Abstract
BACKGROUND/OBJECTIVES
Combination hepatic artery infusion (HAI) and systemic (SYS) chemotherapy for unresectable CRLM results in high tumor-response rates. This study represents an update of long-term survival and conversion to resectability in patients with unresectable CRLM treated with HAI and SYS chemotherapy in a phase II study.
METHOD
The primary endpoint was complete resection. Multivariate and landmark analysis assessed the effect of complete resection on progression-free (PFS) and overall survival (OS).
RESULTS
From 2007 to 2012, 64 patients with median of 13 tumors were enrolled; 67% had prior chemotherapy. 33 patients (52%) were converted to resection. Median follow-up among survivors was 81 months. Median PFS and OS were 13 and 38 months, respectively, with 5-year-OS of 36%. Chemotherapy-naïve patients had 5-year-OS of 51%. Conversion to resection was the only independent factor prognostic of improved PFS and OS. Nine of 64 patients (14%) are NED (5 since initial resection, 3 after resection of recurrent disease, 1 from chemotherapy alone) at median follow-up of 86 months from treatment initiation, and 72 months from last operative intervention.
CONCLUSION
Combination HAI and SYS is an effective therapy for high-volume unresectable CRLM, resulting in a high rate of resection, long-term survival and the potential for cure.
TRIAL REGISTRATION
#NCT00492999, https://clinicaltrials.gov/ct2/show/NCT00492999
Keywords: colorectal liver metastases, hepatic artery infusion chemotherapy, hepatectomy
INTRODUCTION
Colorectal cancer is the third most commonly diagnosed cancer worldwide, with over one million new cases annually, of which nearly 150,000 occur in the United States[1, 2]. Thirty-five to 55% of colorectal cancer patients will develop liver metastases, which is often the only site of distant disease [3–5]. Complete resection of colorectal liver metastases (CRLM) is the only potentially curative treatment option and is associated with prolonged survival [6, 7]. Unfortunately, up to 85% of patients with CRLM are unresectable at presentation [8–10]. With current systemic chemotherapy options, a small minority of these patients will have sufficient response to allow complete resection and clearance of disease [7, 11, 12].
Hepatic artery infusion (HAI) chemotherapy allows precise delivery of high-dose regional hepatic chemotherapy. Taking advantage of the anatomic dependence of metastatic liver tumors on hepatic arterial blood supply, HAI chemotherapy effectively delivers a high-concentration of drug to the liver disease, and minimizes any adverse effect on normal parenchyma[9, 13, 14]. 5-fluoro-2-deoxyuridine (FUDR) is a particularly suitable chemotherapeutic drug for intrahepatic delivery, with a high first-pass hepatic extraction of 94–99% [15]. The limited systemic exposure of HAI FUDR provides the opportunity for combinatory regimens with systemic agents, which has been demonstrated to be safe and effective, even in patients with multiple prior lines of therapy[16]. With conventional systemic (SYS) chemotherapy alone, the median survival for patients with unresectable CRLM is approximately 30 months [17–24]. In patients whose tumors are RAS-wild-type, a higher median survival of over three years has been reported [25–28]. Conversion to complete resection with SYS chemotherapy has been reported, but conversion rates are low, ranging from 13% to 27% [6, 7, 12, 29]. Furthermore, reports of conversion are methodologically flawed due to lacking or vague definitions of resectability.
Prior randomized trials of combination HAI and SYS chemotherapy have demonstrated promising results with response rates of 55–88% with nearly half of patients converting to complete resection[16, 30–32]. In our initial report of a phase II trial evaluating patients with unresectable CRLM, 47% were converted to resection after treatment with HAI and SYS chemotherapy. The present study represents the long-term results of this trial and includes an expansion cohort not originally reported [33]. We have also included an analysis of outcomes stratified by tumor KRAS mutational status. The primary objective of this study was to assess conversion to resection and to evaluate the long-term survival and curative potential of patients with unresectable CRLM in this expanded phase II of HAI FUDR in combination with best SYS chemotherapy.
METHODS
This is a prospective, non-randomized, single-institution phase II trial (Registration #NCT00492999) approved by the MSKCC institutional review board. Patients presenting with unresectable CRLM were evaluated for trial eligibility from July 2007 to July 2012. All patients had histologically-confirmed colorectal carcinoma and no evidence of extrahepatic metastatic disease on cross-sectional imaging performed within six weeks of trial enrollment. This protocol involved a two-step registration process. Patients were registered to Step 1 prior to pump placement, and then eligible patients were registered to Step 2 after pump placement. Only patients registered to both Step 1 and Step 2 were included. As previously reported, irresectability was determined by 2 hepatobiliary surgeons and 1 radiologist and primarily defined as technical irresectability (a margin-negative resection would require resection of all 3 hepatic veins, both portal veins, or the retrohepatic vena cava, or a resection would result in <2 adequately perfused and drained liver segments); a small group of patients were also defined as biologically irresectable due to burden of disease (>6 metastases in a single lobe, with 1 lesion ≥5cm; or ≥6 bilobar metastases) and also included[33]. The definition of irresectability used in this study was the result of the consensus among our institution’s medical and surgical oncologists [34, 35]. Patients were excluded from this study if they had prior liver resection or radiation, any evidence of end organ insufficiency, or Karnofsky performance score <60%. Prior to treatment initiation, all patients underwent detailed cross-sectional imaging, HAI pump insertion, and tissue biopsy, as detailed in the original trial publication [33].
