Abstract
Background
Although dysphagia represents a hallmark manifestation of oculopharyngeal muscular dystrophy (OPMD), limited knowledge exists regarding the underlying nature of oropharyngeal swallowing impairments in this patient population. We aimed to delineate global pharyngeal dysphagia profiles in OPMD and identify the prevalence and physiologic associations of impairments in swallowing safety and efficiency.
Methods
Twenty-two individuals with OPMD completed a videofluoroscopic swallowing evaluation. Blinded raters completed validated scales of global dysphagia (dynamic imaging grade of swallowing toxicity, DIGEST), efficiency (normalized residue ratio scale, NRRS), and safety (penetration aspiration scale, PAS). Degree of laryngeal vestibule closure and aspiration events were described. Descriptives and chi-squared analyses were conducted with alpha set at p<0.05.
Key Results
134 swallowing trials were analyzed. DIGEST scores revealed that 96% (n=21) of participants demonstrated pharyngeal dysphagia (score >1). Presence of a cricopharyngeal bar was noted in 10 individuals. The predominant swallowing categorization across patients was safe and inefficient (51%) followed by unsafe and inefficient (32%). 77.3% demonstrated vallecular residue (NRRSv>0.07) and 90.1% piriform sinus residue (NRRSp>0.20). Thirty-three percent (n=54) of swallows were unsafe (PAS>3) with 45 episodes of penetration and 9 episodes of aspiration. Aspiration occurred during the swallow in 100% of identified occurrences. Incomplete epiglottic inversion was associated with airway compromise and post-swallow residue (p<0.05).
Conclusions and Inferences
These findings highlight the high prevalence of oropharyngeal swallowing impairments in both swallowing efficiency and safety. A high proportion of physiologic impairments in epiglottic inversion and laryngeal vestibule closure were noted that related to functional impairments in swallow safety and inefficiency.
Keywords: deglutition, dysphagia, muscular dystrophy, swallowing disorders
Graphical Abstract
Introduction
Oculopharyngeal muscular dystrophy (OPMD) is a rare, adult-onset dominant muscular dystrophy with hallmark manifestations of dysphagia, dysphonia and ptosis. Disease onset typically occurs in the fifth and sixth decades of life and a clinical diagnosis of OPMD can be made in cases of late-onset ptosis and dysphagia, with a family history of the syndrome in two or more generations 1. A diagnosis of OPMD can be confirmed by genetic testing for a short triplet repeat expansion in the PABPN1 gene on chromosome 14 2. Ptosis results from weakness of the levator palprebrae superioris and dysphagia from weakness of the pharyngeal musculature 1, 3. With time, other voluntary muscles may also become affected 1. Disease progression in OPMD is slow with normal life expectancy, although quality of life is reported to be significantly reduced 4.
Current Knowledge of Swallowing Impairment and Treatments in OPMD
Dysphagia can be broadly classified by impairments in swallowing efficiency and safety. Swallow inefficiency refers to incomplete bolus clearance and contributes to weight loss and malnutrition while impairments in airway safety result in aspiration, or material entering the trachea, contributing to significant pulmonary sequelae5. In addition to physiologic changes in swallow function, swallowing-related quality of life is moderately impacted characterized by prolonged mealtime durations and increased burden4. Prior studies suggest that decreased pharyngeal pressure generation and impaired relaxation and hypertonicity of the upper esophageal sphincter (UES) represent the primary mechanisms contributing to dysphagia in OPMD 6, 7. Fibrosis and atrophy of the cricopharyngeus muscle have also been documented 8. Not surprisingly, current treatments for dysphagia in individuals with OPMD focus on mitigating UES hypertonicity. Surgical interventions include cricopharyngeal myotomy, cricopharyngeal dilatation and/or cricopharyngeal botulinum toxin injection 3, 9. Because UES opening is the target of these interventions, these procedures do not impact other mechanisms that contribute to oropharyngeal dysphagia including impaired lingual propulsion, pharyngeal contraction and laryngeal vestibule closure10. As a result, current interventions are reported to yield disappointing functional improvements in oropharyngeal swallowing 7, 9, 11, 12 with percutaneous endoscopic gastronomy (PEG) tubes recommended for means of alternative nutrition in end stages of disease as swallow function progressively declines 13. This is particularly impactful as severity of dysphagia is associated with pulmonary sequelae and exacerbated disease prognosis3.
