Abstract
Background
The molecular changes that occur in the stomach that are associated with idiopathic gastroparesis are poorly described. The aim of this study was to use quantitative analysis of mRNA expression to identify changes in mRNAs encoding proteins required for the normal motility functions of the stomach.
Methods
Full thickness stomach biopsy samples were collected from non-diabetic control subjects that exhibited no symptoms of gastroparesis and from patients with idiopathic gastroparesis. mRNA was isolated from the muscularis externa and mRNA expression levels were determined by quantitative reverse transcriptase (RT)-PCR.
Key Results
Smooth muscle tissue from idiopathic gastroparesis patients had decreased expression of mRNAs encoding several contractile proteins, such as MYH11 and MYLK1. Conversely, there was no significant change in mRNAs characteristic of interstitial cells of Cajal (ICCs) such as KIT or ANO1. There was also a significant decrease in mRNA encoding platelet derived growth factor receptor α (PDGFRα) and its ligand PDGFB and in Heme oxygenase 1 in idiopathic gastroparesis subjects. In contrast, there was a small increase in mRNAs characteristic of neurons. Although there was not an overall change in KIT expression in gastroparesis patients, KIT expression showed a significant correlation with gastric emptying whereas changes in MYLK1, ANO1 and PDGFRα showed weak correlations to the fullness/satiety subscore of patient assessment of upper gastrointestinal disorders-symptom severity index scores.
Conclusions and Inferences
Our findings suggest that idiopathic gastroparesis is associated with altered smooth muscle cell contractile protein expression and loss of PDGFRα+ cells without a significant change in ICCs.
Keywords: gastroparesis, interstitial cells of Cajal, smooth muscle, fibroblasts, neurons, macrophages, transcription
Abbreviated abstract
qRT-PCR analysis revealed that smooth muscle tissue from idiopathic gastroparesis patients had decreased expression of mRNAs encoding several contractile and regulatory proteins, PDGFRalpha and its ligand PDGFB and Heme oxygenase 1. Conversely, there was no significant change in mRNAs characteristic of interstitial cells of Cajal (ICCs), such as KIT or ANO1. These findings suggest that idiopathic gastroparesis is most commonly associated with transcriptional changes that are indicative of altered smooth muscle cells and PDGFRalpha positive fibroblast cells rather than ICCs.
Introduction
Gastroparesis defined as delayed gastric emptying in the absence of a physical obstruction, is estimated to occur in about 1.8% of the adult population, with the majority of cases being in women.1, 2 Patients with gastroparesis have a marked decrease in their quality of life, with symptoms including nausea, vomiting, early satiety, bloating and abdominal pain. Treatment of gastroparesis is difficult as there are usually multiple symptoms to treat and the symptoms do not accurately reflect the underlying pathophysiology. Treatment of gastroparesis is further hampered by the lack of understanding of the underlying molecular changes that cause or contribute to the disease pathology. Of patients with gastroparesis approximately 36% are idiopathic 29% diabetic and 13% postsurgical.3, 4 As gastric emptying is dependent on an intricate interaction of neural cells, interstitial cells of Cajal (ICC), platelet derived growth factor α positive (PDGFRα+) fibroblasts and smooth muscle cells (SMCs), it is likely that defects in any of these cell types can contribute to this disease.5–10 ICC are the pacemaker cells in the stomach that transmit slow wave depolarization to the gastric smooth muscle cells.7, 11 The PDGFRα+ fibroblasts are a more recently recognized component of the electrical syncytium that controls gastric contractility. These cells transmit inhibitory neural signals to gastric smooth muscle cells.5 The major goal of our study was to use transcriptional profiling to identify changes in any of these cell types that may be associated with idiopathic gastroparesis.
Histopathological analyses have suggested decreases in numbers of ICCs and increases in numbers of CD45 and CD68 positive blood cells in gastric body biopsies from diabetic and idiopathic gastroparesis patients.12 A similar decrease in ICCs has been observed in mouse models of diabetes.13 In contrast, it has been reported that there are no changes in the PDGFRα+ cell number in either group of gastroparesis patients.6 It has also been proposed that an increased ratio of M1 relative to M2 macrophages may contribute to delayed gastric emptying in diabetic gastroparesis.14 In the previous human studies ICCs have been identified by their immunoreactivity with anti-KIT antibodies (CD117). KIT is a receptor tyrosine kinase found on the surface of ICC that is required for ICC survival and proliferation.12 Mouse models have suggested that diabetes-induced loss of ICC may result, at least in part, from decreased expression of KIT ligand (KITLG or Stem Cell Factor) from atrophied gastric SMCs.15 It is not clear if similar changes occur in humans with either diabetic or idiopathic gastroparesis.
All the previous human studies have relied on histological analyses, there have been no definitive quantitative studies that have identified molecular changes that could initiate or contribute to the dysmotility observed in patients with gastroparesis. The goal of the current study was to begin to address this gap in our knowledge by using real time RT-PCR to quantitate changes in mRNA expression that occur within the muscularis layer of the stomach of patients with idiopathic gastroparesis.
Materials and Methods
Clinical Assessment
All procedures were conducted under protocol approved by the Indiana University Institutional Review Board. Informed consents were obtained from control subjects and patients with idiopathic gastroparesis. Idiopathic gastroparesis was defined as patients with symptoms of gastroparesis ≥ 3 months with delayed gastric emptying by 4-hour gastric scintigraphy or gastric bezoar with undigested foods by upper endoscopy after an overnight fast. Gastric scintigraphy was performed following consumption of a low fat egg-white meal with imaging at 0, 2 and 4hrs as described previously.16, 17 Abnormal gastric emptying was defined as 2-hour retention ≥60% or 4-hour retention ≥10%. Symptom presentation was classified as vomiting-predominant, dyspepsia-predominant, or regurgitation-predominant, based on a clinical classification we previous reported.18 The Patient Assessment of Gastrointestinal Disorders-Symptom Severity Index (PAGI-SYM) was administered.19 PAGI-SYM is a 20-item severity scale from 0 (none) to 5 (very severe) with six subscales: nausea/vomiting, postprandial fullness/early satiety, bloating, heartburn/regurgitation, upper abdominal pain, and lower abdominal pain. The Gastroparesis Cardinal Symptom Index (GCSI) was defined as the average of the nausea/vomiting, postprandial fullness/early satiety and bloating PAGI-SYM subscale scores.20 Clinical severity were defined using a classification suggested by the Gastroparesis Clinical Research Consortium: grade 1, mild (symptoms relatively easily controlled and able to maintain weight and nutrition on a regular diet); grade 2, compensated (moderate symptoms with only partial control with use of daily medications, able to maintain nutrition with dietary adjustments); grade 3, gastric failure (refractory symptoms that are not controlled, with the patient having emergency department visits, frequent physician visits, or hospitalizations and/or inability to maintain nutrition via the oral route).3 Gastroparesis patients included in the study did not have diabetes as determined by normal fasting glucose or normal hemoglobin A1c (HbA1c) by blood test. We also excluded subjects with other systemic diseases such as surgery with vagal trauma, scleroderma, mixed connective tissue disorder and paraneoplastic syndrome.