All patients were assigned to received HAI FUDR in combination with best SYS chemotherapy (either oxaliplatin/irinotecan or 5-FU/leucovorin/irinotecan) as determined by their prior chemotherapy history, in addition to Bevacizumab (Bev) [33]. Bev was given to the first 24 patients but subsequently discontinued due to unexpected biliary toxicity (reported below); after IRB authorization and protocol amendment, the subsequent 40 patients did not received Bev treatment. Toxicities and complications were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v 3.0[36]. Biliary toxicity was graded as previously reported [30, 37]. Patients were evaluated at least every two weeks during treatment with physical examination, complete blood count, complete metabolic panel, and carcinoembryonic antigen. Molecular genotyping for the KRAS mutational status was performed, as previously described [38]. Mutations in KRAS (codons 12 and 13) were identified using the iPLEX assay (Sequenom Inc, San Diego, Calif.).
The primary endpoint evaluated in this study was conversion to complete gross resection. Patients with a sufficient response to treatment were explored for potential resection. Treatment response was evaluated using World Health Organization criteria[39]. Resectability was re-assessed after the 4th cycle of therapy and then every 2 cycles after that. Surgical strategies for resection included ablation and 2-stage resections. All attempts were made to resect all sites of prior disease; however, sites of radiographic complete response that were not visualized at surgery were not required to be resected. Secondary endpoints included overall survival (OS), progression-free survival (PFS), hepatic progression-free survival (HPFS), tumor response rate, and treatment-related toxicities and complications.
The initial study was powered using the historical conversion rate with systemic chemotherapy at the time of trial design of 15%. In order to detect at least a doubling of the historical rate with 90% power, a sample size of 49 patients was determined. Fifteen patients not reported in the original publication were added as an expansion cohort to further clarify and confirm our initial findings.
OS, HPFS, and PFS were measured from initiation of HAI and SYS chemotherapy. Patients who did not experience the event of interest by the end of the study were censored at time of last available follow-up. OS, HPFS, and PFS were estimated using Kaplan-Meier methods. A Cox proportional-hazards model was used to evaluate the association between clinical characteristics and survival outcomes. All identified KRAS mutations were classified as KRAS mutant (MUT), and, in the absence of any identified mutations, KRAS wild-type (WT). Conversion to resection was treated as a time-dependent covariate. The models were adjusted for age (for OS) and other significant variables on univariate analysis.
We conducted a landmark analysis using resection at 12 months as a pre-defined landmark time, in order to examine the effect of resection on OS using Kaplan-Meier methods[40]. The landmark analysis attempts to account for the inherent bias in comparing patients with favorable responses to those with less favorable responses. Patients who had less than 12-month follow-up were excluded from landmark analysis and patients resected after 12 months were counted in the non-resection group.
All p values were based on 2-tailed statistical analysis and p<0.05 was considered statistically significant. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina) and R (13.0).
RESULTS
Patient Characteristics
A total of 117 patients with unresectable CRLM were reviewed for study eligibility between July 2007 and July 2012. Fifty-three patients were excluded from the study (34 had extrahepatic disease; 1 had extrahepatic perfusion after hepatic pump placement; 8 were resected at the time of pump placement; 2 had significant comorbidities; 4 developed post-operative complications; 1 had FUDR started early; 1 had no evidence of malignancy on pathology; 1 patient declined study enrollment; and 1 was enrolled in a different study). 64 eligible patients were enrolled. All but three patients were defined as technically unresectable. Patient characteristics are summarized in Table 1. The majority of patients had received prior chemotherapy (43/64, 67%); 30 patients had one prior therapy, 12 had two prior therapies, and 1 had three. Five patients did not have adequate specimen to determine KRAS status. Of the 59 patients who underwent KRAS genotyping, 39 had KRAS WT and 20 had KRAS MUT tumors. Upon study enrollment, the systemic protocol for 37 patients (58%) included systemic 5-FU/leucovorin/irinotecan while 27 (42%) received systemic oxaliplatin/irinotecan.
Table 1.
Patient Demographics and Presenting Characteristics
| All Patients N (%) |
|
|---|---|
|
| |
| Total | 64 |
|
| |
| Age, years | |
| Median (IQR) | 55.5 (47–63) |
|
| |
| Sex | |
| Male | 36 (56%) |
|
| |
| Synchronous disease | |
| Yes | 61 (95%) |
|
| |
| Bilobar disease | |
| Yes | 61 (95%) |
|
| |
| Number of liver tumors | |
| Median (IQR) | 13 (8–24) |
|
| |
| Prior chemotherapy | |
| Yes | 43 (67%) |
|
| |
| Clinical risk score | |
| 3, 4, 5 | 58 (91%) |
|
| |
| Size of largest liver tumor | |
| >5 cm | 36 (56%) |
|
| |
| LN positivity of primary tumor | |
| Yes | 52 (81%) |
|
| |
| CEA >200 ng/ml | |
| Yes | 19 (30%) |
|
| |
| Disease-free interval | |
| <12 months | 61 (95%) |
|
| |
| KRAS Status | |
| KRAS WT | 39 (61%) |
| KRAS MUT | 20 (31%) |
| Unknown | 5 (8%) |
|
| |
| Criteria for irresectability | |
| Technical* | 61 (95%) |
| Biological | 3 (5%) |
IQR indicates interquartile range; LN, lymph node; CEA, carcinoembryonic antigen; Bev, bevacizumab; WHO, World Health Organization response criteria; PR, partial response; CR, complete response
Defined as a margin-negative resection which would require resection of 3 hepatic veins, both portal veins, or the retrohepatic vena cava, or a resection would result in <2 adequately perfused and drained liver segments
Toxicity
Grade 3/4 diarrhea occurred in 13% of patients and neutropenia occurred in 2% (Table 2). Elevated liver function tests was the most common toxicity with 20% of patients showing an increase in liver enzymes.
Table 2.
