roux S, Tillement O. Gadolinium-Based Nanoparticles for Theranostic MRI-Radiosensitization. Nanomedicine. 2015;10:1801–1815. doi: 10.2217/nnm.15.30. [DOI] [PubMed] [Google Scholar]
- 22.Coughlin AJ, Ananta JS, Deng N, Larina IV, Decuzzi P, West JL. Gadolinium-Conjugated Gold Nanoshells for Multimodal Diagnostic Imaging and Photothermal Cancer Therapy. Small. 2014;10:556–565. doi: 10.1002/smll.201302217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan D, Caruthers SD, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Revisiting an Old Friend: Manganese-Based MRI Contrast Agents. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2011;3:162–173. doi: 10.1002/wnan.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro EM. Biodegradable, Polymer Encapsulated, Metal Oxide Particles for MRI-Based Cell Tracking. Magn Reson Med. 2015;73:376–389. doi: 10.1002/mrm.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfbeis OS. An Overview of Nanoparticles Commonly Used in Fluorescent Bioimaging. Chem Soc Rev. 2015;44:4743–4768. doi: 10.1039/c4cs00392f. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Qiu H, Prasad PN, Chen X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem Rev (Washington, DC, U S) 2014;114:5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv R, Wang D, Xiao L, Chen G, Xia J, Prasad PN. Stable ICG-Loaded Upconversion Nanoparticles: Silica Core/Shell Theranostic Nanoplatform for Dual-Modal Upconversion and Photoacoustic Imaging Together with Photothermal Therapy. Sci Rep. 2017;7:15753. doi: 10.1038/s41598-017-16016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rzigalinski BA, Strobl JS. Cadmium-Containing Nanoparticles: Perspectives on Pharmacology and Toxicology of Quantum Dots. Toxicol Appl Pharmacol. 2009;238:280–288. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu G, Lin G, Lin S, Wu N, Deng Y, Feng G, Chen Q, Qu J, Chen D, Chen S, Niu H, Mei S, Yong KT, Wang X. The Reproductive Toxicity of CdSe/ZnS Quantum Dots on the in vivo Ovarian Function and in vitro Fertilization. Sci Rep. 2016;6:37677. doi: 10.1038/srep37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill TK, Kelkar SS, Wojtynek NE, Souchek JJ, Payne WM, Stumpf K, Marini FC, Mohs AM. Near Infrared Fluorescent Nanoparticles Derived from Hyaluronic Acid Improve Tumor Contrast for Image-Guided Surgery. Theranostics. 2016;6:2314–2328. doi: 10.7150/thno.16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill TK, Abdulahad A, Kelkar SS, Marini FC, Long TE, Provenzale JM, Mohs AM. Indocyanine Green-Loaded Nanoparticles for Image-Guided Tumor Surgery. Bioconjugate Chem. 2015;26:294–303. doi: 10.1021/bc5005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradbury MS, Phillips E, Montero PH, Cheal SM, Stambuk H, Durack JC, Sofocleous CT, Meester RJ, Wiesner U, Patel S. Clinically-Translated Silica Nanoparticles as Dual-Modality Cancer-Targeted Probes for Image-Guided Surgery and Interventions. Integr Biol (Camb) 2013;5:74–86. doi: 10.1039/c2ib20174g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gönen M, Kalaigian H, Schöder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Clinical Translation of an Ultrasmall Inorganic Optical-PET Imaging Nanoparticle Probe. Sci Transl Med. 2014;6:260ra149–260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivatsan A, Chen X. Recent Advances in Nanoparticle-Based Nuclear Imaging of Cancers. Adv Cancer Res. 2014;124:83–129. doi: 10.1016/B978-0-12-411638-2.00003-3. [DOI] [PubMed] [Google Scholar]
- 35.Goel S, Chen F, Ehlerding EB, Cai W. Intrinsically Radiolabeled Nanoparticles: An Emerging Paradigm. Small. 2014;10:3825–3830. doi: 10.1002/smll.201401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of Tumor-Specific Targeting on the Biodistribution and Efficacy of siRNA Nanoparticles Measured by Multimodality In Vivo Imaging. Proc Natl Acad Sci U S A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo P. The Emerging Field of RNA Nanotechnology. Nat Nanotechnol. 2010;5:833–842. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schluep T, Hwang J, Hildebrandt IJ, Czernin J, Choi CHJ, Alabi CA, Mack BC, Davis ME. Pharmacokinetics and Tumor Dynamics of the Nanoparticle IT-101 from PET Imaging and Tumor Histological Measurements. Proc Natl Acad Sci U S A. 2009;106:11394–11399. doi: 10.1073/pnas.0905487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polyak A, Ross TL. Nanoparticles for SPECT and PET Imaging: Towards Personalized Medicine and Theranostics. Curr Med Chem. 2017 doi: 10.2174/0929867324666170830095553. [DOI] [PubMed] [Google Scholar]
- 40.Kazuma SM, Sultan D, Zhao Y, Detering L, You M, Luehmann HP, Abdalla DS, Liu Y. Recent Advances of Radionuclide-Based Molecular Imaging of Atherosclerosis. Curr Pharm Des. 2015;21:5267–5276. doi: 10.2174/1381612821666150915104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, Gambhir SS. Clinically Approved Nanoparticle Imaging Agents. J Nucl Med. 2016;57:1833–1837. doi: 10.2967/jnumed.116.181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doepker MP, Yamamoto M, Applebaum MA, Patel NU, Jaime Montilla-Soler M, Sarnaik AA, Wayne Cruse C, Sondak VK, Zager JS. Comparison of Single-Photon Emission Computed Tomography-Computed Tomography (SPECT/CT) and Conventional Planar Lymphoscintigraphy for Sentinel Node Localization in Patients with Cutaneous Malignancies. Ann Surg Oncol. 2017;24:355–361. doi: 10.1245/s10434-016-5590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashton JR, West JL, Badea CT. In Vivo Small Animal Micro-CT using Nanoparticle Contrast Agents. Front Pharmacol. 2015;6:256. doi: 10.3389/fphar.2015.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curry T, Kopelman R, Shilo M, Popovtzer R. Multifunctional Theranostic Gold Nanoparticles for Targeted CT Imaging and Photothermal Therapy. Contrast Media Mol Imaging. 2014;9:53–61. doi: 10.1002/cmmi.1563. [DOI] [PubMed] [Google Scholar]