There are a number of validated outcomes to index degree of oropharyngeal swallowing impairment including indices of swallowing and bolus efficiency (Normalized Residue Ratio Scale, NRRS), airway safety (Penetration Aspiration scale, PAS), and global pharyngeal swallow function (Dynamic Imaging Grade of Swallowing Toxicity, DIGEST). To date, these validated measures have not yet been applied to delineate swallowing impairment profiles in individuals with OPMD. As such, little is known regarding the underlying physiologic impairments leading to dysphagia to guide efficacious intervention strategies in this challenging patient population.
We therefore aimed to delineate global pharyngeal dysphagia profiles in OPMD and identify the prevalence and physiologic mechanisms of identified impairments in swallowing safety and efficiency. Due to the pathophysiology of the disease, we anticipated that swallowing impairments would be specific to muscle weakness and hypertonicity. Specifically, we hypothesized that inefficient swallowing would predominate characterized by gross pharyngeal residue due to UES dysfunction, with impairments in airway safety characterized by incomplete laryngeal vestibule closure and aspiration during and after the swallow.
Methods
Participants
OPMD patients attending the University of New Mexico OPMD clinic were recruited and included in this study (convenience sample). Inclusion criteria were: 1) ≥18 years of age, 2) English-speaking, 3) genetically confirmed OPMD, and 4) self-reported swallowing difficulty. Exclusion criteria included: 1) prior cricopharyngeal myotomy, 2) cricopharyngeal dilatation in the past 12 months, 3) cricopharyngeal botulinum toxin injection in the past 6 months, 4) history of head or neck cancer, or radiation therapy, 5) history of any medical condition that resulted in dysphagia, and 6) pregnancy. Patients with a history of cricopharyngeal myotomy, dilatation, and injections were excluded in effort to analyze swallowing impairment in OPMD prior to surgical intervention. Four subjects were excluded from VFSE analyses due to poor quality and the patient moving from frame of view. Twenty-two individuals with genetically confirmed OPMD were included and completed a standardized videofluoroscopic swallowing evaluation as part of a larger OPMD research study at the University of New Mexico. Within this cohort, 82% identified themselves as hispanic (n=18) and 18% as non-hispanic (n=4). Genetic testing revealed that 100% (n=22) had the GCN13GCN10 genotype. Mean age was 62.5 years (+/−11.3) and 50% were female (N=11). Disease severity was classified as mild (n=6, 27%), moderate (n=15, 68%) and severe (n=1, 5%) by the primary neurologist. Mean self-reported dysphagia duration was 9.3 (+/− 5.4) and self-reported dysphagia onset was 53.2 years (+/−9.1). This study was approved by the Institutional Review Board at the University of New Mexico and all subjects provided written informed consent to participate.
Procedures
A standardized videofluoroscopic swallowing evaluation (VFSE) was completed with the patient seated in lateral viewing plane (Philips Easy Diagnostic Eleva DRF Digital Radiography system). The following barium bolus presentations were administered: ×2 5cc thin liquid, single cup sip of thin liquid barium, 5cc nectar, single cup sip of nectar, 5cc honey, 5cc pudding, ½ shortbread cookie coated in 3cc pudding (Varibar, EZ-EM, Westbury, NY). A bailout criterion was enforced by the speech pathologist and larger volume consistencies were only administered after small volumes were deemed safe. The VFSE studies were de-identified and downloaded onto an external computer for data analysis. A blinded speech pathologist analyzed 22 complete videofluoroscopic swallowing studies, and a total of 163 individual swallowing clips. Thirty-nine individual swallowing clips were excluded from bolus efficiency analyses due to poor video quality. Thus, 163 clips were included for airway safety, and 124 for bolus efficiency analyses.
