Abstract
In adult rats, erythropoietin improved outcomes early and late after traumatic brain injury, associated with increased levels of Brain Derived Neurotrophic Factor. Using our model of pediatric traumatic brain injury, controlled cortical impact in 17-day old rats, we previously showed that erythropoietin increased hippocampal neuronal fraction in the first two days after injury. Erythropoietin also decreased activation of caspase3, an apoptotic enzyme modulated by Brain Derived Neurotrophic Factor, and improved Novel Object Recognition testing 14 days after injury. Data on long-term effects of erythropoietin on Brain Derived Neurotrophic Factor expression, histology and cognitive function after developmental traumatic brain injury are lacking. We hypothesized that erythropoietin would increase Brain Derived Neurotrophic Factor and improve long-term object recognition in rat pups after controlled cortical impact, associated with increased neuronal fraction in the hippocampus. Methods: Rats pups received erythropoietin or vehicle at 1, 24, and 48 hours and 7 days after injury or sham surgery followed by histology at 35 days, Novel Object Recognition testing at adulthood, and Brain Derived Neurotrophic Factor measurements early and late after injury. Results: Erythropoietin improved Novel Object Recognition performance and preserved hippocampal volume, but not neuronal fraction, late after injury. Conclusions: Improved object recognition in erythropoietin treated rats was associated with preserved hippocampal volume late after traumatic brain injury. Erythropoietin is approved to treat various pediatric conditions. Coupled with exciting experimental and clinical studies suggesting it is beneficial after neonatal hypoxic ischemic brain injury, our preliminary findings support further study of erythropoietin use after developmental traumatic brain injury.
Keywords: controlled cortical impact, erythropoietin, developmental, apoptosis, neurocognitive impairment
Pediatric traumatic brain injury (TBI) is the leading cause of traumatic death and disability in children. [1] Neurocognitive deficits in these young survivors most commonly manifest as impairments in learning and executive function domains, such as processing speed and declarative memory. [2, 3] No therapies in use today directly improve neurologic outcome after pediatric TBI.
Erythropoietin (EPO) is a pleiotropic cytokine produced in the kidney and central nervous system. It mediates cell survival and proliferation in erythrocytes systemically and in neurons, astrocytes, and immune cells of the developing and mature central nervous system. EPO also modulates inflammation and angiogenesis. Upregulation of cerebral EPO and its receptor in various animal models led to the hypothesis that exogenous EPO could exert neuroprotection in the setting of brain injury. [4] A number of subsequent studies support EPO administration in models of adult and developmental brain injury. [5, 6]
In adult rats, EPO improved functional and histologic outcome at short- and long-term time points after TBI. [7, 8] In our developmental TBI model using controlled cortical impact (CCI) in 17-day old rats, EPO improved performance on the Novel Object Recognition (NOR) task at post injury day (PID) 14 and decreased caspase activation, associated with increased neuronal fraction in the PID 1 and 2 hippocampus. [9]Data on EPO’s effects on long-term cognitive function or histology after developmental TBI are lacking.
EPO appears to exert some of its neuroprotection via increased Brain Derived Neurotrophic Factor (BDNF) transcription in the injured brain. BDNF, a neurotrophin that regulates neuronal development, survival and function, is important for normal learning and memory throughout the lifespan. [10] Using an in vitro neurotoxicity model, Viviani et al found that EPO time-dependently increased neuronal BDNF mRNA and greatly increased neuronal survival, the latter abrogated by pretreatment with a neutralizing anti-BDNF antibody. Viviani et al also demonstrated that EPO administration increased BDNF transcription in vivo in the adult rodent brain. [11] In the developing rat brain, intraperitoneal (IP) co-administration of EPO reduced MK-801-induced apoptotic neurodegeneration and preserved BDNF mRNA at 24 hours after injection, relative to co-administration of vehicle. [12] Finally, IP administration of EPO improved spatial learning and increased BDNF brain protein levels in the adult rat 35 days after experimental TBI using CCI. [13]
Declarative memory, the conscious memory for facts and events, is an important executive function. Declarative memory is commonly impaired in adults and children after TBI. [14, 15] In rodents, the Novel Object Recognition (NOR) task is the preferred method by which to test a critical component of declarative memory, object recognition. [16]
We hypothesized that, in rat pups after CCI, EPO would increase hippocampal BDNF mRNA early after administration and improve object recognition at adulthood (PID 50, or age 9–10 weeks old) as shown by the NOR task. Based on published histologic outcomes in adult rats treated with EPO after CCI, we measured brain volumes, hippocampal neuronal fraction and astrocytosis at PID 35. We measured BDNF mRNA at time points after EPO dosing, namely at PID 1, 2, 3 and 7.
Methods
Animal Model
The Animal Care and Use Committee at the University of Utah approved protocols, according to NIH guidelines. Male Sprague-Dawley rats were obtained from Charles Rivers Laboratories (Raleigh, NC) on post-natal day (P) 7–10 and housed in litters of 10 with the dam until weaning on P 21–23 in a temperature- and light-controlled environment.