- 45.Badea CT, Athreya KK, Espinosa G, Clark D, Ghafoori AP, Li Y, Kirsch DG, Johnson GA, Annapragada A, Ghaghada KB. Computed Tomography Imaging of Primary Lung Cancer in Mice Using a Liposomal-Iodinated Contrast Agent. PLoS One. 2012;7:e34496. doi: 10.1371/journal.pone.0034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JY, Chang Y, Lee GH. Multi-Modal Imaging and Cancer Therapy Using Lanthanide Oxide Nanoparticles: Current Status and Perspectives. Curr Med Chem. 2015;22:569–581. doi: 10.2174/0929867322666141128162843. [DOI] [PubMed] [Google Scholar]
- 47.Cormode DP, Naha PC, Fayad ZA. Nanoparticle Contrast Agents for Computed Tomography: A Focus on Micelles. Contrast Media Mol Imaging. 2014;9:37–52. doi: 10.1002/cmmi.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao Y, Hernandez C, Yuan HX, Lilly J, Kota P, Zhou H, Wu H, Exner AA. Ultrasound Molecular Imaging of Ovarian Cancer with CA-125 Targeted Nanobubble Contrast Agents. Nanomedicine. 2017;13:2159–2168. doi: 10.1016/j.nano.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Uemura H, Sano F, Nomiya A, Yamamoto T, Nakamura M, Miyoshi Y, Miki K, Noguchi K, Egawa S, Homma Y, Kubota Y. Usefulness of Perflubutane Microbubble-Enhanced Ultrasound in Imaging and Detection of Prostate Cancer: Phase II Multicenter Clinical Trial. World J Urol. 2013;31:1123–1128. doi: 10.1007/s00345-012-0833-1. [DOI] [PubMed] [Google Scholar]
- 50.Willmann JK, Bonomo L, Testa AC, Rinaldi P, Rindi G, Valluru KS, Petrone G, Martini M, Lutz AM, Gambhir SS. Ultrasound Molecular Imaging With BR55 in Patients With Breast and Ovarian Lesions: First-in-Human Results. J Clin Oncol. 2017;35:2133–2140. doi: 10.1200/JCO.2016.70.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zackrisson S, van de Ven S, Gambhir SS. Light In and Sound Out: Emerging Translational Strategies for Photoacoustic Imaging. Cancer Res. 2014;74:979–1004. doi: 10.1158/0008-5472.CAN-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, Yuan Z, Wu C. Nanoparticle Probes for Structural and Functional Photoacoustic Molecular Tomography. BioMed Res Int. 2015;2015:757101. doi: 10.1155/2015/757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Chen X. Gold Nanoparticles for Photoacoustic Imaging. Nanomedicine (London, U K) 2015;10:299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum Dots as Multimodal Photoacoustic and Photothermal Contrast Agents. Nano Lett. 2008;8:3953–3958. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pu K, Mei J, Jokerst JV, Hong G, Antaris AL, Chattopadhyay N, Shuhendler AJ, Kurosawa T, Zhou Y, Gambhir SS, Bao Z, Rao J. Diketopyrrolopyrrole-Based Semiconducting Polymer Nanoparticles for In Vivo Photoacoustic Imaging. Adv Mater. 2015;27:5184–5190. doi: 10.1002/adma.201502285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maji SK, Sreejith S, Joseph J, Lin M, He T, Tong Y, Sun H, Yu SWK, Zhao Y. Upconversion Nanoparticles as a Contrast Agent for Photoacoustic Imaging in Live Mice. Adv Mater. 2014;26:5633–5638. doi: 10.1002/adma.201400831. [DOI] [PubMed] [Google Scholar]
- 57.Weber J, Beard PC, Bohndiek SE. Contrast Agents for Molecular Photoacoustic Imaging. Nat Methods. 2016;13:639–650. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 58.Hannah A, Luke G, Wilson K, Homan KA, Emelianov S. Indocyanine Green-Loaded Photoacoustic Nanodroplets – Dual Contrast Nanoconstructs for Enhanced Photoacoustic and Ultrasound Imaging. ACS Nano. 2014;8:250–259. doi: 10.1021/nn403527r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eberhardt K, Stiebing C, Matthäus C, Schmitt M, Popp J. Advantages and Limitations of Raman Spectroscopy for Molecular Diagnostics: An Update. Expert Rev Mol Diagn. 2015;15:773–787. doi: 10.1586/14737159.2015.1036744. [DOI] [PubMed] [Google Scholar]
- 60.Vo-Dinh T, Liu Y, Fales AM, Ngo H, Wang HN, Register JK, Yuan H, Norton SJ, Griffin GD. SERS Nanosensors and Nanoreporters: Golden Opportunities in Biomedical Applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:17–33. doi: 10.1002/wnan.1283. [DOI] [PubMed] [Google Scholar]
- 61.Wilson AJ, Willets KA. Surface-Enhanced Raman Scattering Imaging using Noble Metal Nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:180–189. doi: 10.1002/wnan.1208. [DOI] [PubMed] [Google Scholar]
- 62.Harmsen S, Huang R, Wall MA, Karabeber H, Samii JM, Spaliviero M, White JR, Monette S, O’Connor R, Pitter KL, Sastra SA, Saborowski M, Holland EC, Singer S, Olive KP, Lowe SW, Blasberg RG, Kircher MF. Surface-Enhanced Resonance Raman Scattering Nanostars for High Precision Cancer Imaging. Sci Transl Med. 2015;7:271ra7. doi: 10.1126/scitranslmed.3010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jalani G, Lee S, Jung CW, Jang H, Choo J, Lim DW. Controlled Biohybrid Nanoprobes with Silver Nanoparticle Clusters for Raman Imaging. Analyst. 2013;138:4756–4759. doi: 10.1039/c3an00943b. [DOI] [PubMed] [Google Scholar]
- 64.Wang YXJ, Idee JM, Corot C. Scientific and Industrial Challenges of Developing Nanoparticle-Based Theranostics and Multiple-Modality Contrast Agents for Clinical Application. Nanoscale. 2015;7:16146–16150. doi: 10.1039/c5nr03887a. [DOI] [PubMed] [Google Scholar]