Outcome Measures
Dynamic Imaging Grade of Swallowing Toxicity
The Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) is a validated five-point ordinal scale created to assess both efficiency and safety of bolus flow 14. The DIGEST yields a global grade of pharyngeal dysphagia evaluated on bolus transport during the entirety of the videofluoroscopic swallow study (vs. frame-by-frame analysis) 14. DIGEST analyses were completed on all studies to determine clinically relevant categories of overall pharyngeal dysphagia severity levels. DIGEST total scores are a composite of two subscores (scored 0–4) addressing: 1) swallowing efficiency based on degree of bolus clearance, and 2) airway safety rated by severity and frequency of laryngeal penetration and aspiration. Total and subscore grades of zero indicate functional swallowing while total and subscore grades of 4 indicate life-threatening dysphagia, with the need for immediate intervention.
Bolus Efficiency Profiles
Objective measures of bolus efficiency were analyzed and calculated for each swallow in effort to corroborate DIGEST findings with objective measures of pharyngeal swallowing efficiency. Bolus efficiency was calculated on the first swallow of each bolus trial and analyzed using the validated Normalized Residue Ratio Scale scores 15. The NRRS is a pixel-based measure of vallecular and piriform sinus residue, and were calculated as previously described in Steele et al., 2010 15. NRRS valleculae (NRRSv) ratings ≥ 0.07 and NRRS piriform (NRRSp) ratings of ≥0.20 were characterized as inefficient 16.
Airway Safety Profiles
The primary outcome for airway safety was the penetration aspiration scale (PAS), 17 an eight point ordinal scale indexing degree airway compromise during swallowing. Unsafe swallowing was characterized as Penetration-Aspiration scale (PAS) score of ≥3. Instances of unsafe swallowing were further classified to understand the timing of the aspiration event (before, during or after the swallow). Airway invasion before the swallow was defined as occurring prior to hyoid burst, during the swallow defined as occurring between hyoid burst and maximum hyoid elevation, and after the swallow defined as occurring after maximum hyoid elevation.
Swallowing Kinematics and Anatomical Ratings
Degree of laryngeal vestibule closure was rated as either: 1) complete and protective, 2) complete and not protective, 3) incomplete and protective, or 4) incomplete and not protective.
Epiglottic inversion was rated as complete vs. incomplete.
Presence of cricopharyngeal bar was noted and was defined by a visual observation of a narrowing of the cricopharyngeal segment.
Statistical Analysis
Descriptive statistics were first conducted for overall swallowing safety and efficiency profiles based on DIGEST scores (measures of central tendency and frequency counts). Chi-squared analyses across binary outcomes (complete vs. incomplete epiglottic inversion) were conducted to identify associations with 1) airway safety and 2) bolus efficiency. Alpha was set at p<0.05.
Results
1. Global Pharyngeal Dysphagia Grade: DIGEST in OPMD Patients
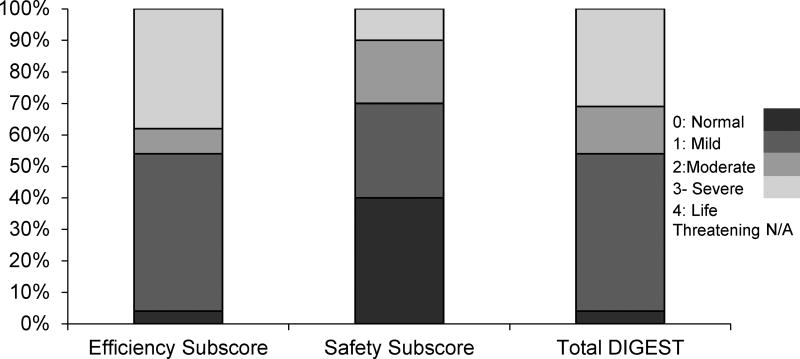
DIGEST scores revealed that 96% (n=21) of participants demonstrated pharyngeal dysphagia (i.e., total scores ≥1). Specifically, overall DIGEST scores indicated that only 4% (N=1) demonstrated normal pharyngeal swallowing function (i.e. DIGEST score=0), 50% (N=11) exhibited mild pharyngeal dysphagia (DIGEST score=1), 15% (N=3) moderate pharyngeal dysphagia (DIGEST score=2), and 31% (N=7) severe pharyngeal dysphagia (DIGEST score=3). No life-threatening severity levels were analyzed in this cohort (DIGEST score=4). DIGEST efficiency and safety subscores are delineated by severity scores and depicted in Figure 1.