Distributed evenly within litters to control for maternal rearing characteristics, rat pups were randomized to CCI or SHAM craniotomy at P17, followed by EPO or vehicle (VEH) injection to create three experimental groups: EPOCCI, VEHCCI and SHAMVEH. After weaning, rats were placed in cages without segregation by experimental group.
CCI was performed as described, [9] modified only by decreased deformation depth to 1.5 mm rather than 2mm to address the near-obliteration of injured hippocampus found late after CCI. 6–8 rats per group were used at each time point for molecular and functional studies. Rats anesthetized using a Vet Equip Bench Top Isoflurane Anesthesia System (Pleasanton, CA) were placed into a stereotaxic frame (David Kopf, Tujunga, CA). Rectal temperature was kept at 37±0.5 °C via servo-controlled heating pad. A 6-mm×6-mm craniotomy was performed over the left parietal cortex (centered 4-mm posterior and 4-mm lateral to Bregma) with care taken not to perforate the dura. Isoflurane was reduced to 1% for 5-min followed by delivery of CCI (Pittsburgh Precision Instruments, Pittsburgh, PA) to the left parietal cortex via a 5-mm rounded tip to create a 1.5-mm at 4 m/s velocity during 100 ms. Isoflurane was returned to 2–2.5%, and the bone flap replaced and secured (Patterson Dental, Salt Lake City, UT). SHAM rats underwent identical surgical craniotomy, equilibration, and closure procedures without CCI.
Drug Dosing
RhEPO, recombinant human erythropoietin (Procrit®, Amgen Inc, CA) 5000 U/kg or an equal volume of vehicle was dosed intraperitoneally (IP) at 1, 24, and 48 hours after CCI and again at PID 7. [9] As described previously, the rationale for RhEPO dosing was based on its pharmacokinetics and on published dosing regimens successfully used in adult rats after TBI and in neonatal rats after hypoxic ischemic brain injury. In the 7 day old and adult rat, 5000 U/kg Rh EPO given IP reliably produces measurable brain levels of EPO. [17, 18] Rh EPO takes between 3 and 9 hours to peak in the brain. Therefore, we chose to give the first dose one hour after injury so it would be both clinically relevant and likely to produce an effect on early cell death. [18] We gave two more doses, one at 24 and the other at 48 hours after injury, to coincide with the expected peak in apoptotic cell death in the immature brain and to match typical regimens used in adult rat TBI. [13, 19, 20] Finally, we added a fourth dose based on data that an additional dose of Rh EPO at PID 7 improved long-term outcome in a rat model of neonatal stroke. [21]
Cognitive Outcome
NOR was tested at PID 50 to evaluate object recognition at adulthood (9–10 weeks old or post-natal day 67). As appropriate for adult rats, we used a larger chamber (70 × 70 × 30cm) as described previously.[22] [9] We used a black painted wooden box, rather than the white Plexiglas described in Reger et al, to minimize glare and to decrease distractions. Object interaction was defined as the time the rat directed its nose at a distance less than 2cm from the object and measured using EthoVision® v 7.1, confirmed by dual observer videotape analysis when needed. Non-exploration was defined as described in Reger et al. Rats underwent Habituation (exposure to the empty chamber) for ten minutes per day for two days after which none showed stress-associated behaviors. Two objects, equally spaced from diagonally-facing corners, were then introduced into the empty chamber. Each rat was sequentially placed in the chamber, at the same location and facing away from the objects, and allowed to explore the two objects for five minutes (Familiarization) before returning to its home cage. After a 22-hour delay, one object was changed and a Novel object placed in its location. Rats were again sequentially placed in the chamber in the same order and location as during the Familiarization phase. Rats were allowed explore these two objects, one Familiar and one Novel, for five minutes (Testing). Total exploration time, the sum of time spent exploring each of the two objects during the Familiarization and Testing phases was recorded for each rat. During Familiarization, time spent on each object should not vary by object and should equal approximately 50% of total exploration time. On the other hand, during Testing, rats with normal memory should spend more than 50% of the total exploration time on the Novel object. NOR performance was quantified using the Discrimination Ratio (N/N+F), where N is time spent exploring Novel and F is time spent exploring Familiar.[22–24] Values for the Discrimination ratio range from 0 to 1, where a score closer to 1 indicates greater preference for the Novel object while a score closer to 0 indicates preference for the familiar. Ratios greater than 0.5 support normal object recognition; generally, higher ratios suggest improved performance on the NOR. [23]
BDNF mRNA and Protein
Dissected hippocampi from the left (ipsilateral to injury) was labeled CCI or SHAM Ipsi, while the right (contralateral to injury) was labeled CCI or SHAM Con. Hippocampal BDNF mRNA and protein were measured using real-time RT-PCR and ELISA, respectively, at PID1,2,3, and 7. BDNF levels were also measured at PID 35 to coincide with the time of histologic testing, as was one in the study by Mahmood et al. [13]
Total RNA was extracted from frozen homogenized hippocampi using Nucleospin II (Macherey-Nagel Inc. Bethlehem, PA). cDNA was synthesized from 1 microgram of RNA and run on 7900HT Sequence Detection System (Applied Biosystems Foster City, CA) using the Rn 02531967_ s1 (Applied Biosystems) primer/probe set for BDNF using GAPDH for internal control and quantified by the comparative threshold cycle method.