- 65.Rieffel J, Chitgupi U, Lovell JF. Recent Advances in Higher-Order, Multimodal, Biomedical Imaging Agents. Small. 2015;11:4445–4461. doi: 10.1002/smll.201500735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lv G, Guo W, Zhang W, Zhang T, Li S, Chen S, Eltahan AS, Wang D, Wang Y, Zhang J, Wang PC, Chang J, Liang XJ. Near-Infrared Emission CuInS/ZnS Quantum Dots: All-in-One Theranostic Nanomedicines with Intrinsic Fluorescence/Photoacoustic Imaging for Tumor Phototherapy. ACS Nano. 2016;10:9637–9645. doi: 10.1021/acsnano.6b05419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chanda N, Shukla R, Zambre A, Mekapothula S, Kulkarni RR, Katti K, Bhattacharyya K, Fent GM, Casteel SW, Boote EJ, Viator JA, Upendran A, Kannan R, Katti KV. An Effective Strategy for the Synthesis of Biocompatible Gold Nanoparticles Using Cinnamon Phytochemicals for Phantom CT Imaging and Photoacoustic Detection of Cancerous Cells. Pharm Res. 2011;28:279–291. doi: 10.1007/s11095-010-0276-6. [DOI] [PubMed] [Google Scholar]
- 68.Shin TH, Choi Y, Kim S, Cheon J. Recent Advances in Magnetic Nanoparticle-Based Multi-Modal Imaging. Chem Soc Rev. 2015;44:4501–4516. doi: 10.1039/c4cs00345d. [DOI] [PubMed] [Google Scholar]
- 69.Chen F, Ellison PA, Lewis CM, Hong H, Zhang Y, Shi S, Hernandez R, Meyerand ME, Barnhart TE, Cai W. Chelator-free synthesis of a dual-modality PET/MRI agent. Angew Chem, Int Ed. 2013;52:13319–13323. doi: 10.1002/anie.201306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S, Goins B, Zhang L, Bao A. Novel Multifunctional Theranostic Liposome Drug Delivery System: Construction, Characterization, and Multimodality MR, Near-Infrared Fluorescent, and Nuclear Imaging. Bioconjugate Chem. 2012;23:1322–1332. doi: 10.1021/bc300175d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panagiotopoulos N, Duschka RL, Ahlborg M, Bringout G, Debbeler C, Graeser M, Kaethner C, Lüdtke-Buzug K, Medimagh H, Stelzner J, Buzug TM, Barkhausen J, Vogt FM, Haegele J. Magnetic Particle Imaging: Current Developments and Future Directions. Int J Nanomed. 2015;10:3097–3114. doi: 10.2147/IJN.S70488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khandhar AP, Ferguson RM, Arami H, Krishnan KM. Monodisperse Magnetite Nanoparticle Tracers for In Vivo Magnetic Particle Imaging. Biomaterials. 2013;34:3837–3845. doi: 10.1016/j.biomaterials.2013.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song G, Chen M, Zhang Y, Cui L, Qu H, Zheng X, Wintermark M, Liu Z, Rao J. Janus Iron Oxides @ Semiconducting Polymer Nanoparticle Tracer for Cell Tracking by Magnetic Particle Imaging. Nano Lett. 2018;18:182–189. doi: 10.1021/acs.nanolett.7b03829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng W, Goldys EM. Chemical Sensing with Nanoparticles as Optical Reporters: From Noble Metal Nanoparticles to Quantum Dots and Upconverting Nanoparticles. Analyst. 2014;139:5321–5334. doi: 10.1039/c4an01272k. [DOI] [PubMed] [Google Scholar]
- 75.Chen Q, Liu X, Zeng J, Cheng Z, Liu Z. Albumin-NIR Dye Self-Assembled Nanoparticles for Photoacoustic pH Imaging and pH-Responsive Photothermal Therapy Effective for Large Tumors. Biomaterials. 2016;98:23–30. doi: 10.1016/j.biomaterials.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 76.Gurka MK, Pender D, Chuong P, Fouts B, Sobelov A, McNally M, Mezera M, Woo S, McNally LR. Identification of Pancreatic Tumors In Vivo with Ligand-Targeted, pH Responsive Mesoporous Silica Nanoparticles by Multispectral Optoacoustic Tomography. J Controlled Release. 2016;231:60–67. doi: 10.1016/j.jconrel.2015.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng M, Wang Y, Shi H, Hu Y, Feng L, Luo Z, Zhou M, He J, Zhou Z, Zhang Y, Ye D. Redox-Mediated Disassembly to Build Activatable Trimodal Probe for Molecular Imaging of Biothiols. ACS Nano. 2016;10:10075–10085. doi: 10.1021/acsnano.6b05030. [DOI] [PubMed] [Google Scholar]
- 78.Mezghrani O, Tang Y, Ke X, Chen Y, Hu D, Tu J, Zhao L, Bourkaib N. Hepatocellular Carcinoma Dually-targeted Nanoparticles for Reduction Triggered Intracellular Delivery of Doxorubicin. Int J Pharm. 2015;478:553–568. doi: 10.1016/j.ijpharm.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 79.Ye D, Shuhendler AJ, Pandit P, Brewer KD, Tee SS, Cui L, Tikhomirov G, Rutt B, Rao J. Caspase-Responsive Smart Gadolinium-Based Contrast Agent for Magnetic Resonance Imaging of Drug-Induced Apoptosis. Chem Sci. 2014;5:3845–3852. doi: 10.1039/C4SC01392A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen LJ, Sun SK, Wang Y, Yang CX, Wu SQ, Yan XP. Activatable Multifunctional Persistent Luminescence Nanoparticle/Copper Sulfide Nanoprobe for in Vivo Luminescence Imaging-Guided Photothermal Therapy. ACS Appl Mater Interfaces. 2016;8:32667–32674. doi: 10.1021/acsami.6b10702. [DOI] [PubMed] [Google Scholar]
- 81.Morris RT, Joyrich RN, Naumann RW, Shah NP, Maurer AH, Strauss HW, Uszler JM, Symanowski JT, Ellis PR, Harb WA. Phase II Study of Treatment of Advanced Ovarian Cancer with Folate-Receptor-Targeted Therapeutic (Vintafolide) and Companion SPECT-Based Imaging Agent (99mTc-etarfolatide) Ann Oncol. 2014;25:852–858. doi: 10.1093/annonc/mdu024. [DOI] [PubMed] [Google Scholar]
- 82.Yang K, Zhu L, Nie L, Sun X, Cheng L, Wu C, Niu G, Chen X, Liu Z. Visualization of Protease Activity In Vivo Using an Activatable Photo-Acoustic Imaging Probe Based on CuS Nanoparticles. Theranostics. 2014;4:134–141. doi: 10.7150/thno.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu L, Yang Y, Farquhar K, Wang J, Tian C, Ranville J, Boyes SG. Surface Modification of Gd Nanoparticles with pH-Responsive Block Copolymers for Use As Smart MRI Contrast Agents. ACS Appl Mater Interfaces. 2016;8:5040–5050. doi: 10.1021/acsami.5b12463. [DOI] [PubMed] [Google Scholar]
