Abstract
Modulation of nitric oxide (NO) activity through blockade of CD47 signaling has been shown to reduce ischemia-reperfusion injury (IRI) in various models of tissue ischemia. Here, we evaluate the potential effect of an antibody-mediated CD47 blockade in a syngeneic and an allogeneic DCD rat kidney transplant model. The donor organ was subjected to 1 hr of warm ischemia time after circulatory cessation, then flushed with a CD47 monoclonal antibody (CD47mAb) in the treatment group, or an isotype-matched immunoglobulin in the control group. We found that CD47mAb treatment improved survival rates in both models. Serum markers of renal injury were significantly decreased in the CD47mAb treated group compared with the control group. Histologically the CD47mAb treated group had significantly reduced scores of acute tubular injury and acute tubular necrosis. The expression of biomarkers related to mitochondrial stress and apoptosis also were significantly lower in the CD47mAb treated groups. Overall, the protective effects of CD47 blockade were greater in the syngeneic model. Our data show that CD47mAb blockade decreased the IRI of DCD kidneys in rat transplant models. This therapy has the potential to improve DCD kidney transplant outcomes in the human setting.
Introduction
Kidney transplantation is still the optimal choice for patients with end-stage renal disease (ESRD). However, the shortage of donor kidneys is a severe problem worldwide (1), and wait list times continue to grow in the United States. Using kidneys from donation after cardiac death (DCD) is an important way to expand the donor pool. Based on Organ Procurement and Transplantation Network (OPTN) data as of December 31. 2015, the number of DCD donors increased each year in the last two decades in the United States, from 64 in 1995 to 1,494 in 2015. However, one of the major potential complications with DCD kidneys is the higher incidence of delayed graft function (DGF) compared with kidneys from donation after brain death (DBD) (2–4).
Ischemia-reperfusion injury (IRI) is one of the most important causes of DGF (5). Compared with DBD donors, longer warm ischemia times after cardiac arrest in DCD donor reduce oxygen and nutrient supply to the tissues, leading increased IRI and reduced kidney quality. A recent study reported that greater warm ischemia times are associated with higher rates of graft failure and mortality after kidney transplantation (6, 7). IRI can also lead to progressive graft dysfunction with chronic fibroinflammatory changes that impacts long-term graft survival (8, 9). It is therefore essential to develop strategies to prevent or ameliorate IRI and improve the quality of DCD allografts.
Thrombospondin-1 (TSP1), a ligand of CD47, is a protein secreted by cells throughout the vascular system in response to hypoxia, thrombosis, and other stressors. TSP1 induced CD47 receptor activation inhibits the nitric oxide (NO) signaling pathway that reduces blood flow, and that can ultimately result in necrosis, apoptosis, thrombosis and inflammation (10–12). Therefore, we hypothesized that the use of anti-CD47 blocking antibody could reduce IRI and improve organ preservation. In our previous studies, we demonstrated the effectiveness of CD47 blockade in reducing IRI in standard criteria donor (SCD) rat kidney model (13). Because DCD kidneys are more susceptible to the deleterious effects of IRI, we evaluated the potential effect of an antibody-mediated CD47 blockade in both syngeneic and allogeneic DCD rat kidney transplant models.
Materials and methods
Animals
Male Lewis and Brown Norway rats (275–300 g; Charles River Laboratories, Wilmington, MA) were acclimated for at least 72 hours prior to the experiments. They were given free access to standard rodent food and water ad libitum before and after transplantation except for fasting 12 hours before surgery. Animal experimental protocols were approved by the Animal Studies Committee at Washington University School of Medicine in St. Louis.
Rat DCD kidney transplant IRI model and CD47mAb treatment
The syngeneic (Lewis) or an allogeneic (Brown Norway) donor animal was anesthetized with 2% isoflurane. After intravenous heparinization (200U), cardiac arrest was induced by opening the chest. The cessation of the heart beat was observed within 2–3 min, and the donor animals were kept for 1 hr on a 37-degree pad. The left kidney was mobilized, the aorta clamped proximal and distal to the renal arteries and the kidneys perfused using a 25-gauge needle with 5 mL of UW solution containing 50 µg of control IgG isotype or a humanized anti-CD47 monoclonal antibody (CD47mAb, Tioma Therapeutics, Inc., St. Louis, MO) into the renal artery. The infrarenal inferior vena cava was transected distal to the renal veins. The left kidney was then placed in cold storage for 6 hours.
A Lewis recipient was then anesthetized and a left nephrectomy performed. The transplant procedure was performed as previously described (13). The allogeneic recipients received one dose of tacrolimus (0.2mg/kg, i.v.) after reperfusion of the kidney. The ureter was anastomosed to the bladder, and a right nephrectomy was performed. The first set of experiments were performed to assess post-transplant survival (syngeneic model n=20, 10 for each group and allogeneic model n=12, 6 for each group). Transplanted rats that survived to day 7 were euthanized. A second set of experiments (syngeneic model n=10, 5 for each group and allogeneic model n=10, 5 for each group) were performed using the same experimental design to assess renal function, histological injury and apoptosis and were euthanized 48 hours after transplantation.
Renal Function Assays
Blood samples of the recipients were collected at 48 hours after kidney transplantation. Serum creatinine, blood urea nitrogen (BUN), potassium and phosphorus were measured using a Beckman AU480 Chemistry Analyzer.
Histology and Immunohistochemistry
The recipient animals were euthanized on post-operative day 2, at which time, the graft was recovered, and portions were preserved in 10% buffered neutral formalin solution or snap frozen in liquid nitrogen for further analysis. The formalin fixed and paraffin embedded (FFPE) tissues were cut into a thickness of 5µm and stained with Periodic acid-Schiff (PAS). Acute tubular injury (ATI) was defined as tubular dilatation, epithelial flattening, cell sloughing, or coagulative necrosis. Acute tubular necrosis (ATN) was defined as the death of tubular epithelial cells. This was scored as percent total kidney damage in a blinded manner by a renal pathologist (J.P.G). Kidney tissue samples at day 2 after transplantation were snap frozen in liquid nitrogen. Subsequently, the frozen tissue was cut to a thickness of 5um and assessed immunohistochemically. Sections were incubated with primary antibodies (1:100 diluted) at 4 °C overnight, secondary antibody (1:200 diluted) 1 hour at room temperature, subsequently subjected to fluoroshield mounting medium with DAPI (Abcam 104139) at room temperature for 1 hour. CD3 (Abcam ab16669), Keratin 18 (K18, Novus NBP2-44929), aquaporin 2 (AQP2, Abbexa 121278), a voltage-dependent anion-selective channel protein 1 (VDAC-1, Abcam 191440), poly (ADP-ribose) polymerase (PARP, Abcam 194586), and cleaved caspase-3 (Cell signaling 9961) were tested. Photomicrographs were taken with a Zeiss Observer Z1 microscope and images were captured by Axiovision 4.8.2 software.