Figure 1.
Stacked bar chart depicting the distribution of Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) Efficiency and Safety subscores, and Total DIGEST scores within the cohort.
2. Anatomical Observations during VFSE analyses
Presence of a cricopharyngeal bar was identified during VFSE analyses in 45% (N=10) of OPMD patients. No differences in demographics, airway safety or bolus efficiency indices were revealed between patients with or without a cricopharyngeal bar (p>.05).
3. Severity of pharyngeal dysphagia analyzed by bolus trial
Analysis of 134 bolus trials using binary outcomes to rate for swallow safety and efficiency (safe vs. unsafe, inefficient vs. efficient) revealed in rank order that approximately half (51%) of swallows in this cohort were characterized as safe but inefficient; 32% were both unsafe and inefficient; 16% were both safe and efficient; and 1% of trials classified as efficient but unsafe (see Table1). When stratified by bolus consistency, a total of 58 thin liquid trials, 43 nectar trials, 22 honey trials, 21 pudding trials and 19 solid consistency trials were administered during VFSE. Table 2 depicts all unsafe and inefficient analyzed swallows by bolus type.
Table 1.
Contingency table depicting swallowing safety and efficiency profiles across bolus types. Inefficient=Normalized Residue Ratio scores of ≥0.07 and ≥0.20 for valleculae and piriform sinus, respectively. Unsafe= Penetration-aspiration score > 2.
| Safe | Unsafe | |
|---|---|---|
| Efficient | 16% (N=21) | 1% (N=1) |
| Inefficient | 51% (N=69) | 32% (N=43) |
Table 2.