Toxicity Profile Stratified by Bevacizumab Administration
| Toxicity | All Patients n = 64 N (%) |
Bevacizumab Treated n = 24 N (%) |
No Bevacizumab n = 40 N (%) |
|---|---|---|---|
| Gr 3/4 Diarrhea | 13 (20%) | 8 (20%) | 5 (13%) |
| Gr 3 Alk | 13 (20%) | 8 (33%) | 5 (13%) |
| Gr 3 AST | 13 (20%) | 5 (21%) | 8 (20%) |
| Gr 3 Abdominal Pain | 7 (11%) | 5 (21%) | 2 (5%) |
| Gr 3/4 Neuro | 6 (9%) | 4 (17%) | 2 (5%) |
| Gr 3 HGB | 5 (8%) | 1 (4%) | 4 (10%) |
| Biliary Stent | 4 (6%) | 3 (13%) | 1 (3%) |
| Gr 3 Bilirubin | 4 (6%) | 2 (8%) | 2 (5%) |
| Gr 3 Vomiting | 4 (6%) | 1 (4%) | 3 (8%) |
| Gr 3 Nausea | 3 (5%) | 1 (4%) | 2 (5%) |
| Gr 3 WBC | 2 (3%) | 1 (4%) | 1 (3%) |
| Gr 4 Bilirubin | 2 (3%) | 1 (4%) | 1 (3%) |
| Gr 3 ANC | 1 (2%) | 0 (0%) | 1 (3%) |
| Gr 4 Platelets | 1 (2%) | 0 (0%) | 1 (3%) |
| Gr 3 Mucositis | 1 (2%) | 1 (4%) | 0 (0%) |
Alk indicates alkaline phosphatase; AST, aspartate aminotransferase; HGB, hemoglobin; WBC, white blood cell count; ANC, absolute neutrophil count
Patients who received Bev with HAI and SYS regimens had unexpectedly significant biliary toxicity, with three of 24 patients (13%) requiring biliary stenting. Among the remaining 40 patients treated without Bev, 1 (2.5%) required biliary stenting.
Response and Conversion to Resection
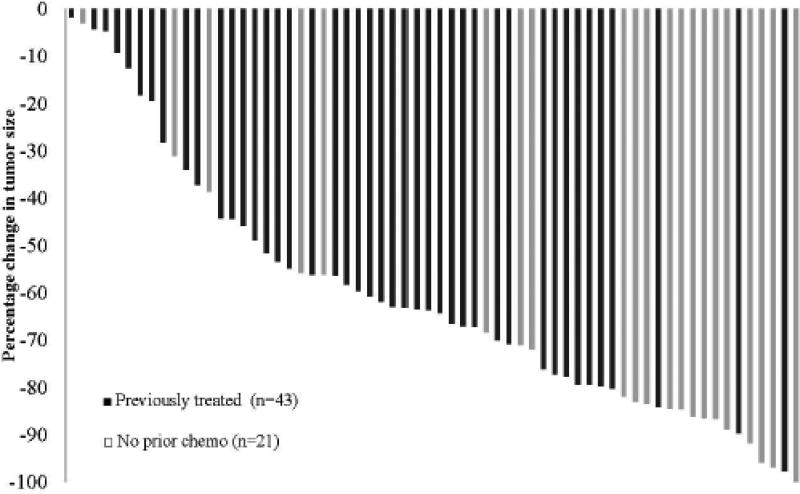
The overall response rate (RR) was 73%, with 47 patients having a complete or partial response (1 CR, 46 PR). Seventeen patients had stable disease (SD), and no patients had progression of disease. Chemotherapy-naïve patients had RR of 86%, with median percent volumetric response of 83%, including 1 CR in a patient with a KRAS WT tumor. Patients treated with prior chemotherapy had a 67% RR, with median volumetric percent response of 61%. Patients who did not receive Bev had similar RR to those who did (73% versus 75%, p = .83). Patients with KRAS WT tumors had improved RR compared to KRAS MUT (68% versus 56%, p=.009). Volumetric responses are illustrated for all patients in Figure 1.
Figure 1.
Waterfall plot demonstrating percentage decrease in tumor size in response to therapy. Dark bars represent patients previously treated with chemotherapy (n = 43); light bars represent patients without prior treatment (n = 21).
Thirty-three patients (52%) were converted to resection at a median of 5 months from treatment initiation (2–22 months), meeting the primary endpoint of doubling the historical conversion rate. Twenty-eight of these 33 resected patients (85%) experienced PR to protocol treatment while 5 had a minimal response that allowed resection. Twelve underwent portal venous embolization (PVE) to increase the volume of their future liver remnant prior to resection. Seventeen patients underwent hemi-hepatectomy accompanied by contralateral wedge resections and/or ablations. Sixteen patients underwent a combination of wedge and segmental resections, of which 12 also had concurrent ablation. Eight of these 33 patients underwent a 2-stage resection. An additional 8 patients underwent the initial stage of a planned 2-stage resection but did not reach the second stage of resection (7 due to progression of disease and 1 due to death secondary to unrelated cardiac arrhythmia). These 8 patients were not considered resected. Of the 33 resected patients, 25 demonstrated significant pathologic response to chemotherapy (≥75% necrosis), with 3 demonstrating pathologic CR. Eight resected patients (24%) had a positive margin on pathology. Thirty-one patients (48%) did not reach resection; 1 had a sustained CR, 18 had PR, and 12 had SD.
There were no post-operative mortalities following HAI pump insertion or subsequent resection and/or ablation. There were no grade 3 or 4 adverse events following HAI pump insertion. Three of the 41 patients who underwent resection developed grade 3 intra-abdominal fluid collections, abscesses, or bilomas that resolved with operative or percutaneous drainage. There were no other grade 3 or 4 perioperative adverse events.
Long-term Follow up and Survival
The median follow-up for surviving patients was 81 months (58–114 months). There were 17 patients alive at last follow-up. Nine of these patients were NED, of whom 8 underwent resection and 1 had a durable CR and never had surgery for liver metastases.