For protein, hippocampi were homogenized in ice-cold lysis buffer. Supernatants obtained after centrifugation were tested using ELISA (BDNF Emax® ImmunoAssay System, Promega Corporation, WI) based on antibody raised against the 119 amino acid BDNF (accession# P23560). Optical density was measured using the GENiosPro reader (Tecan, Research Triangle Park, NC) and results expressed as picograms BDNF/milligrams of total protein loaded.
Histology
We evaluated histologic outcomes at PID 35 guided by results from TBI studies in adult rats that used similar EPO dosing regimens. [20] [13]
At PID 35, brains were fixed and cryoprotected using graded sucrose solutions, embedded in gelatin and frozen for immunofluorescence (IF). Frozen brains (n=4 per SHAM and n=5 per each CCI groups) were sliced into 20 micron thick coronal sections from −3 to −3.84 Bregma encompassing contused cortex. For lesion volume and astrocyte/neuron analyses, six and three sections 200 microns apart from each other were used, respectively. Sections were taken from comparable anatomical levels as by The Rat Brain Atlas in Stereotaxic Coordinates. [25] All slides were processed on the same day using freshly made reagents.
For lesion volume, slides were incubated with DiD’ oil 1:1000 (1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindodicarbocyanine Perchlorate, Catalog Number D307, Invitrogen Molecular Probes). Slides were imaged using Nikon A1 confocal (Melville, NY) microscope. Left and right hemispheric and hippocampal areas were drawn manually by a blinded observer (DR) as illustrated in Figure 3B using Image J (National Institutes of Health, Bethesda, Maryland, USA). We used average right sided hemispheric and hippocampal volumes for SHAMVEH rats to normalize corresponding volumes for VEHCCI and EPOCCI rats because CCI produces variable histologic injury on both hemispheres.
Figure 3. EPO Preserved Hippocampal Volume Late after CCI.
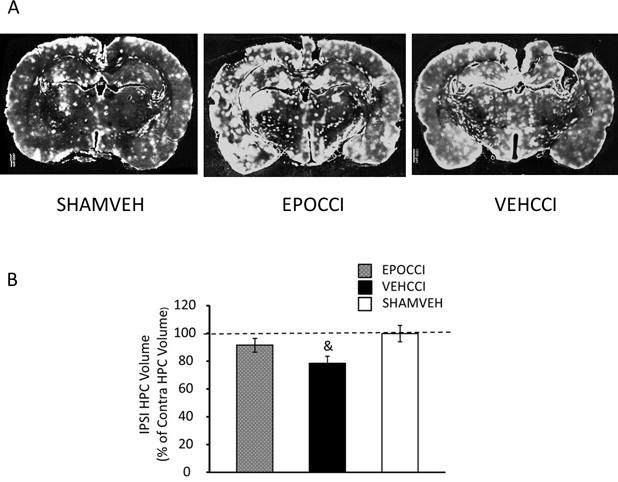
Fig 3A: Representative DiD’oil-stained brain slices (left to right) are from SHAMVEH, EPOCCI and VEHCCI rats at PID 35. Fig 3B: PID35 hippocampal volume ipsilateral to injury decreased in VEHCCI compared to EPOCCI. In contrast, PID35 hippocampal volume ipsilateral to injury did not differ between EPOCCI and SHAM. Ipsilateral hippocampal volumes are shown normalized to the average sham contralateral volume. Scale bar: 1000 μm
For IF, slides were blocked using 3% Normal Goat Serum (NGS, Immuno Jackson Technology). After primary and secondary antibody staining, slides were incubated with Orange Nucleic Acid Stain 1:30,000 (SYTOX® Molecular Probes OR). For hippocampal neuronal analyses, sections were incubated with anti-NeuN 1:500 (mouse monoclonal MAB377, Millipore, MA) followed by secondary goat anti-mouse 1:250 (AlexaFluor633®, Molecular Probes, OR).
Hippocampi were imaged using a Nikon A1 confocal (Melville, NY) microscope and 10× PLANAPO NA 1.45 Objective. Automated XY scanning and stitching of multiple fields was done using Nikon Elements software v4.1 Scan large Image module with 10% overlap. The CA1, CA3, DG sub-regions of interest (ROIs) ipsilateral and contralateral to injury were drawn manually (as shown schematically in Figure 1A in Supplementary Materials) after thresholding the SYTOX channel to separate dense nuclear areas from the surrounding tissue. For DG, area extended peripherally to the granule cell body layer by as much as one cell body layer, while for CA1 and CA3 the areas encompassed the width of one cell body layer on both sides of the stratum pyramidale. The box areas for CA1, CA3 and DG were 0.7, 0.55 and 0.47 mm2, respectively.