Airway safety and efficiency profiles expressed as % of unsafe/inefficient swallows, and stratified by bolus type. Unsafe=Penetration-Aspiration score ≥3; Inefficient= Normalized residue ratio: valleculae ≥ 0.07; piriform sinus ≥ 0.20.
| Bolus Type | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Thin | Nectar | Honey | Pudding | Solid | Total Unsafe/ Inefficient Swallows |
|
|
| ||||||
| Unsafe (%) | 35% | 37% | 15% | 9% | 4% | 54 |
| (n=19) | (n=20) | (n=9) | (n=5) | (n=1) | ||
|
| ||||||
| Inefficient (%) | 33% | 30% | 19% | 11% | 7% | 107 |
| (n=35) | (n=32) | (n=20) | (n=12) | (n=8) | ||
4. Mechanisms of bolus inefficiency
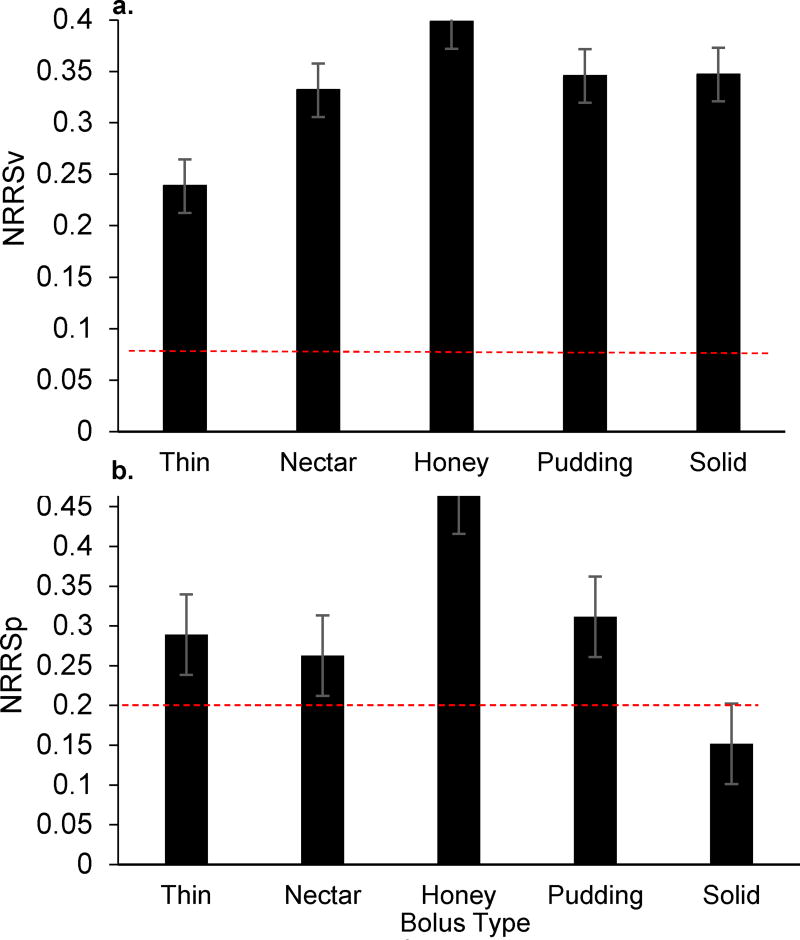
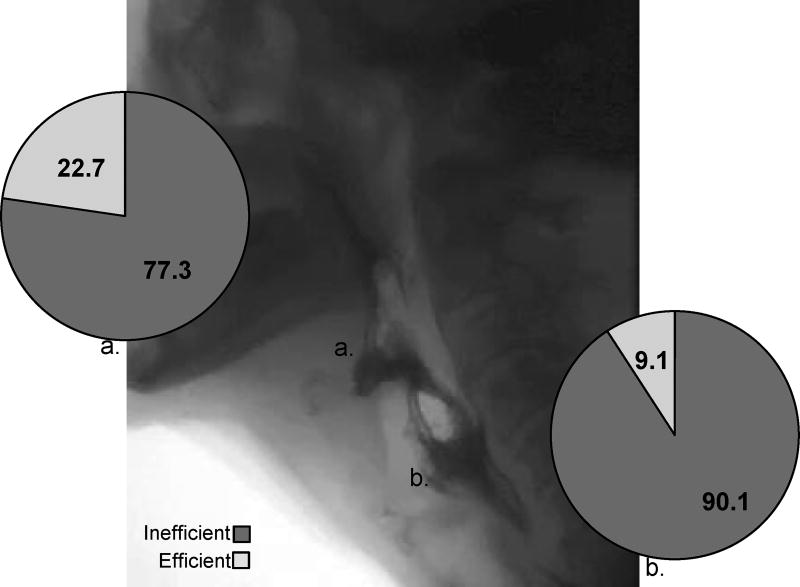
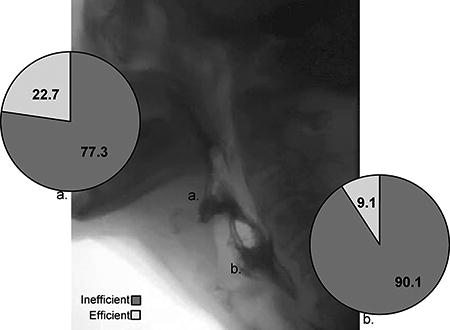
Of the 124 individual clips suitable for analysis of bolus efficiency, 86.3% (N=107) were characterized as inefficient (NRRSv ≥ 0.07; NRRSp ≥ 0.20). Specifically, mean NRRSv values across all bolus trials and consistencies were .385 (+/−.293) and mean NRRSp values were .436 (+/− .429) (Figure 2). When delineating profiles by specific anatomical site across each participant’s most severe bolus trial, 77.3% of patients demonstrated vallecular residue (NRRSv ≥0.07) and 90.1% piriform sinus residue (NRRSp ≥0.20) (see Figure 3). An association was observed between epiglottic inversion and bolus efficiency [χ2(1,N=136)=7.57, p<0.05] with a moderate effect size observed (Cramer’s V=.24), and incomplete epiglottic inversion noted to contribute to post-swallow residue in 95.1% of inefficient swallows.
Figure 2.
Bar graphs depicting mean Normalized Residue Ratio Score values for the a) valleculae and b) piriform sinus across bolus types. Dashed lines indicate established normative values for liquid thin only (NRRSv<0.07; NRRSp<0.20).
Figure 3.
