Abstract
Background
Frailty, including low muscle mass, is an emerging risk factor for poor outcomes after lung transplant. The sarcopenia index (SI)—(serum creatinine value/cystatin C value) × 100—is a novel blood test to approximate muscle mass. We sought to validate SI among lung transplant patients.
Methods
We retrospectively identified adult lung transplant recipients from 2000 through 2012 at our institution who underwent computed tomography within 1 year before transplant and had preserved blood samples. Creatinine and cystatin C values were measured using the samples and used to calculate SI. Muscle mass was estimated by computed tomographic measurement of skeletal muscle cross-sectional surface area (SA) at the L1 to L3 vertebral levels. Correlation between SI and SA was evaluated.
Results
Of 28 patients meeting eligibility criteria, most were white (96%) and men (54%). Median (interquartile range) body mass index, SI, and SA were 25.9 (22–30) kg/m2, 106 (91–119), and 157 (113–195) cm2, respectively. The Pearson correlation coefficient between SI and SA was significant at L2 (0.43; P=.02 and L3 (0.41; P=.03).
Conclusion
SI is a potentially objective measure for estimating muscle mass that is noninvasive and less expensive. SI could be considered in lung transplant candidate selection following prospective validation in larger cohorts.
Keywords: creatinine, cystatin C, lung transplant, muscle mass, nutrition, sarcopenia index
Introduction
Reduced muscle mass and physical function are increasingly being recognized as associated with limitations in functional reserve, frailty and poor health outcomes in lung transplant patients (1–4). Reduced pre-transplant muscle index as quantified by single-slice computed tomography (CT) is associated with a higher mortality rate and increased hospital length of stay (LOS) after lung transplant (1). Furthermore, lower pre-transplant psoas muscle mass has been linked to increased mechanical ventilation days, need for tracheostomy, and intensive care unit (ICU) LOS after transplant (3).
The combined loss of skeletal muscle mass and of function defines sarcopenia (5, 6). Declined muscle mass and functional status is prevalent particularly in lung transplant candidates (35%) (2) and patients with chronic lung disease (7). Sarcopenia is associated with frailty, poor surgical outcomes, prolonged need for mechanical ventilation, increased hospital cost, depression, decreased quality of life, increased risk of fall, nursing home residence, and a higher risk of death (8–12). Sarcopenia among patients with end-stage lung disease leads to impaired exercise capacity, worse quality of life, and higher mortality rates (13).
Evaluation of patients with sarcopenia can be difficult as often physical function assessments are not accessible and the measurement of muscle mass requires advanced, expensive, and complex radiologic imaging techniques (12). The imaging studies validated for muscle mass assessments include dual-energy absorptiometry, CT, and magnetic resonance imaging (14). Most of the current non-radiologic measures of nutritional status (i.e., body mass index (BMI), serum albumin levels, prealbumin levels, and physical examination) lack sensitivity and specificity to be used as surrogates for muscle mass, especially among patients who require lung transplant (15).
Researchers have therefore sought other laboratory markers as surrogate measures of muscle mass. Serum creatinine originates from skeletal muscle cells and is eliminated via the kidney. Low baseline serum creatinine value, therefore, has been proposed as an indicator of low muscle mass and is associated with significantly worse outcomes (16–19). In a recent report, Kashani et al (20) introduced the sarcopenia index (SI), a method to estimate muscle mass using the differential origin of 2 molecules that are cleared by the kidney (creatinine generated by skeletal muscle cells and cystatin C originating from all nucleated cells) (21, 22), assuming steady kidney function. The muscle mass estimation by the SI significantly correlated with the findings of abdominal CT among critically ill patients and demonstrated superior performance compared with serum creatinine alone in the estimation of muscle mass (20).
Given the promise of SI as a surrogate clinical marker for muscle mass and the importance of low muscle mass as a key element of identifying lung transplant candidates at risk for or with sarcopenia, we sought to evaluate the diagnostic performance of SI in this population.