For neuronal fraction, images were imported into Imaris v4.5 (Bitplane, South Windsor, CT) for Spots analysis. [26, 27] [9] Spots analysis involves replacement of image data with a place marker (colored sphere) whenever two signals coincide, followed by counting of all spheres present in the ROI. NeuN- positive cells within each ROI were counted using colocalization of NeuN (specific for neuronal nuclei) and SYTOX (all nuclei) using Spots analysis. The region target size (2uM) used for Spots analysis was based on the average size of SYTOX positive labeling in NeuN positive cells. Neuron fraction was calculated as the ratio of NeuN+ cells to total cells in the ROI. A representative Image of Spots analysis is shown in Figure 1B (Supplementary Materials).
Figure 1. EPO Improved Performance on Novel Object Recognition Testing Late after CCI.
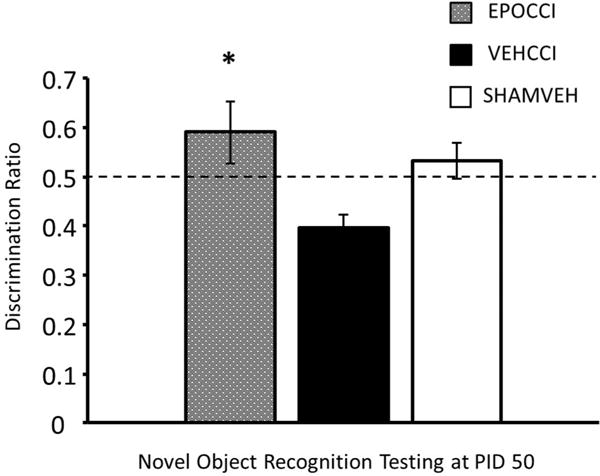
Results are presented as percent Novel Exploration Time ±SEM, for EPOCCI (gray bars), VEHCCI (black bars) and SHAMVEH (white bars); n=6–7/group and *p<0.05 relative to VEHCCI while & p<0.05 relative to SHAMVEH.
For hippocampal astrocyte counts and area, sections were incubated with anti-GFAP 1:250 (rabbit polyclonal ab 48050, Abcam, Cambridge MA) followed by secondary goat anti-rabbit 1:250 (Alexa Fluor 488®, Molecular Probes, OR). Low-magnification imaging and manual drawing of boxes for the hippocampal CA1, CA3 and DG ROIs were performed as for neuronal fraction (Supplementary Materials Figure 1A). GFAP + area was assessed using the same box for each of the 3 ROIs across groups, placing each box such that it encompassed the corresponding ROI at similar anatomic landmarks. After thresholding for GFAP + signal using a negative and positive control slice, the GFAP+ area was measured using Image J (National Institutes of Health, Bethesda, Maryland, USA) to assess area covered by the astrocyte bodies in the ROI. For astrocyte cell count, one cell were defined as the nucleus surrounded by GFAP signal. We performed pilot testing to compare manual counts of astrocytes in the entire ROI to that obtained by estimation based on manual counts within an increasing number of subdivisions in a fixed grid. Pilot results revealed that accurate total astrocyte counts in the CA1, CA3 and DG ROIs resulted from counting 50%, 60% and 75% of the grid boxes when these were chosen randomly (RAND function in Excel® by Microsoft). ROI astrocyte counts were performed by a blinded observer (DR) for each study group.
Statistics
All data are expressed as mean ± SE and/or as percentage of control. One-way ANOVA was performed followed by Sidak’s multiple comparisons test using GraphPad Prism version 7.03 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com). For histologic and molecular analyses, comparisons between the three groups were limited to samples from the same hemisphere using ANOVA. Comparisons within the same group for contralateral and ipsilateral hemispheres were done only for BDNF mRNA and protein, using a paired two-tailed t-test.
Results
EPO improved Object Recognition
During Familiarization, total Exploration time did not differ between groups, as expected: the average time per group that rats spent exploring was 32 ± 6, 35 ± 4, and 45 ± 8 seconds for the EPOCCI, VEHCCI and SHAMVEH groups, respectively. No rats exhibited lack of exploration as defined in the Methods. Similarly, rats did not manifest object preference: EPOCCI, VEHCCI and SHAMVEH rats spent 46 ± 4, 42 ± 5, and 40 ± 7% of total Exploration time, respectively, interacting with the object later to become the Familiar (during Testing) when exposed to it during the Familiarization phase.
In contrast, the Discrimination Ratio differed significantly between CCI groups at PID 50 as shown in Figure 1 (F (2, 17) = 4.97, p=0.02). EPOCCI explored longer than did VEHCCI (adjusted p=0.007). The Discrimination Ratio for each group time averaged 0.59 ± 0.06, 0.39 ± 0.03, and 0.53 ± 0.04 for EPOCCI, VEHCCI and SHAMVEH, respectively. Both EPOCCI and SHAMVEH rats demonstrated normal performance on the NOR, as shown by Discrimination Ratios greater than 0.5, while VEHCCI rats did not perform normally.