Western Blot assay
Cytokeratin 18, PARP, VDAC1 caspase-3, caspase-8, and caspase-9 protein levels in the kidneys were measured using Western blot assay. Ice-cold lyses buffer was used to homogenize renal tissues. After collecting supernatants by centrifuging homogenized liquid, the Bradford assay (BioRad, Hercules, CA) was used to quantify the protein. Twenty micrograms of total protein were resolved by SDS-PAGE and transferred onto nitrocellulose (BioRad). Antibodies against K18 (1:1000 dilution, Novus NBP2-44929), AQP2 (1:1000 dilution, Abbexa 121278), VDAC-1 (1:1000 dilution; Abcam 191440), PARP (1:1000 dilution; Abcam 194586), cleaved caspase-3 (1:1000 dilution, Cell signaling 9961), caspase-8 (1:1000 dilution; SIGMA C2976), caspase-9 (1:1000 dilution; SIGMA C4356) and β-actin (1:2000 dilution; SIGMA A5316) were used as primary antibodies, and horseradish peroxidase-conjugated secondary antibodies were used for detection. Membranes were developed with an ECL Kit (CST6883S) at different time points.
Statistical Analysis
Statistical analyses were performed using Prism (GraphPad Software, San Diego, CA). Survival analysis was performed using Kaplan-Meier analysis and the log-rank test. Data are expressed as mean ± the standard error of the mean. Comparisons between groups were performed using Student’s t-test. Differences with p-values less than 0.05 were considered to be statistically significant.
Results
Treatment with CD47mAb improved rat DCD kidney transplant survival
To assess the ability of antibody-mediated CD47 blockade to improve DCD graft function, we performed experiments in both syngeneic and allogeneic rat transplant models of kidney transplant in bilaterally nephrectomized recipients. Our first set of experiments were designed to assess the survival benefit of CD47 blockade after transplantation. We found that treatment with CD47mAb significantly improved the survival rate in both the syngeneic and allogeneic DCD kidney models (Figure 1, p<0.001 and p<0.002, respectively). However, no recipients survived more than seven days in the allogeneic model.
Figure 1. Treatment of the kidney graft with CD47mAb improves posttransplant survival rate in two rat DCD renal transplant models.
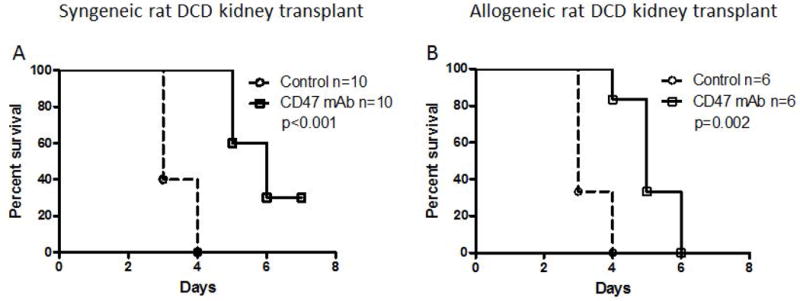
(A) Syngeneic kidney transplant model, Kaplan-Meir survival analysis of the study group showed an increase in CD47mAb group survival. (B) Allogeneic renal transplant model, also showed an increase in CD47mAb group survival.
Treatment with CD47mAb improved graft renal function
Renal function was evaluated 48 hr after kidney transplantation. We measured serum creatinine, blood urea nitrogen (BUN), potassium, and phosphorus. Creatinine (1.0 ± 0.1 mg/dL; vs. 5.8 ± 0.1 mg/dL; p<0.001, Figure 2A), BUN (50.1 ± 7.9 mg/dL; vs. 207.0 ± 2.0 mg/dL; p<0.001, Figure 2B), potassium (4.5 ± 0.2 mmol/L; vs. 7.4 ± 0.2 mmol/L; p<0.001, Figure 2C) and phosphorus (6.1 ± 0.6 mg/dL; vs. 19.4 ± 0.3 mg/dL; p<0.001, Figure 2D) levels were significantly lower in the CD47mAb treated group compared with the IgG-treated control group after kidney transplantation in the syngeneic model. In the allogeneic model, creatinine (4.0 ± 0.7 mg/dL; vs. 6.9 ± 0.3 mg/dL; p=0.005, Figure 2A), BUN (140.4 ± 11.1 mg/dL; vs. 191.5 ± 4.8 mg/dL; p=0.003, Figure 2B), potassium (5.0 ± 0.4 mmol/L; vs. 6.6 ± 0.3 mmol/L; p=0.016, Figure 2C) and phosphorus (12.0 ± 2.0 mg/dL; vs.18.3 ± 1.0 mg/dL; p=0.024, Figure 2D) levels were also significantly lower in CD47mAb treated group compared with the IgG-treated control group. Comparing the results of the two different models, the reductions in creatinine and BUN levels in the CD47mAb-treated group were greater in the syngeneic than in the allogenic model (Figure 2A and 2B, p<0.001, respectively).
Figure 2. Serum markers of kidney function were reduced by treatment of donor kidneys with CD47mAb.