Methods
Participants and Measurements
We retrospectively searched our patient database for the records of all adult recipients of a single lung, bilateral lung, or heart-lung transplant at Mayo Clinic, Rochester, Minnesota, from January 1, 2000, through January 13, 2012. We included those with biobanked pre-transplant plasma samples and those with CT that covered the L3 vertebral level, performed within 365 days before transplant. We excluded those who did not provide Minnesota Research Authorization or were undergoing repeat transplant.
The Mayo Clinic Institutional Review Board approved this project and waived consent because of minimal risk. This study complies with the ethical Declarations of Helsinki and Istanbul.
We abstracted all demographic and patient-related information from the electronic medical records. Weight and height measurements were abstracted from the pretransplant period as close to the time of transplant as possible. We used the banked plasma samples from each patient to measure both serum creatinine concentration (in mg/dL) with the Roche enzymatic method (Roche Hitachi Modular P analyzer with the Roche Creatinine plus assay; Hoffmann–La Roche) and cystatin C concentration (in mg/L) with the cystatin C immunoturbidimetric assay (Gentian). If multiple pretransplant samples were available, we chose the sample closest to the date of the CT. SI was calculated as (serum creatinine value/cystatin C value) × 100.
To measure muscle mass, we used sliceOmatic software, version 5.0 (TomoVision) with the available CT images to determine the abdominal skeletal muscle cross-sectional surface area (SA) at the level of the first to third lumbar vertebrae (L1 to L3) (23, 24). The software uses tissue radiodensity to identify muscle (Hounsfield units of −40 to +170) and can be manually corrected if needed. Muscles measured at this site include the rectus abdominis, internal and external obliques, psoas, quadratus lumborum, erector spinae, and the transversus abdominis. Prior studies have demonstrated that measurements at the L3 level are precise surrogates for total body muscle mass (25, 26). Measurements were performed in duplicate and averaged. The interrater agreement has previously been demonstrated to be excellent. Kelm et al (1) reported the interrater Pearson correlation coefficient (r) for muscle index measurements to be 0.998 (95% CI, 0.997–0.999) (20). We calculated body surface area with the Mosteller formula: body surface area in m2 = (height in cm × weight in kg/3,600)1/2. The skeletal muscle index (cm2/m2) was computed as the ratio of the SA at the L3 level to body surface area.
Definitions
The primary study aim was to assess the correlation between SI and SA. The secondary aims were to assess ICU, hospital, and 1-year follow-up mortality rates, duration of mechanical ventilation, and ICU and hospital LOS. Frailty Deficit Index was defined based on a 32-item scale that has been previously validated in the lung transplant population (4, 27). Lung allocation score (LAS) was obtained at the time of lung transplant (28).
Statistical Analysis
We summarized all demographic information as median and interquartile range (IQR) or mean (SD) for continuous variables, and as counts and percentages for discrete variables. We used the t test and χ2 test to compare means and proportions, respectively. In order to evaluate the relevant differences in the secondary outcomes we used the SI and SA as independent variables and each outcome as a dependent variable in separate t-tests or chi-square tests (or their non-parametric version, when appropriate). All statistical analyses were performed with JMP software (version 10.0.0; SAS Institute Inc). For all analyses, P<.05 was considered statistically significant.
