Abstract
Previous research on activational effects of testosterone on spatial memory has produced mixed results, possibly because the effects of testosterone on memory are dose-dependent effects. We tested a wide range of testosterone doses using two spatial memory tasks: a working-reference memory version of the radial-arm maze (RAM) and an object location memory task (OLMT). Adult male Sprague-Dawley rats were castrated or sham-castrated and given daily injections of drug vehicle (Oil Sham and Oil GDX) or one of four doses of testosterone propionate (0.125, 0.250, 0.500, and 1.000 mg T) beginning seven days before the first day of behavioral tests and continuing throughout testing. For the RAM, four arms of the maze were consistently baited on each day of testing. Testosterone had a significant effect on working memory on the RAM, with the Oil Sham, 0.125 mg T, and 0.500 mg T groups performing better than the Oil GDX group. In contrast, there was no significant effect of testosterone on spatial reference memory on the RAM. For the OLMT, we tested long-term memory using a 2 h inter-trial interval between first exposure to two identical objects and re-exposure after one object had been moved. Only the 0.125 and 0.500 mg T groups showed a significant increase in exploration of the moved object during the testing trials, indicating better memory than all other groups. Testosterone replacement restored spatial memory among castrated male rats on both behavioral tasks, but there was a complex dose-response relationship; therefore, the therapeutic value of testosterone is likely sensitive to dose.
Keywords: testosterone, androgen, spatial memory, radial arm maze, object location memory, rat
1. Introduction
Spatial memory, the ability to recall the locations and relative spatial relationships of stimuli in one's environment, shows a robust sex difference in humans (Voyer et al., 1995), with men performing better than women on a variety of spatial tasks (Kaufman, 2007; Woolley et al., 2010). Similarly, male rats have better spatial memory than females based on performance in the Morris water maze (MWM) (Markowska, 1999), radial-arm maze (RAM) (Gibbs and Johnson, 2008), and object location memory task (OLMT) (Cost et al., 2012). Considerable evidence indicates that male sex steroids (androgens) play an important role in producing sex differences in spatial memory. Experiments with humans and rats have shown that testosterone has organizational effects upon the brain early in development that enhance spatial learning and memory (Bull et al., 2010; Isgor and Sengelaub, 1998). Whether androgens have activational effects that enhance spatial memory in adult men remains controversial (Holland et al., 2011; Ulubaev et al., 2009), but some studies have shown a positive correlation between circulating testosterone levels and performance on a variety of spatial memory tasks both in younger men, under 30 years old (Christiansen and Knussmann, 1987; Silverman et al., 1999; Thilers et al., 2006), and in older men, over 50 years old (Barrett-Connor et al., 1999; Moffat et al., 2002; Yaffe et al., 2002). Among patients with prostate cancer, androgen deprivation therapy caused significant impairments in spatial ability (Cherrier et al., 2009). However, testosterone replacement given to healthy elderly men has produced mixed effects on spatial cognition (Emmelot-Vonk et al., 2008), so the therapeutic benefits of testosterone replacement remain unclear.
Studies with rats have shown that castration in adulthood impairs spatial memory (Gibbs and Johnson, 2008; Kritzer et al., 2001; Spritzer et al., 2008), and exogenous testosterone replacement restores the spatial memory of castrated adult male rats to normal (Bimonte-Nelson et al., 2003; Spritzer et al., 2011). Additionally, testosterone seems to differentially influence the working and reference components of spatial memory. Within this context, working memory is defined as a form of short-term memory that involves storage of information from a particular task for as long as it is useful to complete the task, and reference memory is defined as the long-term storage of memories that are used from one task to the next (Cowan, 2008; Olton and Papas, 1979). Although most studies show no effect of castration on reference memory on the RAM (Gibbs and Johnson, 2008; Spritzer et al., 2008), some experiments involving the MWM indicate that testosterone replacement can improve spatial reference memory in adult male rats (Khalil et al., 2005; Spritzer et al., 2011). In contrast, testosterone replacement has consistently been shown to improve spatial working memory on various versions of the RAM (Bimonte-Nelson et al., 2003; Gibbs and Johnson, 2008; Spritzer et al., 2008). However, the reference vs working memory dichotomy does not fully capture the variety of spatial memory tasks that have been employed with rodents. For example, two experiments have shown that testosterone injections improve performance on the OLMT with a 2 h delay interval (Jacome et al., 2016; McConnell et al., 2012), suggesting that testosterone improves reference (i.e., long-term) memory when the retention period is relatively short. Thus, although the most consistent activational benefits of testosterone have been demonstrated using working-memory tasks, testosterone may also improve some forms of long-term memory.
It has been speculated that the relationship between testosterone and spatial memory is curvilinear, with both unusually low and high levels of testosterone causing memory impairments. In support of this hypothesis, long-term use of anabolic-androgenic steroids has been shown to impair memory in humans (Kaufman et al., 2015). Similarly, intra-hippocampal injections of supra-physiological doses of testosterone impair spatial memory in male rats (Emamian et al., 2010). Some studies with men have supported the optimum-testosterone hypothesis (Muller et al., 2005; Nowak et al., 2014), but few studies have tested a broad range of testosterone doses on spatial memory using rodents. In our previous work, we found that a broad range of testosterone doses improved performance on the MWM relative to castrated control rats, and certain doses (0.250 and 1.00 mg/rat) impaired reversal learning (Spritzer et al., 2011). Another study using the MWM demonstrated a curvilinear relationship between testosterone and spatial reference memory, with a high physiological dose (0.750 mg/rat) of testosterone optimal for performance (Jia et al., 2013).
In the current study, we tested the effects of both testosterone elimination and replacement, using four doses of testosterone, ranging from physiological to supra-physiological, on the performance of adult male rats on two different tasks: a working-reference memory version of the eight-arm RAM and an OLMT. For our RAM experiment, we predicted that testosterone replacement would restore working memory in castrated male rats while having minimal impact on reference memory (Spritzer et al., 2011). The OLMT has been used in relatively few studies testing the relationship between testosterone and memory (Jacome et al., 2016; McConnell et al., 2012). Two advantages of this task are that it avoids the stressors associated with other tasks and the inter-trial interval can be easily varied to test different memory durations. We used a 2 h inter-trial interval on the OLMT for comparison with the longer retention period (24 h) that we used for RAM testing. Thus, these experiments replicate and expand upon past research designed to assess the cognitive benefits of androgen replacement.
2. Materials and methods
2.1. Subjects
Adult male Sprague-Dawley rats (approximately 60 days old) were acquired from Charles River Laboratories (St. Constant, Quebec, Canada). Animals were individually housed in opaque, polypropylene bins (21 × 42 × 21 cm) with metal lids and Tek-Fresh Bedding (Harlan Laboratories, Indianapolis, IN, USA). Rats had free access to water (glass water bottles) and soy protein-free food (Harlan Teklad Diet 2020X), except during periods of food restriction for RAM testing. The housing and testing rooms were temperature and humidity controlled at 21°C, ± 1°C and 50%, respectively, with a 12:12 h light-dark cycle (lights on at 0700 h). All animal procedures were approved by the Middlebury College Animal Care and Use Committee and were carried out in accordance with ethical guidelines set by the National Institutes of Health.
Subjects underwent bilateral castrations or sham-castrations 7-9 days after arriving in the animal facility. All surgeries were performed with aseptic technique under isoflurane anesthesia (3.5% in oxygen during induction; 2.0-3.0% in oxygen during maintenance). The analgesic Ketofen (5 mg/kg body mass, s.c.) was administered just prior to surgery and again within 24 h of surgery. For castration surgeries, both testes were removed through a single incision at the posterior end of the scrotum and ligated with chromic gut suture material (Ethicon, Somerville, NJ, USA). Sham castrations involved incisions into the skin and muscular sheath without removal of the testes. For all surgeries, the muscle and skin layers were closed with chromic gut sutures and nylon sutures (Ethicon), respectively. Immediately after surgery, topical antibiotic (vetryopolycin) was applied to the surgical site, and lactated Ringer's solution (10 ml/kg body mass, s.c.) was administered as replacement fluids. To ensure full recovery, rats were checked daily for one week after surgery. During the final four days of recovery, subjects were handled for 4-5 min per day to acclimate to the researchers.