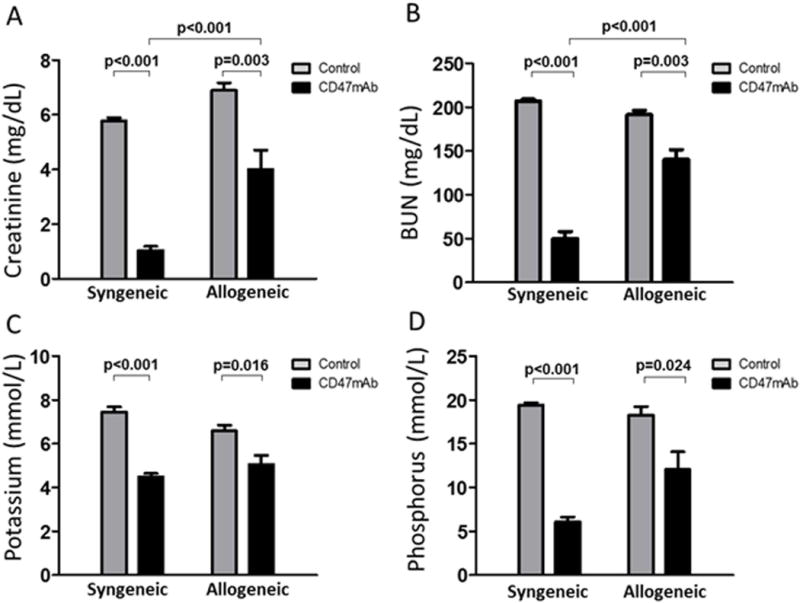
Serum samples were obtained at 48 hours after renal transplantation from recipients that received IgG-treated kidneys or CD47mAb-treated kidneys. (A) Creatinine, (B) BUN, (C) potassium, and (D) phosphorus were measured using an autoanalyzer. Two days after transplantation, serum creatinine, BUN, potassium and phosphorus were significantly lower in rats that received the CD47mAb-treated kidneys than the IgG-treated control kidneys.
Treatment with CD47mAb reduced ATI and ATN
Because we were not able to collect serum and graft tissue from the survival experiments, we performed another set of experiments under identical conditions and concluded the experiments at 48 hours, at which time, serum and the transplanted kidneys were collected for further study. We first evaluated the grafts for histological evidence of IRI. ATI and ATN were characterized as tubular dilatation, epithelial flattening, cell sloughing, or coagulative necrosis. Histological sections were evaluated and scored in a blinded manner by a renal pathologist. Histological evaluation showed that the CD47mAb treated group had significantly decreased scores of ATI (7±1% vs 48±6%, p<0.001) and ATN (17±2% vs 57±8%, p=0.001) in the syngeneic model. In the allogeneic model, ATI (11 ± 3% vs 50 ± 8%, p=0.002) and ATN (19 ± 4% vs 62 ± 8%, p=0.002) were also significantly reduced in the CD47mAb-treated kidneys (Figure 3).
Figure 3. CD47mAb-treated donor kidneys exhibit histological evidence of IRI protection.
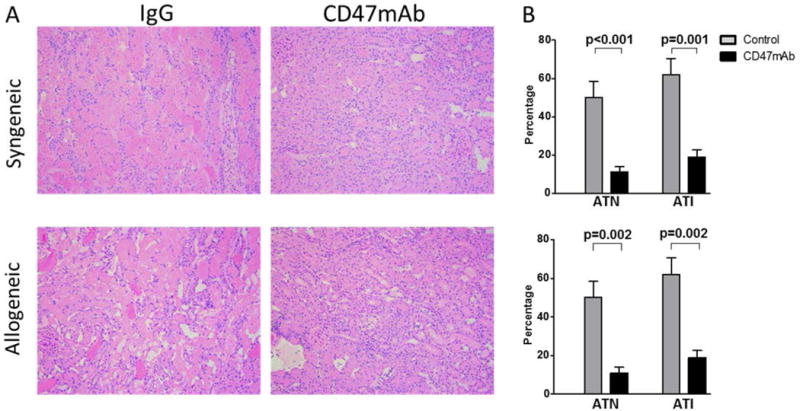
(A) In the syngenic model and the allogeneic model, CD47mAb-treated donor kidneys showed less histological injury when examined 48 hours after transplantation (magnification ×200) than kidneys receiving control IgG antibody. (B) Histological sections were scored in a blinded manner for ATI and ATN. The CD47mAb-treated groups had significantly decreased scores of ATI and ATN in both models.
CD47mAb treatment decreased the inflammatory response
We next investigated the extent of CD3+ T-cell infiltration in the graft tissue to assess the inflammatory response after kidney transplantation. As shown in Figure 4A and 4B, Immunofluorescence staining using anti-CD3 antibodies indicated that CD47mAb treatment remarkably lowered the CD3+ T-cell infiltration in the syngeneic renal grafts compared with that in the control group (39.2 ± 2.0/field vs 7.8 ± 1.8/field, p<0.001). In the allogeneic model, Immunofluorescent staining also showed that CD3+ T-cell infiltration was significantly lower in the CD47mAb treatment group compared to control group (55.2 ± 3.1/field vs 30.2 ± 7.3/field, p=0.009).
Figure 4. CD47mAb treatment decreased the inflammatory response of the DCD renal grafts.
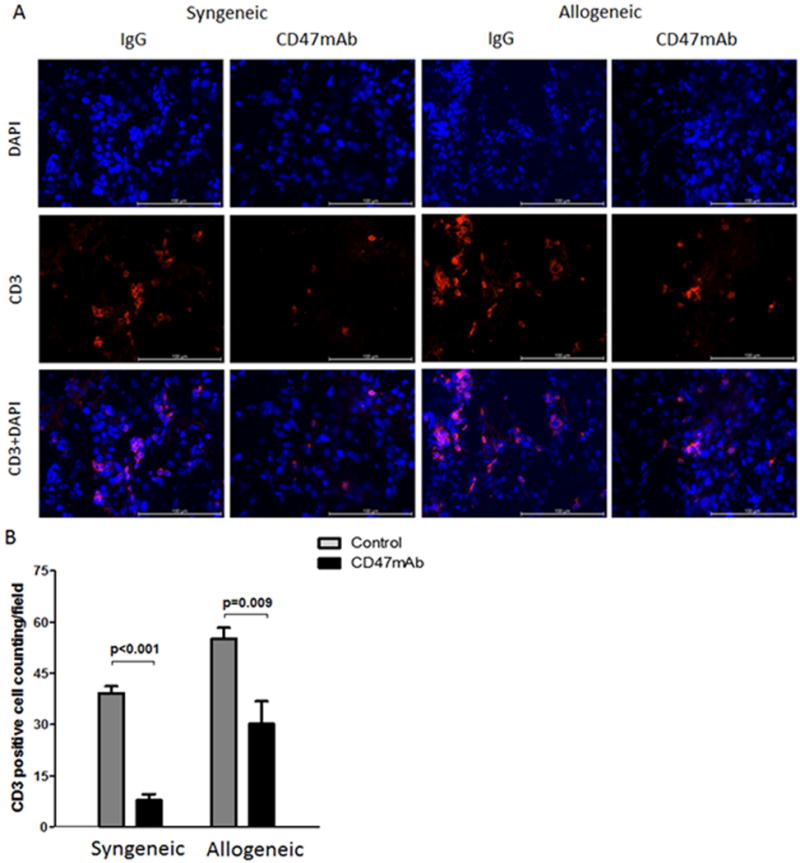
(A) CD3+ T lymphocytes were visualized by immunofluorescence staining in renal tissue at 48 hours after transplantation (magnification ×400). (B) The number of infiltrating CD3+ cells were quantified and found to be significantly decreased in the CD47mAb treated renal grafts versus the control group in both the syngeneic and allogeneic models.