Full-thickness stomach biopsies
Biopsies from the control group were obtained from non-diabetic individuals without gastroparesis symptoms that were undergoing bariatric weight loss surgery (normal HbA1c level < 1 month prior to surgery). Control and gastroparesis subjects were age and sex matched (Table 1). Surgical full-thickness biopsies from the idiopathic gastroparesis patients were obtained at the time of routine pathological analysis in patients with severe symptoms or at the time of gastric stimulator placement. In each case (control and gastroparesis patients), the biopsy was taken about 10cm from the pylorus on the anterior aspect midway between the greater and lesser curvatures of the stomach. All biopsies were obtained using linear cutting staplers. For sleeve gastrectomy subjects the sample was obtained after the stomach was removed from the abdomen, for all other subjects the samples were obtained laparoscopically. Tissues were collected into ice cold DMEM (Dullbeco’s modified eagle media) or RNAlater (Ambion) and immediately transferred to the investigator’s laboratory. Muscle, submucosal and mucosal layers were separated by dissection, flash frozen in liquid nitrogen and ground to a powder under liquid nitrogen prior to storage at −80°. A small amount of frozen muscle tissue powder (~150mg) was homogenized in Trizol (Invitrogen) and a Purelink Micro RNA isolation kit was used to isolate total RNA. Contaminating DNA was removed by on-column DNase treatment. The integrity and purity of the isolated RNA was determined using an Agilent Bioanalyzer. Samples used had an RNA integrity number of >7.
Table 1.
Clinical characteristics of patients with idiopathic gastroparesis
| Idiopathic Gastroparesis (n=16) | |
|---|---|
|
| |
| Duration of symptoms | 47.1±34.7 months |
| Gastroparesis severity | |
| Grade 3 | 16 (100%) |
| Symptoms presentation | |
| Vomiting-predominant | 9 (56%) |
| Dyspepsia-predominant | 2 (12.5%) |
| Regurgitation-predominant | 5 (31%) |
| Weight loss >10% of body weight past 6 months | 8 (50%) |
| Gastric emptying: % remaining at 4-hour* | 29±19% |
| Gastric emptying: % remaining at 2-hour* | 52±27% |
| PAGI-SYM Total Score* | 3.0±0.8 |
| PAGI-SYM Subscale Scores* | |
| Nausea/Vomiting | 3.3±1.2 |
| Post prandial fullness/Early satiety | 4.1±0.8 |
| Bloating | 3.2±1.6 |
| Upper Abdominal Pain | 3.1±1.6 |
| Lower Abdominal Pain | 2.3±1.6 |
| GCSI Total Score* | 3.6±0.9 |
Mean±SD
qRT-PCR
500ng of RNA was used in reverse transcription reactions (High Capacity RT-cDNA Kit, Life Technologies). Real time PCR was performed using Syber Green (Roche). Levels of mRNA expression were normalized to expression of TATA binding protein (TBP) as an internal control and are expressed relative to the mean values seen in samples from control patients. The primers used for qRT-PCR are listed in Supplemental Table 1. These primers detect mRNAs for the following smooth muscle-specific markers: MYH11 (smooth muscle myosin heavy chain), ACTA2 (smooth muscle α-actin), ACTG2 (smooth muscle γ-actin) and CCN1 (calponin).21; In addition primers used to detect mRNAs for ATPA4 (which encodes the H+/K+ pump) and GAST (gastrin) were used as markers of parietal and G cells, respectively, within the mucosa.22 Primers to detect mRNAs for TH (tyrosine hydroxylase), SLC18A2 (VMAT2, a monoamine transporter), VIP (vasoactive intestinal peptide), CHAT (choline O-acetyltransferase) and SLC18A3 (solute carrier protein 18A3, which transports acetyl choline into secretory vesicles) and UCHL1 (PGP9.5, ubiquitin carboxylterminal esterase L1) were used as neural markers.23, 24
Isolation and polarization of human macrophages
Whole blood (~8 mL) was collected from consenting healthy donors using Vacutainer CPT Mononuclear Cell Preparation Tubes (CPT; BD) containing sodium citrate. Tubes were processed according to the manufacturer’s instructions to separate peripheral blood mononuclear cells (PBMCs). PBMCs were washed twice in PBS (5 min at 500 × g) at room temperature before being resuspended in complete media (RPMI, 10%FBS, 1% penicillin-streptomycin, and 10 ng mL−1 recombinant human macrophage colony stimulating factor (M-CSF; [R&D Systems]). Isolated PBMCs (1x106 mL−1) were plated on 12 well tissue culture-treated plates in complete media. Complete media was refreshed every 48 hours. After 7 days, adherent M-CSF-derived cells were treated with 100 ng mL−1 lipopolysaccharide from E. coli 055:B5 (LPS; Sigma) and 5 ng mL−1 recombinant human interferon gamma (IFN-γ; R&D Systems) to induce M1 macrophage polarization or with 20 ng mL−1 recombinant human interleukin 4 (IL-4; R&D Systems) to induce M2 macrophage polarization. Cells were harvested and RNA extracted 24 hrs later.
Statistical analysis
Significant differences between the means values of the gastroparesis and control patients were determined using a two-tailed, Welch corrected student T-test in order to account for potential differences in the variance in values obtained from control and gastroparesis subjects. P values <0.05 were considered significant. Correlations between mRNA levels and clinical parameters were determined using Spearman correlations. Where significant correlations were found, linear regression analysis was performed in order to visualize the correlations graphically. For analysis in which we divided the gastroparesis patients into symptom predominant groups, one way Anova was performed using a Kruskal-Wallis test with a Duns Multiple Comparison post-test.