Results
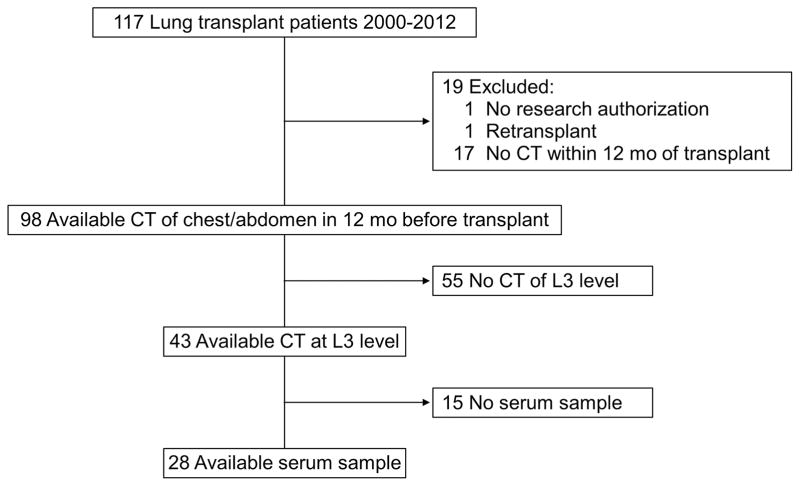
We identified 117 recipients of single lung, bilateral lung, or heart-lung transplants during the study period. After application of eligibility criteria, 28 patients were included in the final analysis (Figure 1); 15 (54%) were men, 27 (96%) were white, and the median age was 58 (IQR, 53–63) years (Table 1). The majority of patients received lung transplant because of end-stage chronic obstructive pulmonary disease (n=19, 68%). Median (IQR) baseline serum creatinine and cystatin C levels were 0.9 (0.8–1.1) mg/dL and 0.9 (0.78–1.1) mg/L, respectively. Among the enrolled patients 12 (43%) patients received a single lung transplant, and although they had a comparable length of mechanical ventilation and ICU days, they had significantly shorter total ischemic time (47 (95%CI 4–90) minutes; p-value .03). The median time to death was 3.2 (IQR, 1.1–7.9) years after transplant among the 18 patients (64%) who died during the follow-up period. The median duration of follow-up was 5.2 (IQR, 1.7–7.7) years. The CT scan that was used for muscle mass measurement was acquired 65 (IQR −31 to 309) days from SI measurement and was performed primarily for pre-transplant evaluation.
Figure 1.
Patient Flowchart. CT indicates computed tomography; L3, third lumbar vertebra.
Table 1.
Patient Characteristics
| Characteristic | Value (N=28)a |
|---|---|
| Men | 15 (54) |
| White | 27 (96) |
| Age, y | 58 (53–63) |
| BMI, kg/m2 | 25.9 (22–30) |
| Barthel score | 55 (45–65) |
| 6MWD (meter) | 291 (227–384) |
| LAS | 34 (33–35) |
| Charlson Comorbidity Index | 2 (1–2) |
| Sarcopenia index | 106 (91–119) |
| SA at L3, cm2 | 157 (113–195) |
| Reason for transplant | |
| COPD | 19 (68) |
| IPF | 4 (14) |
| Lymphangiomyomatosis | 2 (7) |
| Bronchiolitis obliterans | 1 (4) |
| Sarcoidosis | 1 (4) |
| PAH | 1 (4) |
| Single lung transplantation | 12 (43) |
| Total ischemic time (minutes) | 235 (187–270) |
| Length of Mechanical ventilation, Days | 4 (3–9) |
| ICU LOS after transplant, d | 4.5 (3–7.8) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IPF, interstitial pulmonary fibrosis; 6MWD, 6-minute walk distance; LAS, lung allocation score; LOS, length of stay; PAH, pulmonary artery hypertension; SA, muscle cross-sectional surface area.
Values are No. of patients (%) or median (interquartile range).
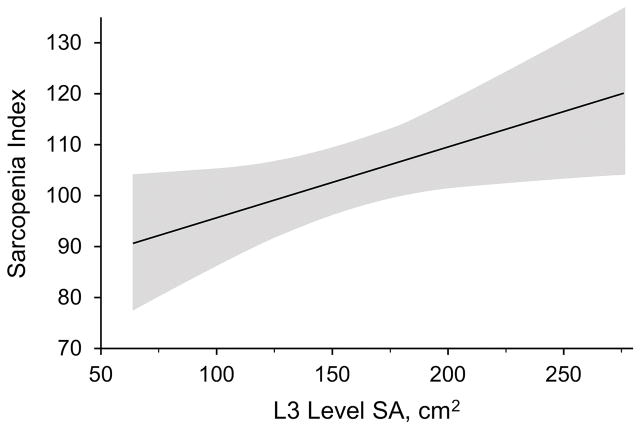
Median (IQR) BMI and SI among these patients were 25.9 (22-kg/m2 and 106 (91–119), respectively. Among the included patients, median (IQR) SA was 157 (113–195) cm2. The median skeletal muscle index was 39.6 (IQR, 34.6–54.3) cm2/m2. Among the 3 vertebral levels at which SA was measured, there was a significant correlation between SA and SI at L3 (Pearson r=0.41; P=.03) (Figure 2) and L2 (Pearson r=0.43; P=.02). Table 2 shows correlation of SA and SI with other surrogate scores and measurements.