EPO increased hippocampal BDNF mRNA relative to VEHCCI at PID 3
In contralateral hippocampi, BDNF mRNA differed between groups at PID 1, 3 and 7. At PID3, EPO increased BDNF mRNA after injury ((F (2, 17) = 3.73, p=0.04). At PID1, increased BDNF mRNA in EPOCCI relative to VEHCCI approached significance (adjusted p=0.07) as shown in Figure 2A. At PID7, differences between groups (F (2, 21) =58, p<0.0001) were driven by injury but not treatment: BDNF mRNA decreased in both CCI hippocampi (p<0.0001 for EPOCCI and VEHCCI) relative to SHAMVEH as shown in Figures 2A and B. In ipsilateral hippocampi, differences in BDNF mRNA between groups at PID2 and PID7 were driven by injury and not treatment ((F (2, 21) = 5.49, p=0.01 and (F (2, 21) = 9.5, p=0.0015, at PID 2 and 7, respectively.
Figure 2. Effects of EPO on Early and Late Hippocampal BDNF mRNA Levels.
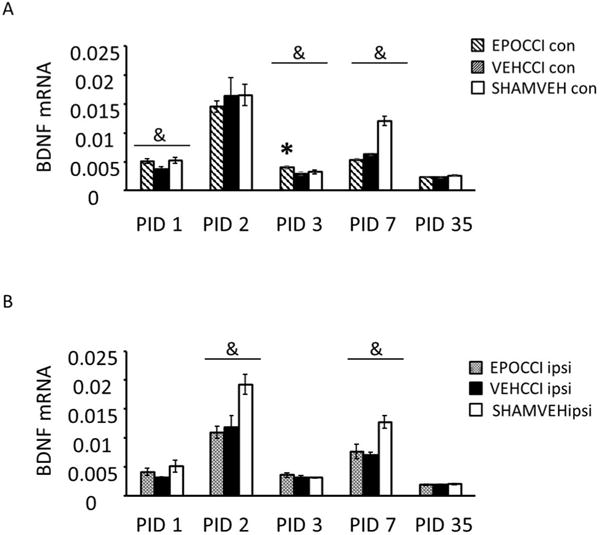
Hippocampal BDNF mRNA Levels in EPOCCI and VEHCCI rats obtained at post injury days (PID) 1, 2, 3, 7 and 35 presented as percentage of the corresponding SHAM mRNA. Contralateral and Ipsilateral hemispheres, abbreviated as Con and Ipsi, are shown in Figures 2A and 2B. EPOCCI and VEHCCI Con are shown as hatched gray and black bars, respectively (2A); EPOCCI and VEHCCI Ipsi are shown as solid gray and solid black bars, respectively (2B). * =p<0.05 relative to VEHCCI; &= p<0.05 relative to the corresponding SHAMVEH
In SHAMVEH rats, BDNF mRNA levels did not differ between ipsilateral and contralateral hippocampi at PID1, 2, 3, 7 nor 35. In CCI rats, BDNF mRNA levels behaved similarly except at PID 2, at which time BDNF mRNA decreased in ipsilateral relative to contralateral hippocampi in VEH-treated CCI rats (p=0.03) but did not differ from each other in EPO-treated CCI rats.
Injury decreased EPOCCI and VEHCCI BDNF protein in the ipsilateral hippocampus at PID35 (F (2, 15) = 26.1, adjusted p<0.0001: 0.05 ± 0.04, 0.1± 0.03 and 1± 0.17 pg /mg protein for EPOCCI, VEHCCI and SHAMVEH, respectively). Contralateral CCI hippocampal BDNF protein levels showed a trend in the same direction relative to SHAMVEH (F (2, 15) = 2.8, p=0.1) BDNF protein levels did not vary between groups on PID1 nor on PID3.
EPO preserved PID 35 hippocampal volume ipsilateral to injury
PID35 hippocampal volume ipsilateral to injury decreased in VEHCCI compared to SHAM (F (2, 11) =4.07, p<0.05: 78.6 ± 5% vs 99.8± 6 % of contralateral hippocampus). In contrast, PID35 hippocampal volume ipsilateral to injury did not differ between EPOCCI and SHAM (91.5 ± 5 and 99.8± 6 % of contralateral hippocampus), as shown in the graph (Figure 3B) with representative images shown in Fig 3A. EPO did not affect hemispheric volumes ipsilateral to injury (89 ± 3% and 88 ± 5% of contralateral volumes for EPOCCI and VEHCCI, respectively). CCI effects on hippocampal neuronal fraction, GFAP+ area and astrocyte number at PID 35 were not statistically different between groups. Representative images of GFAP staining in the PID35 CA3 hippocampus are shown in Supplementary Figure 2.