For both experiments, rats were divided into six groups (n = 13-15/ group for Experiment 1; n = 11-12/group for Experiment 2). Sham-castrated and castrated control groups received daily s.c. injections of 0.1 ml sesame oil (referred to as Oil Sham and Oil GDX). The other treatment groups received daily s.c. injections of one of four doses of testosterone propionate (Sigma-Aldrich, St. Louis, MO) dissolved in 0.1 ml of sesame oil: 0.125, 0.250, 0.500, and 1.000 mg T. We previously found that these doses produced serum testosterone concentrations ranging from physiological levels typical of adult male rats to supra-physiological levels (Spritzer et al., 2011b). Injections began 8-9 days after surgery and were performed in the morning (0800-1000 h) approximately 4 h before the start of each testing session each day through to the day of euthanasia. Seven days of injections were given prior to the start of any behavioral testing, as our past research suggested this was necessary to observe treatment effects on spatial memory (Spritzer et al., 2011b). All rats had similar weights at the start of each of the experiments (Experiment 1: 293.1 ± 2.0 g; Experiment 2: 261.5 ± 2.3 g).
2.2. Apparatus
For Experiment 1, testing was conducted in a small room (280 × 250 cm) with dim lighting (50 lux) on a grey-painted polycarbonate eight-arm radial maze elevated 65 cm above the floor, with arms (56 × 10 cm) projecting at equal angles from a central platform (115 cm diameter). Small polycarbonate reward cups (1.5 cm height) were affixed at the end of each arm, forming troughs that prevented rats from seeing their contents until they reached the end of an arm. Each rat was randomly assigned four arms to be baited with 45 mg dustless reward pellets (Bio-Serv, Frenchtown, NJ). To minimize intra-maze cues, the maze was randomly rotated to multiples of 45° increments at the start of each day.
Testing in Experiment 2 was conducted in a larger dimly lit room (85 lux) in an open field arena, which was an open-topped grey-painted polycarbonate box (100 × 100 × 46 cm) elevated 33 cm above the floor, with painted black lines forming a grid of 25 uniform squares. A curtain separated the testing area (210×420 cm) from the rest of the room. Each trial was recorded by a digital USB camera mounted on the ceiling directly above the maze and interfaced with a laptop computer running a video tracking program (ANY-maze™ Video Tracking System, ver. 4.115, Stoelting Co., Wood Dale, IL, USA). Different objects were used for the two days of OLMT testing: two black-and-white cylindrical containers for OLMT day 1, and two green cylindrical containers for OLMT day 2. All objects were filled with gravel to prevent dislocation.
For both experiments, two large, high-contrast cues were mounted on adjacent walls to make them visually distinct. Several other heterogeneous visual stimuli (cart, table, broom, etc.) were also present and remained in the same location for all testing. For Experiment 1, the observer was visible in the same location for all trials, whereas for Experiment 2 the observer quickly left the testing area to operate the computer on the other side of the curtain. Rats were placed in the center of the maze in Experiment 1 and the center of the arena in Experiment 2, facing the same direction for all trials. To minimize odor cues, the RAM was wiped with 70% ethanol between trials for Experiment 1. Similarly, for Experiment 2 the open field and the objects were wiped with ethanol between trials.
2.3. Experiment 1: RAM
Experiment 1 assessed the effects of testosterone dose on spatial ability in a RAM protocol designed to distinguish between working and reference memory. From the start of testosterone injections through euthanasia, rats were maintained at 85% of free-feeding body mass. Two free-feeding reference rats were weighed daily to determine expected growth rates, and the target masses for the food-restricted rats were adjusted accordingly. Subjects received 10 reward pellets in their home cages during each day of habituation and shaping to reduce neophobia.
Our protocol consisted of three habituation days, three shaping days, and 25 consecutive training days. On all days, testing was conducted between 1300–1600 h and rats were brought into the testing room 5 min prior to the start of testing. Maze habituation began one day after the 4-day handling period. During maze habituation, rats were placed in the center of the maze and allowed to explore for 5 min on the first day and 10 min on the second and third days. No reward pellets were present on the maze during habituation. During the first day of shaping, three reward pellets were placed at equidistant intervals along each of the four assigned arms, and one pellet was placed in each assigned trough. On subsequent shaping days, the number of reward pellets along each arm was reduced by one each day leading up to training. On training days, reward pellets were placed only in the troughs of assigned arms.
Shaping and training trials ended either when the rat reached the end of all four baited arms or when 10 min had elapsed. An arm entry was scored if the rat's hind legs passed from the center of the maze into an arm. For a training trial in which a rat failed to enter all four arms within 10 min, the average number of errors made on the completed trial before and after the incomplete trial was assigned. This was necessary to correct for the fact that a rat that remained relatively immobile in the maze for 10 min could make relatively few errors, inaccurately suggesting that it had good memory. Fortunately, there were only three testing trials during which three different rats reached the 10 min maximum.
Two types of memory errors were scored during training trials: reference memory errors (RME), defined as first entries into non-baited arms, and working memory errors (WME), defined as repeated entries into arms within a trial. We also scored working-reference errors (WRE), a sub-set of WMEs defined as within-trial reentries into arms that were never-baited. Latency to enter the first arm and average time spent on the maze per arm entry were used as indices of motivation and motor ability.
2.4. Experiment 2: OLMT
Experiment 2 tested spatial memory in an open field arena using an OLMT adapted from McConnell et al. (2012). Testing consisted of four habituation days, one OLMT day, four more habituation days, and a second OLMT day. On all days, testing occurred between 1200-1600 h, and rats were brought into the testing room 10 min prior to the start of testing. During habituation days, each rat was allowed to explore the vacant arena for 10 min. OLMT days consisted of an exposure trial followed by a testing trial (Fig. 1). During each exposure trial, two identical objects were placed 20 cm from the back wall and nearest side wall. Each rat was then placed in the center of the arena and allowed to explore for 5 min. Following the exposure trial, rats were placed back in their home cages for a 2 h inter-trial interval. During each testing trial, one of the objects was moved to a quadrant diagonal from the other object, with the object that was moved (left or right) counterbalanced across treatments. Each rat was then returned to the arena and allowed to explore for 3 min.
Fig. 1.
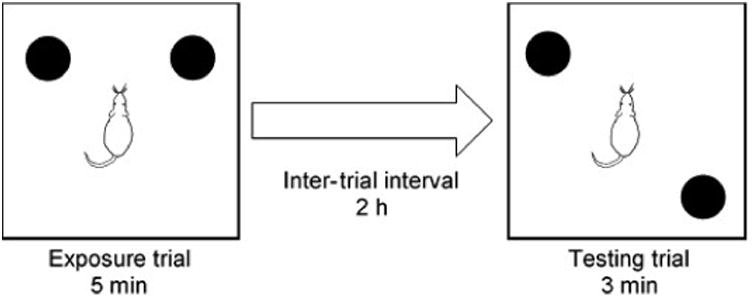
Diagram of the procedure used for the OLMT. Each rat was placed in the center of the open field and allowed to investigate two identical objects for 5 min during the exposure trial. This was followed by a 2 h inter-trial interval in the rat's home cage. One of the two objects was moved, and the rat was placed back in the open field for a 3 min testing trial. Which object was moved (left or right) was counterbalanced within each group of rats.
A rat was considered to be exploring an object when its nose was within 2 cm from the object for a least 1 s. Touching and sniffing activities were counted as exploration, while sitting on or turning around an object were not. Rats that failed to explore both objects during exposure trials or at least one object during testing trials were excluded from analyses. Trials in which rats failed to meet these criteria were excluded from all analyses. Most rats explored the objects on both of the OLMT days, so the data from the two days were averaged for each rat.