CD47mAb treatment reduces oxidative stress
Under DCD conditions, the donor organs are subjected to prolonged warm ischemia times during which renal cellular mitochondria remain hypoxic. To evaluate mitochondrial stress, we queried the expression of keratin K18, a sensitive marker of renal tubular cell stress (14), in the graft tissue. Immunofluorescent (IF) histological staining showed that K18 was much lower in the CD47mAb-treated groups in both syngeneic and allogeneic models (Figure 5A). Western blot and densitometry analysis found that K18 levels were markedly lower in the CD47mAb treated group in the syngeneic model (p<0.002). Similarly, in the allogeneic model, the levels of K18 were numerically lower in the CD47mAb treated group but did not reach significance (Figure 5B, p=0.110).
Figure 5. CD47mAb treatment reduced renal tubular cell stress after kidney transplantation.
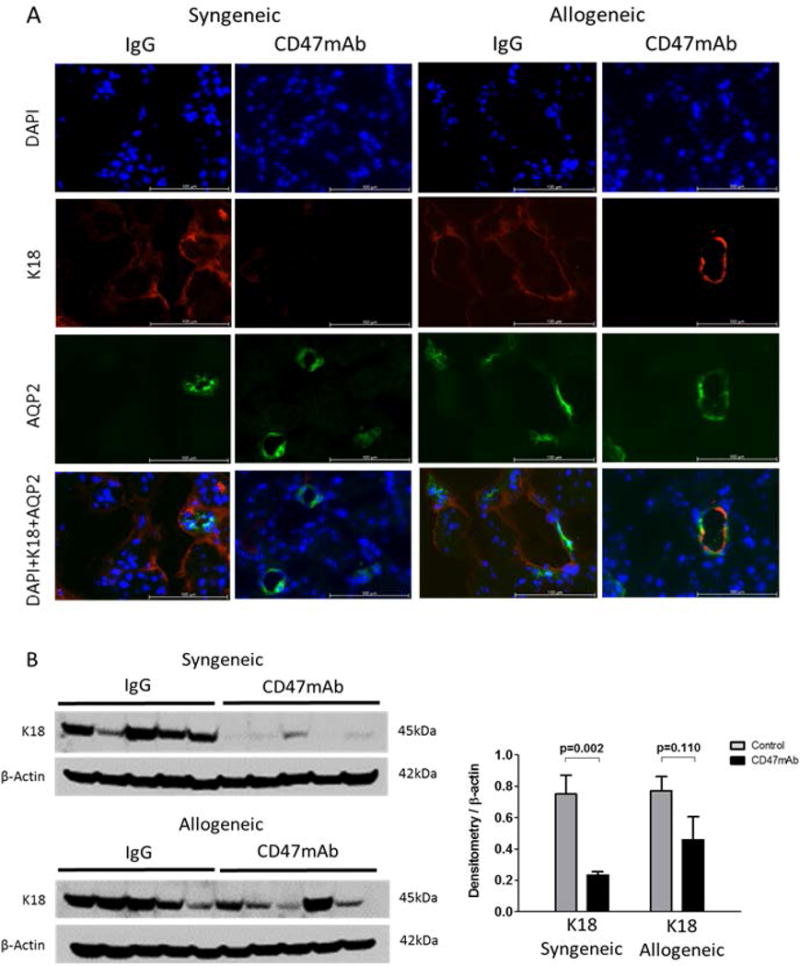
(A) IF histology staining of K18 (magnification x400), colocalized with AQP2 as a marker of the collecting ducts, indicated that CD47mAb treatment reduced expression of K18 in both syngeneic and allogeneic models. (B) Western blot and densitometry analysis found that K18 levels were markedly lower in the CD47mAb treated group in the syngeneic model, while in the allogeneic model, there was no significant difference.
We next examined the expression of poly (ADP-ribose) polymerase (PARP) which is involved in the cellular response to DNA and mitochondrial damage. IF staining indicated that PARP was much lower in the graft tissues of the CD47mAb-treated group, both in the syngeneic and allogeneic models (Figures 6A and Figure 7A). Western blot and densitometry analysis showed that in the CD47mAb treated groups, PARP expression was significantly reduced when compared with IgG control groups (Figures 6B and 7B, p<0.001 and p=0.044, respectively). The voltage-dependent anion-selective channel protein 1 (VDAC1) is also involved in the regulation of cell metabolism and mitochondrial stress. IF staining of VDAC1 was also lower in the CD47mAb-treated group in both syngeneic and allogeneic models (Figure 6A, Figure 7A). Western blot and densitometry analysis revealed that in the syngeneic model, VDAC1 expression was significantly reduced in CD47mAb treated group when compared with IgG control group (Figure 6B, p=0.001). However, there was no significant difference in the allogeneic model (Figure 7B).
Figure 6. Syngeneic rat DCD renal transplant model, CD47mAb treatment reduced oxidative stress.
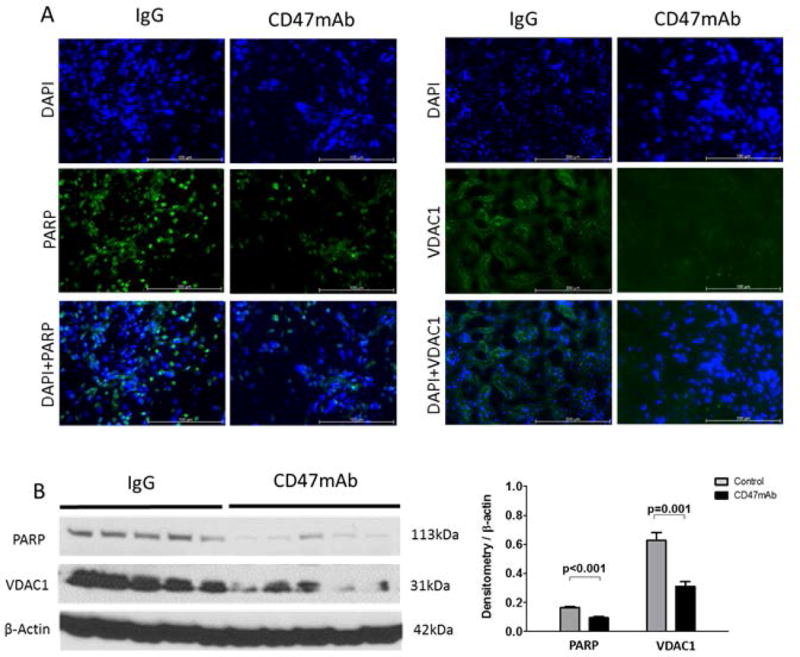
(A) IF staining of PARP and VDAC1 (magnification ×200) showed lower in the CD47mAb-treated group. (B) Western blot and densitometry analysis showed that expression of activated PARP and VDAC1 were markedly lower in CD47mAb treated group compared to IgG control group.