- 84.Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Activatable Cell Penetrating Peptides Linked to Nanoparticles as Dual Probes for In Vivo Fluorescence and MR Imaging of Proteases. Proc Natl Acad Sci U S A. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J, Zhang WJ, Guo Z, Wang HB, Wang DD, Zhou JJ, Chen QW. pH-Responsive Iron Manganese Silicate Nanoparticles as T1-T2* Dual-Modal Imaging Probes for Tumor Diagnosis. ACS Appl Mater Interfaces. 2015;7:5373–5383. doi: 10.1021/acsami.5b00727. [DOI] [PubMed] [Google Scholar]
- 86.Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T, Aoki I, Nishiyama N, Kataoka K. A pH-Activatable Nanoparticle with Signal-Amplification Capabilities for Non-Invasive Imaging of Tumour Malignancy. Nat Nanotechnol. 2016;11:724–730. doi: 10.1038/nnano.2016.72. [DOI] [PubMed] [Google Scholar]
- 87.Rosenthal EL, Warram JM, Bland KI, Zinn KR. The Status of Contemporary Image-Guided Modalities in Oncologic Surgery. Ann Surg. 2015;261:46–55. doi: 10.1097/SLA.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hill TK, Mohs AM. Image-Guided Tumor Surgery: Will There be a Role for Fluorescent Nanoparticles? Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2016;8:498–511. doi: 10.1002/wnan.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang K, Luo D, Huang M, Long M, Peng X, Li H. Protection of Parathyroid Function using Carbon Nanoparticles During Thyroid Surgery. Otolaryngol –Head Neck Surg. 2013;149:845–850. doi: 10.1177/0194599813509779. [DOI] [PubMed] [Google Scholar]
- 90.Schaafsma BE, Verbeek FP, Rietbergen DD, van der Hiel B, van der Vorst JR, Liefers GJ, Frangioni JV, van de Velde CJ, van Leeuwen FW, Vahrmeijer AL. Clinical Trial of Combined Radio- and Fluorescence-Guided Sentinel Lymph Node Biopsy in Breast Cancer. Br J Surg. 2013;100:1037–1044. doi: 10.1002/bjs.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaque D, Martinez Maestro L, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, Martin Rodriguez E, Garcia Sole J. Nanoparticles for Photothermal Therapies. Nanoscale. 2014;6:9494–9530. doi: 10.1039/c4nr00708e. [DOI] [PubMed] [Google Scholar]
- 92.Lucky SS, Soo KC, Zhang Y. Nanoparticles in Photodynamic Therapy. Chem Rev (Washington, DC, U S) 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 93.Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew Chem, Int Ed. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 94.Retif P, Pinel S, Toussaint M, Frochot C, Chouikrat R, Bastogne T, Barberi-Heyob M. Nanoparticles for Radiation Therapy Enhancement: the Key Parameters. Theranostics. 2015;5:1030–1044. doi: 10.7150/thno.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-Based Immunotherapy for Cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 96.Dutz S, Hergt R. Magnetic Nanoparticle Heating and Heat Transfer on a Microscale: Basic Principles, Realities and Physical Limitations of Hyperthermia for Tumour Therapy. Int J Hyperthermia. 2013;29:790–800. doi: 10.3109/02656736.2013.822993. [DOI] [PubMed] [Google Scholar]
- 97.Yhee J, Son S, Lee H, Kim K. Nanoparticle-Based Combination Therapy for Cancer Treatment. Curr Pharm Des. 2015;21:3158–3166. doi: 10.2174/1381612821666150531165059. [DOI] [PubMed] [Google Scholar]
- 98.Chen F, Ehlerding EB, Cai W. Theranostic Nanoparticles. J Nucl Med. 2014;55:1919–1922. doi: 10.2967/jnumed.114.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang H, Mintri S, Menon AV, Lee HY, Choi HS, Kim J. Pharmacokinetics, Pharmacodynamics and Toxicology of Theranostic Nanoparticles. Nanoscale. 2015;7:18848–18862. doi: 10.1039/c5nr05264e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma H, Mishra PK, Talegaonkar S, Vaidya B. Metal Nanoparticles: A Theranostic Nanotool Against Cancer. Drug Discovery Today. 2015;20:1143–1151. doi: 10.1016/j.drudis.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 101.Charron DM, Chen J, Zheng G. Theranostic Lipid Nanoparticles for Cancer Medicine. In: Mirkin CA, Meade TJ, Petrosko SH, Stegh AH, editors. Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer. Springer International Publishing; Cham: 2015. pp. 103–127. [DOI] [PubMed] [Google Scholar]
- 102.Kumar R, Shin WS, Sunwoo K, Kim WY, Koo S, Bhuniya S, Kim JS. Small Conjugate-Based Theranostic Agents: An Encouraging Approach for Cancer Therapy. Chem Soc Rev. 2015;44:6670–6683. doi: 10.1039/c5cs00224a. [DOI] [PubMed] [Google Scholar]
- 103.Huang H, Lovell JF. Advanced Functional Nanomaterials for Theranostics. Adv Funct Mater. 2017;27:1603524. doi: 10.1002/adfm.201603524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xing H, Hwang K, Lu Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics. 2016;6:1336–1352. doi: 10.7150/thno.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen ML. Lipid Excipients and Delivery Systems for Pharmaceutical Development: A Regulatory Perspective. Adv Drug Delivery Rev. 2008;60:768–777. doi: 10.1016/j.addr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 106.Kodama T, Tomita N, Yagishita Y, Horie S, Funamoto K, Hayase T, Sakamoto M, Mori S. Volumetric and Angiogenic Evaluation of Antitumor Effects with Acoustic Liposome and High-Frequency Ultrasound. Cancer Res. 2011;71:6957. doi: 10.1158/0008-5472.CAN-11-2389. [DOI] [PubMed] [Google Scholar]