2.5. Testosterone assays
Within two days after testing was completed, blood samples were collected from all subjects to assay circulating testosterone levels. Morning testosterone or oil injections continued for 1-2 days after behavioral testing was completed, and blood samples were collected in the afternoon (1300-1600 h) to correspond with the timing of behavioral testing relative to injections. Rats were euthanized with a lethal injection of sodium pentobarbital (approximately 150 mg/kg, i.p.) and blood was collected via heart puncture into LoBind Eppendorf tubes (Fisher, Pittsburgh, PA, USA) on ice. Blood was refrigerated overnight at 4 °C to coagulate before centrifugation (15 min, 2,000 g). Serum was extracted and stored at -20 °C in LoBind microcentrifuge tubes (Fisher, USA) until assaying.
For Experiment 1, total serum testosterone was assayed using coated-tube radioimmunoassay (RIA) kits (Siemens Healthcare Diagnostics, Los Angeles, CA, USA), with all samples run in duplicate. The manufacturer-reported detection limit for the kit was 0.04 ng/ml, and the testosterone antibody had some cross-reactivity with dihydrotestosterone (3.3%), other androgens (< 1.0%), and very low cross-reactivity with progesterone, estrogens, or glucocorticoids (< 0.1%). Serum of subjects in the 1.000 mg T group was diluted by half using the “zero calibrator” to avoid exceeding the kit's maximum detection limit (16.67 ng/ml). Based on samples run in duplicate, the intra-assay coefficient of variation was 6.8%. Based on four samples run on all assays, the inter-assay coefficient of variation was 11.5%.
For Experiment 2, an ELISA was performed using a testosterone EIA Kit (MP Biomedicals, Santa Anna, CA, USA), with all samples run in duplicate. The manufacturer-reported detection limit was 0.05 ng/ml, and the testosterone antibody had limited cross-reactivity with dihydrotestosterone (0.86%), androstenedione (0.89%), androsterone (1.0%), and very low cross-reactivity with all other steroids (< 0.05%). Based on samples run in duplicate, the intra-assay coefficient of variation was 13.5%. Based on two samples run on all assays, the inter-assay coefficient of variation was 6.14%.
2.6. Statistical analysis
For Experiment 1, RAM data were divided into five 5-day training blocks for analyses. The various types of memory errors (WME, RME, and WRE), time to first arm entry, and average time per arm were analyzed using repeated measures analysis of variance (ANOVA), with trial block as the within-subjects factor and treatment as the between-subjects factor. Analyses of the treatment effects were split into two separate sets of analyses: 1) Oil Sham vs. the Oil GDX and 2) Oil GDX vs. all the testosterone-treated groups. Each of the five blocks of trials was analyzed individually using a either univariate ANOVA or t-tests. For Experiment 2, the total distance traveled by rats during the habituation trials was analyzed by repeated measures ANOVA, with habituation day as the within-subjects factor and treatment as the between-subjects factor. Each day of habituation was analyzed individually using a univariate ANOVA with treatment as the fixed factor. As for Experiment 1, the analysis of the habituation data were split into a comparison of the Oil Sham vs. Oil GDX and a separate comparison of the GDX with the testosterone-treated groups. An exploration ratio (time exploring to-be-moved object/total time exploring both objects) was calculated for each rat during both the exposure trials and the testing trials. Paired t-tests were used to compare the exploration ratios between the two trials and univariate ANOVA was used to compare the exploration ratios among groups. For both experiments, serum testosterone levels were analyzed using the univariate ANOVA. The Oil GDX group was not included in these analyses because most of the samples for both experiments had testosterone levels below the detection limit of the assays. Fisher's LSD was used for all post-hoc comparisons. All statistical analyses were performed using SPSS 23.0 software (SPSS, Chicago, IL, USA), with α = 0.05.
3. Results
3.1. Experiment 1: Testosterone had dose-dependent effects on RAM performance
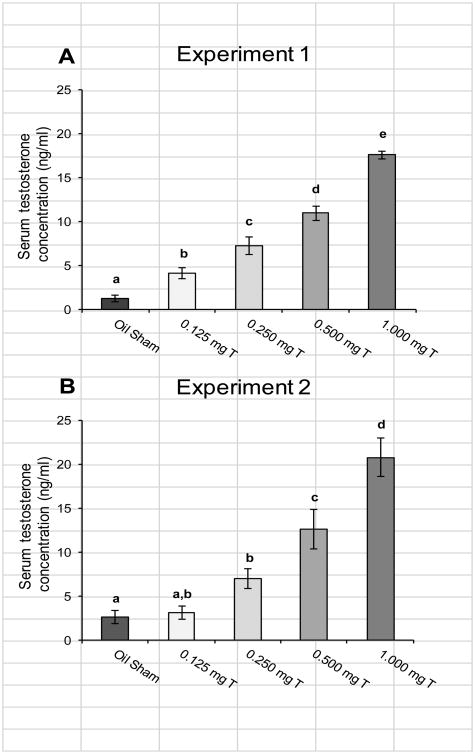
All of the rats from the Oil GDX group had serum testosterone concentrations below the detection limit for the assay (0.05 ng/ml) except one sample that was just above the detection limit (0.057 ng/ml). Due to low variance, this group was not included in statistical comparisons. Serum testosterone concentrations differed significantly among the other groups (Fig. 2A; F4,67 = 83.47, p < 0.0005). Post-hoc comparisons showed that each group had significantly different serum testosterone concentrations that all the others (all p < 0.01). The Oil Sham group had the lowest serum testosterone concentration of any of the groups on average (mean ± SEM = 1.26 ± 0.37 ng/ml) with a range of 0.20-4.2 ng/ml.
Fig. 2.
Mean (± SEM) testosterone concentration in serum samples collected from male rats injected daily with sesame oil (Sham) or one of four doses of testosterone propionate. Blood samples were collected via heart puncture at the time of euthanasia, within two days of completing behavioral testing. For Experiment 1 (A) all groups had mean serum testosterone concentrations that differed from each other, and for Experiment 2 (B) most groups differed from each other except that the 0.125 mg T group did not differ significantly from the Oil Sham or the 0.250 mg T groups. Letters indicate groups that differed significantly from each other (p < 0.05).
There was no evidence that motivation to perform the task varied among the groups for Experiment 1. Comparing the Oil Sham to the Oil GDX group, latency to first entry showed no significant block × treatment interaction (p = 0.62). Rats showed significant reductions in latency over the five testing blocks (F4, 104 = 3.77, p = 0.007), but there was no significant effect of treatment (p = 0.41). Comparing latency to first entry among the Oil GDX group to the testosterone-treated groups produced similar results for, with no significant block × treatment interaction (p = 0.96) or treatment effect (p=0.95), but a significant decline over the testing blocks (F4, 264 = 9.85, p < 0.0005). Comparing the Oil Sham to the Oil GDX group, there was also no significant block × treatment interaction for time per arm entry (p = 0.94). Rats showed significant reductions in time per arm entry over the five testing blocks (F4, 104 = 7.93, p < 0.0005), but there was no significant effect of treatment (p = 0.058). Time per arm entry also showed no significant block × treatment interaction when the Oil GDX group was compared to the testosterone-treated groups (p = 0.85). Comparing these groups also showed no effect of treatment on time per arm entry (p=0.85), whereas there was a significant decline over the testing blocks (F4, 264 = 19.20, p < 0.0005).