Figure 2.
Linear regression between Sarcopenia Index and L3 Level Skeletal Muscle Cross-Sectional Surface Area (SA). Shaded area shows the 95% CI.
Table 2.
Pearson Correlation Coefficient (r) Among Sarcopenia Index and Other Measures of Severity of Illness, Muscle Mass, and Function
| Variable 1 | Variable 2 | Variable 2 Median (IQR) |
r (95% CI) | P |
|---|---|---|---|---|
| Sarcopenia index | SA at L1, cm2 | 131 (89–166) | 0.27 (−0.11–0.58) | .18 |
| SA at L2, cm2 | 147 (103–178) | 0.43 (0.07–0.69) | .02 | |
| SA at L3, cm2 | 157 (113–195) | 0.41 (0.04–0.68) | .03 | |
| CCI | 2 (1–2) | −0.39 (−0.7–0.07) | .09 | |
| Frailty deficit index score | 7.75 (6–9.87) | −0.36 (−0.7–0.11) | .1 | |
| BMI, kg/m2 | 26 (22–30) | 0.05 (−0.4–0.76) | .7 | |
| LAS | 33 (34–35) | −0.08 (−0.5–0.36) | .7 | |
| 6MWD | 291 (227–384) | 0.39 (0.03–0.67) | .04 | |
| SA at L3 | CCI | 2 (1–2) | −0.1 (−0.5–0.35) | .6 |
| Frailty deficit index score | 7.75 (6–9.87) | −0.1 (−0.5–0.36) | .7 | |
| BMI, kg/m2 | 26 (22–30) | 0.45 (0.1–0.7) | .02 | |
| LAS | 33 (34–35) | −0.01 (−0.44–0.42) | .9 | |
| 6MWD | 291 (227–384) | 0.06 (−0.32–0.42) | .8 | |
| BMI | CCI | 2 (1–2) | −0.02 (−0.46–0.44 | .9 |
| Frailty deficit index score | 7.75 (6–9.87) | 0.37 (−0.1–0.7) | .1 | |
| LAS | 33 (34–35) | 0.43 (0.07–0.73) | .047a | |
| 6MWD | 291 (227–384) | 0.09 (−0.29–0.45) | .7 |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; IQR, interquartile range; LAS, lung allocation score; 6MWD, 6-minute walk distance; SA, muscle cross-sectional surface area.
Because height and weight are used in LAS calculation, the reported statistically significant correlation could be due to collinearity between BMI and LAS.
We did not find statistically significant differences in average or proportion of any of the secondary outcomes (ICU, hospital, and 1-year follow-up mortality rates, length of mechanical ventilation, ICU and hospital LOS) when the muscle mass measured by CT or estimated by SI was used as an independent variable (data not shown).
Discussion
To our knowledge, the current study is the first to show that SI, calculated using simultaneously measured serum creatinine and cystatin C values, is significantly correlated with measured SA at the level of the L3 vertebrae by abdominal CT among patients being evaluated for lung transplant (Figure 2). Our group previously reported the correlation between radiologic- measured muscle mass and the SI among a large cohort of critically ill patients (20). The SI potentially provides an estimate of muscle mass as a part of lung transplant evaluation. It would, indeed, be reasonable to evaluate the use of SI as a predictor of lung transplant outcomes in a larger cohort. This measurement is appealing because it is relatively noninvasive, easy to do, and inexpensive. In addition, trends in the SI could be followed during the pretransplant waiting period as a monitor for developing frailty or after a frailty intervention, such as pulmonary rehabilitation, to gauge the response. Given that many candidates waiting for lung transplant are geographically dispersed, the SI offers an advantage compared with other measures of frailty because it is amenable to remote monitoring.