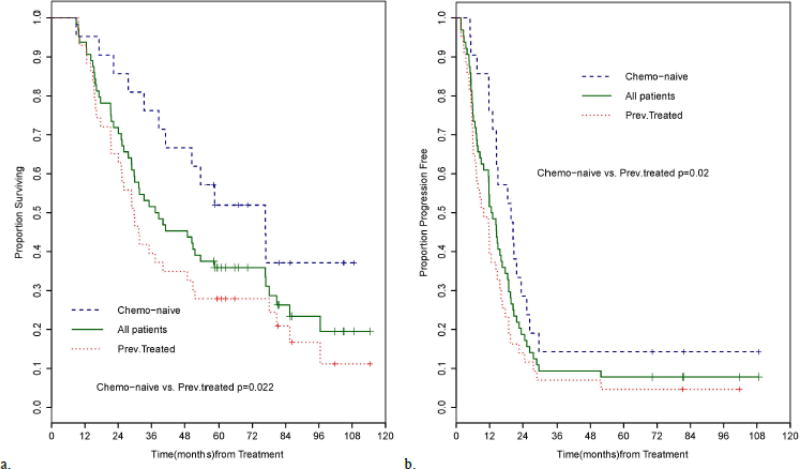
Median OS from initial diagnosis for all patients was 46 months (95%CI 32.3–59.7 months). Median OS from treatment initiation for all patients was 38 months (95%CI 28.8–53.7 months) and 5-year OS was 35.8% [95%CI: 24.3%–47.5%] (Figure 2A). There was a significant difference in OS between chemotherapy-naive and previously-treated patients (median 76.6 months [95%CI: 38.6-NR] versus 29.7 months [95%CI: 21.5–40.2 months], p = 0.022). The 5-year OS was 51.9% for chemotherapy-naïve patients [95%CI: 29.1%–70.6%] and 27.9% for previously-treated patients [95%CI: 15.6%–41.6%]. Patients who received Bev had similar 5-year OS compared to those who did not (41.6% [95%CI: 22.2%–60.0%] versus 35.0% [95%CI: 20.8%–49.5%]). Patients with KRAS WT tumors had similar 5-year-OS (41.0% [95%CI: 25.6%–55.7%]) to those with KRAS MUT tumors (35.0% [95%CI: 15.6%–55.2%]). Median PFS for the entire cohort was 13 months (95%CI: 9–16 months) (Figure 2B). However, 38 patients with initial progression of disease were able to undergo subsequent salvage resections, of whom four are currently NED and eight are AWD. Chemotherapy-naïve patients experienced longer PFS than previously-treated patients (median 19.7 months [95%CI: 13.1–23.4 months] versus 10 months [95%CI: 6.2–14.3 months], p = 0.021); Bev treatment did not have a significant effect on PFS (p = 0.46). Median HPFS for all patients was 16 months (95%CI: 11.9–19.7 months). At last follow-up, there were 5 patients with no evidence of hepatic progression. The KRAS mutational status did not affect either PFS or HPFS. On univariate analysis, the absence of previous chemotherapy treatment and conversion to resection were associated with improved OS. Only conversion to resection remained significantly associated with OS on multivariate analysis (Table 3). Age, prior chemotherapy, and conversion to resection were associated with PFS on univariate analysis. On multivariate analysis, only age and conversion to resection remained a significant predictor of PFS (Table 3).
Figure 2.
A. Overall survival of 64 patients treated with systemic chemotherapy and HAI FUDR, stratified by prior chemotherapy exposure. Median survival was 38 months for all patients (n=64) (95% CI: 28.8–53.7). The median survival for chemo-naïve patients (n=21) was 76.6 months (95% CI: 38.6-NR) and 29.7 months (95% CI: 21.5–40.2) for previously treated patients (n=43) (p=0.022).
B. Progression-free survival of all patients stratified by prior chemotherapy exposure. Progression-free survival for all patients (n=64) was 13 months (95% CI: 9–16 months). Progression-free survival for chemotherapy-naïve patients was 19.7 months (95% CI: 13.1–23.4) and 10 months (95% CI: 6.2–14.3) for previously treated patients (p=0.020).
Table 3.
Univariate and Multivariate Cox Regression Analysis for Overall and Progression-Free Survival
| Overall Survival | Progression-Free Survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | P | HR | 95% CI | p | HR | 95% CI | P | HR | 95% CI | p | |
| Age of diagnosis >55 | 0.659 | 0.370–1.175 | 0.157 | 0.698 | 0.367–1.328 | 0.272 | 0.533 | 0.317–0.895 | 0.017 | 0.56 | 0.316–0.995 | 0.048 |
| Gender | 0.845 | 0.473–1.509 | 0.568 | 1.024 | 0.612–1.714 | 0.928 | ||||||
| Prior chemotherapy Yes | 2.122 | 1.098–4.103 | 0.025 | 1.812 | 0.893–3.684 | 0.100 | 1.912 | 1.093–3.344 | 0.023 | 1.563 | 0.858–2.847 | 0.144 |
| Clinical risk score ≥3 | 1.139 | 0.408–3.181 | 0.803 | 1.039 | 0.415–2.603 | 0.935 | ||||||
| CEA >200 ng/ml | 0.963 | 0.507–1.831 | 0.909 | 1.044 | 0.591–1.842 | 0.882 | ||||||
| LN positivity | 1.810 | 0.804–4.075 | 0.151 | 1.387 | 0.717–2.681 | 0.331 | ||||||
| Largest tumor >5 cm | 1.123 | 0.626–2.012 | 0.697 | 1.320 | 0.782–2.229 | 0.298 | ||||||
| Synchronous disease | 0.582 | 0.180–1.888 | 0.367 | 0.815 | 0.253–2.630 | 0.732 | ||||||
| Bilobar disease | 1.696 | 0.409–7.028 | 0.466 | 0.874 | 0.271–2.824 | 0.822 | ||||||
| Number of liver tumors | 1.000 | 0.998–1.001 | 0.729 | 1.000 | 0.999–1.001 | 0.750 | ||||||
| KRAS MUT | 1.120 | 0.586–2.139 | 0.732 | 1.209 | 0.690–2.120 | 0.507 | ||||||
| Bevacizumab | 0.924 | 0.505–1.693 | 0.798 | 0.887 | 0.522–1.506 | 0.656 | ||||||
| Conversion to resection | 0.269 | 0.145–0.500 | <.001 | 0.255 | 0.134–0.485 | <.001 | 0.534 | 0.297–0.930 | 0.036 | 0.469 | 0.253–0.870 | 0.016 |
CEA indicates carcinoembryonic antigen; LN, lymph node. Bold values represent a statistically significant difference.