Results
Control full thickness biopsy samples of the stomach were obtained from 14 patients undergoing bariatric weight loss surgery, 5 patients from sleeve gastrectomy and 9 patients from Roux-en-Y bypass surgery. None of the 14 controls subjects had diabetes (normal HbA1c < 1 month prior to surgery). Patient samples were obtained from 16 patients with idiopathic gastroparesis, 6 patients during laparoscopic implantations of gastric electrical stimulator, 5 patients during surgical jejunostomy feeding tube placements (1 of these patients also had gastric electrical stimulator), and 6 patients during diagnostic laparoscopies with clinical intent of full-thickness biopsy of the stomach to look for underlying neuromuscular pathologies. None of the patients with gastroparesis had diabetes. The average ages of the control and gastroparesis patients were 42.8±7.8 years and 34.6±10.1 years, respectively. In addition, 11 of 14 (79%) control subjects were female compared to 14 out of 16 (87%) patients with gastroparesis. Finally, 12 of the 14 control patients were Caucasian and all of the gastroparesis group were Caucasian. Body mass index (BMI) was significantly greater in the control subjects (47.8±8.9 compared to 25.1±6.9). The clinical characteristics of the 16 patients with idiopathic gastroparesis are provided in Table 1.
Dissected muscle layer is free of contaminating mucosa
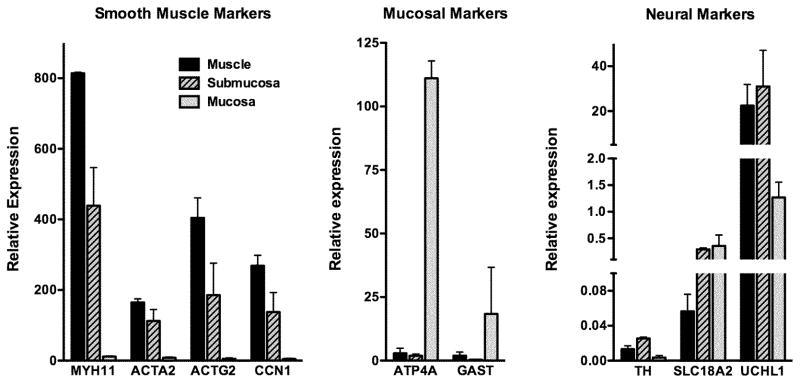
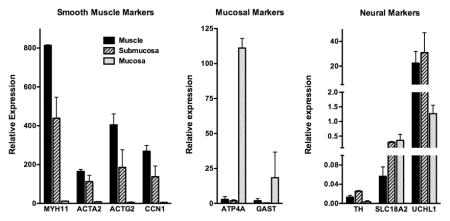
To confirm that we effectively separated the muscle, submucosal and mucosal layers during dissection we performed a focused quantitative real time reverse transcriptase-PCR (qRT-PCR) analysis of mRNAs whose expression are expected to be localized to specific cell types. This analysis revealed that the muscle layer has high levels of expression of the smooth muscle markers and low to undetectable levels of expression of the mucosal markers, as expected (Figure 1). In contrast, the mucosal layer has high levels of expression of mucosal markers ATPA4 and GAST and very low expression of smooth muscle markers (Figure 1). Neuronal markers are most abundant in the muscle and submucosal layers, consistent with the presence of the myenteric and submucosal nerve plexi, respectively, in these layers (Figure 1). The submucosal layer is rich in blood vessels, which likely accounts for the relatively high levels of expression of smooth muscle markers that were also observed in this layer, as these proteins would be expected to be expressed in vascular SMCs. These data suggest that the dissected muscularis layer is highly enriched with smooth muscle cells.
Figure 1. Separation of muscle, submucosa and mucosa layers from biopsies.
Full thickness biopsies of stomach were obtained from two control subjects and the muscle, submucosa and mucosa, separated as described in ‘Methods’. RNA was isolated from each layer and the expression of mRNAs encoding proteins characteristic of each layer was quantitated by qRT-PCR. Expression levels relative to an internal control mRNA encoding TATA binding protein (TBP) are indicated. The mean±SEM of two samples are shown.
Loss of PDGFRα+ fibroblasts but not ICCs in idiopathic gastroparesis
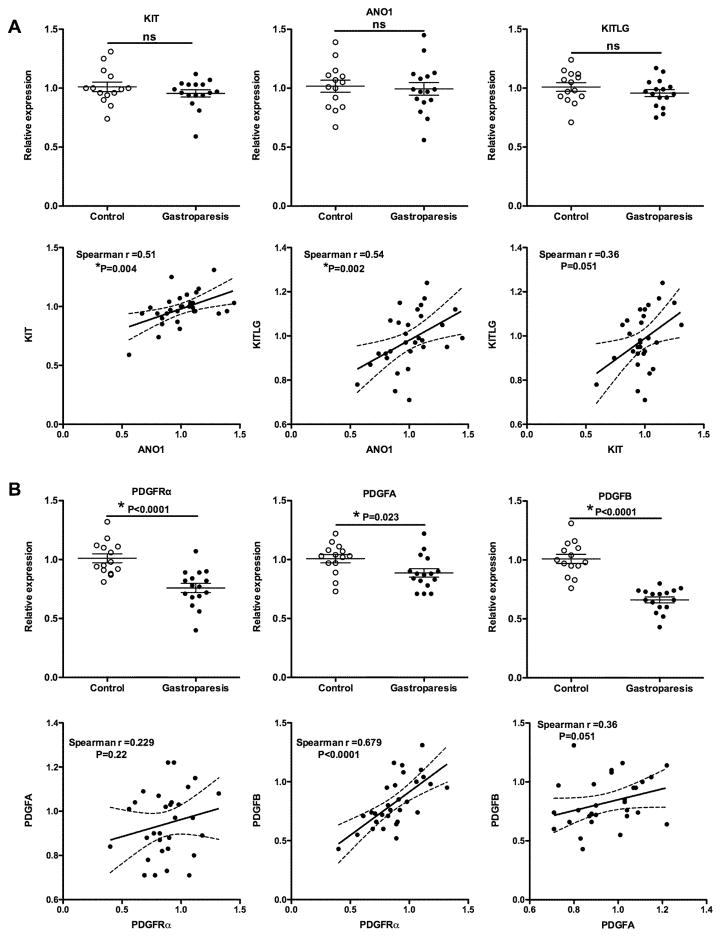
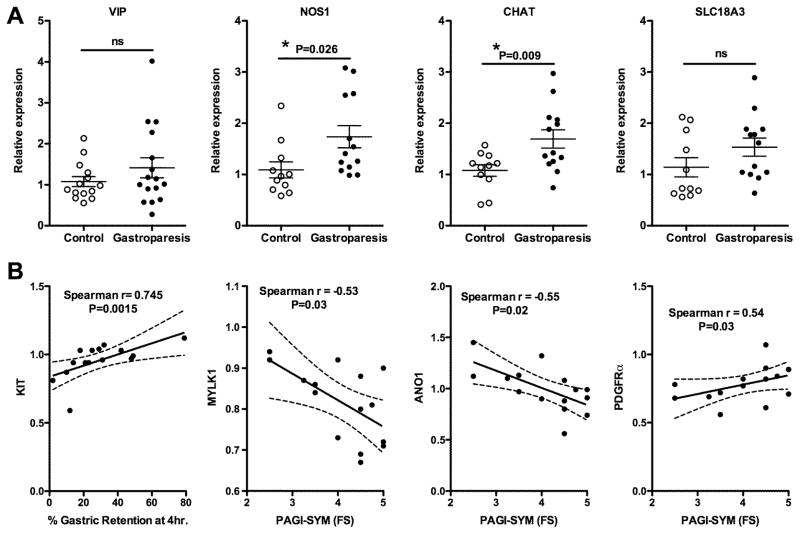