Sarcopenia is a complex syndrome that is associated with muscle mass loss, alone or in conjunction with increased fat mass. Sarcopenia is thought to be mainly due to deranged regulation of protein synthesis and myogenesis and increased skeletal muscle cell proteolysis and apoptosis. It is essential to emphasize that sarcopenia is defined on the basis of two distinct characteristics of muscle: function and mass. These two characteristics are often correlated but can also be dissociated. Some patients with low muscle mass have reasonable muscle function, and some with age-appropriate muscle mass have substantial decreases in their daily functions. In addition, gaining muscle mass does not automatically translate to improved muscle strength and endurance. Sarcopenia index is designed for estimation of muscle mass rather than muscle function. Although we were able to confirm a statistically significant correlation between SI and functional capacity (6MWD: r = 0.39 (95% CI 0.03–0.67); P=.04), we did not have data available to evaluate the correlation of SI and muscle function.
Among patients with advanced lung disease, several factors such as muscle disuse, hypoxemia, malnutrition, inflammation, and chronic use of glucocorticoids contribute to muscle wasting and sarcopenia (13). In a study of patients with chronic obstructive pulmonary disease, forced expiratory volume in 1 second, sex, plasma level of tumor necrosis factor α, and physical inactivity were independently correlated with muscle strength and endurance (29). Conversely, lung structure and functional changes have been commonly reported among patients with sarcopenia (30, 31). Dyspnea is a common consequence of sarcopenia among patients with advanced lung diseases (32, 33). A systematic review of 18 studies in lung transplant patients found that muscle mass and strength are reduced in both the pretransplant and posttransplant periods (34). The prevalence of sarcopenia in lung transplant candidates on the waiting list has been estimated to be 35% (2).
On the basis of the current literature, it is clear that muscle mass and sarcopenia among lung transplant candidates is clinically relevant.
Multiple studies have demonstrated that measured muscle mass is associated with post–lung transplant outcomes. As previously stated, low muscle mass has been associated with increased mortality rates and LOS (1), as well as increased duration of mechanical ventilation and ICU LOS in lung transplant patients (3). Likewise, Lee et al (35) reported longer posttransplant recovery among lung transplant recipients who had lower thoracic muscle SA. In another study of measured thoracic muscle SA in lung transplant candidates (N=527), increased muscle mass was associated with increased 6-minute walk distance and shorter posttransplant hospital LOS (36). Finally, an investigation of lung transplant candidates (N=50) showed that deficits in muscle mass occurred less frequently than did shortcomings of muscle strength or physical performance (37). In addition, patients who had more than one deficit (muscle mass or functional domains), had lower pretransplant 6-minute walk distance and longer posttransplant hospital LOS, although there were no differences in posttransplant 6-minute walk distance or mortality rate. Although most of the current evidence indicates that lower muscle mass is associated with worse outcomes, Lee et al. (35) reported a significantly higher 1-year posttransplant mortality rate among those who had higher thoracic muscle SA measured by CT (54% in the fourth quartile vs. 33.3% in the first quartile; P=.04). The authors attributed this finding to a higher BMI among those with larger muscle mass, which is considered a pretransplant risk factor for increased posttransplant mortality rates although other factors like the higher rate of interstitial pulmonary fibrosis among those with the greatest cross-sectional skeletal muscle surface area (85% in the 4th quartile in comparison with 36% in the first quartile) could explain their findings (35, 38).
Balancing pretransplant risk factors is an important part of lung transplant candidate screening (39). In the United States, the patients are listed based on the computation of the LAS, which is a composite score of multiple variables to predict transplant urgency (28). Among these variables are height and weight—surrogate indicators of nutritional status. Neither these variables nor the calculated BMI, however, correlate consistently with muscle mass (40). Although muscle mass appears to be a unique predictor of posttransplant outcomes, it is currently not a factor in transplant selection, nor is it reflected in the LAS.