Figure 7. Allogeneic rat DCD renal transplant model, CD47mAb treatment reduced mitochondrial oxidative stress.
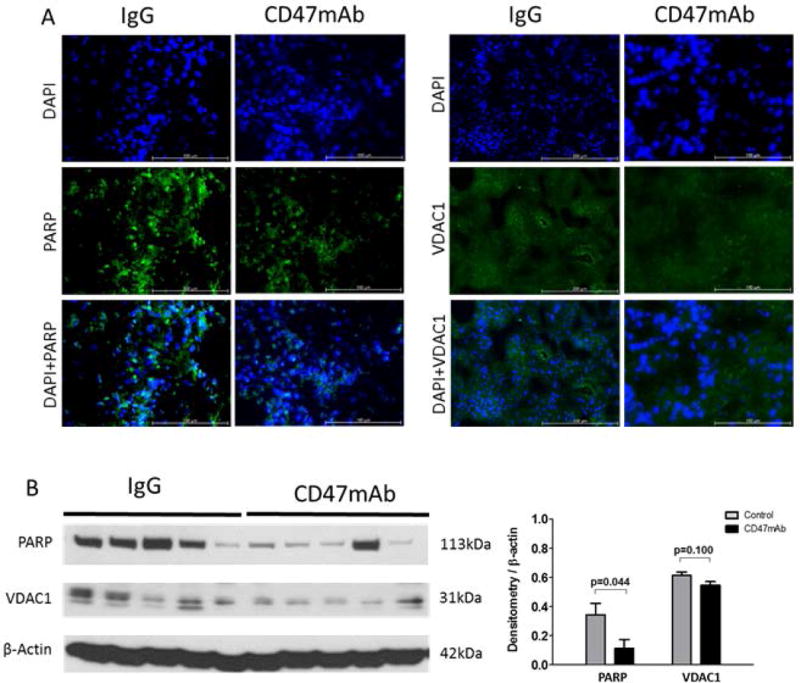
(A) IF staining of PARP and VDAC1 (magnification ×200) showed lower in the CD47mAb-treated group. (B) Western blot and densitometry analysis showed that expression of activated PARP was significantly lower in CD47mAb treated group compared to IgG control group, however, there was no significant difference in VDAC1 expression.
CD47mAb treatment reduces kidney cell apoptosis
IRI has been shown to induce apoptosis in kidney cells (15). We therefore examined the effect of CD47 blockade on the apoptotic response to IRI using both western blot and IF analysis of caspase-9, caspase-8, and caspase-3 expression. IF staining indicated that the expression of Caspase-3 was much lower in the CD47mAb treatment group when compared with the control group in both syngeneic and allogeneic models (Figure 8A, Figure 9A). Western blotting and densitometry analysis confirmed the findings of IF staining, and it also showed caspase-9 and caspase-8 expression were significantly reduced in CD47mAb treated recipients (Figure 8B, Figure 9B).
Figure 8. Syngeneic rat DCD renal transplant model, CD47mAb treatment reduced cell apoptosis.
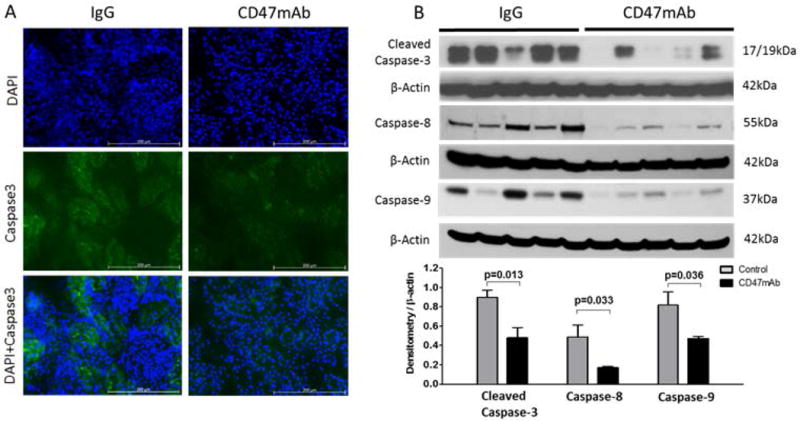
(A) IF staining of caspase-3 (magnification ×200) showed lower in the CD47mAb-treated group. (B) Western blot and densitometry analysis showed that expression of activated caspase-3, caspase-8, and caspase-9 were significantly lower in CD47mAb treated group compared to IgG control group.
Figure 9. Allogeneic rat DCD renal transplant model, CD47mAb treatment reduced cell apoptosis.
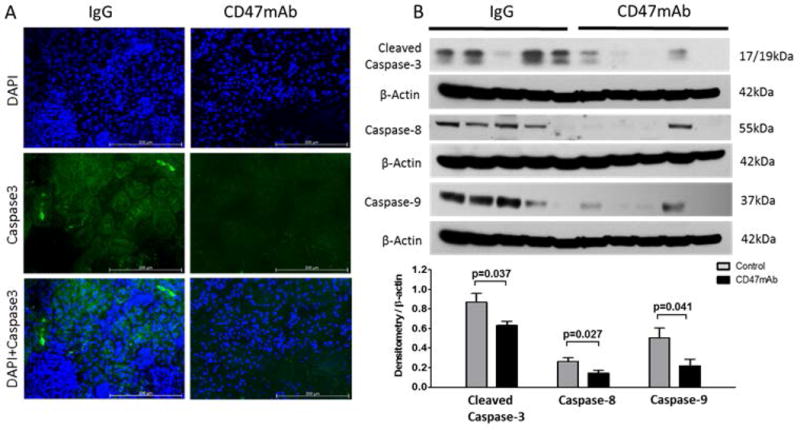
(A) IF staining of caspase-3 (magnification ×200) was reduced in the CD47mAb-treated group compared to control. (B) Western blot and densitometry analysis showed that expression of activated caspase-3, caspase-8, and caspase-9 were significantly reduced in CD47mAb treated group compared to IgG control group.