- 107.Tagami T, Foltz WD, Ernsting MJ, Lee CM, Tannock IF, May JP, Li SD. MRI Monitoring of Intratumoral Drug Delivery and Prediction of the Therapeutic Effect with a Multifunctional Thermosensitive Liposome. Biomaterials. 2011;32:6570–6578. doi: 10.1016/j.biomaterials.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 108.Chen D, Dougherty CA, Zhu K, Hong H. Theranostic Applications of Carbon Nanomaterials in Cancer: Focus on Imaging and Cargo Delivery. J Controlled Release. 2015;210:230–245. doi: 10.1016/j.jconrel.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 109.Yu W, Cao X, Xu G, Song Y, Li G, Zheng H, Zhang N. Potential Role for Carbon Nanoparticles to Guide Central Neck Dissection in Patients with Papillary Thyroid Cancer. Surgery. 2016;160:755–761. doi: 10.1016/j.surg.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 110.Wu X, Lin Q, Chen G, Lu J, Zeng Y, Chen X, Yan J. Sentinel Lymph Node Detection Using Carbon Nanoparticles in Patients with Early Breast Cancer. PLoS One. 2015;10:e0135714. doi: 10.1371/journal.pone.0135714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao H, Chao Y, Liu J, Huang J, Pan J, Guo W, Wu J, Sheng M, Yang K, Wang J, Liu Z. Polydopamine Coated Single-Walled Carbon Nanotubes as a Versatile Platform with Radionuclide Labeling for Multimodal Tumor Imaging and Therapy. Theranostics. 2016;6:1833–1843. doi: 10.7150/thno.16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gong H, Peng R, Liu Z. Carbon Nanotubes for Biomedical Imaging: The Recent Advances. Adv Drug Delivery Rev. 2013;65:1951–1963. doi: 10.1016/j.addr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Hu D, Zhang J, Gao G, Sheng Z, Cui H, Cai L. Indocyanine Green-Loaded Polydopamine-Reduced Graphene Oxide Nanocomposites with Amplifying Photoacoustic and Photothermal Effects for Cancer Theranostics. Theranostics. 2016;6:1043–1052. doi: 10.7150/thno.14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y, Chen H, Shi J. Drug Delivery/Imaging Multifunctionality of Mesoporous Silica-Based Composite Nanostructures. Expert Opin Drug Delivery. 2014;11:917–930. doi: 10.1517/17425247.2014.908181. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y, Xu M, Chen Q, Guan G, Hu W, Zhao X, Qiao M, Hu H, Liang Y, Zhu H, Chen D. Gold Nanorods/Mesoporous Silica-Based Nanocomposite as Theranostic Agents for Targeting Near-Infrared Imaging and Photothermal Therapy Induced with Laser. Int J Nanomed. 2015;10:4747–4761. doi: 10.2147/IJN.S82940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang G, Gong H, Liu T, Sun X, Cheng L, Liu Z. Two-Dimensional Magnetic WS2@Fe3O4 Nanocomposite with Mesoporous Silica Coating for Drug Delivery and Imaging-Guided Therapy of Cancer. Biomaterials. 2015;60:62–71. doi: 10.1016/j.biomaterials.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 117.Chen F, Hong H, Goel S, Graves SA, Orbay H, Ehlerding EB, Shi S, Theuer CP, Nickles RJ, Cai W. In Vivo Tumor Vasculature Targeting of CuS@MSN Based Theranostic Nanomedicine. ACS Nano. 2015;9:3926–3934. doi: 10.1021/nn507241v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chakravarty R, Goel S, Hong H, Chen F, Valdovinos HF, Hernandez R, Barnhart TE, Cai W. Functionalized Hollow Mesoporous Silica Nanoparticles for Tumor Vasculature Targeting and PET Image-Guided Drug Delivery. Nanomedicine (London, U K) 2015;10:1233–1246. doi: 10.2217/nnm.14.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Milgroom A, Intrator M, Madhavan K, Mazzaro L, Shandas R, Liu B, Park D. Mesoporous Silica Nanoparticles as a Breast-Cancer Targeting Ultrasound Contrast Agent. Colloids Surf, B. 2014;116:652–657. doi: 10.1016/j.colsurfb.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cabral RM, Baptista PV. Anti-Cancer Precision Theranostics: A Focus on Multifunctional Gold Nanoparticles. Expert Rev Mol Diagn. 2014;14:1041–1052. doi: 10.1586/14737159.2014.965683. [DOI] [PubMed] [Google Scholar]
- 121.Deng H, Zhong Y, Du M, Liu Q, Fan Z, Dai F, Zhang X. Theranostic Self-Assembly Structure of Gold Nanoparticles for NIR Photothermal Therapy and X-Ray Computed Tomography Imaging. Theranostics. 2014;4:904–918. doi: 10.7150/thno.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Pietro P, Strano G, Zuccarello L, Satriano C. Gold and Silver Nanoparticles for Applications in Theranostics. Curr Top Med Chem. 2016;16:3069–3102. doi: 10.2174/1568026616666160715163346. [DOI] [PubMed] [Google Scholar]
- 123.Yamada M, Foote M, Prow TW. Therapeutic Gold, Silver, and Platinum Nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:428–445. doi: 10.1002/wnan.1322. [DOI] [PubMed] [Google Scholar]
- 124.Alric C, Taleb J, Duc GL, Mandon C, Billotey C, Meur-Herland AL, Brochard T, Vocanson F, Janier M, Perriat P, Roux S, Tillement O. Gadolinium Chelate Coated Gold Nanoparticles As Contrast Agents for Both X-ray Computed Tomography and Magnetic Resonance Imaging. J Am Chem Soc. 2008;130:5908–5915. doi: 10.1021/ja078176p. [DOI] [PubMed] [Google Scholar]
- 125.Chen W, Ayala-Orozco C, Biswal NC, Perez-Torres C, Bartels M, Bardhan R, Stinnet G, Liu X-D, Ji B, Deorukhkar A, Brown LV, Guha S, Pautler RG, Krishnan S, Halas NJ, Joshi A. Targeting of Pancreatic Cancer with Magneto-Fluorescent Theranostic Gold Nanoshells. Nanomedicine (London, U K) 2014;9:1209–1222. doi: 10.2217/nnm.13.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duran N, Duran M, de Jesus MB, Seabra AB, Favaro WJ, Nakazato G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomedicine. 2016;12:789–799. doi: 10.1016/j.nano.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 127.Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S, BV, Bhadra MP, Sreedhar B, Patra CR. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System) Theranostics. 2014;4:316–335. doi: 10.7150/thno.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu X, Chen G, Shen J, Li Z, Zhang Y, Han G. Upconversion Nanoparticles: A Versatile Solution to Multiscale Biological Imaging. Bioconjugate Chem. 2015;26:166–175. doi: 10.1021/bc5003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan X, Wang J, Pang X, Liu L, Sun Q, You Q, Tan F, Li N. Indocyanine Green-Loaded Silver Nanoparticle@Polyaniline Core/Shell Theranostic Nanocomposites for Photoacoustic/Near-Infrared Fluorescence Imaging-Guided and Single-Light-Triggered Photothermal and Photodynamic Therapy. ACS Appl Mater Interfaces. 2016;8:34991–35003. doi: 10.1021/acsami.6b11262. [DOI] [PubMed] [Google Scholar]