To gain a more accurate measure of muscle mass, clinicians could use more advanced radiologic tests such as dual-energy absorptiometry, magnetic resonance imaging, or abdominal CT (14, 41, 42), but these tests are costly, can be time-consuming, and involve radiation. Indeed, the standard cost of Sarcopenia Index is 25.68 US dollars and the cost of abdominal CT scan without contrast is 151.81 US dollars. Therefore, despite their promise, they have not been routinely adopted into transplant selection.
In a kidney transplant cohort, low serum creatinine (as a surrogate for muscle mass) has been used in transplant selection to predict posttransplant death and graft failure (43). The lung transplant population differs from the kidney transplant population, however, in that they will clear creatinine at a higher rate. Therefore, creatinine value alone is unlikely to be a sufficient surrogate for muscle mass.
Our study has several limitations. First, the study was retrospective and was conducted at a single center, and many patients were excluded because they did not have available CT images. In addition, the CT and measured serum creatinine and cystatin C were not necessarily synchronous. It is possible that some patients had changes in muscle mass between the CT and the blood tests. Most of our patients were white, and the proportion of patients with COPD was higher than previously reported rates (68% vs. 46% reported by the International Society of Heart and Lung Transplantation (44)) therefore our results may not be generalizable to all patients. Finally, our sample size was small, and, hence, our results may be subject to type II error. Larger prospective multicenter studies are needed to validate our findings and evaluate the associations between different SI levels and clinical outcomes. In this study, we were not able to link any of the measures of muscle mass with clinical outcomes. This most likely is because our sample was small and not powered to evaluate the secondary objectives of the study.
Conclusion
In this retrospective study of lung transplant candidates, we found a significant correlation between muscle mass measured by CT and estimated skeletal muscle mass via the SI. Hence, following prospective validation in a larger cohort of lung transplant candidates, SI could potentially be used as a replacement for muscle mass measurement by the abdominal CT scan. Given the importance of frailty, muscle mass, and sarcopenia in predicting lung transplant outcomes, practitioners are seeking a method to screen for frailty during lung transplant evaluations. The SI will need to be studied further in larger cohorts to examine its utility in predicting lung transplant outcomes. If our results are confirmed, the SI may be useful in clinical practice as a readily available, inexpensive, safe measurement that is amenable to repeated tests over time.
Acknowledgments
Financial Support: CCK and EFB are supported by the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, Minnesota. CCK is also supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL128859. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- BMI
body mass index
- CT
computed tomography
- ICU
intensive care unit
- IQR
interquartile range
- LAS
lung allocation score
- LOS
length of stay
- SA
skeletal muscle cross-sectional surface area
- SI
sarcopenia index
Footnotes
Conflict of interest: None of the authors have any conflict of interest.
Author Contributions
KK was responsible for concept and design, data collection, statistical analysis, data interpretation, and wrote the first draft of the manuscript. CCK was also responsible for concept and design, data collection, assisted with data interpretation, and critical revisions of the manuscript. EFB assisted with concept and design and critical revisions of the manuscript. KS assisted with data collection and critical revision of the manuscript. NLP assisted with biobank sample processing/data collection and critical revision of the manuscript.