Discussion
This study contributes novel data on long-term effects of EPO administration in a rat model of pediatric traumatic brain injury. EPO improved object recognition at adulthood and preserved long-term hippocampal volume in our rat pup model of developmental CCI. We previously found that EPO exerted anti-apoptotic effects and increased neuronal fraction in the hippocampus early after injury. [9] We had hypothesized that EPO’s anti-apoptotic effects are associated with BDNF upregulation, as shown in various brain injury models. BDNF mRNA increased early after EPO administration, but in a limited and inconsistent fashion. In contrast to BNDF effects reported after EPO in adult rat TBI models, EPO did not blunt long-term BDNF protein loss in the injured hippocampus after developmental CCI. [13]
EPO improved object recognition at adulthood in rats after CCI at P17, as shown by NOR testing. Object recognition is a critical component of declarative memory. A study of nearly 300 children tested at 3 years or later after TBI showed that declarative memory impairments correlate with TBI severity, highlighting the importance of object recognition after developmental TBI. [14] Both the peri-rhinal cortex and hippocampus are needed for adequate memory acquisition and retrieval in the NOR test, but only the hippocampus is required to maintain strong novel object preference after long delays such as the 22 hours used in our study. [28] NOR results suggest, then, that EPO preserved hippocampal function at adulthood in rats after developmental CCI.
Imaging studies in adults and children after TBI commonly report decreased hippocampal volume and widespread white matter injury. Generally, declarative memory impairments correlate best with white matter volume loss and indices of white matter injury. [15, 29, 30] We thus plan to explore the effects of EPO on hippocampal connectivity and/or white matter integrity in our model, also prompted by the findings of van de Looij et al in a premature rat model of ischemic stroke. They reported that EPO preserved cortical function and white matter diffusivity indices without affecting histology. [31]
EPO preserved long-term hippocampal volume, but not hemispheric volume, in rats exposed to CCI at P17. CCI produces a progressive loss of brain tissue over time in rats to an extent proportional to severity of impact. [32] Indeed, near-obliteration of the hippocampus and extensive cortical volume loss late after CCI in our model obligated a reduction in injury severity for the present study, as noted in the Methods. Injury modification thus allowed assessment of hippocampal volume, but the lesser severity abrogated any differences in neuronal fraction and astrocytosis between CCI and SHAM rats and thus the ability to determine EPO effects on either after developmental CCI. In future studies, we will assess white matter integrity and connectivity using magnetic resonance for diffusion tensor imaging and tractography to determine if EPO’s effects on hippocampal volume result from decreased axonal injury and loss.
At the time of this writing, published data on long-term effects of EPO on the developing brain after experimental TBI are limited to one study. Robinson et al showed that EPO improved subtle gait impairments and reversed magnetic resonance indices of bilateral white matter and cortical abnormalities two to three weeks after CCI, but did not report on cognitive nor histologic outcomes. [33] Their model was comparable to ours but differed in a few aspects: the rat pups were younger at injury (P12 vs P17); impact depth was less (0.6 versus 1.5mm), and EPO doses used were greater in number (6 rather than 4) albeit the cumulative EPO dose was quite similar. The preponderance of publications on EPO and experimental injury to the developing brain involve neonatal hypoxia-ischemia models and support neuroprotection in this condition. [34] For example, EPO improved spatial working memory at PID 55 after experimental stroke in P10 rats. [21]
We had hypothesized that EPO would increase hippocampal BDNF mRNA as a putative mechanism for its anti-apoptotic effects in our model early after injury, particularly at PID1 and 2. However, EPO did not consistently increase BDNF mRNA. EPO increased BDNF mRNA in the contralateral hippocampus at PID3, with a trend in the same direction at PID1. At PID2, EPO blunted the CCI-associated decrease in BDNF mRNA levels in ipsilateral hippocampi relative to contralateral. In conclusion, BDNF upregulation does not appear to be a key mechanism for EPO’s protection against apoptosis in our model. However, these results do not refute the possibility that EPO increases BDNF transcription in a dose-dependent fashion. The doses used in studies showing BDNF upregulation after EPO dosing were four times higher than those in our model. [11, 12]
EPO’s efficacy in clinical adult TBI has recently come into question in spite of the abundant evidence supporting its use in adult animals and the suggestion of benefit found in small clinical studies. [35] Results of a large, multicenter randomized placebo-controlled trial (RCT) in Europe did not support neuroprotection from EPO after adult TBI. [36] Further, Robertson et al found that dosing EPO earlier after TBI, a strategy that improved outcomes in a small, single center RCT, was not neuroprotective in their large, two-center study. [37, 38]
EPO may have more benefit in the developing brain after injury than in the adult. EPO is important for normal neurodevelopment. [6] In addition, an abundance of published experimental and clinical literature on neonatal hypoxic ischemic injury and neurologic injury of prematurity supports EPO’s neuroprotection in these settings. [34, 39] A phase II clinical study in human infants of EPO administration after neonatal hypoxic ischemic injury showed safety of EPO and suggested efficacy, as demonstrated by magnetic resonance injury and long-term neurodevelopmental scores in these patients. [40, 41] Phase III studies building on this work are currently underway in the U. S. (NCT 02811263) and in Australia (ANZCTR.org.au).