To determine if idiopathic gastroparesis is associated with loss of ICCs from the muscle layer we quantitated expression of mRNAs encoding ICC markers KIT and ANO1 as well as the KIT ligand (KITLG). This analysis revealed no significant change in expression of any of these markers in samples from idiopathic gastroparesis patients (Figure 2A). Spearman correlation analysis revealed a small but significant correlation between KIT and ANO1 expression levels, which would be expected if these mRNAs are indicative of ICC number (Figure 2A). There was also a weak, although significant correlation between ANO1 expression and KITLG expression, but the correlation between KIT and KITLG expression did not reach significance (Figure 2A). In contrast to the lack of changes in ICC markers, we observed a significantly decreased expression of mRNAs encoding PDGFRα and its ligands PDGFA and PDGFB, suggesting that there may be a loss of the PDGFRα-positive (PDGFRα+) fibroblasts (Figure 2B). The expression of PDGFRα mRNA displayed a strong positive correlation to PDGFB mRNA levels but no significant correlation to PDGFA mRNA levels using a Spearman correlation analysis (Figure 2B).
Figure 2. qRT-PCR analysis of mRNA encoding proteins characteristic of ICCs and PDGFRα+ fibroblasts.
Total RNA was isolated from the muscle layer of biopsies obtained from 14 control subjects and 16 subjects with idiopathic gastroparesis. qRT-PCR was used to measure the expression of each of the mRNAs indicated. Data presented were normalized to an internal control encoding TBP and are expressed relative to levels in the control subjects. Relative expression =2−ΔΔCt, where ΔΔCt = (CtGastroparesis-CtTBP) – (Ctcontrol-CtTBP). Each circle represents an individual subject with the control subjects represented as open circles and the gastroparesis subjects as closed circles. The mean±SEM are also indicated. Student T-tests were used to identify statistical significance. ns, not significant. Data from both control and gastroparesis subjects, were analyzed by spearman correlation and linear regression analyses to examine the relationship between expression of KIT, ANO1 and KITLG (A) and PDGFRα and its ligands PDGFA and PDGFB (B). Plots of the linear regression analysis are shown together with the indicated Spearman r values and P values.
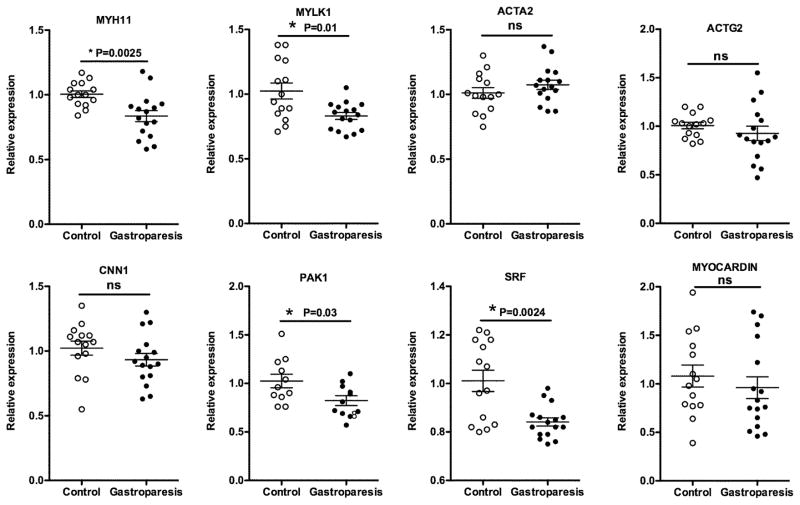
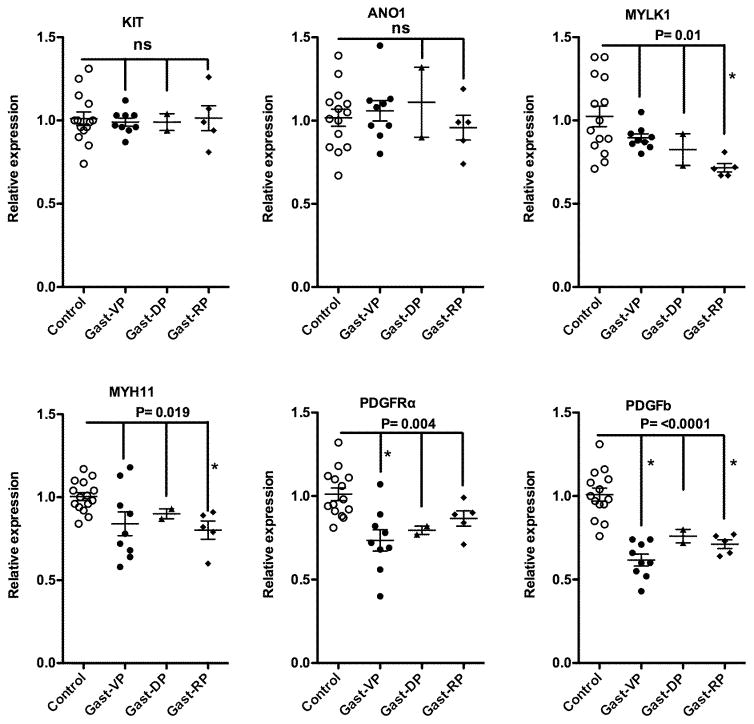
Attenuated expression of mRNAs encoding some proteins characteristic of differentiated contractile SMCs in gastroparesis subjects
To determined if alterations in SMCs may also occur in gastroparesis patients we examined expression of mRNAs encoding proteins characteristic of differentiated contractile SMCs. This analysis revealed a specific decrease in expression of MYH11, MYLK1 and PAK1 without any significant change in ACTA2, ACTG2 or CCN1 (Figure 3). Transcription of both MYLK1 and MYH11 is known to be dependent on SRF complexes, whose activity can be modulated by myocardin.25–27 We thus analyzed the expression of mRNAs encoding these proteins and found that the changes in mRNAs encoding contractile proteins were accompanied by significantly decreased expression of SRF but not myocardin transcripts (Figure 3). The expression levels of MYLK and MYH11 transcripts were significantly correlated with expression of SRF transcripts, across all of our human samples (Figure 4).