Discussion
In this study, we evaluated the potential effect of CD47mAb treatment in a syngeneic and an allogeneic DCD rat kidney transplant model. CD47mAb treatment administered by flush to the donor organ improved overall survival of recipients and reduced kidney damage (ATN and ATI). In both models, the graft function improved significantly in the CD47mAb-treated group when compared with control group. However, possibly due to the confounding effects of the immune response in the allogeneic model, serum biomarkers such as creatinine and BUN were not reduced to the same magnitude as in the syngeneic model. We also demonstrated that CD47mAb could reduce mitochondrial stress as well as apoptosis.
In our previous studies, we demonstrated the effectiveness of CD47 blockade in reducing IRI in standard criteria donor (SCD) rat kidney and liver transplant models (13, 16). TSP1-induced CD47 receptor activation inhibits the NO signaling pathway which can lead to vasoconstriction, apoptosis, and thrombosis, and inflammation. Therefore, modulation of NO signaling through blockade of CD47 signaling has been shown to be an effective mitigator of IRI in various models of tissue ischemia. Other studies also found that therapies targeting the thrombospondin-1 receptor CD47 offer the potential for increasing tissue survival and decreasing tissue damage in IRI (17–19). A recent study showed that treatment with a CD47 blocking antibody increased self-renewal transcription factor expression and alleviated IRI in a syngeneic kidney transplant model (20). These studies suggest that CD47 blockade may have therapeutic potential to reduce IRI in organ transplantation. Whereas these studies used either transient ischemia or SCD syngeneic transplant models, we extended our evaluation using allogeneic and DCD transplant models.
The incidence of DGF is much higher in DCD kidneys than DBD kidneys (2, 5, 21) which is attributed to exposure of DCD kidneys to hypoperfusion, hypoxia, and inflammation during prolonged warm ischemia and reperfusion (22, 23). In our study, we used 1 hr of warm ischemia time after circulatory arrest to induce a measure of damage from which we could investigate the protective effects of CD47mAb treatment. Although we demonstrate that both syngeneic and allogeneic DCD kidney transplant recipients benefited from CD47mAb therapy, no recipients survived beyond 7 days in the allogeneic model, and 30% CD47mAb treated recipients were alive at 7 days, indicating that the DCD model with 1 hr of warm ischemia after circulatory arrest resulted in severe tissue damage. This is in contrast to the human transplant setting with DCD donors, where “donor warm ischemia time” usually denotes the time after withdrawal of treatment (22, 24). Although perfusion is reduced during this period, oxygen delivery continues to occur, and the actual ischemic time after complete cessation of circulation is typically less than 10 minutes. Despite the severity of our DCD model, we were able to demonstrate meaningful improvement of transplant outcomes with CD47mAb treatment of the graft.
It has been reported that inflammatory cells including T helper cells, neutrophils, and macrophages play an important role in renal IRI (25, 26). Several studies showed that blocking CD47 could inhibit IL-12-driven Th1 cell development and IL-2 response of activated naïve T cells (27, 28). Our previous study also found that CD47mAb treatment reduced the proinflammatory response in the transplanted rat liver (29). In this study, outcome of immunofluorescence staining indicated that CD47mAb treatment remarkably lowered the CD3+ T-cell infiltration of the renal grafts in both syngeneic and allogeneic models, although it was less striking in the allogeneic model. These results provide evidence that the improved transplant outcomes following CD47mAb treatment could be due in part to the decreased inflammatory response in the DCD kidney grafts. Although in the allogeneic model, the BUN and creatinine were significantly lower in the CD47mAb treated group than the control group, they were higher than in the syngeneic model. One limitation of our model was the use of just one dose of immunosuppressive drugs that was administered just after reperfusion in the allogeneic model as more aggressive immunosuppression was not well tolerated. This lower dose of immunosuppression likely resulted in some increased graft damage due to the remaining, but reduced immune response that was not present in the syngeneic model.
In the current study, we evaluated oxidative stress and apoptosis in DCD kidney transplantation by several biomarkers of IRI. Keratins are up-regulated in stress situations and recognized as novel markers of renal epithelial cell injury (14). In this study, we found that K18 was much lower in the CD47 treated groups compared to controls. However, the western blotting analysis showed that there was no significant difference in the allogeneic model. This may be due to the more severe damage in the allogeneic recipients. PARP activation induces ATP depletion by promoting mitochondrial damage and then induces ROS production, elevates intracellular Ca2+ and destabilizes the mitochondrial membrane system leading to mitochondrial permeability transition and cell death (30–32). A recent study found that PARP increases the expression of inflammatory mediators such as cytokines, inducible nitric oxide synthase and adhesion molecules (33). In our study, we found that CD47mAb-treated recipients had significantly lower PARP expression compared with the IgG-treated control group. VDAC1 is a protein involved in the regulation of cell metabolism and mitochondrial apoptosis. Some studies have demonstrated that downregulated VDAC1 leads to prevention of mitochondrial permeability transition pore opening and cell apoptosis (34–37). We also found that CD47mAb treated recipients had significantly lower VDAC1 expression compared with IgG-treated control group in the syngeneic model.
Caspases are a family of protease enzymes playing essential roles in programmed cell death and inflammation. Caspase-3 is a classic biomarker of apoptosis. Caspase-8 and caspase-9 are initiators, while caspase-3 is an executioner. In the intrinsic apoptotic pathway, during cellular stress, active capase-9 will cleave and activate caspase-3, then lead to degradation of cellular components for apoptosis (38–40). In the extrinsic apoptotic pathway, activated caspase-8 can lead to either downstream activation of the intrinsic pathway by inducing mitochondrial stress, or direct activation of caspase-3 activation and apoptosis (41–43). Our study showed that caspase-3, caspase-8, and caspase-9 were all significantly lower in CD47mAb treated group in both allogeneic and syngeneic models. All these biomarkers demonstrated that CD47mAb therapy could decrease oxidative stress, mitochondrial damage, and apoptosis, reducing IRI and providing renal protection in DCD kidney transplantation.