Landmark Analysis
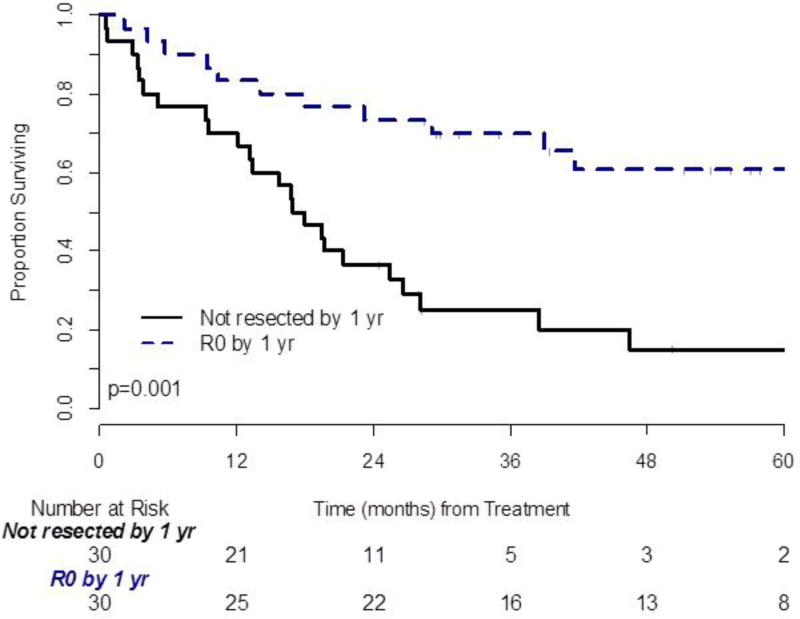
Thirty patients had undergone resection by the prespecified landmark time of 1 year. Three patients who underwent resection after 1 year were grouped for analysis with patients who never underwent resection. Four patients died without undergoing resection prior to the 1-year mark and were excluded from analysis. Patients who converted to resection had significantly prolonged OS compared to those who did not (p = 0.001, Figure 3). The 5-year OS of resected patients was 63.3% [95%CI: 43.6%–77.7%] and 12.5% for unresected patients [95%CI: 3.5%–27.3%] (p≤.001). The median number of tumors was the only factor that was significantly different between the resected and non-resected groups (10 versus 14 tumors, p = 0.043). KRAS status was not associated with conversion to resection (p=0.400). No other patient, disease, or clinical characteristics were significantly associated with conversion to resection.
Figure 3.
Landmark analysis of overall survival: 4 patients without 12 months followup were excluded. Time zero means 12 months from start of treatment.
*HAI FUDR indicates hepatic artery infusional 5-fluoro-2-deoxyuridine.
Curative Potential
Overall, nine of 64 patients (14%) are free of cancer at a median follow-up of 94 months from initial diagnosis (range 65–120 months), 86 months from protocol treatment initiation (range 61–109 months), and 72 months from time of last operative/ablative procedure (range 21–105 months) (Table 4). Of the 33 resected patients, five never had any evidence of disease recurrence at a median follow-up from liver resection of 77 months (64–105 months). Of the 9 patients currently free of disease, another three underwent successful resection/ablation procedures for recurrences after hepatic resection. The follow-up time from the last salvage procedure for these 3 patients is 21, 39, and 53 months. One additional patient with radiologic CR to the study regimen never required liver resection but did undergo removal of an abdominal wall metastasis and pelvic lymphadenectomy for a single nodal recurrence and has since remained NED at follow-up time of 74 months from this procedure and 105 months from treatment initiation. Of the 9 NED patients, six were KRAS WT and three were KRAS MUT. An additional 8 of 64 patients (13%) are AWD at median follow-up time of 69 months (59–129 months) from initial diagnosis and 63 months from treatment initiation (58–114 months).
Table 4.
Long-Term Surgical Treatment and Outcomes of 9 NED patients.
| # of Salvage Attempts |
Time from Last Salvage |
OS (months) |
Previously Treated |
# of Liver Tumors |
Resected | Time from Liver Resection (months) |
|
|---|---|---|---|---|---|---|---|
| 1 | 0 | 109 | No | 7 | Yes | 105 | |
| 2 | 0 | 100 | Yes | 27 | Yes | 92 | |
| 3 | 0 | 81 | No | 8 | Yes | 77 | |
| 4 | 0 | 81 | Yes | 6 | Yes | 72 | |
| 5 | 0 | 69 | No | 3 | Yes | 64 | |
| 6 | 2 | 53 | 105 | No | 14 | Yes | 92 |
| 7 | 2 | 39 | 84 | No | 7 | Yes | 78 |
| 8 | 6 | 21 | 61 | Yes | 10 | Yes | 57 |
| 9* | 1 | 74 | 105 | No | 27 | No | - |
NED indicates no evidence of disease
Patient exhibited complete response to combination HAI and systemic chemotherapy and achieved NED without need for resection.