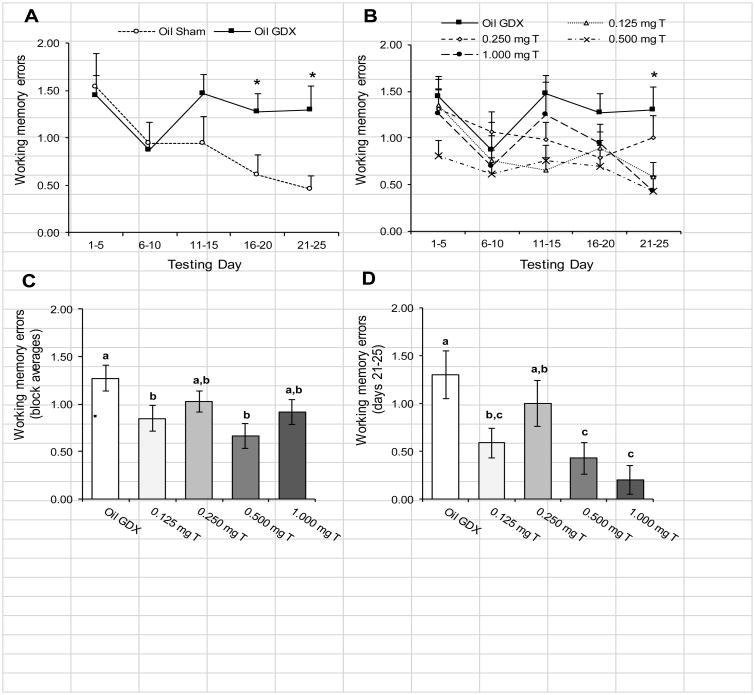
Rats showed dose-dependent enhancement of spatial working memory on the RAM task. Comparing the Oil Sham to the Oil GDX group (Fig. 3A), there was no significant block × treatment interaction for WMEs (p = 0.13). Rats showed significant improvements in working memory over the five testing blocks (F4, 104 = 2.77 p = 0.031). There were also a nearly significant difference in WMEs between the two groups (p = 0.068). Comparisons within blocks revealed that that Oil GDX group performed significantly more WMEs than the Oil Sham group during the last two testing blocks (Fig. 3A: both p < 0.04). Comparing the Oil GDX group to the testosterone-treated groups (Fig. 3B), there was no significant block × treatment interaction for WMEs (p = 0.48). Again, rats showed significant improvements in working memory over the five testing blocks (F4, 264 = 5.06 p = 0.001). There was also a significant effect of testosterone treatment for WMEs (Fig. 3C; F4, 66 = 3.09 p = 0.021). Post-hoc comparisons indicated that the Oil GDX group performed significantly more WMEs than the 0.125 mg T, and 0.500 mg T groups (both p < 0.025). None of the other pair-wise comparisons were significant (all p > 0.05), indicating that the performance of both the 0.250 mg T and the 1.00 mg T groups were between that observed in the Oil GDX group and the other groups. Additionally, the 0.500 mg T group performed nearly significantly fewer WMEs than did the 0.250 mg T group (p = 0.052). The final testing block (days 21-25) was the only block to show a significant treatment effect for WMEs (Fig. 3D; F4, 70 = 3.79, p = 0.008). Within this block, the Oil GDX and 0.250 mg T groups performed significantly more WMEs than the 0.500 mg T and 1.00 mg T groups (all p < 0.05), while the 0.125 mg T group performed fewer WMEs than the Oil GDX group (p = 0.014) but not the 0.250 mg T group (p = 0.14).
Fig. 3.
Mean (± SEM) number WMEs performed by male rats injected daily with sesame oil (Sham and GDX) or one of four doses of testosterone propionate during 5-day blocks of trials over 25 days of testing in an 8-arm radial maze (n = 13-15/group). (A) Comparing the Oil Sham and Oil GDX groups showed significant improvements over the testing blocks (p = 0.031), and the Oil GDX group performed significantly more WMEs than the Oil Sham group during the last two testing blocks (*p < 0.05). (B) Comparing the Oil GDX group to the testosterone-treated groups also showed significant improvements over the testing blocks (p = 0.001). Analyses within blocks showed significant differences between groups during the final block of testing (*p < 0.05). (C) There was a significant main effect of treatment, with the Oil GDX group performing significantly more WME's than did the 0.125mg T and the 0.500 mg T groups. (D) Analyzing only the final testing block (days 21-25) showed that the Oil GDX group performed significantly more WMEs than did the 0.125 mg T, 0.500 mg T, and the 1.00 mg T groups. Letters designate groups that differed significantly from each other (p < 0.05).
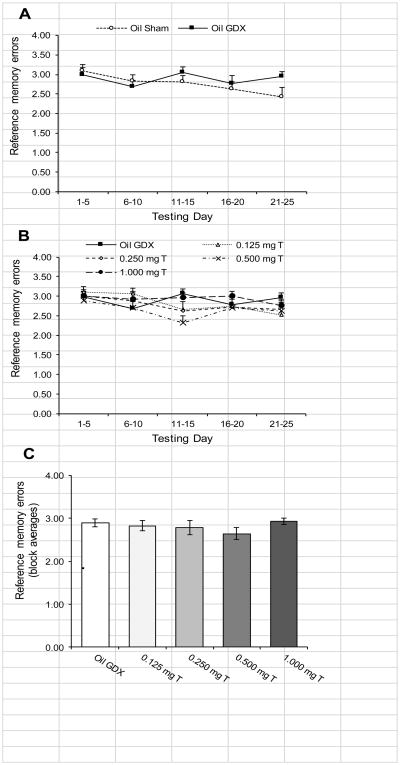
Unlike working memory, there was no evidence that castration or testosterone treatments influenced reference memory. Comparing the Oil Sham group to the Oil GDX group (Fig. 4A), there was no significant block × treatment interaction for RMEs (p = 0.14). For this comparison, there was also no significant effect of block (p = 0.069) or treatment (p = 0.39). Comparing the Oil GDX group to the testosterone-treated groups (Fig. 4B), there was no significant block × treatment interaction for RMEs (p = 0.35). Rats showed significant differences over the five testing blocks (F4, 264 = 3.26, p = 0.012), but there was no significant effect of treatment (Fig. 4C; p = 0.55). Additionally, there were no significant differences among groups in RMEs when each testing block was analyzed separately (all p > 0.07).
Fig. 4.
Mean (± SEM) number RMEs performed by male rats injected daily with sesame oil (Sham and GDX) or one of four doses of testosterone propionate during 5-day blocks of trials over 25 days of testing in an 8-arm radial maze (n = 13-15/group). (A) Comparing the Oil Sham to the Oil GDX group revealed no effects of blocks or castration. (B) Comparing the Oil GDX group to the testosterone-treated groups demonstrated a significant changes over the five testing blocks (p = 0.012). (C) There was no significant effect of treatment on RMEs. There were also no significant differences between groups when the testing blocks were analyzed separately.