Figure 3. qRT-PCR analysis of mRNA encoding proteins and transcription factors characteristic of contractile SMCs.
Total RNA was isolated from the muscle layer of biopsies obtained from 14 control subjects and 16 subjects with idiopathic gastroparesis and analyzed by qRT-PCR as described in figure 2.
Figure 4. Relationship between expression of contractile proteins and the transcription factors that regulate their expression.
Data from both control and gastroparesis subjects, shown in figure 3, were analyzed by Spearman’s correlation and linear regression analyses to examine the relationship between expression of MYKL, MYH11 and transcription factors SRF and myocardin. These analyses revealed a highly significant positive correlation between the expression of SRF and MYLK1 and MYH11.
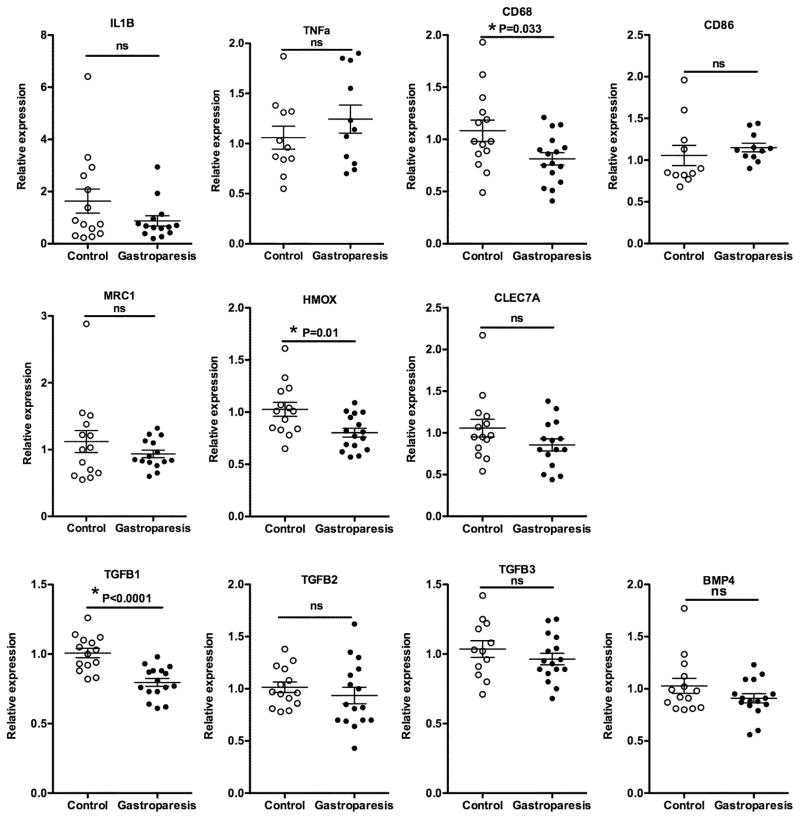
Reduced expression of mRNAs encoding anti-inflammatory proteins in gastroparesis subjects
To evaluate potential changes in inflammation and macrophage activation in idiopathic gastroparesis we examined the expression of mRNAs encoding inflammatory cytokines IL1β and TNFα, markers of macrophages, CD68, CD86, MRC1(CD206), HMOX1(HO-1) and CLEC7A and anti-inflammatory cytokines such as TGFβ (Figure 5). In order to try to distinguish M1 from M2 macrophages, we first examined the relative expression of several macrophage markers in human macrophages derived from peripheral blood mononuclear cells (PBMCs) from healthy donors. Macrophages were polarized into M1 or M2 macrophages as described in methods. This analysis identified MRC1, MRC2, HMOX1 and CLEC7A as relatively selective markers of M2 macrophages compared to M1 macrophages (Supplemental Figure 1). However, each of these markers is also expressed at relatively high levels in unstimulated macrophages (M0; Supplemental Figure 1). Of the genes examined, only TNFα was selectively expressed by M1 macrophages as compared to M2 macrophages (Supplemental Figure 1). Analysis of mRNA isolated from the gastric muscle layer of control and gastroparesis subjects did not reveal any significant changes in mRNAs encoding proinflammatory cytokines IL1β and TNFα, although we did observe decreased expression of the immunosuppressive cytokine TGFβ1 (Figure 5). We also observed decreased expression of the pan monocyte/macrophage marker CD68 and decreased expression of HMOX1, which encodes heme oxygenase 1 (HO-1), without any significant change in other M2 macrophage selective markers, CLEC7A and MRC1 (Figure 5).
Figure 5. qRT-PCR analysis of mRNA encoding proteins related to inflammation and macrophage polarity.
Total RNA was isolated from the muscle layer of biopsies obtained from 14 control subjects and 16 subjects with idiopathic gastroparesis and analyzed by qRT-PCR as described in figure 2.
Altered expression of mRNAs encoding neural markers in gastroparesis subjects
Previous studies have observed decreases in inhibitory nerves in individual patients with gastroparesis, although as a group there was no statistical difference between control and gastroparesis patients.12 To evaluate neuronal changes in our samples we assessed levels of mRNAs encoding markers of inhibitory nerves, VIP (vasoactive intestinal peptide), NOS1 (neural nitric oxide synthase), and excitatory nerves, CHAT (choline O-acetyltransferase) and SLC18A3 (solute carrier protein 18A3, (VAChT) which transports acetyl choline into secretory vesicles)(Figure 6A). This analysis revealed a small but significant increase in NOS1 and CHAT mRNA in samples from gastroparesis patients as compared to controls (Figure 6A).
Figure 6. qRT-PCR analysis of mRNA encoding proteins related to neurons and correlations between mRNA expression and clinical symptoms.
A. Total RNA was isolated from the muscle layer of biopsies obtained from 14 control subjects and 16 subjects with idiopathic gastroparesis and analyzed by qRT-PCR as described in Figure 2. B. Linear regression analysis of the indicated mRNAs and PAGI-SYM subscores, or gastric retention, together with the Spearman r values and P values obtained from correlation analysis shown in Supplemental Table 2.