DISCUSSION
This study reached its primary endpoint with a conversion rate to complete resection of well over the anticipated doubling of the historical rate. Thirty-three of the 64 patients (52%) presenting with unresectable CRLM had sufficient radiologic response after treatment with combination HAI and SYS chemotherapy to undergo resection, demonstrating a significantly promising rate of conversion. Despite a high rate of prior treatment, the overall RR was 73% and median OS of all patients was 38 months with a significant difference in OS between those who were able to undergo resection and those who were not.
Studies on the use of SYS chemotherapy alone in patients with unresectable CRLM have reported a much lower conversion rate to resection, ranging from 13–38%[6, 12, 29, 41–43]. While these rates have improved since the initiation of our study, very few studies have reported results comparable to our 52% conversion to resection rate. As resection is the only potentially curative treatment option, this remains a critical outcome to consider. In these prior studies, resectability was often not clearly defined and thus, it is difficult to determine how truly unresectable these patients were at presentation. In comparison, the criteria for irresectability in our study were prospectively designed and defined at the initiation of this study. The relevance of our definition is demonstrated by the median number of tumors per patient in this study (13 tumors, IQR 8–24 tumors). In addition, in order to meet study eligibility, patients had to be determined to have unresectable disease by two hepatobiliary surgeons and one radiologist to ensure the maximum level of standardization throughout the study.
This trial also demonstrated significantly longer survival for patients who were converted to resection than the previously reported range of 35.2 to 48.4 months for converted patients from SYS chemotherapy alone[44–46]. Although our study patients all presented with an extensive burden of disease, the predicted 5-year OS for patients who were able to convert to complete resection was 63%, which is similar to the rate seen in patients with initially resectable disease, and higher than that demonstrated among patients converted to resection with SYS chemotherapy alone[12, 29]. This may represent an additional survival benefit from improved targeted local disease control with the addition of HAI chemotherapy [47]. These findings strongly support the use of combination HAI and SYS chemotherapy as a therapeutic strategy to improve the rate of conversion to resection and optimize long-term disease control. As SYS chemotherapy regimens continue to evolve, additional studies are needed to evaluate the benefit of the potential benefit of the addition of HAI chemotherapy in combination with these novel treatment protocols.
Interestingly, when we evaluated the KRAS mutational status of study patients, we identified an improved RR to the study regimen for patients with KRAS WT tumors compared with KRAS MUT (68% versus 56%, p=.009). While KRAS has been demonstrated as a predictive marker of response of anti-epidermal growth factor receptor treatment for metastatic colorectal cancer, it is unclear whether if this also applies to other chemotherapy regimens [48, 49]. We did not find a significant association in our study between KRAS mutational status and conversion to resection or survival outcomes, which is potentially due to the relatively small size of the study population compared to prior studies and as this study was not powered to perform this subgroup analysis[50, 51]. However, the improved response among patients with KRAS WT tumors indicates a potential predictive biomarker role for KRAS when evaluating patients for combination HAI and SYS chemotherapy.
Additional methods to increase conversion to resectability can be used in conjunction with HAI and SYS chemotherapy. We employed surgical techniques such as intraoperative ablation and two-stage liver resections to achieve complete resection in patients with complex, bilobar metastases[52–56]. PVE can also performed for patients who are deemed unresectable due to lack of sufficient volume in the future liver remnant; however, PVE does not address the issue of irresectability due to excessive tumor burden or unfavorable tumor location relative to major vessels or biliary structures [57]. Other loco-regional therapies such as Yttrium-90 radioembolization can also be considered for patients with liver-only, or liver-predominant, colorectal metastases. A few case reports have shown conversion to resection with Y-90 therapy; however, at our institution, Y-90 radioembolization has not been used as a downstaging strategy due to concerns over hepatic toxicity [58–60].
The possibility of cure for patients presenting with unresectable CRLM has only become a reality within the past decade due to development of effective chemotherapeutic regimens and combination surgical treatment strategies. Adam et al proposed the use of 5-year disease-free survival (DFS) as the most appropriate definition for cure among patients presenting with advanced disease requiring a combinatorial treatment approach, and identified a 16% cure rate among 184 patients with initially unresectable CRLM who were converted to resection with SYS chemotherapy alone [61]. Within our study population, six of 64 patients have achieved 5-year DFS, representing a potential cure rate of 9% overall, and of 15% among resected patients. This demonstrates the significant potential for combination HAI FUDR and SYS chemotherapy and resection in patients presenting with a substantial burden of biologically aggressive disease and failure of disease control on prior chemotherapy treatments.
The main limitation of this study is the rigorous patient selection process that resulted in patients with good functional status, which may not be an accurate representation of the overall population of patients with CRLM. In addition, while criteria for irresectability were prospectively defined, these are not standardized and may reflect an institutional bias. However, as a prospective trial, this study demonstrates the promise that combination HAI and SYS chemotherapy holds in dramatically improving outcomes for patients presenting with unresectable CRLM with otherwise limited therapeutic options. The current availability of HAI chemotherapy is also limited to certain centers worldwide; however, we hope the high rates of conversion to resection and the survival benefits of HAI and SYS chemotherapy demonstrated in the present study will encourage more centers to incorporate this combinatorial regimen into their practice.
CONCLUSIONS
Combination HAI and SYS chemotherapy is an effective treatment regimen in patients with unresectable CRLM with a high rate of conversion to resection and long-term survival. Despite presenting with an advanced high burden of disease and failure on prior conventional therapies, prolonged disease-free survival and potential cure can still be achieved. Large-scale, prospective, randomized trials investigating this combinatory therapy are indicated to further define its long-term benefits and delineate the appropriate criteria for patient selection.
SYNOPSIS.