In conclusion, our data showed that CD47mAb treatment could significantly reduce IRI after kidney transplantation in both syngeneic and allogeneic DCD models. This therapy has the potential to increase use of DCD kidneys and improve DCD kidney transplant outcomes in the human setting.
Acknowledgments
This project was funded in part by the Barnes-Jewish Hospital Foundation Project Award, Transplant Research Support and by the National Institutes of Health grant 5R44DK092078. The content is solely the responsibility of the authors and does necessarily represent the official views of the National Institutes of Health. Thanks to Tioma Therapeutics, Inc, St. Louis for providing the CD47mAb and the Digestive Diseases Research Core Centers (DDRCC, NIDDK P30 DK052574) at WUSM for sharing equipment and core facility support.
Abbreviations
- ESRD
end-stage renal disease
- DCD
donation after cardiac death
- DBD
donation after brain death
- SCD
standard criteria donor
- DGF
delayed graft function
- IRI
ischemia reperfusion injury
- CD47mAb
anti-CD47 monoclonal antibody
- TSP-1
thrombospondin-1
- NO
nitric oxide
- PAS
Periodic acid-Schiff
- IF
Immunofluorescent
- K18
Keratins 18
- PARP
Poly (ADP-ribose) polymerase
- VDAC-1
Voltage-dependent anion-selective channel protein 1
Footnotes
DR YIING LIN (Orcid ID : 0000-0002-0317-7608)
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. William C. Chapman is a founder of Pathfinder Therapeutics (no equity interests) and an advisory board of Novartis Pharmaceutical. Pamela T. Manning is an employee and stockholder of Tioma Therapeutics, Inc. The other authors have no conflicts of interest.
References
- 1.Sayegh MH, Carpenter CB. Transplantation 50 years later–progress, challenges, and promises. The New England journal of medicine. 2004;351(26):2761–2766. doi: 10.1056/NEJMon043418. [DOI] [PubMed] [Google Scholar]
- 2.Nagaraja P, Roberts GW, Stephens M, Horvath S, Fialova J, Chavez R, et al. Influence of delayed graft function and acute rejection on outcomes after kidney transplantation from donors after cardiac death. Transplantation. 2012;94(12):1218–1223. doi: 10.1097/TP.0b013e3182708e30. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, Farney AC, Rogers J, Zuckerman J, Reeves-Daniel A, Hartmann E, et al. Kidney transplantation from donation after cardiac death donors: lack of impact of delayed graft function on post-transplant outcomes. Clinical transplantation. 2011;25(2):255–264. doi: 10.1111/j.1399-0012.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- 4.Paloyo S, Sageshima J, Gaynor JJ, Chen L, Ciancio G, Burke GW. Negative impact of prolonged cold storage time before machine perfusion preservation in donation after circulatory death kidney transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2016;29(10):1117–1125. doi: 10.1111/tri.12818. [DOI] [PubMed] [Google Scholar]
- 5.Ponticelli C. Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(6):1134–1140. doi: 10.1093/ndt/gft488. [DOI] [PubMed] [Google Scholar]
- 6.Heylen L, Pirenne J, Samuel U, Tieken I, Naesens M, Sprangers B, et al. The Impact of Anastomosis Time During Kidney Transplantation on Graft Loss: A Eurotransplant Cohort Study. Am J Transplant. 2016 doi: 10.1111/ajt.14031. [DOI] [PubMed] [Google Scholar]
- 7.Tennankore KK, Kim SJ, Alwayn IP, Kiberd BA. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016;89(3):648–658. doi: 10.1016/j.kint.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Denecke C, Tullius SG. Innate and adaptive immune responses subsequent to ischemia-reperfusion injury in the kidney. Progres en urologie : journal de l’Association francaise d’urologie et de la Societe francaise d’urologie. 2014;24(Suppl 1):S13–19. doi: 10.1016/S1166-7087(14)70058-2. [DOI] [PubMed] [Google Scholar]
- 9.Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004;66(2):523–527. doi: 10.1111/j.1523-1755.2004.761_11.x. [DOI] [PubMed] [Google Scholar]
- 10.Rogers NM, Yao M, Novelli EM, Thomson AW, Roberts DD, Isenberg JS. Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am J Physiol Renal Physiol. 2012;303(8):F1117–1125. doi: 10.1152/ajprenal.00359.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soto-Pantoja DR, Isenberg JS, Roberts DD. Therapeutic Targeting of CD47 to Modulate Tissue Responses to Ischemia and Radiation. J Genet Syndr Gene Ther. 2011;2(2) doi: 10.4172/2157-7412.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao M, Rogers NM, Csanyi G, Rodriguez AI, Ross MA, St Croix C, et al. Thrombospondin-1 activation of signal-regulatory protein-alpha stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol. 2014;25(6):1171–1186. doi: 10.1681/ASN.2013040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y, Manning PT, Jia J, Gaut JP, Xiao Z, Capoccia BJ, et al. CD47 blockade reduces ischemia-reperfusion injury and improves outcomes in a rat kidney transplant model. Transplantation. 2014;98(4):394–401. doi: 10.1097/TP.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djudjaj S, Papasotiriou M, Bulow RD, Wagnerova A, Lindenmeyer MT, Cohen CD, et al. Keratins are novel markers of renal epithelial cell injury. Kidney international. 2016;89(4):792–808. doi: 10.1016/j.kint.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nature reviews Drug discovery. 2008;7(12):1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 16.Xiao ZY, Banan B, Jia J, Manning PT, Hiebsch RR, Gunasekaran M, et al. CD47 blockade reduces ischemia/reperfusion injury and improves survival in a rat liver transplantation model. Liver Transpl. 2015;21(4):468–477. doi: 10.1002/lt.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery. 2008;144(5):752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isenberg JS, Shiva S, Gladwin M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide. 2009;21(1):52–62. doi: 10.1016/j.niox.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plastic and reconstructive surgery. 2009;124(6):1880–1889. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers NM, Zhang ZJ, Wang JJ, Thomson AW, Isenberg JS. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int. 2016;90(2):334–347. doi: 10.1016/j.kint.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Weber M, Dindo D, Demartines N, Ambuhl PM, Clavien PA. Kidney transplantation from donors without a heartbeat. The New England journal of medicine. 2002;347(4):248–255. doi: 10.1056/NEJMoa020274. [DOI] [PubMed] [Google Scholar]