- 130.Chen X, Wu Y, Dong H, Zhang CY, Zhang Y. Platinum-Based Agents for Individualized Cancer Treatment. Curr Mol Med. 2013;13:1603–1612. doi: 10.2174/1566524013666131111125515. [DOI] [PubMed] [Google Scholar]
- 131.Cheng Q, Liu Y. Multifunctional Platinum-Based Nanoparticles for Biomedical Applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1410. doi: 10.1002/wnan.1410. [DOI] [PubMed] [Google Scholar]
- 132.Hariri G, Wellons MS, Morris WH, Lukehart CM, Hallahan DE. Multifunctional FePt Nanoparticles for Radiation-Guided Targeting and Imaging of Cancer. Ann Biomed Eng. 2011;39:946–952. doi: 10.1007/s10439-010-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yue L, Wang J, Dai Z, Hu Z, Chen X, Qi Y, Zheng X, Yu D. pH-Responsive, Self-Sacrificial Nanotheranostic Agent for Potential In Vivo and In Vitro Dual Modal MRI/CT Imaging, Real-Time, and In Situ Monitoring of Cancer Therapy. Bioconjugate Chem. 2017;28:400–409. doi: 10.1021/acs.bioconjchem.6b00562. [DOI] [PubMed] [Google Scholar]
- 134.Ho D, Sun X, Sun S. Monodisperse Magnetic Nanoparticles for Theranostic Applications. Acc Chem Res. 2011;44:875–882. doi: 10.1021/ar200090c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Santhosh PB, Ulrih NP. Multifunctional Super-paramagnetic Iron Oxide Nanoparticles: Promising Tools in Cancer Theranostics. Cancer Lett. 2013;336:8–17. doi: 10.1016/j.canlet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 136.Yallapu MM, Othman SF, Curtis ET, Gupta BK, Jaggi M, Chauhan SC. Multi-functional Magnetic Nanoparticles for Magnetic Resonance Imaging and Cancer Therapy. Biomaterials. 2011;32:1890–1905. doi: 10.1016/j.biomaterials.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou H, Qian W, Uckun FM, Wang L, Wang YA, Chen H, Kooby D, Yu Q, Lipowska M, Staley CA, Mao H, Yang L. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano. 2015;9:7976–7991. doi: 10.1021/acsnano.5b01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Estelrich J, Escribano E, Queralt J, Busquets MA. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int J Mol Sci. 2015;16:8070–8101. doi: 10.3390/ijms16048070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilson MW, Kerlan RK, Jr, Fidelman NA, Venook AP, LaBerge JM, Koda J, Gordon RL. Hepatocellular Carcinoma: Regional Therapy with a Magnetic Targeted Carrier Bound to Doxorubicin in a Dual MR Imaging/Conventional Angiography Suite–Initial Experience with Four Patients. Radiology. 2004;230:287–293. doi: 10.1148/radiol.2301021493. [DOI] [PubMed] [Google Scholar]
- 140.Wang C, Cheng L, Liu Z. Upconversion Nanoparticles for Photodynamic Therapy and Other Cancer Therapeutics. Theranostics. 2013;3:317–330. doi: 10.7150/thno.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tian G, Zhang X, Gu Z, Zhao Y. Recent Advances in Upconversion Nanoparticles-Based Multifunctional Nanocomposites for Combined Cancer Therapy. Adv Mater. 2015;27:7692–7712. doi: 10.1002/adma.201503280. [DOI] [PubMed] [Google Scholar]
- 142.Tripathi SK, Kaur G, Khurana RK, Kapoor S, Singh B. Quantum Dots and their Potential Role in Cancer Theranostics. Crit Rev Ther Drug Carrier Syst. 2015;32:461–502. doi: 10.1615/critrevtherdrugcarriersyst.2015012360. [DOI] [PubMed] [Google Scholar]
- 143.Liu X, Que I, Kong X, Zhang Y, Tu L, Chang Y, Wang TT, Chan A, Lowik CWGM, Zhang H. In Vivo 808 nm Image-Guided Photodynamic Therapy Based on an Upconversion Theranostic Nanoplatform. Nanoscale. 2015;7:14914–14923. doi: 10.1039/c5nr03690a. [DOI] [PubMed] [Google Scholar]
- 144.Yong Y, Cheng X, Bao T, Zu M, Yan L, Yin W, Ge C, Wang D, Gu Z, Zhao Y. Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for In Vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy. ACS Nano. 2015;9:12451–12463. doi: 10.1021/acsnano.5b05825. [DOI] [PubMed] [Google Scholar]
- 145.Thakur M, Mewada A, Pandey S, Bhori M, Singh K, Sharon M, Sharon M. Milk-Derived Multi-Fluorescent Graphene Quantum Dot-Based Cancer Theranostic System. Mater Sci Eng, C. 2016;67:468–477. doi: 10.1016/j.msec.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 146.Park YI, Lee KT, Suh YD, Hyeon T. Upconverting Nanoparticles: A Versatile Platform for Wide-Field Two-Photon Microscopy and Multi-Modal In Vivo Imaging. Chem Soc Rev. 2015;44:1302–1317. doi: 10.1039/c4cs00173g. [DOI] [PubMed] [Google Scholar]
- 147.Agarwal A, Ressler D, Snyder G. The Current and Future State of Companion Diagnostics. Pharmacogenomics Pers Med. 2015;8:99–110. doi: 10.2147/PGPM.S49493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roscoe DM, Hu YF, Philip R. Companion Diagnostics: A Regulatory Perspective from the Last 5 Years of Molecular Companion Diagnostic Approvals. Expert Rev Mol Diagn. 2015;15:869–880. doi: 10.1586/14737159.2015.1045490. [DOI] [PubMed] [Google Scholar]
- 149.Myers MB. Targeted Therapies with Companion Diagnostics in the Management of Breast Cancer: Current Perspectives. Pharmacogenomics Pers Med. 2016;9:7–16. doi: 10.2147/PGPM.S56055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Conde E, Hernandez S, Prieto M, Martinez R, Lopez-Rios F. Profile of Ventana ALK (D5F3) Companion Diagnostic Assay for Non-Small-Cell Lung Carcinomas. Expert Rev Mol Diagn. 2016;16:707–713. doi: 10.1586/14737159.2016.1172963. [DOI] [PubMed] [Google Scholar]