References
- 1.Kelm DJ, Bonnes SL, Jensen MD, Eiken PW, Hathcock MA, Kremers WK, et al. Pre-transplant wasting (as measured by muscle index) is a novel prognostic indicator in lung transplantation. Clin Transplant. 2016;30(3):247. doi: 10.1111/ctr.12683. [DOI] [PubMed] [Google Scholar]
- 2.Singer JP, Diamond JM, Gries CJ, McDonnough J, Blanc PD, Shah R, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. Am J Respir Crit Care Med. 2015;192(11):1325. doi: 10.1164/rccm.201506-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weig T, Milger K, Langhans B, Janitza S, Sisic A, Kenn K, et al. Core Muscle Size Predicts Postoperative Outcome in Lung Transplant Candidates. Ann Thorac Surg. 2016;101(4):1318. doi: 10.1016/j.athoracsur.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplant. 2016;35(2):173. doi: 10.1016/j.healun.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. Journal of the American Medical Directors Association. 2011;12(4):249. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg IH. Summary comments. The American Journal of Clinical Nutrition. 1989;50(5):1231. [Google Scholar]
- 7.Luo Y, Zhou L, Li Y, Guo S, Li X, Zheng J, et al. Fat-Free Mass Index for Evaluating the Nutritional Status and Disease Severity in COPD. Respir Care. 2016;61(5):680. doi: 10.4187/respcare.04358. [DOI] [PubMed] [Google Scholar]
- 8.Beaudart C, Rizzoli R, Bruyère O, Reginster J-Y, Biver E. Sarcopenia: burden and challenges for public health. Archives of Public Health. 2014;72:45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43(6):748. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of Sarcopenia in Major Surgery. Nutrition in Clinical Practice. 2015 doi: 10.1177/0884533615569888. [DOI] [PubMed] [Google Scholar]
- 11.Hanna JS. Sarcopenia and Critical Illness: A Deadly Combination in the Elderly. Journal of Parenteral and Enteral Nutrition. 2015;39(3):273. doi: 10.1177/0148607114567710. [DOI] [PubMed] [Google Scholar]
- 12.Muscaritoli M, Lucia S, Molfino A. Sarcopenia in critically ill patients: the new pandemia. Minerva Anestesiol. 2013;79(7):771. [PubMed] [Google Scholar]
- 13.Langen RCJ, Gosker HR, Remels AHV, Schols AMWJ. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. The International Journal of Biochemistry & Cell Biology. 2013;45(10):2245. doi: 10.1016/j.biocel.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 14.VP, EAS Methods, Diagnostic Criteria, Cutoff Points, and Prevalence of Sarcopenia among Older People. The Scientific World Journal. 2014;2014(231312):11. doi: 10.1155/2014/231312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer JP, Peterson ER, Snyder ME, Katz PP, Golden JA, D’Ovidio F, et al. Body Composition and Mortality after Adult Lung Transplantation in the United States. Am J Respir Crit Care Med. 2014;190(9):1012. doi: 10.1164/rccm.201405-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartin-Ceba R, Afessa B, Gajic O. Low baseline serum creatinine concentration predicts mortality in critically ill patients independent of body mass index*. Crit Care Med. 2007;35(10):2420. doi: 10.1097/01.ccm.0000281856.78526.f4. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The Obesity Paradox and Mortality Associated With Surrogates of Body Size and Muscle Mass in Patients Receiving Hemodialysis. Mayo Clinic Proceedings. 2010;85(11):991. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. Journal of Thoracic Disease. 2016;8(5):E305. doi: 10.21037/jtd.2016.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udy AA, Scheinkestel C, Pilcher D, Bailey M, et al. Australian ft, Outcomes NZICSCf. The Association Between Low Admission Peak Plasma Creatinine Concentration and In-Hospital Mortality in Patients Admitted to Intensive Care in Australia and New Zealand*. Crit Care Med. 2016;44(1):73. doi: 10.1097/CCM.0000000000001348. [DOI] [PubMed] [Google Scholar]
- 20.Kashani KBMD, Frazee ENPR, Kukralova LMD, Sarvottam KMD, Herasevich VMDP, Young PMMD, et al. Evaluating Muscle Mass by Using Markers of Kidney Function: Development of the Sarcopenia Index. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 21.Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindström V, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clinical Chemistry. 1994;40(10):1921. [PubMed] [Google Scholar]