This study represents an update of long-term survival and conversion to resectability in patients with unresectable CRLM in a phase II study treated with hepatic artery infusion (HAI) and systemic (SYS) chemotherapy. 33 patients (52%) were converted to resection and 9 patients (14%) are free of disease at median follow-up of 86 months from treatment initiation. Combination HAI and SYS is an effective therapy for high-volume unresectable CRLM, resulting in a high rate of resection, long-term survival and the potential for cure.
Acknowledgments
Sources of support: Dr. Linda Pak received support from the Clinical and Translation Science Center at Weill Cornell Medical Center and MSKCC (grant number UL1TR00457). This work was supported in part by NIH/NCI P30 CA008748 Cancer Center Support Grant.
Footnotes
Disclaimers: None.
This study was presented as an oral presentation at the American College of Surgeons (ACS) Clinical Congress 2016 in Washington, D.C.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(8):1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Mayo SC, Pawlik TM. Current management of colorectal hepatic metastasis. Expert review of gastroenterology & hepatology. 2009;3(2):131–44. doi: 10.1586/egh.09.8. [DOI] [PubMed] [Google Scholar]
- 4.Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(3):651–7. doi: 10.1093/annonc/mdt591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu M, Hu J, Yang D, et al. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6(36):38658–66. doi: 10.18632/oncotarget.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Annals of surgery. 2009;249(3):420–5. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 7.Galizia G, De Vita F, Lieto E, et al. Conversion chemotherapy followed by hepatic resection in colorectal cancer with initially unresectable liver-limited metastases. Oncology reports. 2013;30(6):2992–8. doi: 10.3892/or.2013.2795. [DOI] [PubMed] [Google Scholar]
- 8.Chua TC, Saxena A, Chu F, et al. Predictors of cure after hepatic resection of colorectal liver metastases: an analysis of actual 5- and 10-year survivors. Journal of surgical oncology. 2011;103(8):796–800. doi: 10.1002/jso.21864. [DOI] [PubMed] [Google Scholar]
- 9.Pwint TP, Midgley R, Kerr DJ. Regional hepatic chemotherapies in the treatment of colorectal cancer metastases to the liver. Seminars in oncology. 2010;37(2):149–59. doi: 10.1053/j.seminoncol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Nikfarjam M, Shereef S, Kimchi ET, et al. Survival outcomes of patients with colorectal liver metastases following hepatic resection or ablation in the era of effective chemotherapy. Annals of surgical oncology. 2009;16(7):1860–7. doi: 10.1245/s10434-008-0225-3. [DOI] [PubMed] [Google Scholar]
- 11.Beppu T, Miyamoto Y, Sakamoto Y, et al. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Annals of surgical oncology. 2014;21(Suppl 3):S405–13. doi: 10.1245/s10434-014-3577-x. [DOI] [PubMed] [Google Scholar]
- 12.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Annals of surgery. 1996;224(4):509–20. doi: 10.1097/00000658-199610000-00009. discussion 20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leal JN, Kingham TP. Hepatic artery infusion chemotherapy for liver malignancy. Surgical oncology clinics of North America. 2015;24(1):121–48. doi: 10.1016/j.soc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Seminars in oncology. 1983;10(2):176–82. [PubMed] [Google Scholar]
- 15.Ensminger WD, Rosowsky A, Raso V, et al. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2'-deoxyuridine and 5-fluorouracil. Cancer research. 1978;38(11 Pt 1):3784–92. [PubMed] [Google Scholar]
- 16.Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(21):3465–71. doi: 10.1200/JCO.2008.20.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet (London, England) 2011;377(9783):2103–14. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(5):672–80. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 19.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(31):4697–705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 20.Borner M, Koeberle D, Von Moos R, et al. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19(7):1288–92. doi: 10.1093/annonc/mdn058. [DOI] [PubMed] [Google Scholar]
- 21.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. The New England journal of medicine. 2009;360(14):1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 22.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. The New England journal of medicine. 2009;360(6):563–72. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 23.Tomasello G, Petrelli F, Ghidini M, et al. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA oncology. 2017;3(7):e170278. doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oki E, Emi Y, Miyamoto Y, et al. Phase II Trial of S-1 and Oxaliplatin Plus Cetuximab for Colorectal Cancer Patients with Initially Unresectable or Not Optimally Resectable Liver Metastases (KSCC1002) Annals of surgical oncology. 2015;22(Suppl 3):S1067–74. doi: 10.1245/s10434-015-4771-1. [DOI] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(15):2011–9. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 26.Venook AP, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) Journal of Clinical Oncology. 2014;32(5s) Abstract LBA3. [Google Scholar]
- 27.Carrato A, Abad A, Massuti B, et al. First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: A randomised, phase II trial (PLANET-TTD) European journal of cancer (Oxford, England : 1990) 2017;81:191–202. doi: 10.1016/j.ejca.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. The New England journal of medicine. 2014;371(17):1609–18. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 29.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Annals of surgery. 2004;240(4):644–57. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 57-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemeny N, Jarnagin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(22):4888–96. doi: 10.1200/JCO.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 31.Goere D, Deshaies I, de Baere T, et al. Prolonged survival of initially unresectable hepatic colorectal cancer patients treated with hepatic arterial infusion of oxaliplatin followed by radical surgery of metastases. Annals of surgery. 2010;251(4):686–91. doi: 10.1097/SLA.0b013e3181d35983. [DOI] [PubMed] [Google Scholar]
- 32.Maeda Y, Shinohara T, Nagatsu A, et al. Long-Term Outcomes of Conversion Hepatectomy for Initially Unresectable Colorectal Liver Metastases. Annals of surgical oncology. 2015 doi: 10.1245/s10434-015-4460-0. [DOI] [PubMed] [Google Scholar]
- 33.D'Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Annals of surgery. 2015;261(2):353–60. doi: 10.1097/SLA.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huiskens J, van Gulik TM, van Lienden KP, et al. Treatment strategies in colorectal cancer patients with initially unresectable liver-only metastases, a study protocol of the randomised phase 3 CAIRO5 study of the Dutch Colorectal Cancer Group (DCCG) BMC cancer. 2015;15:365. doi: 10.1186/s12885-015-1323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devaud N, Kanji ZS, Dhani N, et al. Liver resection after chemotherapy and tumour downsizing in patients with initially unresectable colorectal cancer liver metastases. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2014;16(5):475–80. doi: 10.1111/hpb.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006 [Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 37.Kemeny NE, Jarnagin WR, Capanu M, et al. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(7):884–9. doi: 10.1200/JCO.2010.32.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014;120(24):3965–71. doi: 10.1002/cncr.28954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. WHO handbook for reporting results of cancer treatment. 1979 [Available from: http://apps.who.int/iris/bitstream/10665/37200/1/WHO_OFFSET_48.pdf.