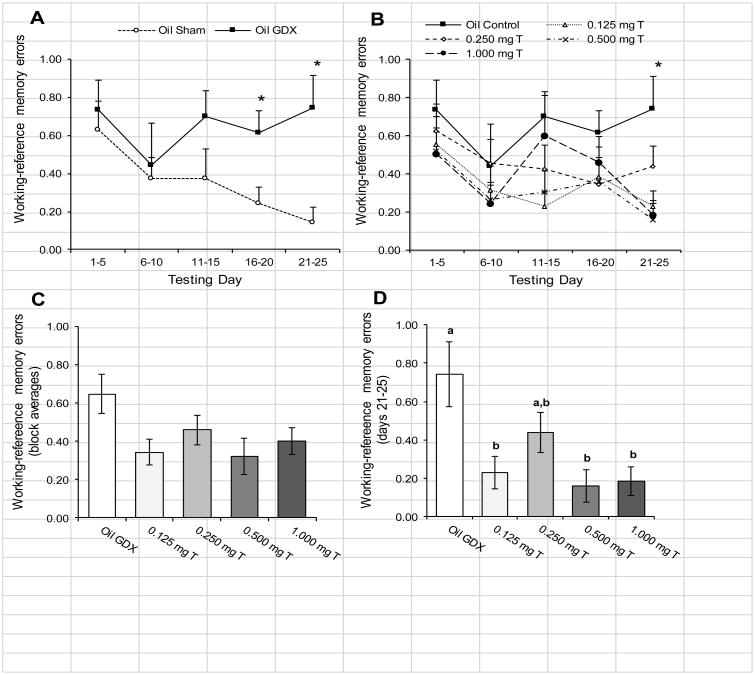
Finally for WREs, there was no significant block × treatment interaction when comparing Oil Sham to the Oil GDX group (Fig. 5A; p = 0.24). There was also no significant effect of block (p = 0.19), but the Oil GDX group did perform significantly more WREs than did the Oil Sham group (F1, 26 = 3.08, p = 0.022). Pairwise comparisons within days showed significant differences on days 16-20 (t26 = 2.59, p = 0.015) and days 21-25 (t26 = 3.18, p = 0.004). Comparing the Oil GDX group to the testosterone-treated groups (Fig. 5B), there was no significant block × treatment interaction for WMEs (p = 0.61). Rats showed significant reductions in the number of WREs over the five testing blocks (F4, 264= 4.04, p = 0.003). The effect of testosterone treatment was marginally significant (Fig. 5C; F1,66 = 2.37, p = 0.061), with differences between groups showing a pattern similar to that observed for WMEs. The final testing block (days 21-25) was the only block to show a significant treatment effect for WREs (Fig. 5D; F4, 70 = 4.98, p = 0.001). Within this block, the Oil GDX group performed significantly more WREs than all of the other groups (all p < 0.05) except the 0.250 mg T (p = 0.052).
Fig. 5.
Mean (± SEM) number WREs performed by male rats injected daily with sesame oil (Sham and GDX) or one of four doses of testosterone propionate during 5-day blocks of trials over 25 days of testing in an 8-arm radial maze (n = 13-15/group). (A) The Oil Sham and Oil GDX groups showed no significant effect of testing blocks. The Oil GDX group performed significant more WREs overall than did the Oil Sham group (p = 0.022), and this difference was particularly apparent during the last two testing blocks (* p < 0.05). (B) Comparing the Oil GDX group to the testosterone-treated groups showed significant improvements over the testing blocks (p = 0.003). Analyses within blocks showed significant differences between groups during the final block of testing (*p < 0.05). (C) There was no significant main effect of treatment (p = 0.061). (D) Analyzing only the final testing block (days 21-25) showed that the Oil GDX group performed significantly more WREs than did the 0.125 mg T, 0.500 mg T, and the 1.00 mg T groups. Letters designate groups that differed significantly from each other (p < 0.05).
3.2. Experiment 2: Testosterone had dose-dependent effects on OLMT performance
Seven rats from the Oil GDX group had serum testosterone concentrations below the detection limit for the assay (0.05 ng/ml), while the other four rats from this group had relatively low levels (0.23-0.40 ng/ml). Again, due to relatively low variance, this group was not included in statistical comparisons. Serum testosterone concentrations differed significantly among the other groups (Fig. 2B; F4, 53 = 23.85, p < 0.0005). Post-hoc comparisons showed that serum testosterone levels did not differ between the 0.125 mg T and the 0.250 mg T groups (p = 0.086) or between the Oil Sham and the 0.125 mg T groups (p = 0.83). All of the other pairwise comparisons were significant (all p < 0.005). The Oil Sham group had serum testosterone concentrations with a range of 0.40-8.4 ng/ml.
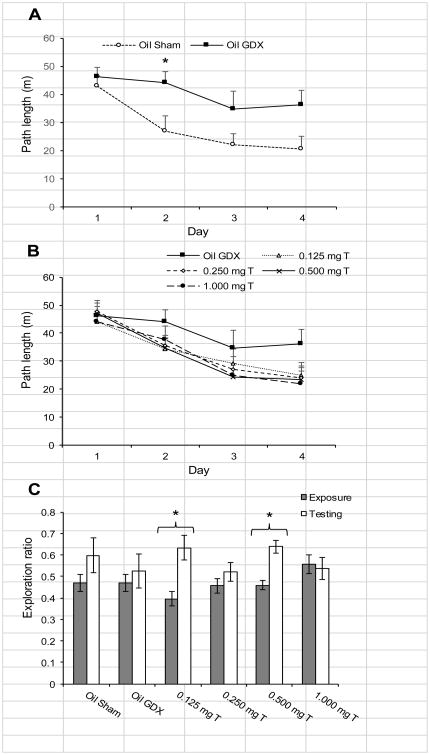
Comparing the Oil Sham to the Oil GDX group, there was a significant decrease in the path lengths over the first four days of habituation to the open field (Fig. 6A; F3,60 = 816.82; p < 0.0005), but there was no significant day × treatment interaction effect (p = 0.057) or treatment effect (p = 0.073). Comparisons within each day revealed that the Oil GDX group has significantly longer path lengths on day 2 of habituation than did the Oil Sham group (t20 = 2.22, p = 0.038). Comparing the Oil GDX group to the testosterone-treated groups (Fig. 6B), also showed a significant decrease in path lengths over course of habituation (F3, 156 = 64.81, p < 0.0005), and there was no significant day × treatment interaction effect (p = 0.24) or treatment effect (p = 0.54). Analyses within each of the days separately showed no significant effect of treatment (all p > 0.18).
Fig. 6.
Mean (± SEM) behavioral data for male rats injected daily with sesame oil (Sham and GDX) or one of four doses of testosterone propionate during habituation and testing on the OLMT (n = 11-12). (A) Over the four days of habituation to the open field prior to testing, the Oil Sham and Oil GDX groups showed a significant decline in the distance traveled (p < 0.0005). There was no effect of treatment on path length (p = 0.073), but the Oil GDX group traveled further on day 2 of habituation than did the Oil Sham group. (B) Comparing the Oil GDX group to the testosterone-treated groups showed a significant decrease in path lengths over course of habituation (p < 0.0005) but no effect of treatment. (C) During OLMT testing, only the 0.125 mg T and 0.500 mg T groups showed a significant increase in exploration ratios from the exposure phase to the testing phase (*p < 0.05).
The exploration ratios during the testing phase did not differ significantly among the groups (Fig. 4B; p = 0.43). Note that an exploration ratio above 0.5 indicates a bias toward exploring the moved object. Only the 0.125 mg T and 0.500 mg T groups had a significantly higher exploration ratio during the testing phase than during the exposure phase (Fig. 3B; t10 = 3.27, p = 0.008 and t10 = 4.09, p = 0.002, respectively), whereas none of the other groups demonstrated significant differences in the exploration ratios for the two phases of testing (all p > 0.16).
4. Discussion
Although the cognitive effects of testosterone on men remain ambiguous (Puts et al., 2010; Young et al., 2010), substantial research has shown that androgen replacement therapy provides cognitive benefits for some clinical populations (Cherrier et al., 2009; Nead et al., 2017). Our results demonstrated that testosterone had some dose-dependent effects on spatial memory in male rats. On the 8-arm RAM, castration impaired spatial working memory and some doses of testosterone restored working memory. On the OLMT, some doses of testosterone improved long-term spatial memory. However, castration and testosterone replacement had no effect on spatial reference memory as measured on the RAM. These results are consistent with past findings using these tasks (McConnell et al., 2012; Spritzer et al., 2011). Importantly, using two separate populations of rats, we found that the same doses of testosterone improved spatial memory on both the RAM and OLMT. The dose-response relationship was complex, with a low physiological dose of testosterone (0.125 mg) and a high physiological dose (0.500 mg) improving memory while an intermediate dose (0.250 mg) did not improve memory relative to that observed in the castrated control group (Oil GDX). The effects of the supra-physiological dose of testosterone (1.00 mg) were less consistent, with some evidence of working memory improvements on the RAM but memory impairment on the OLMT. The complexity of this dose-response relationship may help explain why some past studies with humans have failed to observe effects of testosterone on spatial memory (Alexander et al., 1998; Young et al., 2010).