- 151.Harbison CT, Horak CE, Ledeine JM, Mukhopadhyay P, Malone DP, O’Callaghan C, Jonker DJ, Karapetis CS, Khambata-Ford S, Gustafson N, Trifan OC, Chang SC, Ravetto P, Iv GAG. Validation of Companion Diagnostic for Detection of Mutations in Codons 12 and 13 of the KRAS Gene in Patients with Metastatic Colorectal Cancer: Analysis of the NCIC CTG CO.17 Trial. Arch Pathol Lab Med. 2013;137:820–827. doi: 10.5858/arpa.2012-0367-OA. [DOI] [PubMed] [Google Scholar]
- 152.Ocana A, Ethier JL, Díez-González L, Corrales-Sánchez V, Srikanthan A, Gascón-Escribano MJ, Templeton AJ, Vera-Badillo F, Seruga B, Niraula S, Pandiella A, Amir E. Influence of Companion Diagnostics on Efficacy and Safety of Targeted Anti-Cancer Drugs: Systematic Review and Meta-Analyses. Oncotarget. 2015;6:39538–39549. doi: 10.18632/oncotarget.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.St Pierre TG, El-Beshlawy A, Elalfy M, Al Jefri A, Al Zir K, Daar S, Habr D, Kriemler-Krahn U, Taher A. Multicenter Validation of Spin-Density Projection-Assisted R2-MRI for the Noninvasive Measurement of Liver Iron Concentration. Magn Reson Med. 2014;71:2215–2223. doi: 10.1002/mrm.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van de Watering FC, Rijpkema M, Perk L, Brinkmann U, Oyen WJ, Boerman OC. Zirconium-89 Labeled Antibodies: A New Tool for Molecular Imaging in Cancer Patients. BioMed Res Int. 2014;2014:203601. doi: 10.1155/2014/203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wright BD, Lapi SE. Designing the Magic Bullet? The Advancement of Immuno-PET into Clinical Use. J Nucl Med. 2013;54:1171–1174. doi: 10.2967/jnumed.113.126086. [DOI] [PubMed] [Google Scholar]
- 156.Mortimer JE, Bading JR, Park JM, Frankel PH, Carroll MI, Tran TT, Poku EK, Rockne RC, Raubitschek AA, Shively JE, Colcher DM. Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J Nucl Med. 2018;59:38. doi: 10.2967/jnumed.117.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and Key Considerations of the Enhanced Permeability and Retention (EPR) Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maeda H, Nakamura H, Fang J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging. Adv Drug Delivery Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 159.Stapleton S, Allen C, Pintilie M, Jaffray DA. Tumor Perfusion Imaging Predicts the Intra-Tumoral Accumulation of Liposomes. J Controlled Release. 2013;172:351–357. doi: 10.1016/j.jconrel.2013.08.296. [DOI] [PubMed] [Google Scholar]
- 160.Spence T, De Souza R, Dou Y, Stapleton S, Reilly RM, Allen C. Integration of Imaging into Clinical Practice to Assess the Delivery and Performance of Macromolecular and Nanotechnology-Based Oncology Therapies. J Controlled Release. 2015;219:295–312. doi: 10.1016/j.jconrel.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 161.Karageorgis A, Dufort S, Sancey L, Henry M, Hirsjärvi S, Passirani C, Benoit JP, Gravier J, Texier I, Montigon O, Benmerad M, Siroux V, Barbier EL, Coll JL. An MRI-Based Classification Scheme to Predict Passive Access of 5 to 50-nm Large Nanoparticles to Tumors. Sci Rep. 2016;6:21417. doi: 10.1038/srep21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH, Yang KS, Laughney AM, Wojtkiewicz G, Kamaly N, Bhonagiri S, Pittet M, Farokhzad OC, Weissleder R. Predicting Therapeutic Nanoparticle Efficacy Using a Companion MR Imaging Nanoparticle. Sci Transl Med. 2015;7:314ra183–314ra183. doi: 10.1126/scitranslmed.aac6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 164.Hansen AE, Petersen AL, Henriksen JR, Boerresen B, Rasmussen P, Elema DR, Rosenschöld PMa, Kristensen AT, Kjær A, Andresen TL. Positron Emission Tomography Based Elucidation of the Enhanced Permeability and Retention Effect in Dogs with Cancer Using Copper-64 Liposomes. ACS Nano. 2015;9:6985–6995. doi: 10.1021/acsnano.5b01324. [DOI] [PubMed] [Google Scholar]
- 165.Edmonds S, Volpe A, Shmeeda H, Parente-Pereira AC, Radia R, Baguna-Torres J, Szanda I, Severin GW, Livieratos L, Blower PJ, Maher J, Fruhwirth GO, Gabizon A, de Rosales TMR. Exploiting the Metal-Chelating Properties of the Drug Cargo for In Vivo Positron Emission Tomography Imaging of Liposomal Nanomedicines. ACS Nano. 2016;10:10294–10307. doi: 10.1021/acsnano.6b05935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee H, Shields AF, Siegel BA, Miller KD, Krop I, Ma CX, LoRusso PM, Munster PN, Campbell K, Gaddy DF, Leonard SC, Geretti E, Blocker SJ, Kirpotin DB, Moyo V, Wickham TJ, Hendriks BS. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin Cancer Res. 2017;23:4190–4202. doi: 10.1158/1078-0432.CCR-16-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ekdawi SN, Stewart JMP, Dunne M, Stapleton S, Mitsakakis N, Dou YN, Jaffray DA, Allen C. Spatial and Temporal Mapping of Heterogeneity in Liposome Uptake and Microvascular Distribution in an Orthotopic Tumor Xenograft Model. J Controlled Release. 2015;207:101–111. doi: 10.1016/j.jconrel.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 168.Karathanasis E, Suryanarayanan S, Balusu SR, McNeeley K, Sechopoulos I, Karellas A, Annapragada AV, Bellamkonda RV. Imaging Nanoprobe for Prediction of Outcome of Nanoparticle Chemotherapy by Using Mammography. Radiology. 2009;250:398–406. doi: 10.1148/radiol.2502080801. [DOI] [PubMed] [Google Scholar]