A still frame of an OPMD videofluoroscopy depicting significant vallecular and piriform sinus residue accompanied by pie charts illustrating efficiency profiles delineated by anatomical region of the a) valleculae and b) piriform sinus across each participants most severe bolus trial.
Mechanisms of compromised airway protection
Of the 163 total swallows analyzed for airway safety, 54 swallows (33%) were unsafe with material entering the laryngeal vestibule (PAS ≥3). Of these unsafe episodes, 9 (17%) were classified as aspiration (PAS≥6). Specifically, episodes of aspiration were characterized as: material entering the airway below the level of the vocal folds with effective clearance (N=3, PAS=6), material entering the airway with ineffective effort to expel (N=1, PAS=7), and silent aspiration with no apparent effort to expel material from the airway (N=5, PAS=8). Compromised airway protection occurred across all timing zones (before vs. during vs. after), however, 93% (N=50) of penetration or aspiration occurred during the swallow, 5% (N=3) after the swallow and 1% (N=1) before the swallow. Aspiration episodes (PAS≥6, N=9) occurred with thin (N=5, 55.5%) or nectar thickened liquids (N=4, 44.5%), and all (100%) of aspiration events occurred during the swallow. An association between epiglottic inversion and airway safety was noted [χ2(1,N=136)=36.154, p<0.001] with complete epiglottic inversion occurring in 90.9% of swallows that had adequate airway protection and a large effect size (Cramer’s V=.516).
Discussion
In this group of 22 OPMD patients, swallowing was both unsafe and inefficient. Inefficient pharyngeal stage swallowing was noted in almost all (96.2%) of these OPMD individuals while airway safety was noted to be compromised in two-thirds (65.4%). Objective measures of post-swallow residue and swallowing safety supported the finding that efficient swallowing was more impacted than airway safety, resulting in significant post-swallow pharyngeal residue, in both the valleculae and piriform sinus for almost all patients studied. Timing of airway invasion occurred primarily during the swallow and was attributable to incomplete epiglottic inversion and thus, incomplete laryngeal vestibule closure.
Previous studies focusing on changes within cricopharyngeal muscle tissue in OPMD report muscle fibrosis and atrophy in this muscle, leading to impairments in muscle movement and coordination 18. Specifically, cricopharyngeal muscle biopsies of OPMD individuals indicate the presence of cytokines and growth factors associated with increased muscle fibrosis, atrophy, and impaired muscle regeneration compared to age and gender matched controls 8. Further, abnormal histological and morphological changes have been identified in the cell nuclei containing PABPN1 protein aggregates within symptomatic and asymptomatic muscle tissue, although the relationship between these aggregates and muscle function in OPMD is not fully known 19. Thus, bolus inefficiency is likely attributable to the targeted involvement of the primary muscle of the UES, the cricopharyngeus muscle, resulting in dystrophic and atrophic changes in muscle fiber composition contributing to impaired UES relaxation and extent and duration of opening 7, 8.
Although predilection of the cricopharyngeus has been the focus of previous study in OPMD, pharyngeal skeletal muscle is also impacted with similar findings of muscle fibrosis and atrophy 3. Thus, mechanisms crucial for complete bolus transport and adequate airway protection, including pharyngeal contraction, epiglottic inversion and arytenoid to base of epiglottis contact, are adversely impacted by limitations in movement and coordination. Identifying timing of airway invasion and subsequent aspiration events elucidates mechanistic impairments in swallow function and facilitates the use of rationale-based compensatory and treatment recommendations.
The results of the current study indicate physiological impairments in epiglottic inversion, and arytenoid to base of epiglottis approximation, contributing to incomplete laryngeal vestibule closure and subsequent airway invasion and post-swallow residue. Previous studies identified oral phase swallowing impairments characterized by reduced lingual pressure generation 20. As well, studies focusing on UES function have identified impairments in cricopharyngeal relaxation and hypertonicity of the UES 6. Analyses of specific mechanisms contributing to dysphagia throughout the oropharyngeal swallow using instrumental evaluation facilitates implementation of evidence-based treatment regimens, such as rehabilitative treatment recommendations and compensatory meal-time modifications. Considering these findings, appropriate compensatory strategies may include: 1) the effortful swallow to increase pressure generation of the base of tongue to posterior pharyngeal wall, pharyngeal constriction, laryngeal vestibule closure, and UES opening duration 21, 22 and 2) the supraglottic swallow to improve arytenoid to base of epiglottis approximation and laryngeal vestibule closure 23. Additionally, evidence-based rehabilitative strategies, such as the Mendelsohn maneuver, aim to improve extent and duration of UES opening and may facilitate bolus clearance 22.