4.1. Testosterone replacement improves spatial memory
Our results using the RAM add to past experiments indicating that testosterone enhances spatial working memory. Consistent with our current findings, we previously demonstrated that castrated male rats had increased WMEs and WREs on the RAM relative to intact males (Spritzer et al., 2008) and that a testosterone dose of 0.500 mg restored working memory in castrated males (Spritzer et al., 2011). Other researchers have shown that castration impairs working memory on the RAM (Daniel et al., 2003; Gibbs and Johnson, 2008). Likewise, some past experiments have demonstrated that testosterone replacement restores spatial working memory in castrated male rats (Gibbs, 2005; Locklear and Kritzer, 2014). Although we did not observe a significant day × treatment interaction, within-block analyses showed that the strongest differences between groups occurred during the last five days of testing on the RAM. This suggests that the Oil GDX group was unable to learn the working memory component of the task over the 25 days of testing, whereas some of the testosterone-treated groups displayed very few WMEs by the final block of testing.
Past studies with the RAM indicate that testosterone has no effect on reference memory. For example, we previously found no effect of a 0.500 mg dose of testosterone on RMEs relative to the castrated control group (Spritzer et al., 2011). This may be due to a ceiling effect, because with only four arms baited, rats can commit a maximum of only four RMEs per day. The reference memory component of the RAM seems to be difficult for rats to learn, as we have previously found minimal change in the number of RMEs over the course of testing (Spritzer et al., 2011, 2008). The reference memory component of the RAM involves remembering the rewarded cups for about 24 h. Rats can readily learn the reference memory version of the MWM, which also typically uses a 24 h delay between trials. Some past studies with the MWM have shown that testosterone replacement improves reference memory (Jia et al., 2013; Spritzer et al., 2011). The difference between RAM and MWM results for reference memory may be due to differences in the nature of the motivation for these two tasks—appetitive vs. aversive motivation, respectively. The term “reference memory” was developed by researchers working with rodents in order to operationally define memory errors after a 24 h delay (Olton and Papas, 1979), but reference memory is simply a specific case of long-term memory (Cowan, 2008). Our results for the OLMT showed that testosterone replacement can restore spatial memory on a task involving a 2 h inter-trial interval in castrated male rats. Other experiments with the OLMT, employing a 2 h interval (Jacome et al., 2016) and a 30 min interval (McConnell et al., 2012), have also shown that testosterone replacement restored spatial memory in castrated male rats. Thus, in spite of our finding that testosterone replacement does not influence the number of RMEs on the RAM, there is evidence that testosterone influences long-term memory on other tasks.
One noteworthy difference between our results obtained with the RAM and those obtained with OLMT was that the Oil Sham group out-performed the Oil GDX group on the working memory component of the RAM, whereas both the Oil Sham and Oil GDX groups both failed to distinguish between the moved and unmoved objects on the OLMT task. This suggests that while testosterone may restore normal working memory following castration, it may actually improve long-term memory above pre-castration levels. A study of aged male rats using a radial-arm water maze demonstrated that testosterone implants improved both working and reference memory beyond that observed in an aged sham group (Bimonte-Nelson et al., 2003). Some human studies have shown that androgen deprivation impairs spatial memory in young men (Cherrier et al., 2009). One recent study demonstrated that testosterone replacement therapy improved memory in hypogonadal young men (Lašaitė et al., 2017), but two other studies found that short-term testosterone supplementation given to eugonadal young men had no effect on spatial memory (Alexander et al, 1998; Young et al., 2010). Therefore, beyond our findings, there is little evidence that supplementary testosterone can improve spatial memory above baseline abilities in males, but more work is needed to determine the relative effects of testosterone on different forms of memory.
4.2. Dose-dependent effects of testosterone
We observed a complex dose-response relationship between testosterone and spatial memory. The 0.125 and 0.500 mg testosterone doses improved spatial memory, but the 0.250 dose did not. Among the two experiments, serum testosterone levels in the Oil Sham group ranged from 0.2-8.4 ng/ml. The average testosterone levels of the 0.125 and 0.250 mg T groups fell within this range, while the 0.500 mg T group had averages that were slightly above this range. Past work indicates that serum testosterone levels in young adult male rats range from 0.50-15.0 ng/ml, with considerable circadian variability (Bartke et al., 1973; Mock et al., 1978). Therefore, our doses ranged from mid-range physiological (0.125 mg T group) to high physiological (0.500 mg T group). Our highest dose (1.00 mg T group) resulted in testosterone levels that were about 5 ng/ml higher than the highest values previously reported for adult male rats, and they were 13 × and 8 × greater than the mean values that we observed in the Oil Sham group for Experiment 1 and 2, respectively. Thus, our highest dose was in the supra-physiological range.
A review of previous experiments with male rats provides some support for dose-dependent effects of testosterone and spatial memory. For instance, Hawley et al. (2013) gave castrated rats two doses of testosterone followed by testing on a Y-maze, and they found that both doses of testosterone restored long-term spatial memory (24 h inter-trial interval). Based on serum testosterone levels, their high dose was comparable to our 0.125 mg T group (Hawley et al., 2013). We have not tested the effects of doses below 0.125 mg T on the RAM or OLMT, but we previously found that a lower dose of testosterone (0.0625 mg/rat) was sufficient to restore performance among castrated males on the MWM (Spritzer et al., 2011). Presumably, there is a lower limit to the memory-restoring effects of testosterone replacement, but this has not yet been demonstrated using rats. Jia et al. (2013) documented a negative parabolic relationship between testosterone dose and spatial memory using the MWM. Similar to our findings, they demonstrated that a testosterone dose of 0.250 mg/rat resulted in slightly worse performance than rats receiving 0.500 or 0.750 mg. They also found that supra-physiological doses (1.00 mg and above) impaired performance relative to the lower doses. For our 1.00 mg T group, we found evidence for memory impairment in Experiment 2, but by the final block of testing on the RAM, this group was performing significantly fewer WMEs than the Oil GDX group. This suggests that the supra-physiological doses of testosterone may selectively impair long-term memory while still restoring spatial working memory. Interestingly, when we tested rats using reversal-learning trials on the MWM, rats in the 0.250 and 1.000 mg T groups showed significantly elevated perseverative behavior, swimming where the old goal platform had been (Spritzer et al., 2011). Perhaps some of the memory impairments that we observed in the current study among the same groups were due to elevated perseverance. Specifically, poorly performing rats may have been revisiting previously visited arms in the RAM and exploring the previously explored objects on the OLMT.
Some human research supports the conclusion that the effects of testosterone on cognition are dose-dependent. A relatively low threshold for the effects of testosterone might explain why some studies with eugonadal men have failed to demonstrate a correlation between circulating testosterone levels and spatial ability (Puts et al., 2010), whereas studies with hypogonadal men have shown that testosterone replacement improves spatial ability (Cherrier et al., 2007; Lašaitė et al., 2017). Cherrier et al. (2007) found that a moderate increase in serum testosterone improved spatial memory relative to men who experienced no change or a large change in testosterone levels. This result is supported by our finding that the 1.000 mg T group performed the same as the Oil GDX group in Experiment 2. Prolonged use of anabolic-androgenic steroids impairs memory in men, suggesting that chronic exposure to supra-physiological levels of androgens impairs memory (Kaufman et al., 2015). In contrast, another study that gave five different doses of testosterone to healthy elderly men found that spatial memory improved only in the group receiving the highest dose, which increased circulating testosterone levels by nearly 10-fold (Gray et al., 2005). This suggests that the optimal dose of testosterone may differ between elderly and young populations.