Molecular changes correlate with PAGI-SYM subscores
The average percentage of food remaining in the stomachs of our gastroparesis patients 4 hours after ingestion of a standard low fat egg white meal was 29±19% (Table 1). This large variability in gastric emptying allowed us to use Spearman correlation analysis to determine if any of the observed molecular changes correlate with impairment of gastric emptying. This analysis revealed a significant positive correlation between KIT and delayed gastric emptying (Spearman r value of 0.745, Figure 6B) but no correlation between expression of ANO1 or markers of PDGFRα+ fibroblasts, SMCs, inflammatory cell or neural markers and gastric retention (P and r values are shown in Supplemental Table 2). Using a similar approach we determined if changes in markers of SMC (MYLK, MYH11), ICC (KIT, ANO1), PDGFRα+ fibroblasts (PDGFRα, PDGFb), immune cells (MRC1, MHOX) and neurons (NOS1, SLC18A3) correlated with GCSI scores, or PAGI-SYM scores. This analysis revealed a significant correlation between the PAGI-SYM Fullness/Satiety subscores and expression levels of MYLK1, ANO1 and PDGFRα (Supplemental Table 2, with Spearman r values of −0.53, −0.55 and 0.54, respectively, Figure 6B). As our data revealed significant heterogeneity within the gastroparesis patient population we determined if this heterogeneity may be due to different groups of patients that could be identified based on their predominant symptoms (vomiting predominant, VP; dyspepsia predominant, DP; or regurgitation predominant, RP; Table 1). However, when we separated patients based on these predominant symptoms we did not observe any significant differences between the symptom groups (Figure 7).
Figure 7. qRT-PCR analysis of mRNA expression segregated based on symptom predominant phenotype.
Graphs shown are replots of the mRNA expression data shown in Figures 2 and 3, in which the gastroparesis subjects were divided into those whose symptoms were vomiting predominant (VP), dyspepsia predominant (DP) or regurgitation predominant (RP) (Table 1). A one way Anova, using a Kruskal-Wallis test with a Duns Multiple Comparison post-test was performed to evaluate significant differences from control subjects and between gastroparesis groups. P values representing overall differences of the gastroparesis subjects from control subjects are shown, asterisks indicate individual groups that were significantly different from the controls, as determined by the Duns Multiple Comparison test. None of the gastroparesis groups were found to be significantly different from each other.
Discussion
qRT-PCR analysis of RNA isolated from the muscularis layer of gastric biopsies obtained from patients with idiopathic gastroparesis and control subjects revealed changes in mRNAs that would indicate that idiopathic gastroparesis may be associated with changes in PDGFRα+ fibroblasts and SMCs rather than a loss of ICCs. These findings are in contrast to previously published immunohistological studies which showed decreased numbers of ICCs but no change in the number of PDGFRα+ fibroblasts in idiopathic gastroparesis subjects.6, 12 In the current study, we assessed both KIT and ANO1 as markers of ICCs. KIT is a receptor tyrosine kinase expressed on ICCs that is required for their survival. Antibodies to KIT were used in the previous immunohistological studies to quantitate ICC numbers.12 ANO1 encodes a calcium regulated chloride channel that is specifically expressed in ICC and required for slow wave generation in the stomach.28 Previous studies have shown that ANO1 positive ICCs are also decreased in diabetic gastroparesis and this is associated with altered splicing of ANO1 resulting in the generation of an ANO1 transcript lacking exons 1 and 2 that encodes a protein with altered channel activity.29 The qRT-PCR primers used in our study do not distinguish these two splice variants and thus represent total ANO1 mRNA levels. Although we did not observe any significant changes in either KIT or ANO1 mRNA expression in idiopathic gastroparesis patients compared to controls when we compared the relative expression of these two mRNAs in all samples we did observe a statistically significant correlation between the two, which would be expected as they are both markers of ICCs (Figure 2A). Consistent with the lack of changes in ICC markers we also did not observe any significant change in KITLG in the idiopathic gastroparesis patient samples (Figure 2A). However, we did observed a positive correlation between ANO1 and KITLG expression which would support a role of KITLG in maintaining ICCs. Similarly, we observed a strong correlation between PDGFRα and PDGFB mRNA levels across all samples (Figure 2B). This correlation between receptor and ligand may suggest that signaling through the PDGFRα receptor is required to maintain the intramuscular population of PDGFRα+ fibroblasts rather than both receptor and ligand being expressed by the same cells. This would be consistent with the known role of PDGFB in promoting cell proliferation.30, 31
Analysis of mRNAs encoding SMC contractile proteins would suggest that there is not a generalized atrophy of SMCs in most patients with idiopathic gastroparesis but rather there are decreases in mRNAs encoding specific contractile and regulatory proteins (Figure 3). In particular we observed significant decreases in mRNAs encoding smooth muscle myosin heavy chain (MYH11), myosin light chain kinase (MYLK1) and p21 activated kinase 1 (PAK1) (Figure 3). As myosin (MYH11) is required for smooth muscle contraction and phosphorylation of myosin light chain subunit by myosin light chain kinase (MYLK1) is required to stimulate myosin’s ATPase activity in response to contractile agonists, decreases in mRNAs encoding these proteins would be expected to directly impair contractility.32 Similarly, PAK1 also phosphorylates myosin light chains, in addition to the myosin phosphatase targeting subunit MYPT1, to promote smooth muscle contractility. 33 Studies in mice have shown that decreased expression of Myh11, Mylk1 or PAK1 each individually result in impaired SMC contractility and GI motility.25, 32, 34 SRF- myocardin complexes regulate transcription of many smooth muscle contractile proteins including both MYH11 and MYLK1. 25, 26, 35, 36 Although we did not observe a significant change in myocardin mRNA in samples from patients with idiopathic gastroparesis we did see a significant decrease in SRF mRNA (Figure 3). We also noted a tight correlation between levels of SRF and MYLK1 and MYH11 mRNAs (Figure 4), which would be consistent with previous studies that showed that expression of the MYLK1 gene is dependent on an SRF binding site in its first intron25 and that expression of MYH11 is also SRF-dependent.26 Unexpectedly, the decrease in SRF did not result in a significant attenuation of expression of other contractile proteins that are also SRF dependent. This may reflect differences in the sensitivity of each of the genes to levels of SRF.