Limitations of the current study include a small sample size and the cross-sectional manner in which data were collected and analyzed leading to inferences in the presentation of swallowing impairments. Additionally, exclusion of comprehensive timing and kinematic measures of swallow function due to inadequate frame rate capture during the videofluoroscopic swallow study. Future studies will further elucidate the pathophysiology of dysphagia in OPMD in order to develop more effective targeted treatments in a larger group of OPMD individuals studied in a longitudinal fashion to determine onset and development of specific swallowing impairments.
Conclusions
Swallowing efficiency and airway protection in OPMD patients are negatively impacted throughout the course of the disease. Currently, limited evidence is available to support specific dysphagia treatment recommendations for individuals with OPMD. Thus, continued research utilizing objective measurements obtained following instrumental swallowing evaluation to identify and characterize mechanistic impairments in swallow function is crucial. To determine the effectiveness of dysphagia management in OPMD, including cricopharyngeal dilatation and botulinum toxin injections, swallow function must be adequately described using objective outcome measures based on swallowing physiology. In turn, efficacious compensatory and rehabilitative strategies can be implemented based on specific mechanisms contributing to dysphagia.
Supplementary Material
Key Points.
Both swallowing efficiency and safety are affected in OPMD, with residue occurring in almost all patients and penetration/aspiration in almost two-thirds of OPMD patients in this cohort.
Aspiration occurred only with thin and nectar thickened liquids and during the swallow.
Physiological impairments including incomplete epiglottic inversion, and arytenoid to base of epiglottis approximation, were associated with airway invasion and post-swallow residue in OPMD.
Acknowledgments
We would like to acknowledge the participation of the OPMD patients who make this research possible.
Funding. This work was supported by NIH/NCRR/NCATS, CTSA (KL2TR000089) and the National Institute of Health T32 Neuromuscular Plasticity Training Grant.
Footnotes
Contributions.
SY and CR performed the research study and collected the data; LT analyzed the data; LT, EP and SY interpreted the results and wrote the manuscript.
References
- 1.Brais B. Oculopharyngeal muscular dystrophy: a polyalanine myopathy. Curr Neurol Neurosci Rep. 2009;9:76–82. doi: 10.1007/s11910-009-0012-y. [DOI] [PubMed] [Google Scholar]
- 2.Brais B, Bouchard JP, Xie YG, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18:164–167. doi: 10.1038/ng0298-164. [DOI] [PubMed] [Google Scholar]
- 3.Trollet CGT, Klein P, et al. Oculopharyngeal Muscular Dystrophy. GeneReviews. 2014 [Google Scholar]
- 4.Youssof S, Romero-Clark C, Warner T, Plowman E. Dysphagia-Related Quality of Life in Oculopharyngeal Muscular Dystrophy: Psychometric Properties of the SWAL-QOL Instrument. Muscle Nerve. 2016 doi: 10.1002/mus.25441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rommel N, Hamdy S. Oropharyngeal dysphagia: manifestations and diagnosis. Nat Rev Gastroenterol Hepatol. 2016;13:49–59. doi: 10.1038/nrgastro.2015.199. [DOI] [PubMed] [Google Scholar]
- 6.Castell JA, Castell DO, Duranceau CA, Topart P. Manometric characteristics of the pharynx, upper esophageal sphincter, esophagus, and lower esophageal sphincter in patients with oculopharyngeal muscular dystrophy. Dysphagia. 1995;10:22–26. doi: 10.1007/BF00261275. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu J, Lapointe G, Brassard A, et al. A pilot study on upper esophageal sphincter dilatation for the treatment of dysphagia in patients with oculopharyngeal muscular dystrophy. Neuromuscul Disord. 1997;7(Suppl 1):S100–104. doi: 10.1016/s0960-8966(97)00092-8. [DOI] [PubMed] [Google Scholar]