- 22.Shlipak MG, Mattes MD, Peralta CA. Update on Cystatin C: Incorporation Into Clinical Practice. American Journal of Kidney Diseases. 2013;62(3):595. doi: 10.1053/j.ajkd.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairchild B, Webb T, Xiang Q, Tarima S, Brasel K. Sarcopenia and Frailty in Elderly Trauma Patients. World J Surg. 2015;39(2):373. doi: 10.1007/s00268-014-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge M-P, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. Journal of Applied Physiology. 2004;97(6):2333. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Janssen I, Helymsfield SB, Ross R. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr. 2004;80(5):1215. doi: 10.1093/ajcn/80.5.1215. [DOI] [PubMed] [Google Scholar]
- 26.Schweitzer L, Geisler C, Pourhassan M, Braun W, Gluer CC, Bosy-Westphal A, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. 2015;102(1):58. doi: 10.3945/ajcn.115.111203. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ : Canadian Medical Association Journal. 2005;173(5):489. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan TM, Kotloff RM. Pro/Con debate: lung allocation should be based on medical urgency and transplant survival and not on waiting time. Chest. 2005;128(1):407. doi: 10.1378/chest.128.1.407. [DOI] [PubMed] [Google Scholar]
- 29.Ju C, Chen R. Factors Associated with Impairment of Quadriceps Muscle Function in Chinese Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE. 2014;9(2):e84167. doi: 10.1371/journal.pone.0084167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker BL, McKenna S, Mistry V, Pancholi M, Patel H, Haldar K, et al. Systemic and pulmonary inflammation is independent of skeletal muscle changes in patients with chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9:975. doi: 10.2147/COPD.S63568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foong RE, Bosco A, Jones AC, Gout A, Gorman S, Hart PH, et al. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am J Respir Cell Mol Biol. 2015;53(5):664. doi: 10.1165/rcmb.2014-0356OC. [DOI] [PubMed] [Google Scholar]
- 32.Clark BC, Manini TM. Functional Consequences of Sarcopenia and Dynapenia in the Elderly. Curr Opin Clin Nutr Metab Care. 2010;13(3):271. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manini TM, Clark BC. Dynapenia and Aging: An Update. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012;67A(1):28. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozenberg D, Wickerson L, Singer LG, Mathur S. Sarcopenia in lung transplantation: a systematic review. J Heart Lung Transplant. 2014;33(12):1203. doi: 10.1016/j.healun.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Paik HC, Haam SJ, Lee CY, Nam KS, Jung HS, et al. Sarcopenia of thoracic muscle mass is not a risk factor for survival in lung transplant recipients. Journal of Thoracic Disease. 2016;8(8):2011. doi: 10.21037/jtd.2016.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozenberg D, Mathur S, Herridge M, Goldstein R, Schmidt H, Chowdhury NA, et al. Thoracic muscle cross-sectional area is associated with hospital length of stay post lung transplantation: a retrospective cohort study. Transplant International. 2017;30(7):713. doi: 10.1111/tri.12961. [DOI] [PubMed] [Google Scholar]
- 37.Rozenberg D, Singer LG, Herridge M, Goldstein R, Wickerson L, Chowdhury NA, et al. Evaluation of Skeletal Muscle Function in Lung Transplant Candidates. Transplantation. 2017 doi: 10.1097/TP.0000000000001754. [DOI] [PubMed] [Google Scholar]
- 38.Chandrashekaran S, Keller CA, Kremers WK, Peters SG, Hathcock MA, Kennedy CC. Weight loss prior to lung transplantation is associated with improved survival. The Journal of Heart and Lung Transplantation. 2015;34(5):651. doi: 10.1016/j.healun.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation. 2015;34(1):1. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Research Reviews. 2014;15(0):51. doi: 10.1016/j.arr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, et al. Tools in the Assessment of Sarcopenia. Calcif Tissue Int. 2013;93(3):201. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Disease. 2015;17(1):O20. doi: 10.1111/codi.12805. [DOI] [PubMed] [Google Scholar]
- 43.Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, et al. Associations of Pretransplant Weight and Muscle Mass with Mortality in Renal Transplant Recipients. Clinical Journal of the American Society of Nephrology. 2011;6(6):1463. doi: 10.2215/CJN.09131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-seventh official adult lung and heart-lung transplant report—2010. The Journal of Heart and Lung Transplantation. 2010;29(10):1104. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]