Different testosterone doses may be differentially influencing various brain regions to enhance or impair spatial memory. Rats can use either a place strategy or a response strategy to solve spatial tasks (Eichenbaum et al., 1990). A place strategy involves using knowledge about the positions of environmental cues relative to a goal and relative to one's own position to locate the goal, whereas a response strategy involves using stimulus-response relationships to locate a goal (Packard and McGaugh, 1996). A place strategy relies on the hippocampus, and a response strategy relies on the striatum (Packard and McGaugh, 1996), and androgen and estrogen receptors are expressed in both of these brain regions in male rats (Patchev et al., 2004). Therefore, testosterone or its metabolites may influence spatial memory by acting directly on receptors in the hippocampus and/or striatum. A low dose of testosterone (0.125 mg) biased male rats toward using a response strategy, while a high dose (0.500 mg) caused a bias toward a place strategy (Spritzer et al., 2013). The 0.250 mg dose did not cause a strong bias toward using either strategy (Spritzer et al., 2013). This suggests that the 0.250 mg T group performed relatively poorly in the current experiments because it was incapable of employing either a response or a place strategy effectively. A simple turn bias would always result in some WMEs on the RAM, but rats in our 0.125 mg T group could have learned a motor response to effectively minimize errors. In support of this idea, Gibbs and Johnson (2008) found that rats given a testosterone dose comparable to our 0.125 mg T group performed worse than intact control rats on the RAM when they were prevented from using a response strategy. Experiments with female rats have shown that intra-hippocampal infusions of estradiol improved place learning, while intra-striatal infusions of estradiol impaired response learning (Zurkovsky et al., 2007). A reasonable hypothesis is that low physiological doses of testosterone enhance striatal activity to improve response learning, while high physiological doses of testosterone enhance hippocampal activity and impair striatal activity to improve place learning.
Estradiol and dihydrotestosterone (DHT), testosterone's major metabolites, bind to estrogen and androgen receptors, respectively (Edinger et al., 2004). It is possible that differences in the relative effects of estrogens and androgens on the brain could explain the dose-dependent effects that we observed. In support of a role for androgen receptors, studies have shown that both androgen receptor mutation (i.e., testicular feminization) and selective androgen receptor knockout impair spatial memory in adult male rodents (Jones and Watson, 2005; Picot et al., 2016; Rizk et al., 2005). Furthermore, DHT—but not estradiol—has been shown to enhance synaptic density and neurogenesis within the hippocampus of male rats (Leranth et al., 2003; MacLusky et al., 2006; Spritzer and Galea, 2007). DHT replacement has been shown to improve spatial memory in a water radial-arm maze (Bimonte-Nelson et al., 2003) and the OLMT (McConnell et al., 2012). However, estradiol treatments have also been shown to improve performance on the RAM (Luine and Rodriguez, 1994) and OLMT (McConnell et al., 2012). A study with the Barnes maze found that although testosterone and estradiol both improved performance relative to a castrated control group, the estradiol-treated male rats preferred a response strategy and the testosterone-treated male rats preferred a place strategy (Locklear et al., 2015). Interestingly, clinical studies involving men being treated for prostate cancer suggest that estradiol supplementation impairs memory (Beer et al., 2006; Matousek and Sherwin, 2010). Thus, current evidence indicates that testosterone can influence spatial memory through both androgen and estrogen pathways, but the relative impacts of estrogens and androgens on spatial memory in men remains unclear.
Besides possible direct effects of testosterone or its metabolites upon various brain regions, another explanation for our results involves testosterone's indirect effects upon anxiety. Experiments with male rats have shown that stress hormones and sex hormones interact at multiple levels (Viau, 2002). For example, castration leads to elevated corticosterone and this effect is reversed by testosterone replacement (Viau and Meaney, 1996). Whereas testosterone enhances spatial memory, the stress hormone corticosterone impairs spatial memory (Conrad et al., 2004). Furthermore, intra-hippocampal injections of an androgen-receptor antagonist caused an increase in anxiety (Edinger and Frye, 2004), suggesting that testosterone also reduces the stress response by acting directly on the brain. Interestingly, higher testosterone doses (0.500 and 1.00 mg) were found to reduce anxiety on a burying task, whereas a lower dose (0.250 mg) did not (Fernández-Guasti and Martínez-Mota, 2005). Thus the memory-enhancing effects of the higher doses of testosterone that we observed may be partially due to the anxiolytic effects of testosterone. We did not test anxiety directly in our experiment, but we do have some evidence that anxiety did not play a major role in our findings. For Experiment 1, there was no effect of treatment on time to first arm entry or rate of arm entry. For Experiment 2, the Oil GDX group did habituate more slowly to the open field than did the Oil Sham group, but there were no significant differences among the testosterone-treated groups in their exploratory behavior in the open field.
4.3. Conclusion
We demonstrated that certain doses of testosterone given to castrated adult male rats restored working memory and improved one form of long-term memory. This adds to a growing number of studies with rodents and humans that have demonstrated the memory-enhancing effects of testosterone replacement (Hamson et al., 2016). Additionally, we documented a complex dose-response relationship, with an intermediate dose of testosterone causing poorer spatial memory relative to lower and higher doses. This may be due to certain doses of testosterone optimizing the use of particular spatial strategies or differentially influencing perseverative behavior. Recent large-scale clinical studies have demonstrated many potential benefits of androgen-replacement therapy for hypogonadal men (e.g., Konaka et al., 2016), but the dose-dependent effects of chronic testosterone therapies upon cognition should be considered carefully.
Research Highlights.
Testosterone replacement restored spatial working memory in castrated male rats.
Testosterone replacement had no effect on reference memory in castrated male rats.
Testosterone replacement improved long-term memory in castrated male rats.
High and low physiological doses of testosterone had positive effects on memory.
A supra-physiological dose of testosterone had some positive effects on memory.
Acknowledgments
We thank Melissa Childs, Christina Chyr, Emily Goins, Eliza Jaeger, Charlotte Michaelcheck, Cyrus Jalai, Rita Pfeiffer, Amanda Reis, and Summer Spillane for their assistance with data collection. We thank Vicki Major and the animal care staff for their assistance, and we thank Mark Stefani and Tony Desautels for help with the design and construction of the open field and RAM. Jan Thompson helped us develop the OLMT protocol.
Funding: This project was funded by Middlebury College, the Vermont Genetics Network (INBRE grant number 2P20RR016462) from the INBRE Program of the National Center for Research Resources (NCRR), and the National Institute of Aging (NIH AREA grant number 1R15AG042155). The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies. The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the report for publication.
Footnotes
Contributors: B.A Wagner, V.C. Braddick and M.D. Spritzer contributed to the design of this study and to the writing of the manuscript. B.A. Wagner, V.C. Braddick, C.B. Batson, B.H. Cullen, and L.E. Miller all made substantial contributions to data collection. All co-authors contributed to data analysis and interpretation, provided input on revisions of this manuscript, and approved the final version of the submitted article.