Immunohistological studies have suggested that in diabetic gastroparesis a defect in M2 macrophages may be contributing to the disease pathology.14, 37 Alternatively activated M2 macrophages express high levels of heme oxygenase-1 and can thus protect against oxidative stress.38 Although our qRT-PCR analysis did not reveal an overall decrease in M2 macrophages as determined by M2 selective markers MRC1 and CLEC7A we did observe a significant decrease in HMOX1 mRNA that encodes heme oxygenase-1 protein (Figure 5). This finding would suggest that similar to subjects with diabetic gastroparesis, subjects with idiopathic gastroparesis may also be more susceptible to oxidative damage. However, this does not appear to result in marked damage to neurons, to affect stomach motility, as mRNA encoding markers of both inhibitory and excitatory neurons were slightly elevated in gastroparesis patients (Figure 6A). The significance of the elevation in both inhibitory and excitatory neural markers is not immediately apparent, but could represent compensatory changes involving increased neural proliferation/ differentiation that have occurred as a consequence of the disease. This neural plasticity is consistent with the findings of a recent study showing that in adult mice, intestinal neurons are continually dying and being replaced with new neurons.39
Despite the numerous significant changes in mRNA expression that we observed in the stomach muscularis layer of patients with idiopathic gastroparesis, it is not clear how these changes relate to patient symptoms and underlying pathology. Although it is readily apparent how many of the changes could affect gastric motility we did not find significant correlations between individual mRNA expression and gastric emptying (Supplemental Table 2), with the exception of KIT (Figure 6B). Changes in most markers of ICCs, PDGFRα+ fibroblasts, neurons, SMCs or immune cells did not correlate with 4 hour gastric retention values (Supplemental Table 2). The positive correlation we observed between KIT and gastric retention (Spearman r value of 0.745, Figure 6B) is rather puzzling, given that the gastroparesis patients as a group did not have significantly different levels of KIT expression compared to control subjects (Figure 2A). This positive correlation would suggest that higher KIT levels are associated with decreased gastric motility, which is contrary to the expected result.40, 41 There are a number of possible explanations for this observation. As we did not observe an overall decrease in KIT levels in gastroparesis patients compared to control subjects this statistical correlation between the small variations in KIT levels and gastric emptying may not be biologically meaningful. Alternatively, as we did not observe a similar correlation between ANO1 and gastric emptying, and both KIT and ANO1 are markers of ICC, this would suggest that gastric emptying is not correlating to ICC number but more specifically with KIT expression. One possible basis for this is that KIT is not exclusively expressed on ICC but is also expressed on several hematopoietic cells, including mast cells.42 The positive correlation between KIT expression and delayed gastric emptying could thus represent a correlation between increased numbers of mast cells as compared with ICC. We observed significant negative correlations between MYLK1 and ANO1 expression and the fullness/satiety PAGI-SYM subscore (Figure 6B). This suggests that these symptoms are associated with decreased SMC contractility and / or altered activity of ICCs. Conversely, PDGFRα receptor levels showed a significant, although weak, positive correlation with the fullness/satiety PAGI-SYM subscore (Figure 6B). This would suggest that the higher the PDGFRα receptor expression the worse the gastric emptying. This may reflect the role of PDGFRα+ fibroblasts in mediating inhibitory neural input to SMCs, which would be likely to attenuate contractility.
Despite being able to observe significant changes in mRNA expression between the gastroparesis and control subjects our data also revealed significant heterogeneity within the gastroparesis patient population. The different symptom predominant groups of patients do not seem to account for this heterogeneity (Figure 7). Our analyses identified several individual patients that had unique changes in mRNA expression which may provide insight into the specific pathophysiology of these patients. For example, one patient had markedly lower expression of ICC markers and had one of the lowest levels of KITLG expression, suggesting that ICC loss may be contributing to this patients pathology. In contrast, another patient had very low levels of expression of several contractile proteins which would be indicative of a more generalized SMC atrophy in this subject. These findings highlight the need for a more global analysis of the molecular changes that occur in the stomach of subjects with idiopathic gastroparesis in order to be able to design a more personalized rational therapeutic approach for each individual patient.
Although our qRT-PCR data suggest that idiopathic gastroparesis may be associated with changes in PDGFRα+ fibroblasts and SMCs rather than a loss of ICCs, this interpretation must be viewed within the limitations of our study. First, this conclusion is inferred from changes in mRNA expression rather than directly counting the cells themselves within the stomach muscularis. However, this may be both a weakness and a strength of our study as the muscle tissue from our biopsy samples is frozen and ground to a powder before isolation of mRNA this generates a very homogeneous sample. To obtain similar homogeneity using immunohistology it is necessary to analyze a large number of sections taken from many different regions of the biopsy. Counting cells using immunohistology is also greatly affected by the orientation of the sections relative to the different muscle layers. The homogeneity of our sample does however, preclude us from determining in which muscle layer observed changes occur. A second limitation of our study is that the control patient population used in the study consisted of obese patients undergoing bariatric weight loss surgery. This raises the possibility that obesity itself may be affecting the cell populations or cell functions which could mask some of the effects of gastroparesis or could in itself be causing some of the changes we observed. Although this is a possibility, this is unlikely to explain why conclusions from our qRT-PCR analysis differ from those reported based on immunohistology, as these previous studies also used obese control subjects. 6, 12, 40 Unfortunately it is not ethically feasible to obtain full thickness stomach biopsies from any other routinely available patient population, hence identifying possible obesity induced changes will await future studies in which a sufficiently large cohort of other control subjects are identified and analyzed. Finally it is important to note that as all of the gastroparesis patients we analyzed have well established disease it is not possible to determine if the alterations in mRNA expression are causal or a consequence of the disease.
In summary, the data described in this study provide the first detailed quantitative analysis of molecular changes that occur in the stomach of patients with idiopathic gastroparesis. Results from our studies suggest that changes in specific SMC contractile proteins may contribute to the dysmotility seen in idiopathic gastroparesis. Additional changes in numbers of PDGFRα+ fibroblasts may be further contributing to the dysmotility. The studies also highlight the potential of using molecular profiling to better identify the underlying pathology of individual gastroparesis patients which can then be used to design a more rational personalized therapeutic strategy.
Supplementary Material
Total RNA was isolated from unstimulated (M0) PBMC-derived macrophages, M1 polarized macrophages (100ng mL−1 LPS and 5ng mL−1 INFγ) or M2 polarized macrophages (20ng mL−1 IL4). Expression of the indicated mRNAs was determined after 24hrs by qRT-PCR. Expression levels relative to an internal control mRNA encoding TATA binding protein (TBP) are indicated.
Key Points.
Idiopathic gastroparesis is a debilitating disease whose etiology is poorly defined. We aimed to identify transcriptional changes that may contribute to disease pathology.