Our findings are limited to 17-day old male rat pups. We focused on this age group to model the infant/toddler age group, a group at high risk for poor cognitive outcomes after TBI. [42] We also did not evaluate all possible pathways for EPO neuroprotection. Instead, we built on our earlier findings showing that EPO decreased apoptotic neuronal death to state our hypothesis for this study. Finally, our results are limited to a moderate injury. We chose to use a 1.5 mm depression, rather than 2mm, to enable histologic analysis by avoiding the near-total loss of hippocampal tissue we had observed with more severe injury.
In summary, EPO improved object recognition early and late after CCI, associated with preserved hippocampal volume. Exciting results in the neonatal experimental and clinical literature support benefits of EPO for the developing brain after injury. Further study of EPO after developmental TBI is warranted. In light of our present results and that of other research in this area, we plan to explore effects of EPO on white matter integrity after TBI in the developing brain.
Supplementary Material
Supplementary Figure 1
A) Schematic representation of the regions of interest (ROIs: CA1, CA3, DG) drawn for export to Imaris.
B) Representative image of colored spheres resulting from spots analysis of a CA3 ROI. Sytox + cells are shown in this schematic as blue spheres, NeuN +as red spheres; cells positive for both markers are shown as purple spheres, for computerized counting of all spheres present in the ROI.
Supplementary Figure 2 Representative image of Ipsilateral CA3 at higher magnification (20×). Astrocytes are stained using GFAP (green color).
Acknowledgments
Statement of Financial Support: funding for this study provided by the CHRCDA (NIH K12HD001410) and from the Divisions of Neonatology and Pediatric Critical Care Medicine, Department of Pediatrics, University of Utah, Salt Lake City UT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology. 2002;16:514–23. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]
- 3.Yeates KO, Armstrong K, Janusz J, Taylor HG, Wade S, Stancin T, et al. Long-term attention problems in children with traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44:574–84. doi: 10.1097/01.chi.0000159947.50523.64. [DOI] [PubMed] [Google Scholar]
- 4.Sola A, Wen TC, Hamrick SE, Ferriero DM. Potential for protection and repair following injury to the developing brain: a role for erythropoietin? Pediatr Res. 2005;57:110R–7R. doi: 10.1203/01.PDR.0000159571.50758.39. [DOI] [PubMed] [Google Scholar]
- 5.Dame C, Juul SE, Christensen RD. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate. 2001;79:228–35. doi: 10.1159/000047097. [DOI] [PubMed] [Google Scholar]
- 6.Alnaeeli M, Wang L, Piknova B, Rogers H, Li X, Noguchi CT. Erythropoietin in brain development and beyond. Anat Res Int. 2012;2012:953264. doi: 10.1155/2012/953264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Xiong Y, Mahmood A, Meng Y, Qu C, Schallert T, et al. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 2009;1294:153–64. doi: 10.1016/j.brainres.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning R, Xiong Y, Mahmood A, Zhang Y, Meng Y, Qu C, et al. Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Brain Res. 2011;1384:140–50. doi: 10.1016/j.brainres.2011.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schober ME, Requena DF, Block B, Davis LJ, Rodesch C, Casper TC, et al. Erythropoietin improved cognitive function and decreased hippocampal caspase activity in rat pups after traumatic brain injury. J Neurotrauma. 2014;31:358–69. doi: 10.1089/neu.2013.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76(Pt C):628–38. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Viviani B, Bartesaghi S, Corsini E, Villa P, Ghezzi P, Garau A, et al. Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem. 2005;93:412–21. doi: 10.1111/j.1471-4159.2005.03033.x. [DOI] [PubMed] [Google Scholar]
- 12.Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, et al. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177–87. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, et al. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107:392–7. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 14.Brookshire B, Levin HS, Song J, Zhang L. Components of executive function in typically developing and head-injured children. Dev Neuropsychol. 2004;25:61–83. doi: 10.1080/87565641.2004.9651922. [DOI] [PubMed] [Google Scholar]
- 15.Palacios EM, Sala-Llonch R, Junque C, Fernandez-Espejo D, Roig T, Tormos JM, et al. Long-term declarative memory deficits in diffuse TBI: correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex. 2013;49:646–57. doi: 10.1016/j.cortex.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–70. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Lieutaud T, Andrews PJ, Rhodes JK, Williamson R. Characterization of the pharmacokinetics of human recombinant erythropoietin in blood and brain when administered immediately after lateral fluid percussion brain injury and its pharmacodynamic effects on IL-1beta and MIP-2 in rats. J Neurotrauma. 2008;25:1179–85. doi: 10.1089/neu.2008.0591. [DOI] [PubMed] [Google Scholar]