- 8.Gidaro T, Negroni E, Perie S, et al. Atrophy, fibrosis, and increased PAX7-positive cells in pharyngeal muscles of oculopharyngeal muscular dystrophy patients. J Neuropathol Exp Neurol. 2013;72:234–243. doi: 10.1097/NEN.0b013e3182854c07. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Torres A, Abrante Jimenez A, Rivas Infante E, Menoyo Bueno A, Tirado Zamora I, Esteban Ortega F. Cricopharyngeal myotomy in the treatment of oculopharyngeal muscular dystrophy. Acta Otorrinolaringol Esp. 2012;63:465–469. doi: 10.1016/j.otorri.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Logemann JA. Evaluation and Treatment of Swallowing Disorders. Pro-Ed. 1998 [Google Scholar]
- 11.Manjaly JG, Vaughan-Shaw PG, Dale OT, Tyler S, Corlett JC, Frost RA. Cricopharyngeal dilatation for the long-term treatment of dysphagia in oculopharyngeal muscular dystrophy. Dysphagia. 2012;27:216–220. doi: 10.1007/s00455-011-9356-y. [DOI] [PubMed] [Google Scholar]
- 12.Perie S, Eymard B, Laccourreye L, Chaussade S, Fardeau M, Lacau St Guily J. Dysphagia in oculopharyngeal muscular dystrophy: a series of 22 French cases. Neuromuscul Disord. 1997;7(Suppl 1):S96–99. doi: 10.1016/s0960-8966(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 13.Youssof S, Schrader R, Bear D, Morrison L. Hip flexion weakness is associated with impaired mobility in oculopharyngeal muscular dystrophy: A retrospective study with implications for trial design. Neuromuscular Disorders. 25:238–246. doi: 10.1016/j.nmd.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutcheson K, Barringer D, Knott JK, Lin H, Weber RS, Fuller C, Lazarus C, May AH, Patterson JM, Roe J, Starmer HM, Lewin JS. DYNAMIC IMAGING GRADE OF SWALLOWING TOXICITY (DIGEST): SCALE DEVELOPMENT AND VALIDATION. Dysphagia Research Society. 2015 doi: 10.1002/cncr.30283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson WG, Jr, Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: the normalized residue ratio scale. Dysphagia. 2013;28:167–177. doi: 10.1007/s00455-012-9426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molfenter SM, Steele CM. The relationship between residue and aspiration on the subsequent swallow: an application of the normalized residue ratio scale. Dysphagia. 2013;28:494–500. doi: 10.1007/s00455-013-9459-8. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 18.Trollet C, Gidaro T, Klein P, Perie S, Butler-Browne G, Lacau St Guily J. Oculopharyngeal Muscular Dystrophy. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): University of Washington, Seattle; 1993. University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved. [PubMed] [Google Scholar]
- 19.Tome FM, Fardeau M. Nuclear inclusions in oculopharyngeal dystrophy. Acta Neuropathol. 1980;49:85–87. doi: 10.1007/BF00692226. [DOI] [PubMed] [Google Scholar]
- 20.Palmer PM, Neel AT, Sprouls G, Morrison L. Swallow characteristics in patients with oculopharyngeal muscular dystrophy. J Speech Lang Hear Res. 2010;53:1567–1578. doi: 10.1044/1092-4388(2010/09-0068). [DOI] [PubMed] [Google Scholar]
- 21.Ohmae Y, Logemann JA, Kaiser P, Hanson DG, Kahrilas PJ. Effects of two breath-holding maneuvers on oropharyngeal swallow. Ann Otol Rhinol Laryngol. 1996;105:123–131. doi: 10.1177/000348949610500207. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman MR, Mielens JD, Ciucci MR, Jones CA, Jiang JJ, McCulloch TM. High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. 2012;27:418–426. doi: 10.1007/s00455-011-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of Voluntary Maneuvers on Tongue Base Function for Swallowing. Folia Phoniatrica et Logopaedica. 2002;54:171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.