Smooth muscle tissue from idiopathic gastroparesis patients had decreased expression of mRNAs encoding several contractile and regulatory proteins, PDGFRα and its ligand PDGFB and Heme oxygenase 1. Conversely, there was no significant change in mRNAs characteristic of interstitial cells of Cajal (ICCs), such as KIT or ANO1.
Our findings suggest that idiopathic gastroparesis is most commonly associated with transcriptional changes that are indicative of altered smooth muscle cells and PDGFRα+ fibroblast cells rather than ICCs.
Acknowledgments
Funding
The study was supported by a Project Development Team within the ICTSI NIH/NCRR Grant Number UL1TR001108.
Abbreviations
- ICC
Interstitial cells of Cajal
- SMC
smooth muscle cells
- PDGFRα
platelet derived growth factor receptor alpha
- KITLG
kit ligand
Footnotes
Disclosures
Competing interests: all of the authors attest that they have no competing interests.
Author contributions:
BPH conceived and directed the study, performed experiments, analyzed data and wrote the manuscript; AMH performed experiments; AG coordinated sample collection and obtained patient consents; SG performed qRT-PCR analysis, ANA collected surgical biopsies; JNC collected surgical biopsies; MTI helped establish sample collection protocol, reviewed pathology data and reviewed the manuscript; TN performed clinical analysis of patients and identified patients for the study; DLM provided polarized human macrophages; JMW conceived study, performed clinical analysis of patients, identified patients for the study and edited manuscript.
References
- 1.Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34–42. doi: 10.5056/jnm.2012.18.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–33. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–15. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 5.Baker SA, Hennig GW, Salter AK, et al. Distribution and Ca(2+) signalling of fibroblast-like (PDGFR(+)) cells in the murine gastric fundus. J Physiol. 2013;591:6193–208. doi: 10.1113/jphysiol.2013.264747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover M, Bernard CE, Pasricha PJ, et al. Platelet-derived growth factor receptor alpha (PDGFRalpha)-expressing “fibroblast-like cells” in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil. 2012;24:844–52. doi: 10.1111/j.1365-2982.2012.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 8.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602–11. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]
- 9.Bhetwal BP, An C, Baker SA, et al. Impaired contractile responses and altered expression and phosphorylation of Ca(2+) sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil. 2013;34:137–49. doi: 10.1007/s10974-013-9341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu T, Zheng Y, Wang Y, et al. Advanced glycation end products interfere with gastric smooth muscle contractile marker expression via the AGE/RAGE/NF-kappaB pathway. Exp Mol Pathol. 2016;102:7–14. doi: 10.1016/j.yexmp.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Ordog T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–69. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–85. e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–9. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 14.Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis--the missing link? Neurogastroenterol Motil. 2015;27:7–18. doi: 10.1111/nmo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 17.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 18.Harrell SP, Studts JL, Dryden GW, et al. A novel classification scheme for gastroparesis based on predominant-symptom presentation. J Clin Gastroenterol. 2008;42:455–9. doi: 10.1097/MCG.0b013e31815ed084. [DOI] [PubMed] [Google Scholar]
- 19.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–49. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 20.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–50. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 21.Herring BP, Hoggatt AM, Griffith SL, et al. Inflammation and vascular smooth muscle cell dedifferentiation following carotid artery ligation. Physiol Genomics. 2017;49:115–126. doi: 10.1152/physiolgenomics.00095.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi E, Roland JT, Barlow BJ, et al. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711–20. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XY, Sanders KM, Ward SM. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res. 2000;302:331–42. doi: 10.1007/s004410000272. [DOI] [PubMed] [Google Scholar]
- 24.Pasricha PJ, Pehlivanov ND, Gomez G, et al. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Zhang W, Lu X, et al. Regulation of 130-kDa smooth muscle myosin light chain kinase expression by an intronic CArG element. J Biol Chem. 2013;288:34647–57. doi: 10.1074/jbc.M113.510362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zilberman A, Dave V, Miano J, et al. Evolutionarily conserved promoter region containing CArG*-like elements is crucial for smooth muscle myosin heavy chain gene expression. Circ Res. 1998;82:566–75. doi: 10.1161/01.res.82.5.566. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Kitchen CM, Streb JW, et al. Myocardin: a component of a molecular switch for smooth muscle differentiation. Journal of Molecular and Cellular Cardiology. 2002;34:1345–56. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 28.Hwang SJ, Blair PJ, Britton FC, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzone A, Bernard CE, Strege PR, et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem. 2011;286:13393–403. doi: 10.1074/jbc.M110.196089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czochra P, Klopcic B, Meyer E, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–28. doi: 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Stanzel RD, Lourenssen S, Nair DG, et al. Mitogenic factors promoting intestinal smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2010;299:C805–17. doi: 10.1152/ajpcell.00086.2010. [DOI] [PubMed] [Google Scholar]
- 32.He WQ, Peng YJ, Zhang WC, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008;135:610–20. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu J, Pham NT, Olate N, et al. Biphasic regulation of myosin light chain phosphorylation by p21-activated kinase modulates intestinal smooth muscle contractility. J Biol Chem. 2013;288:1200–13. doi: 10.1074/jbc.M112.370718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoover WC, Zhang W, Xue Z, et al. Inhibition of p21 activated kinase (PAK) reduces airway responsiveness in vivo and in vitro in murine and human airways. PLoS One. 2012;7:e42601. doi: 10.1371/journal.pone.0042601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin F, Hoggatt AM, Zhou J, et al. 130-kDa smooth muscle myosin light chain kinase is transcribed from a CArG-dependent, internal promoter within the mouse mylk gene. Am J Physiol Cell Physiol. 2006;290:C1599–609. doi: 10.1152/ajpcell.00289.2005. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida T, Sinha S, Dandre F, et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–64. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 37.Bernard CE, Gibbons SJ, Mann IS, et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil. 2014;26:1275–84. doi: 10.1111/nmo.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–409. 2409 e1. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni S, Micci MA, Leser J, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grover M, Bernard CE, Pasricha PJ, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531–9. e249. doi: 10.1111/j.1365-2982.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–81. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total RNA was isolated from unstimulated (M0) PBMC-derived macrophages, M1 polarized macrophages (100ng mL−1 LPS and 5ng mL−1 INFγ) or M2 polarized macrophages (20ng mL−1 IL4). Expression of the indicated mRNAs was determined after 24hrs by qRT-PCR. Expression levels relative to an internal control mRNA encoding TATA binding protein (TBP) are indicated.