- 169.Kobayashi H, Choyke PL. Super Enhanced Permeability and Retention (SUPR) Effects in Tumors Following Near Infrared Photoimmunotherapy. Nanoscale. 2016;8:12504–12509. doi: 10.1039/c5nr05552k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tam AL, Melancon MP, Abdelsalam M, Figueira TA, Dixon K, McWatters A, Zhou M, Huang Q, Mawlawi O, Dunner K, Li C, Gupta S. Imaging Intratumoral Nanoparticle Uptake after Combining Nanoembolization with Various Ablative Therapies in Hepatic VX2 Rabbit Tumors. J Biomed Nanotechnol. 2016;12:296–307. doi: 10.1166/jbn.2016.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J Cancer Res Clin Oncol. 2015;141:769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Toy R, Bauer L, Hoimes C, Ghaghada KB, Karathanasis E. Targeted Nanotechnology for Cancer Imaging. Adv Drug Delivery Rev. 2014;76:79–97. doi: 10.1016/j.addr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hainfeld JF, O’Connor MJ, Dilmanian FA, Slatkin DN, Adams DJ, Smilowitz HM. Micro-CT Enables Micro-localisation and Quantification of Her2-Targeted Gold Nanoparticles within Tumour Regions. Br J Radiol. 2011;84:526–533. doi: 10.1259/bjr/42612922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kanazaki K, Sano K, Makino A, Shimizu Y, Yamauchi F, Ogawa S, Ding N, Yano T, Temma T, Ono M, Saji H. Development of Anti-HER2 Fragment Antibody Conjugated to Iron Oxide Nanoparticles for In Vivo HER2-Targeted Photoacoustic Tumor Imaging. Nanomedicine. 2015;11:2051–2060. doi: 10.1016/j.nano.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 175.Chen F, Cai W. Tumor Vasculature Targeting: A Generally Applicable Approach for Functionalized Nanomaterials. Small. 2014;10:1887–1893. doi: 10.1002/smll.201303627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Luo Y, Yang J, Yan Y, Li J, Shen M, Zhang G, Mignani S, Shi X. RGD-Functionalized Ultrasmall Iron Oxide Nanoparticles for Targeted T(1)-Weighted MR Imaging of Gliomas. Nanoscale. 2015;7:14538–14546. doi: 10.1039/c5nr04003e. [DOI] [PubMed] [Google Scholar]
- 177.Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Fresta M, Nie G, Chen C, Shen H, Ferrari M, Zhao Y. Safety of Nanoparticles in Medicine. Curr Drug Targets. 2015;16:1671–1681. doi: 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang YXJ. Current Status of Superparamagnetic Iron Oxide Contrast Agents for Liver Magnetic Resonance Imaging. World J Gastroenterol. 2015;21:13400–13402. doi: 10.3748/wjg.v21.i47.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cho EJ, Holback H, Liu KC, Abouelmagd SA, Park J, Yeo Y. Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. Mol Pharmaceutics. 2013;10:2093–2110. doi: 10.1021/mp300697h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA, Adiseshaiah PP, Clogston JD, McNeil SE. Common Pitfalls in Nanotechnology: Lessons Learned from NCI’s Nanotechnology Characterization Laboratory. Integr Biol. 2013;5:66–73. doi: 10.1039/c2ib20117h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial Standards for Efficacy and Toxicity Assessment. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2010;2:99–112. doi: 10.1002/wnan.66. [DOI] [PubMed] [Google Scholar]
- 182.Wicki A, Ritschard R, Loesch U, Deuster S, Rochlitz C, Mamot C. Large-Scale Manufacturing of GMP-Compliant Anti-EGFR Targeted Nanocarriers: Production of Doxorubicin-Loaded Anti-EGFR-Immunoliposomes for a First-in-Man Clinical Trial. Int J Pharm. 2015;484:8–15. doi: 10.1016/j.ijpharm.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 183.Beyer S, Xie L, Gräfe S, Vogel V, Dietrich K, Wiehe A, Albrecht V, Mäntele W, Wacker MG. Bridging Laboratory and Large Scale Production: Preparation and In Vitro-Evaluation of Photosensitizer-Loaded Nanocarrier Devices for Targeted Drug Delivery. Pharm Res. 2015;32:1714–1726. doi: 10.1007/s11095-014-1569-y. [DOI] [PubMed] [Google Scholar]
- 184.Duong AD, Ruan G, Mahajan K, Winter JO, Wyslouzil BE. Scalable, Semicontinuous Production of Micelles Encapsulating Nanoparticles via Electrospray. Langmuir. 2014;30:3939–3948. doi: 10.1021/la404679r. [DOI] [PubMed] [Google Scholar]
- 185.Czapar AE, Steinmetz NF. Plant Viruses and Bacteriophages for Drug Delivery in Medicine and Biotechnology. Curr Opin Chem Biol. 2017;38:108–116. doi: 10.1016/j.cbpa.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Love SA, Maurer-Jones MA, Thompson JW, Lin YS, Haynes CL. Assessing Nanoparticle Toxicity. Annu Rev Anal Chem. 2012;5:181–205. doi: 10.1146/annurev-anchem-062011-143134. [DOI] [PubMed] [Google Scholar]
- 187.Almutary A, Sanderson BJS. The MTT and Crystal Violet Assays: Potential Confounders in Nanoparticle Toxicity Testing. Int J Toxicol. 2016;35:454–462. doi: 10.1177/1091581816648906. [DOI] [PubMed] [Google Scholar]
- 188.Mortelmans K, Zeiger E. The Ames Salmonella/Microsome Mutagenicity Assay. Mutat Res, Fundam Mol Mech Mutagen. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 189.Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ehlerding EB, Chen F, Cai W. Biodegradable and Renal Clearable Inorganic Nanoparticles. Advanced Science. 2016;3:1500223. doi: 10.1002/advs.201500223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Day CP, Merlino G, Van Dyke T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell. 2015;163:39–53. doi: 10.1016/j.cell.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zamboni WC, Torchilin V, Patri A, Hrkach J, Stern S, Lee R, Nel A, Panaro NJ, Grodzinski P. Best Practices in Cancer Nanotechnology – Perspective from NCI Nanotechnology Alliance. Clin Cancer Res. 2012;18:3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li C, Targeted A. Approach to Cancer Imaging and Therapy. Nat Mater. 2014;13:110–115. doi: 10.1038/nmat3877. [DOI] [PMC free article] [PubMed] [Google Scholar]