- 40.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(24):3913–5. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 41.Delaunoit T, Alberts SR, Sargent DJ, et al. Chemotherapy permits resection of metastatic colorectal cancer: experience from Intergroup N9741. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16(3):425–9. doi: 10.1093/annonc/mdi092. [DOI] [PubMed] [Google Scholar]
- 42.Ardito F, Vellone M, Cassano A, et al. Chance of cure following liver resection for initially unresectable colorectal metastases: analysis of actual 5-year survival. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2013;17(2):352–9. doi: 10.1007/s11605-012-2103-3. [DOI] [PubMed] [Google Scholar]
- 43.Giacchetti S, Itzhaki M, Gruia G, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1999;10(6):663–9. doi: 10.1023/a:1008347829017. [DOI] [PubMed] [Google Scholar]
- 44.Nakai T, Okuno K, Kitaguchi H, et al. Unresectable colorectal liver metastases: the safety and efficacy of conversion therapy using hepatic arterial infusion immunochemotherapy with 5-fluorouracil and polyethylene glycol-interferon alpha-2a. World journal of surgery. 2013;37(8):1919–26. doi: 10.1007/s00268-013-2043-4. [DOI] [PubMed] [Google Scholar]
- 45.Levi FA, Boige V, Hebbar M, et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015 doi: 10.1093/annonc/mdv548. [DOI] [PubMed] [Google Scholar]
- 46.Kataoka K, Kanazawa A, Iwamoto S, et al. Does "conversion chemotherapy" really improve survival in metastatic colorectal cancer patients with liver-limited disease? World journal of surgery. 2014;38(4):936–46. doi: 10.1007/s00268-013-2305-1. [DOI] [PubMed] [Google Scholar]
- 47.Kemeny NE, Chou JF, Boucher TM, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. Journal of surgical oncology. 2016;113(5):477–84. doi: 10.1002/jso.24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adelstein BA, Dobbins TA, Harris CA, et al. A systematic review and meta-analysis of KRAS status as the determinant of response to anti-EGFR antibodies and the impact of partner chemotherapy in metastatic colorectal cancer. European journal of cancer (Oxford, England : 1990) 2011;47(9):1343–54. doi: 10.1016/j.ejca.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 49.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22(7):1535–46. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 50.Nash GM, Gimbel M, Shia J, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Annals of surgical oncology. 2010;17(2):572–8. doi: 10.1245/s10434-009-0605-3. [DOI] [PubMed] [Google Scholar]
- 51.Shindoh J, Nishioka Y, Yoshioka R, et al. KRAS Mutation Status Predicts Site-Specific Recurrence and Survival After Resection of Colorectal Liver Metastases Irrespective of Location of the Primary Lesion. Annals of surgical oncology. 2016;23(6):1890–6. doi: 10.1245/s10434-016-5087-5. [DOI] [PubMed] [Google Scholar]
- 52.Narita M, Oussoultzoglou E, Jaeck D, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. The British journal of surgery. 2011;98(10):1463–75. doi: 10.1002/bjs.7580. [DOI] [PubMed] [Google Scholar]
- 53.Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Annals of surgery. 2008;248(6):994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 54.Cardona K, Donataccio D, Kingham TP, et al. Treatment of extensive metastatic colorectal cancer to the liver with systemic and hepatic arterial infusion chemotherapy and two-stage hepatic resection: the role of salvage therapy for recurrent disease. Annals of surgical oncology. 2014;21(3):815–21. doi: 10.1245/s10434-013-3351-5. [DOI] [PubMed] [Google Scholar]
- 55.Evrard S, Poston G, Kissmeyer-Nielsen P, et al. Combined ablation and resection (CARe) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. PloS one. 2014;9(12):e114404. doi: 10.1371/journal.pone.0114404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imai K, Allard MA, Castro Benitez C, et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. The British journal of surgery. 2017;104(5):570–9. doi: 10.1002/bjs.10447. [DOI] [PubMed] [Google Scholar]
- 57.Wicherts DA, de Haas RJ, Adam R. Bringing unresectable liver disease to resection with curative intent. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007;33(Suppl 2):S42–51. doi: 10.1016/j.ejso.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Pini S, Pinto C, Angelelli B, et al. Multimodal sequential approach in colorectal cancer liver metastases: hepatic resection after yttrium-90 selective internal radiation therapy and cetuximab rescue treatment. Tumori. 2010;96(1):157–9. doi: 10.1177/030089161009600126. [DOI] [PubMed] [Google Scholar]
- 59.Van den Eynde M, Flamen P, El Nakadi I, et al. Inducing resectability of chemotherapy refractory colorectal liver metastasis by radioembolization with yttrium-90 microspheres. Clinical nuclear medicine. 2008;33(10):697–9. doi: 10.1097/RLU.0b013e318184b9a0. [DOI] [PubMed] [Google Scholar]
- 60.Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. British journal of cancer. 2010;103(3):324–31. doi: 10.1038/sj.bjc.6605770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(11):1829–35. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]