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M. Androgen–behavior correlations in hypogonadal men and eugonadal men. Horm Behav. 1998;33:85–94. doi: 10.1006/hbeh.1998.1439. https://doi.org/10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92:1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- Beer TM, Bland LB, Bussiere JR, Neiss MB, Wersinger EM, Garzotto M, Ryan CW, Janowsky JS. Testosterone loss and estradiol administration modify memory in men. J Urol. 2006;175:130–135. doi: 10.1016/S0022-5347(05)00049-2. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm ACE. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol. 2003;181:301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Bull R, Davidson WA, Nordmann E. Prenatal testosterone, visual-spatial memory, and numerical skills in young children. Learn Individ Differ. 2010;20:246–250. https://doi.org/10.1016/j.lindif.2009.12.002. [Google Scholar]
- Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. https://doi.org/10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32:72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Sex Drugs. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. https://doi.org/10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Cost KT, Williams-Yee ZN, Fustok JN, Dohanich GP. Sex differences in object-in-place memory of adult rats. Behav Neurosci. 2012;126:457–464. doi: 10.1037/a0028363. https://doi.org/10.1037/a0028363. [DOI] [PubMed] [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? Prog Brain Res. 2008;169:323–338. doi: 10.1016/S0079-6123(07)00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitive enhancing effects may be due in part to actions of its 5a-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5α-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris RGM. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian S, Naghdi N, Sepehri H, Jahanshahi M, Sadeghi Y, Choopani S. Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behav Brain Res. 2010;208:30–37. doi: 10.1016/j.bbr.2009.11.004. https://doi.org/10.1016/j.bbr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJJ, Pour HRN, Aleman A, Lock TMTW, Bosch JLHR, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men. J Am Med Assoc. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Martínez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. https://doi.org/10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PB, Singh aB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, Dzekov C, Sinha-Hikim I, Bhasin S. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90:3838–3846. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- Hamson DK, Roes MM, Galea LAM. Comprehensive Physiology. John Wiley & Sons, Inc; 2016. Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. https://doi.org/10.1002/cphy.c150031. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CLS, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63:559–565. doi: 10.1016/j.yhbeh.2013.02.007. https://doi.org/10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: A review. Maturitas. 2011;69:322–337. doi: 10.1016/j.maturitas.2011.05.012. https://doi.org/10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 cell morphology in rats. Horm Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology. 2016;157:1357–1362. doi: 10.1210/en.2015-1959. https://doi.org/10.1210/en.2015-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Kang L, Li S, Geng D, Fan P, Wang L, Cui H. Amelioratory effects of testosterone treatment on cognitive performance deficits induced by soluble Aβ1-42 oligomers injected into the hippocampus. Horm Behav. 2013;64:477–486. doi: 10.1016/j.yhbeh.2013.08.002. https://doi.org/10.1016/j.yhbeh.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiology Behav. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, Jensen JE, Pope HG. Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015;152:47–56. doi: 10.1016/j.drugalcdep.2015.04.023. https://doi.org/10.1016/j.drugalcdep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SB. Sex differences in mental rotation and spatial visualization ability: Can they be accounted for by differences in working memory capacity? Intelligence. 2007;35:211–223. [Google Scholar]
- Khalil R, King MA, Soliman MRI. Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology. 2005;75:87–92. doi: 10.1159/000087188. [DOI] [PubMed] [Google Scholar]
- Konaka H, Sugimoto K, Orikasa H, Iwamoto T, Takamura T, Takeda Y, Shigehara K, Iijima M, Koh E, Namiki M EARTH study group. Effects of long-term androgen replacement therapy on the physical and mental statuses of aging males with late-onset hypogonadism: a multicenter randomized controlled trial in Japan (EARTH Study) Asian J Androl. 2016;18:25–34. doi: 10.4103/1008-682X.148720. https://doi.org/10.4103/1008-682X.148720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirilis T. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lašaitė L, Čeponis J, Preikša RT, Žilaitienė B. Effects of two-year testosterone replacement therapy on cognition, emotions and quality of life in young and middle-aged hypogonadal men. Andrologia. 2017;49:n/a–n/a. doi: 10.1111/and.12633. https://doi.org/10.1111/and.12633. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Bhamidipaty S, Kritzer MF. Local N-methyl-d-aspartate receptor antagonism in the prefrontal cortex attenuates spatial cognitive deficits induced by gonadectomy in adult male rats. Neuroscience. 2015;288:73–85. doi: 10.1016/j.neuroscience.2014.12.032. https://doi.org/10.1016/j.neuroscience.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav. 2014;66:298–308. doi: 10.1016/j.yhbeh.2014.06.006. https://doi.org/10.1016/j.yhbeh.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. https://doi.org/10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrus cycle. J Neurosci. 1999;15:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek RH, Sherwin BB. A randomized controlled trial of add-back estrogen or placebo on cognition in men with prostate cancer receiving an antiandrogen and a gonadotropin-releasing hormone analog. Psychoneuroendocrinology. 2010;35:215–225. doi: 10.1016/j.psyneuen.2009.06.012. https://doi.org/10.1016/j.psyneuen.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SEA, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm Behav. 2012;61:479–486. doi: 10.1016/j.yhbeh.2012.01.003. https://doi.org/10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Mock EJ, Norton HW, Frankel AI. Daily rhythmicity of serum testosterone concentraion in the male laboratory rat. Endocrinology. 1978;103:1111–1121. doi: 10.1210/endo-103-4-1111. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Muller M, Aleman A, Grobbee DE, de Haan EHF, van der Schouw YT. Endogenous sex hormone levels and cognitive function in aging men. Neurology. 2005;64:866–871. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH. Association between androgen deprivation therapy and risk of dementia. JAMA Oncol. 2017;3:49–55. doi: 10.1001/jamaoncol.2016.3662. https://doi.org/10.1001/jamaoncol.2016.3662. [DOI] [PubMed] [Google Scholar]
- Nowak NT, Diamond MP, Land SJ, Moffat SD. Contributions of sex, testosterone, and androgen receptor CAG repeat number to virtual Morris water maze performance. Psychoneuroendocrinology. 2014;41:13–22. doi: 10.1016/j.psyneuen.2013.12.003. https://doi.org/10.1016/j.psyneuen.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of the hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Schroeder J, Goetz F, Rohde W, Patchev AV. Neurotropic action of androgens: principles, mechanisms and novel targets. Exp Gerontol, Proceedings of the Seventh International Symposium on the Neurobiology and Neuroendocrinology of Aging. 2004;39:1651–1660. doi: 10.1016/j.exger.2004.07.011. https://doi.org/10.1016/j.exger.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Picot M, Billard JM, Dombret C, Albac C, Karameh N, Daumas S, Hardin-Pouzet H, Mhaouty-Kodja S. Neural androgen receptor deletion impairs the temporal processing of objects and hippocampal CA1-dependent mechanisms. PloS One. 2016;11:e0148328. doi: 10.1371/journal.pone.0148328. https://doi.org/10.1371/journal.pone.0148328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts DA, Cardenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM. Salivary testosterone does not predict mental rotation performance in men or women. Horm Behav. 2010;58:282–289. doi: 10.1016/j.yhbeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Rizk A, Robertson J, Raber J. Behavioral performance of tfm mice supports the beneficial role of androgen receptors in spatial learning and memory. Brain Res. 2005;1034:132–138. doi: 10.1016/j.brainres.2004.12.002. https://doi.org/10.1016/j.brainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Silverman I, Kastuk D, Choi J, Phillips K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology. 1999;24:813–822. doi: 10.1016/s0306-4530(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59:484–496. doi: 10.1016/j.yhbeh.2011.01.009. https://doi.org/10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Fox EC, Larsen GD, Batson CG, Wagner BA, Maher J. Testosterone influences spatial strategy preferences among adult male rats. Horm Behav. 2013;63:800–812. doi: 10.1016/j.yhbeh.2013.03.018. https://doi.org/10.1016/j.yhbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LAM. Castration differentially affects spatial working and reference memory in male rats. Arch Sex Behav. 2008;37:19–29. doi: 10.1007/s10508-007-9264-2. [DOI] [PubMed] [Google Scholar]
- Thilers PP, MacDonald SWS, Herlitz A. The association between endogenous free testosterone and cognitive performance: A population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Ulubaev A, Lee DM, Purandare N, Pendleton N, Wu FCW. Activational effects of sex hormone on cognition in men. Clin Endocrinol (Oxf) 2009;71:607–623. doi: 10.1111/j.1365-2265.2009.03562.x. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Jounral Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress in mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Vermaercke B, de Beeck HO, Wagemans J, Gantois I, D'Hooge R, Swinnen SP, Wenderoth N. Sex differences in human virtual water maze performance: Novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 2010;208:408–414. doi: 10.1016/j.bbr.2009.12.019. https://doi.org/10.1016/j.bbr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- Young LA, Neiss MB, Samuels MH, Roselli CE, Janowsky JS. Cognition is not modified by large but temporary changes in sex hormones in men. J Clin Endocrinol Metab. 2010;95:280–288. doi: 10.1210/jc.2009-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. https://doi.org/10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]