- 18.Statler PA, McPherson RJ, Bauer LA, Kellert BA, Juul SE. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res. 2007;61:671–5. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- 19.Bittigau P, Sifringer M, Pohl D, Stadthaus D, Ishimaru M, Shimizu H, et al. Apoptotic neurodegeneration following trauma is markedly enhanced in the immature brain. Ann Neurol. 1999;45:724–35. doi: 10.1002/1531-8249(199906)45:6<724::aid-ana6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Meng Y, Xiong Y, Mahmood A, Zhang Y, Qu C, Chopp M. Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J Neurosurg. 2011;115:550–60. doi: 10.3171/2011.3.JNS101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31:403–11. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reger ML, Hovda DA, Giza CC. Ontogeny of Rat Recognition Memory measured by the novel object recognition task. Dev Psychobiol. 2009;51:672–8. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–11. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- 24.Osborne AL, Solowij N, Babic I, Huang XF, Weston-Green K. Improved Social Interaction, Recognition and Working Memory with Cannabidiol Treatment in a Prenatal Infection (poly I:C) Rat Model. Neuropsychopharmacology. 2017;42:1447–57. doi: 10.1038/npp.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos GaW, Charles The Rat Brain in Stereotaxic Coordinates. 2005 [Google Scholar]
- 26.Marvizon JC, Perez OA, Song B, Chen W, Bunnett NW, Grady EF, et al. Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors. Neuroscience. 2007;148:250–65. doi: 10.1016/j.neuroscience.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce DR, Hayar A, Williams DK, Light KE. Olivary climbing fiber alterations in PN40 rat cerebellum following postnatal ethanol exposure. Brain Res. 2011;1378:54–65. doi: 10.1016/j.brainres.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serra-Grabulosa JM, Junque C, Verger K, Salgado-Pineda P, Maneru C, Mercader JM. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J Neurol Neurosurg Psychiatry. 2005;76:129–31. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palacios EM, Fernandez-Espejo D, Junque C, Sanchez-Carrion R, Roig T, Tormos JM, et al. Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol. 2011;11:24. doi: 10.1186/1471-2377-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Looij Y, Chatagner A, Quairiaux C, Gruetter R, Huppi PS, Sizonenko SV. Multimodal assessment of long-term erythropoietin treatment after neonatal hypoxic-ischemic injury in rat brain. PLoS One. 2014;9:e95643. doi: 10.1371/journal.pone.0095643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 33.Robinson S, Winer JL, Berkner J, Chan LA, Denson JL, Maxwell JR, et al. Imaging and serum biomarkers reflecting the functional efficacy of extended erythropoietin treatment in rats following infantile traumatic brain injury. J Neurosurg Pediatr. 2016;17:739–55. doi: 10.3171/2015.10.PEDS15554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juul SE, Pet GC. Erythropoietin and Neonatal Neuroprotection. Clin Perinatol. 2015;42:469–81. doi: 10.1016/j.clp.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aloizos S, Evodia E, Gourgiotis S, Isaia EC, Seretis C, Baltopoulos GJ. Neuroprotective Effects of Erythropoietin in Patients with Severe Closed Brain Injury. Turk Neurosurg. 2015;25:552–8. doi: 10.5137/1019-5149.JTN.9685-14.4. [DOI] [PubMed] [Google Scholar]
- 36.Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, et al. Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet. 2015;386:2499–506. doi: 10.1016/S0140-6736(15)00386-4. [DOI] [PubMed] [Google Scholar]
- 37.Li ZM, Xiao YL, Zhu JX, Geng FY, Guo CJ, Chong ZL, et al. Recombinant human erythropoietin improves functional recovery in patients with severe traumatic brain injury: A randomized, double blind and controlled clinical trial. Clin Neurol Neurosurg. 2016;150:80–3. doi: 10.1016/j.clineuro.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312:36–47. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Wang Q, Xiang H, Xin Y, Chang M, Lu H. Neuroprotection with erythropoietin in preterm and/or low birth weight infants. J Clin Neurosci. 2014;21:1283–7. doi: 10.1016/j.jocn.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 40.Mulkey SB, Ramakrishnaiah RH, McKinstry RC, Chang T, Mathur AM, Mayock DE, et al. Erythropoietin and Brain Magnetic Resonance Imaging Findings in Hypoxic-Ischemic Encephalopathy: Volume of Acute Brain Injury and 1-Year Neurodevelopmental Outcome. J Pediatr. 2017;186:196–9. doi: 10.1016/j.jpeds.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 41.Wu YW, Mathur AM, Chang T, McKinstry RC, Mulkey SB, Mayock DE, et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics. 2016;137 doi: 10.1542/peds.2016-0191. [DOI] [PubMed] [Google Scholar]
- 42.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–82. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
A) Schematic representation of the regions of interest (ROIs: CA1, CA3, DG) drawn for export to Imaris.
B) Representative image of colored spheres resulting from spots analysis of a CA3 ROI. Sytox + cells are shown in this schematic as blue spheres, NeuN +as red spheres; cells positive for both markers are shown as purple spheres, for computerized counting of all spheres present in the ROI.
Supplementary Figure 2 Representative image of Ipsilateral CA3 at higher magnification (20×). Astrocytes are stained using GFAP (green color).