- 22.Allen MB, Billig E, Reese PP, Shults J, Hasz R, West S, et al. Donor Hemodynamics as a Predictor of Outcomes After Kidney Transplantation From Donors After Cardiac Death. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(1):181–193. doi: 10.1111/ajt.13432. [DOI] [PubMed] [Google Scholar]
- 23.Denecke C, Yuan X, Ge X, Kim IK, Bedi D, Boenisch O, et al. Synergistic effects of prolonged warm ischemia and donor age on the immune response following donation after cardiac death kidney transplantation. Surgery. 2013;153(2):249–261. doi: 10.1016/j.surg.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Woodside KJ, Goldfarb DA, Rabets JC, Sanchez EQ, Lebovitz DJ, Schulak JA, et al. Enhancing kidney function with thrombolytic therapy following donation after cardiac death: a multicenter quasi-blinded prospective randomized trial. Clinical transplantation. 2015;29(12):1173–1180. doi: 10.1111/ctr.12647. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney international. 2004;66(2):486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 26.Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. Journal of immunology (Baltimore, Md : 1950) 2007;178(9):5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 27.Avice MN, Rubio M, Sergerie M, Delespesse G, Sarfati M. CD47 ligation selectively inhibits the development of human naive T cells into Th1 effectors. Journal of immunology (Baltimore, Md : 1950) 2000;165(8):4624–4631. doi: 10.4049/jimmunol.165.8.4624. [DOI] [PubMed] [Google Scholar]
- 28.Avice MN, Rubio M, Sergerie M, Delespesse G, Sarfati M. Role of CD47 in the induction of human naive T cell anergy. Journal of immunology (Baltimore, Md : 1950) 2001;167(5):2459–2468. doi: 10.4049/jimmunol.167.5.2459. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Z, Banan B, Xu M, Jia J, Manning PT, Hiebsch RR, et al. Attenuation of Ischemia-Reperfusion Injury and Improvement of Survival in Recipients of Steatotic Rat Livers Using CD47 Monoclonal Antibody. Transplantation. 2016;100(7):1480–1489. doi: 10.1097/TP.0000000000001186. [DOI] [PubMed] [Google Scholar]
- 30.Halmosi R, Deres L, Gal R, Eros K, Sumegi B, Toth K. PARP inhibition and postinfarction myocardial remodeling. International journal of cardiology. 2016;217(Suppl):S52–59. doi: 10.1016/j.ijcard.2016.06.223. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Wang Y, Parapanov R, Abdelnour E, Gronchi F, Perentes JY, et al. Pharmacological Reconditioning of Marginal Donor Rat Lungs Using Inhibitors of Peroxynitrite and Poly (ADP-ribose) Polymerase During Ex Vivo Lung Perfusion. Transplantation. 2016;100(7):1465–1473. doi: 10.1097/TP.0000000000001183. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Z, Lian P, Wu X, Shi B, Zhuang M, Zhou R, et al. Abate Cytochrome C induced apoptosome to protect donor liver against ischemia reperfusion injury on rat liver transplantation model. American journal of translational research. 2016;8(4):1738–1747. [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Wang W, Li JR, Xu HZ, Peng YC, Fan LF, et al. PARP inhibition attenuates early brain injury through NF-kappaB/MMP-9 pathway in a rat model of subarachnoid hemorrhage. Brain research. 2016;1644:32–38. doi: 10.1016/j.brainres.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Hadj Ayed Tka K, Mahfoudh Boussaid A, Zaouali MA, Kammoun R, Bejaoui M, Ghoul Mazgar S, et al. Melatonin modulates endoplasmic reticulum stress and Akt/GSK3-beta signaling pathway in a rat model of renal warm ischemia reperfusion. Analytical cellular pathology (Amsterdam) 2015;2015:635172. doi: 10.1155/2015/635172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Z, Liu D, Tang L, Yin D, Yin S, Lai S, et al. Long-term oral resveratrol intake provides nutritional preconditioning against myocardial ischemia/reperfusion injury: involvement of VDAC1 downregulation. Molecular nutrition & food research. 2015;59(3):454–464. doi: 10.1002/mnfr.201400730. [DOI] [PubMed] [Google Scholar]
- 36.Paillard M, Tubbs E, Thiebaut PA, Gomez L, Fauconnier J, Da Silva CC, et al. Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation. 2013;128(14):1555–1565. doi: 10.1161/CIRCULATIONAHA.113.001225. [DOI] [PubMed] [Google Scholar]
- 37.Verweij M, Sluiter W, van den Engel S, Jansen E, Ijzermans JN, de Bruin RW. Altered mitochondrial functioning induced by preoperative fasting may underlie protection against renal ischemia/reperfusion injury. Journal of cellular biochemistry. 2013;114(1):230–237. doi: 10.1002/jcb.24360. [DOI] [PubMed] [Google Scholar]
- 38.Ezra-Elia R, Alegro da Silva G, Zanoni DS, Laufer-Amorim R, Vitor Couto do Amaral A, Laus JL, et al. Functional and Structural Evaluation of Sildenafil in a Rat Model of Acute Retinal Ischemia/Reperfusion Injury. Current eye research. 2016:1–10. doi: 10.1080/02713683.2016.1193615. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Yoshidome H, Kimura F, Shimizu H, Ohtsuka M, Takeuchi D, et al. Hepatocyte apoptosis is enhanced after ischemia/reperfusion in the steatotic liver. Journal of clinical biochemistry and nutrition. 2011;48(2):142–148. doi: 10.3164/jcbn.10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tak E, Park GC, Kim SH, Jun DY, Lee J, Hwang S, et al. Epigallocatechin-3-gallate protects against hepatic ischaemia-reperfusion injury by reducing oxidative stress and apoptotic cell death. The Journal of international medical research. 2016 doi: 10.1177/0300060516662735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fauconnier J, Meli AC, Thireau J, Roberge S, Shan J, Sassi Y, et al. Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(32):13258–13263. doi: 10.1073/pnas.1100286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shabanzadeh AP, D’Onofrio PM, Monnier PP, Koeberle PD. Targeting caspase-6 and caspase-8 to promote neuronal survival following ischemic stroke. Cell death & disease. 2015;6:e1967. doi: 10.1038/cddis.2015.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Q, Bian H, Guo L, Zhu H. Pharmacologic preconditioning with berberine attenuating ischemia-induced apoptosis and promoting autophagy in neuron. American journal of translational research. 2016;8(2):1197–1207. [PMC free article] [PubMed] [Google Scholar]


